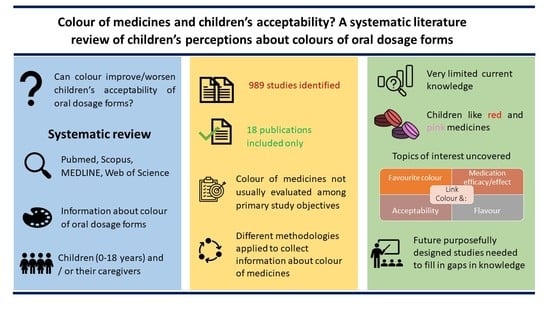
Colour of Medicines and Children’s Acceptability? A Systematic Literature Review of Children’s Perceptions about Colours of Oral Dosage Forms †
Abstract
1. Introduction
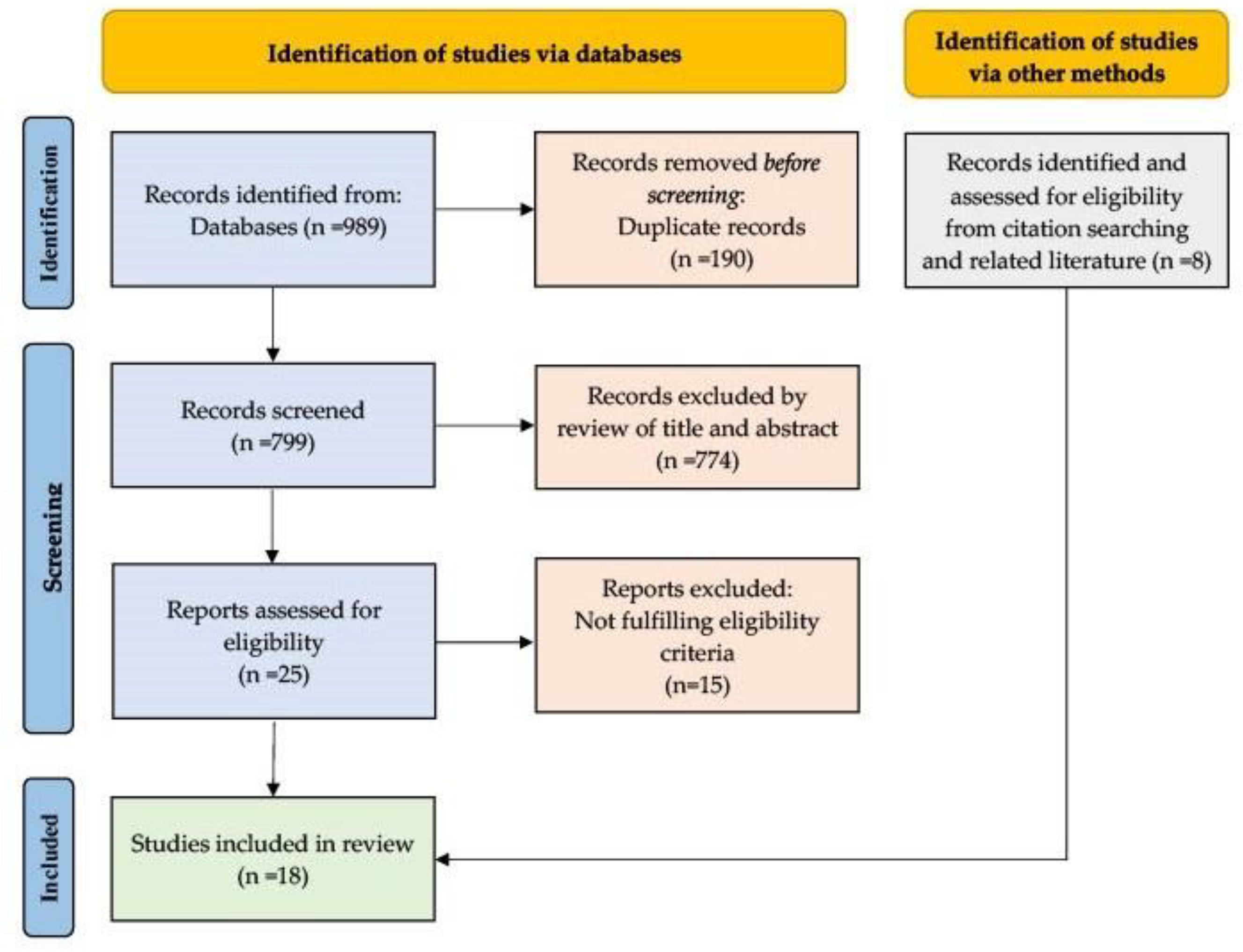
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction
3. Results
3.1. Generality about the Studies
3.2. Information about the Colour of Medicines
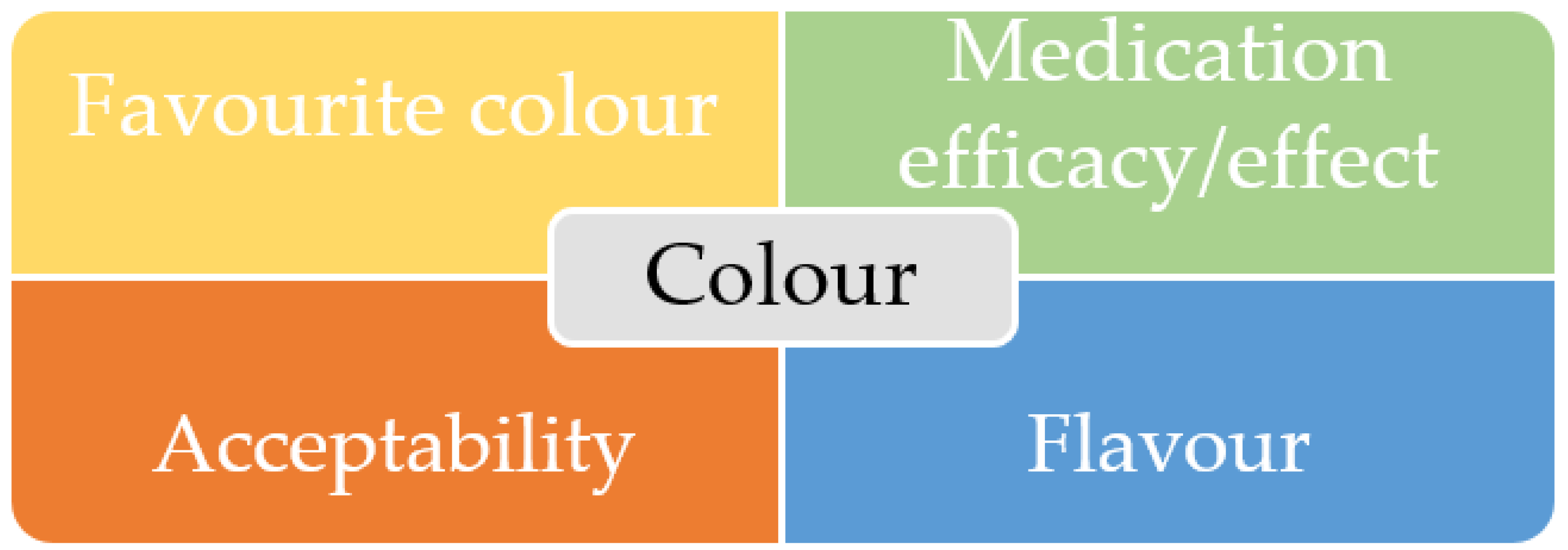
3.3. Assessment of the Most Favourite Colour
3.4. Relationship between Colour and Medication Efficacy/Effect
3.5. Effect of the Colour of Medicines on Acceptability
3.6. Colour and Its Relationship with Flavour
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Search Word | Results |
|---|---|---|
| PubMed | Search: (((colour OR color) AND (medicine *[Title/Abstract] OR dosage form *[Title/Abstract] OR drug *[Title/Abstract])) AND (child * [Title/Abstract] OR paediatric[Title/Abstract] OR pediatric * [Title/Abstract])) AND (accept * [Title/Abstract] OR perception[Title/Abstract] OR preference[Title/Abstract] OR favourite[Title/Abstract] OR compliance[Title/Abstract] OR adherence[Title/Abstract]) | 57 |
| Scopus | (ALL (colour OR color) AND TITLE-ABS-KEY (medicin * OR “dosage form” OR drug) AND TITLE-ABS-KEY (child OR paediatric OR pediatric *) AND TITLE-ABS-KEY (accept * OR perception OR preference OR favourite OR compliance OR adheren *)) | 712 |
| MEDLINE (Ovid) |
| 140 |
| Web of Science | All fields—colour OR color AND topic—child OR paediatric OR pediatric* AND topic—medicin* OR dosage form OR drug AND topic—accept* OR perception OR preference OR favourite OR favorite OR compliance OR adheren* | 80 |
| Total studies identified | 989 | |
References
- Gopikrishna, R.; Kumar, M. A Conceptual Study on Psychology of Colour in Marketing and Branding. Int. J. Econ. Res. 2015, 12, 501–505. [Google Scholar]
- Khattak, S.R.; Ali, H.; Khan, Y.; Shah, M. Color Psychology in Marketing. J. Bus. Tour. 2021, 4, 183–190. [Google Scholar] [CrossRef]
- Aslam, M.M. Are You Selling the Right Colour? A Cross-cultural Review of Colour as a Marketing Cue. J. Mark. Commun. 2006, 12, 15–30. [Google Scholar] [CrossRef]
- Schoneker, D.R. Coloring Agents for Use in Pharmaceuticals. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Informa Healthcare USA, Inc.: Richmond, VA, USA, 2007; p. 648. Available online: http://www.gmpua.com/Process/EncyclopediaPT.pdf (accessed on 11 May 2023).
- European Parliament, Directive 2009/35/EC of the European Parliament and of the Council of 23 April 2009 on the Colouring Matters Which May Be Added to Medicinal Products. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32009L0035 (accessed on 12 May 2023).
- Federal Food, Drug, and Cosmetic Act (FD&C Act), Food and Drug Administration. 2018. Available online: https://www.fda.gov/regulatory-information/federal-food-drug-and-cosmetic-act-fdc-act/fdc-act-chapter-vii-general-authority (accessed on 17 May 2023).
- Pérez-Ibarbia, L.; Majdanski, T.; Schubert, S.; Windhab, N.; Schubert, U.S. Safety and regulatory review of dyes commonly used as excipients in pharmaceutical and nutraceutical applications. Eur. J. Pharm. Sci. 2016, 93, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Morton, J. The Color of Medications Taking the Color of Medications Seriously. Available online: https://www.colormatters.com/color-symbolism/the-color-of-medications (accessed on 27 October 2022).
- Bright, H.R. Why are some tablets coloured? 2018. Available online: https://www.linkedin.com/pulse/why-some-tablets-coloured-heber-rew-bright/ (accessed on 27 October 2022).
- Stegemann, S. Colored capsules—A contribution to drug safety. Pharm. Ind. 2005, 67, 1088–1095. [Google Scholar]
- Branch, E. Oral Solid Dose and the Psychology of Appearance. 2017. Available online: https://www.contractpharma.com/issues/2017-03-01/view_features/oral-solid-dose-and-the-psychology-of-appearance/53366 (accessed on 27 October 2022).
- Spence, C. The multisensory design of pharmaceuticals and their packaging. Food Qual Prefer. 2021, 91, 104200. [Google Scholar] [CrossRef]
- de Craen, A.J.M.; Roos, P.J.; de Vries, A.L.; Kleijnen, J. Effect of colour of drugs: Systematic review of perceived effect of drugs and of their effectiveness. BMJ 1996, 313, 1624–1626. [Google Scholar] [CrossRef]
- Tao, D.; Wang, T.; Wang, T.; Qu, X. Influence of drug colour on perceived drug effects and efficacy. Ergonomics 2018, 61, 284–294. [Google Scholar] [CrossRef]
- Bhakti, P.H. An Overview of Color Additives in Drug Products—Regulation and Enforcement, Office of Cosmetics and Colors Center for Food Safety and Applied Nutrition Food and Drug Administration. 2023. Available online: https://www.fda.gov/media/165718/download (accessed on 20 April 2023).
- Marshall, D.; Stuart, M.; Bell, R. Examining the relationship between product package colour and product selection in preschoolers. Food Qual. Prefer. 2006, 17, 615–621. [Google Scholar] [CrossRef]
- Pope, D.J.; Butler, H.; Qualter, P. Emotional Understanding and Color-Emotion Associations in Children Aged 7–8 Years. Child Dev. Res. 2012, 2012, 975670. [Google Scholar] [CrossRef]
- Zentner, M.R. Preferences for colours and colour-emotion combinations in early childhood. Dev. Sci. 2001, 4, 389–398. [Google Scholar] [CrossRef]
- Ogba, I.; Johnson, R. How packaging affects the product preferences of children and the buyer behaviour of their parents in the food industry. Young Consum. 2010, 11, 77–89. [Google Scholar] [CrossRef]
- Boyatzis, C.J.; Varghese, R. Children’s Emotional Associations with Colors. J. Genet. Psychol. 1994, 155, 77–85. [Google Scholar] [CrossRef]
- Pires, C.; Agante, L. Encouraging children to eat more healthily: The influence of packaging. J. Consum. Behav. 2011, 10, 161–168. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Guideline on Pharmaceutical Development of Medicines for Paediatric Use (EMA/CHMP/QWP/805880/2012 Rev. 2). 2012. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 12 December 2020).
- European Medicines Agency (EMA). Reflection paper: Formulation of Choice for the Paediatric Population (EMEA/CHMP/PEG/194810/2005). 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 9 June 2020).
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Smith, D.; Booth, A. Beyond PICO: The SPIDER tool for qualitative evidence synthesis. Qual. Health Res. 2012, 22, 1435–1443. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme, CASP Qualitative Checklist. 2018. Available online: https://casp-uk.net/images/checklist/documents/CASP-Qualitative-Studies-Checklist/CASP-Qualitative-Checklist-2018.pdf (accessed on 27 October 2022).
- Butler, A.; Hall, H.; Copnell, B. A Guide to Writing a Qualitative Systematic Review Protocol to Enhance Evidence-Based Practice in Nursing and Health Care. Worldviews Evid. Based Nurs. 2016, 13, 241–249. [Google Scholar] [CrossRef]
- Jolly, H.; Forrester, T.R.W. Accidental poisoning in children: An experimental approach to prevention of poisoning by tablets. Lancet 1958, 271, 1308–1309. [Google Scholar] [CrossRef]
- Dyke, T.; Kitembing, R.J. Mothers’ Preference for the Colour of Oral Medication for Their Children in Papua New Guinea. Trop. Doct. 1996, 26, 184–185. [Google Scholar] [CrossRef]
- Powers, J.L. Properties of azithromycin that enhance the potential for compliance in children with upper respiratory tract infections. Pediatr. Infect. Dis. J. 1996, 15, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N. Drug compliance of children and infants with oral antibiotics for pediatric use. Pediatr. Int. 1997, 39, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kairuz, T.; Jordaan, I. Children’s Preferences for Medicines-a Pilot Study in the Eastern Province Region of South Africa. J. Appl. Ther. Res. 2004, 5, 33–37. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-77951551676&origin=resultslist&sort=plf-f&src=s&sid=3d2744106ccb3eb1ee5d138ae77974f4&sot=b&sdt=b&s=TITLE-ABS-KEY%28children+compliance+colour+medicine%29&sl=55&sessionSearchId=3d2744106ccb3eb1ee5d138ae7 (accessed on 9 August 2022).
- Brieger, W.R.; Salami, K.K.; Oshiname, F.O. Perceptions of drug color among drug sellers and consumers in rural southwestern Nigeria. Res. Soc. Adm. Pharm. 2007, 3, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Sammons, H.M.; Fakis, A.; Conroy, S. A prospective study to assess the palatability of analgesic medicines in children. J. Adv. Nurs. 2013, 69, 655–663. [Google Scholar] [CrossRef]
- Whatley, B.; Williams, S.E.; Gard, P.R.; MacAdam, A.B. Healthy children’s identification and risk perception of medicines in England. Res. Soc. Adm. Pharm. 2012, 8, 478–483. [Google Scholar] [CrossRef]
- Dawood, O.T.; Ibrahim, M.I.M.; Abdullah, A.C. Children’s knowledge and beliefs about medicines. J. Child Health Care 2015, 19, 73–83. [Google Scholar] [CrossRef]
- Bryson, S.P. Patient-centred, administration friendly medicines for children—An evaluation of children’s preferences and how they impact medication adherence. Int. J. Pharm. 2014, 469, 257–259. [Google Scholar] [CrossRef]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef]
- Alyami, H.; Dahmash, E.; Alyami, F.; Dahmash, D.; Huynh, C.; Terry, D.; Mohammed, A.R. Dosage form preference consultation study in children and young adults: Paving the way for patient-centred and patient-informed dosage form development. Eur. J. Hosp. Pharm. 2017, 24, 332–337. [Google Scholar] [CrossRef]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Syofyan, S.; Dachriyanus, D.; Masrul, M.; Rasyid, R. Children’s Perception and Belief about Medicines: Effectiveness and Its Autonomy. Open Access Maced J. Med. Sci. 2019, 7, 2556–2562. [Google Scholar] [CrossRef]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef]
- Pereira, M.; Silva, F.C.; Simões, S.; Ribeiro, H.M.; Almeida, A.J.; Marto, J. Innovative, Sugar-Free Oral Hydrogel as a Co-administrative Vehicle for Pediatrics: A Strategy to Enhance Patient Compliance. AAPS PharmSciTech 2022, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Herziger, B.; Jeschke, S.; Müller, R.M.; Neininger, M.P.; Bertsche, T.; Bertsche, A. Drug-handling problems and expectations of the ideal pediatric drug—Reported by children and their parents. Eur. J. Pediatr. 2022, 181, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Alyami, H.; Koner, J.; Huynh, C.; Terry, D.; Mohammed, A.R. Current opinions and recommendations of paediatric healthcare professionals—The importance of tablets: Emerging orally disintegrating versus traditional tablets. PLoS ONE 2018, 13, e0193292. [Google Scholar] [CrossRef]
- Mennella, J.A.; Bobowski, N.K. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol. Behav. 2015, 152, 502–507. [Google Scholar] [CrossRef]
- Liem, D.G.; Mennella, J.A. Sweet and sour preferences during childhood: Role of early experiences. Dev. Psychobiol. 2002, 41, 388–395. [Google Scholar] [CrossRef]
- Spence, C.; Levitan, C.A. Explaining Crossmodal Correspondences Between Colours and Tastes. Iperception 2021, 12, 204166952110182. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-Centered Pharmaceutical Design to Improve Acceptability of Medicines: Similarities and Differences in Paediatric and Geriatric Populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; More, A.T. Some aesthetic considerations for over the-counter (OTC) pharmaceutical products. Int. J. Biotechnol. 2010, 11, 267. [Google Scholar] [CrossRef]
- Mayor, S. Changing color of antiepileptic pills raises risk of patients’ non-adherence, study shows. BMJ 2013, 346, f19. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Misono, A.S.; Shrank, W.H.; Greene, J.A.; Doherty, M.; Avorn, J.; Choudhry, N.K. Variations in Pill Appearance of Antiepileptic Drugs and the Risk of Nonadherence. JAMA Intern. Med. 2013, 173, 202. [Google Scholar] [CrossRef]
- Amawi, R.M.; Murdoch, M.J. Understanding Color Associations and Their Effects on Expectations of Drugs’ Efficacies. Pharmacy 2022, 10, 82. [Google Scholar] [CrossRef]
- Jadva, V.; Hines, M.; Golombok, S. Infants’ Preferences for Toys, Colors, and Shapes: Sex Differences and Similarities. Arch. Sex. Behav. 2010, 39, 1261–1273. [Google Scholar] [CrossRef]
- Ezan, P.; Pantin-Sohier, G.; Lancelot-Miltgen, C. Colour of food as a vector for children’s well-being. Int. J. Retail. Distrib. Manag. 2019, 47, 659–679. [Google Scholar] [CrossRef]
- Wan, X.; Woods, A.T.; van den Bosch, J.J.F.; McKenzie, K.J.; Velasco, C.; Spence, C. Cross-cultural differences in crossmodal correspondences between basic tastes and visual features. Front. Psychol. 2014, 5, 1365. [Google Scholar] [CrossRef]
- Strickley, R.G.; Iwata, Q.; Wu, S.; Dahl, T.C. Pediatric Drugs—A Review of Commercially Available Oral Formulations. J. Pharm. Sci. 2008, 97, 1731–1774. [Google Scholar] [CrossRef]
- Thompson, A.; Reader, S.; Field, E.; Shephard, A. Open-Label Taste-Testing Study to Evaluate the Acceptability of Both Strawberry-Flavored and Orange-Flavored Amylmetacresol/2,4-Dichlorobenzyl Alcohol Throat Lozenges in Healthy Children. Drugs R D 2013, 13, 101–107. [Google Scholar] [CrossRef]
- Tolia, V.; Han, C.; North, J.D.; Amer, F. Taste Comparisons for Lansoprazole Strawberry-Flavoured Delayed-Release Orally Disintegrating Tablet and Ranitidine Peppermint-Flavoured Syrup in Children. Clin. Drug Investig. 2005, 25, 285–292. [Google Scholar] [CrossRef]
- Code of Federal Regulations, 21 CFR Part 70—Color Additives. 1977. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-70 (accessed on 23 January 2023).
- Barrows, J.N.; Lipman, A.L.; Bailey, C.J. Color Additives History. 2017. Available online: https://www.fda.gov/industry/color-additives/color-additives-history#authors (accessed on 23 January 2023).
- Saito, J.; Agrawal, A.; Patravale, V.; Pandya, A.; Orubu, S.; Zhao, M.; Andrews, G.P.; Petit-Turcotte, C.; Landry, H.; Croker, A.; et al. The Current States, Challenges, Ongoing Efforts, and Future Perspectives of Pharmaceutical Excipients in Pediatric Patients in Each Country and Region. Children 2022, 9, 453. [Google Scholar] [CrossRef]
- Swerlick, R.A.; Campbell, C.F. Medication dyes as a source of drug allergy. J. Drugs Dermatol. 2013, 12, 99–102. [Google Scholar] [PubMed]
- Bahramsoltani, R.; Rahimi, R.; Farzaei, M.H. Pharmacokinetic interactions of curcuminoids with conventional drugs: A review. J. Ethnopharmacol. 2017, 209, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Committee on Specifications for Pharmaceutical Preparations Forty-Sixth Report, WHO Technical Report Series No. 970. 2012. Available online: https://cdn.who.int/media/docs/default-source/medicines/norms-and-standards/guidelines/trs970/annex5trs-970.pdf?sfvrsn=699cdb68_6&download=true (accessed on 21 December 2022).
| Scoring System | Score | Paper Quality |
|---|---|---|
| Yes = 1 | 9–10 | High quality |
| Can’t tell = 0.5 | 7.5–9 | Moderate quality |
| No = 0 | <7.5 | Low quality |
| Year & [Ref] | Study Design | Colour Assessed as Primary aim (Yes/NO) | Methodology a | Topic around Colour | Dosage form and Medication Type b | Country | Age (y) | Sample Size | Health Status | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| 1958 [29] | Observational study | Yes | Ten lactose tablets of different colours are showed to the children. Observation of which colours children picked first. | Favourite colour | Tablets NA | United Kingdom | 1–8 | 613 | Inpatients and outpatients | Children like bright colours. Colour ranking order: magenta, pink, blue, orange, brown, yellow, white, green, black, and wine. |
| 1996 [30] | Survey Study | Yes | Bottles with liquids of 5 different colours showed to the mothers. They had to indicate their first and second preferences. | Favourite colour | Liquid formulations NA | Papua New Guinea | NA | 62 (mothers) | Patients attending health clinic | Red most popular colour, followed by yellow. Blue, brown, and white were not popular. |
| 1996 [31] | Acceptability/ preference questionnaire study | Yes | Acceptability/preference questionnaire. | Effect on acceptability | Liquid suspensions Rx—antibiotics | Arizona, USA | 4–12 | 769 | Healthy children | After taking medications, children were asked which had the preferable taste and colour. No mention about the colour of the medicines tested. |
| 1997 [32] | Observational study | No | Not clear how information about colour was collected. | Favourite colour | Granules, dry syrup Rx—antibiotics | Japan | 5–8 | NA | NA | Children and infants prefer orange and pink. Potential reasons: colours agreeable to children, or children associate them with the fruits’ colours, or colours used in preparations taken before. |
| 2004 [33] | Semi structured interview | Yes | A list of 6 non-medicated liquids in different colours was shown to the children. They had to pick their favourite. | Favourite colour | NA OTC | South Africa | 5–6 | 25 | Healthy children | Red was the most popular colour and bubble-gum the favourite flavour. No direct association between colour and flavour observed. Colour ranking order: red, green, white, blue, brown, yellow. |
| 2007 [34] | Focus group discussion | Yes | Open questions about colour preferences/avoidance. | Relationship with efficacy/effect | Tablets Rx—antimalarial | Nigeria, Africa | <5 | 4 (parents) | NA | Respondents associated medicines and their colour with their effects and purpose. Colour for antimalarial drugs: yellow was accepted, blue not accepted. |
| 2012 [35] | Prospective observational study | No | Open question: “If you had to choose what colour you would like your medicine to be which one would it be?” | Favourite colour | Liquid formulations OTC—analgesic | United Kingdom | 5–16 | 159 | Inpatients | Children prefer brightly coloured medicines. Colour ranking order: pink, red, yellow, and blue. Colour is sex dependent: girls significantly prefer pink and boys blue. Preferred flavours: strawberry and banana. |
| 2012 [36] | Questionnaire study | No | Photographed pictures of medicines showed to children. | Relationship with efficacy/effect | Solid dosage forms: tablets and capsules NA | United Kingdom | 4–11 | 182 | Healthy children | Majority of children correctly identified the bicolored capsules as medicines compared to the white or pink tablets. Most of the children identified the white tablets as medicines when the blister pack was next to them. Pink tablets less often identified as medicines: 53% of the children identified the pink tablets as sweets compared with just 3% of the bicolored capsules as sweets. |
| 2013 [37] | Cross-sectional survey | No | Close question: “does the colour of medicines affect their action?” Yes—Sometimes—No relation to the colour of medicine. | Relationship with efficacy/effect | NA | Malaysia | 11–12 | 842 (children) 842 (parents) | Healthy children | 57.3% (n = 482) of the children think the efficacy of medicines is not related to their colours. |
| 2014 [38] | Interview and questionnaire study used to construct a Medication Adherence Prediction Tool (questionnaire) | No | Open and closed question: “Are there any colours of medicines you like?” Yes/No, “which one(s)?” AND “Are there any colours of medicines you do not like?” Yes/No, “which one(s)?” | Effect on acceptability | NA | United Kingdom | 3–11 | 70 | Children with chronic condition | Evidence of the child’s expression of a colour dislike indicative of a potential aversive response to medications of the same colour. This is a contributing factor in acceptability and ultimately adherence. |
| 2015 [39] | Semi-structured face-to-face interviews | No | Anecdotal information about colour reported among the obstacles to medicines administration. | Effect on acceptability | Various (liquids, tablets or capsules, granules, soluble, tablets and melts) NA | United Kingdom | 12–18 | 57 (children) 221 (parents) | Children with chronic condition | An unfavourable colour (“alarming”, off-putting, unappealing, and colourless) associated with 2% (11/542) of medicines prescribed. |
| 2016 [40] | International, multi-site, cross-sectional questionnaire | No | Question not specified. Presumably children had to select their favourite colour from a list of 6 colours. | Favourite colour | Various (liquids, tablets, capsules and ODTs) NA | United Kingdom, Saudi Arabia and Jordan | 6–18 | 104 | Healthy children, previous experience taking medications | Pink was the preferred colour for ODTs followed by white, blue, yellow, orange, and purple. Flavour: strawberry was the most preferred, while orange was the least preferred. Gender and age groups showed different colour preferences for ODTs: girls preferred pink ODTs while boys preferred white. Ranking list of flavours: strawberry, orange, cherry, vanilla, mint, lemon, chocolate, and other. |
| 2016 [41] | Age-adapted questionnaire | No | Not specified. Presumably children had to rank aesthetic attributes. | Effect on acceptability | Solid dosage forms: tablets, capsules, chewable tablets, orodispersible tablets, multiparticulates, and mini-tablets NA | United Kingdom and Canada | 6–18 | 590 | Healthy children | Colour was ranked as the least important attribute, 70.7% rated it as not important. 48.8% of children showed no specific preference for colour, 25.6% preferred white medicines, and 25.6% preferred coloured medicines. |
| 2019 [42] | Single-centre, prospective crossover experimental study | No | Colour and flavour assessed together. Use of a 5-point hedonic scale to rate each physical 3D tablet. (5 = excellent to 1 = inacceptable). | Favourite colour | 3D printed 3D printed tablet RX—metabolic disease | Spain | 3–16 | 4 | Patients with chronic condition | Six types of formulations tested (6 flavour/colour combinations): strawberry-red, orange-orange, lemon-yellow, raspberry-light blue, banana-light green, and coconut-black. Preferred combination (but not statistically significant): orange-orange. Worst rated combination: coconut-black. |
| 2019 [43] | Cross-sectional questionnaire | No | Closed question: “Medicine colour (coloured or white) affects medicine drug efficacy?” Yes—No—I don’t know. | Relationship with efficacy/effect | NA NA | Indonesia | 10–14 | 503 | Healthy children | Medicine colour (coloured or white) affects a drug efficacy: yes 22.5%, no 38.2%, don’t know 39.4%. |
| 2020 [44] | A visual preference semi-structured survey and a short electronic questionnaire | No | Anecdotal records about colour collected from an open question where participants could add their comments. | Effect on acceptability | 3D printed tablets NA | UK | 4–11 | 368 | Healthy children | The majority stated that the 3D printed tablets had a ‘nice colour’. Appearance was closely followed by perceived taste: the 3D printed tablets looked like a gummy/sweet or that they would taste like a lemon/orange. Children show higher preference towards brightly coloured medicines. |
| 2022 [45] | Pre-formulation study with survey about children’s preferences for taste and colour | No | Survey to determine flavour and colour preferences. List of 7 colours used. | Favourite colour | Hydrogel vehicle to improve oral administration of solid dosage forms NA | Portugal | <12 | 157 | Healthy children | Colours more selected: red and pink. Blue was also chosen as one of the favourite colours. Children less likely to choose brown, green, orange, and yellow. Most girls chose pink, most boys preferred green medicines. Strong liking for sweet flavours. Flavour ranking: strawberry, vanilla, caramel, grape, banana, mint, and orange. |
| 2022 [46] | Prospective observational study | No | Colour was assessed indirectly in a questionnaire, and it was one of the characteristics listed that participants had to rank. | Favourite colour | Liquid and solid NA | Germany | 6–17 | 103 | Patients with chronic condition | Red colour was the most mentioned by both parents and children while describing their ideal medicine: ‘bright red’ tablet (child), pink or red liquid (parent), colourful (parent). |
| No. of Studies | Country | Continent |
|---|---|---|
| 1 | Germany | Europe |
| 1 | Portugal | |
| 1 | Spain | |
| 8 | United Kingdom | |
| 1 | Indonesia | Asia |
| 1 | Japan | |
| 1 | Jordan | |
| 1 | Malaysia | |
| 1 | Saudi Arabia | |
| 1 | Canada | North America |
| 1 | United States | |
| 1 | Nigeria | Africa |
| 1 | South Africa | |
| 1 | Papua New Guinea | Oceania |
| Methodology | No. of Studies | Ref |
|---|---|---|
| Evaluation of coloured medicines or photographed medicines | 4 | [29,30,33,36] |
| Hedonic scales/acceptability preference questionnaires | 2 | [31,47] |
| Open or closed questions or mix of the two | 5 | [34,35,37,38,43] |
| List of colours to choose from | 1 | [45] |
| Information not available | 6 | [32,39,41,44,46,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandrini, E.; Gonakova, M.; Batchelor, H.; Gizurarson, S.; Iurian, S.; Klein, S.; Schaufelberger, D.; Turner, R.; Walsh, J.; Tuleu, C. Colour of Medicines and Children’s Acceptability? A Systematic Literature Review of Children’s Perceptions about Colours of Oral Dosage Forms. Pharmaceutics 2023, 15, 1992. https://doi.org/10.3390/pharmaceutics15071992
Alessandrini E, Gonakova M, Batchelor H, Gizurarson S, Iurian S, Klein S, Schaufelberger D, Turner R, Walsh J, Tuleu C. Colour of Medicines and Children’s Acceptability? A Systematic Literature Review of Children’s Perceptions about Colours of Oral Dosage Forms. Pharmaceutics. 2023; 15(7):1992. https://doi.org/10.3390/pharmaceutics15071992
Chicago/Turabian StyleAlessandrini, Elisa, Milena Gonakova, Hannah Batchelor, Sveinbjorn Gizurarson, Sonia Iurian, Sandra Klein, Daniel Schaufelberger, Roy Turner, Jennifer Walsh, and Catherine Tuleu. 2023. "Colour of Medicines and Children’s Acceptability? A Systematic Literature Review of Children’s Perceptions about Colours of Oral Dosage Forms" Pharmaceutics 15, no. 7: 1992. https://doi.org/10.3390/pharmaceutics15071992
APA StyleAlessandrini, E., Gonakova, M., Batchelor, H., Gizurarson, S., Iurian, S., Klein, S., Schaufelberger, D., Turner, R., Walsh, J., & Tuleu, C. (2023). Colour of Medicines and Children’s Acceptability? A Systematic Literature Review of Children’s Perceptions about Colours of Oral Dosage Forms. Pharmaceutics, 15(7), 1992. https://doi.org/10.3390/pharmaceutics15071992