Polymeric Nanoparticles’ Accumulation in Atopic Dermatitis: Clinical Comparison between Healthy, Non-Lesional, and Lesional Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Fluorescent PLGA Nanoparticles
2.2.2. Characterization of the Nanoparticles
2.2.3. Clinical Study
Selection Criteria of Volunteers and Patients
Application of the Fluorescent Nanoparticles
Examination of the Skin Using Confocal Laser Scanning Microscopy (CLSM)
Image Analysis
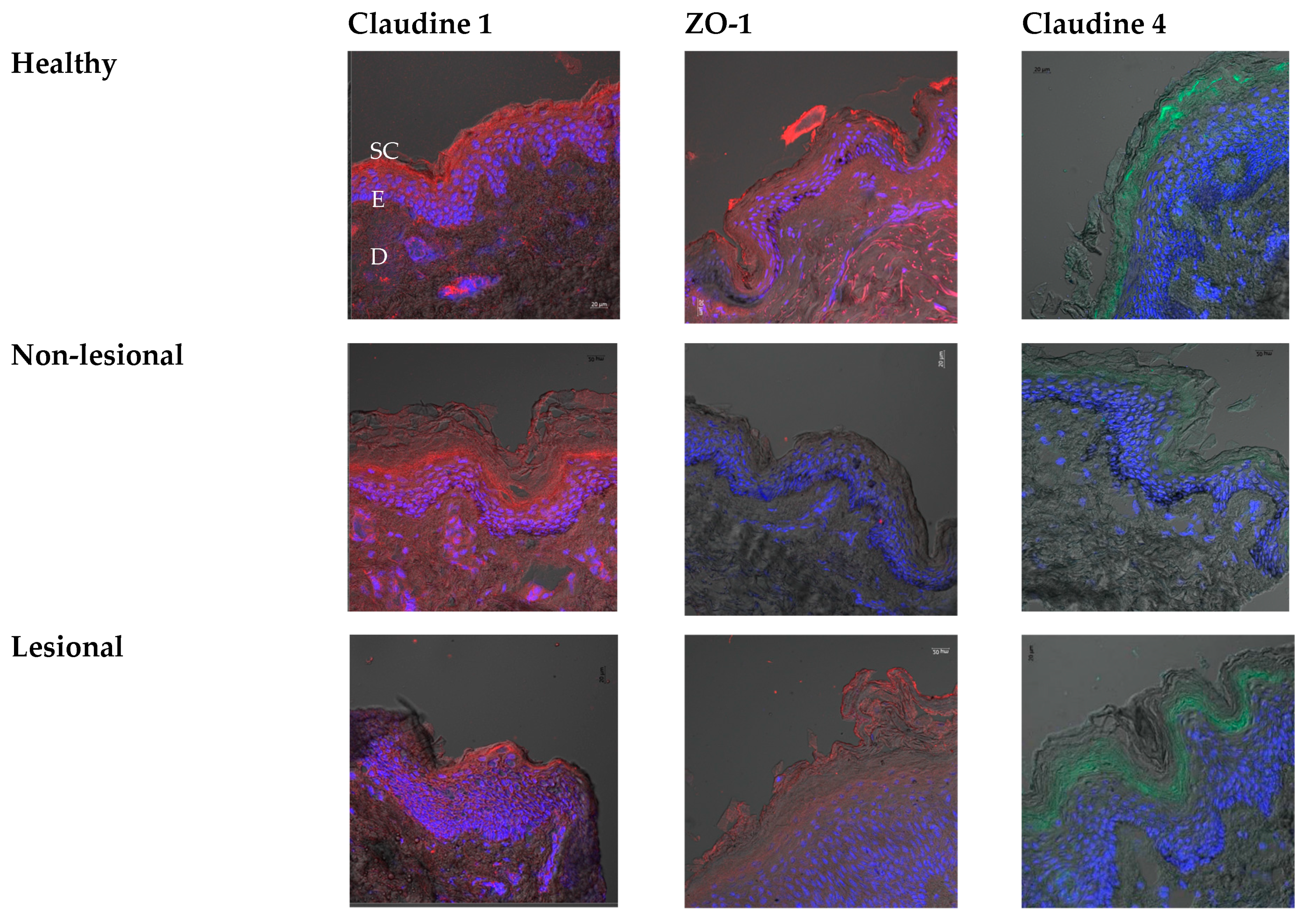
Immunohistochemistry
Statistical Analysis
2.2.4. Application of the Nanoparticles on Porcine Model of Atopic Dermatitis
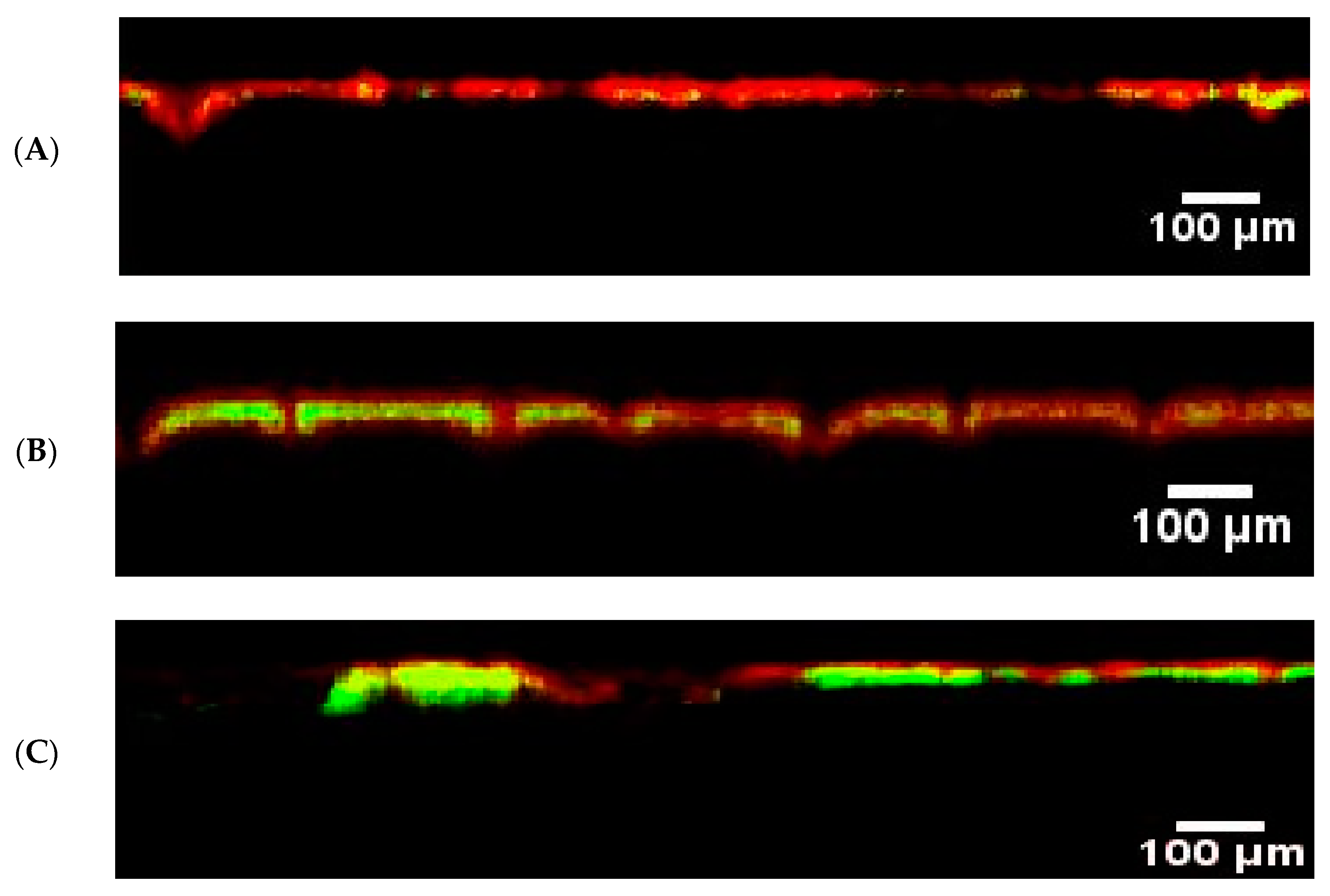
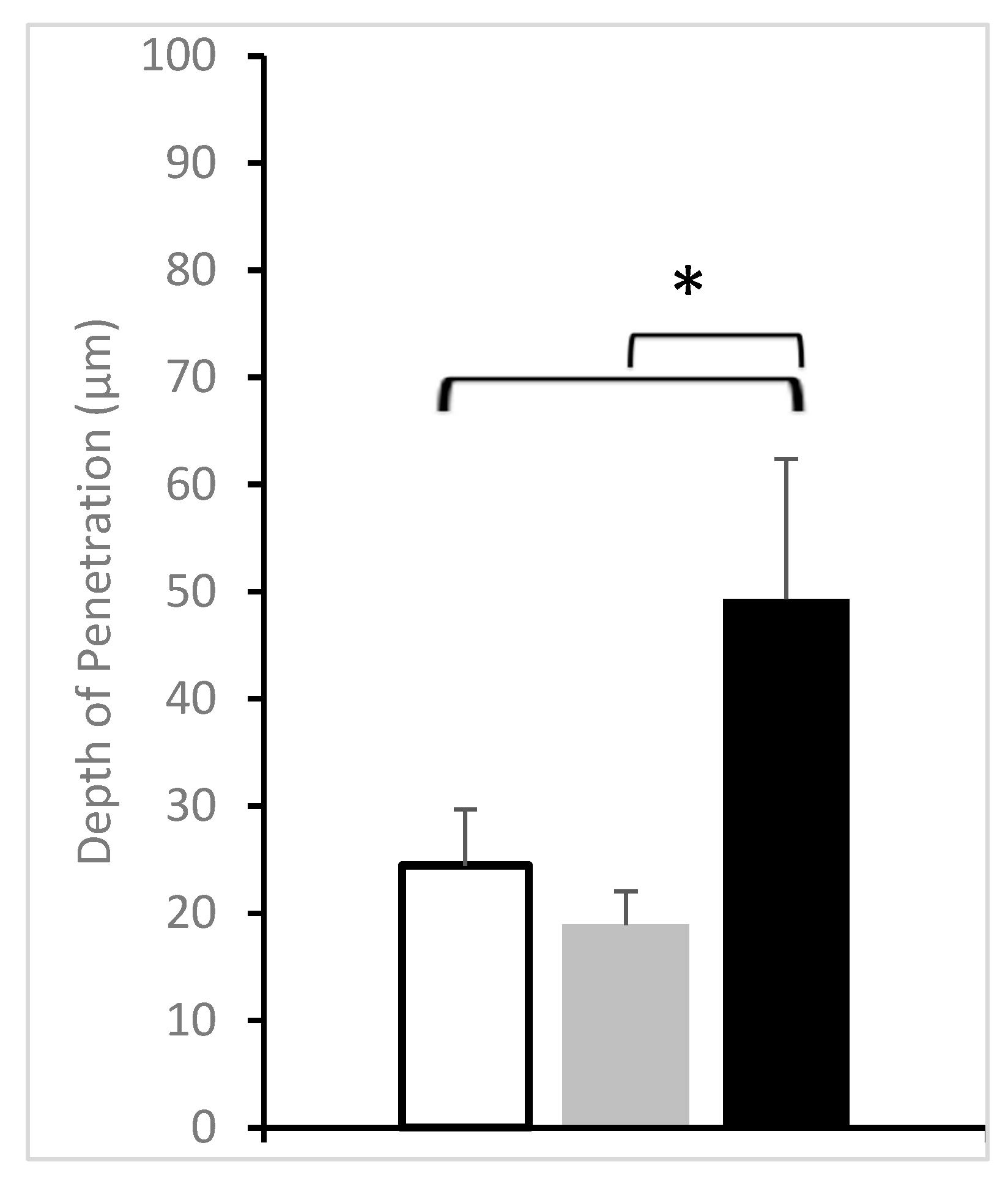
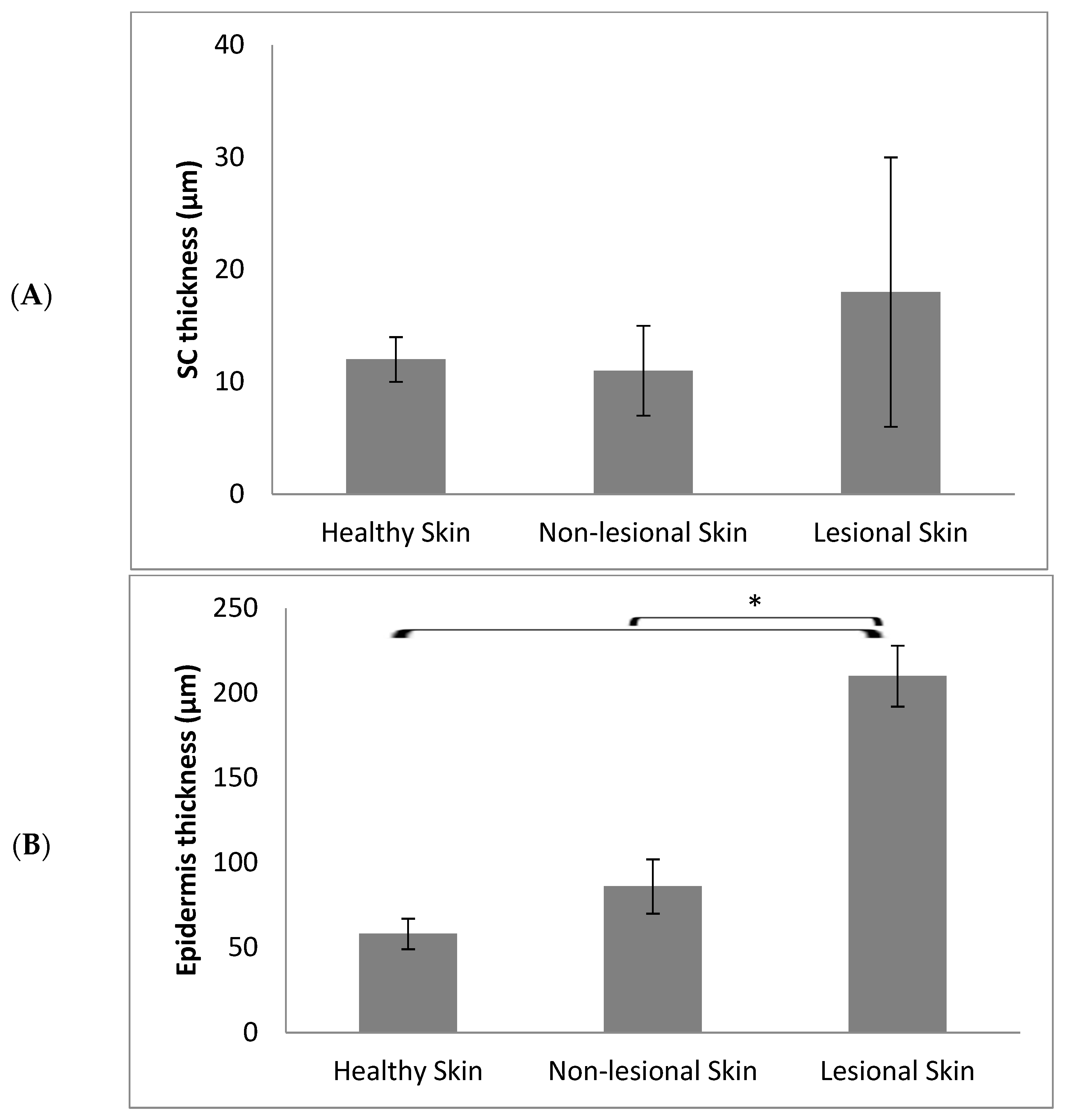
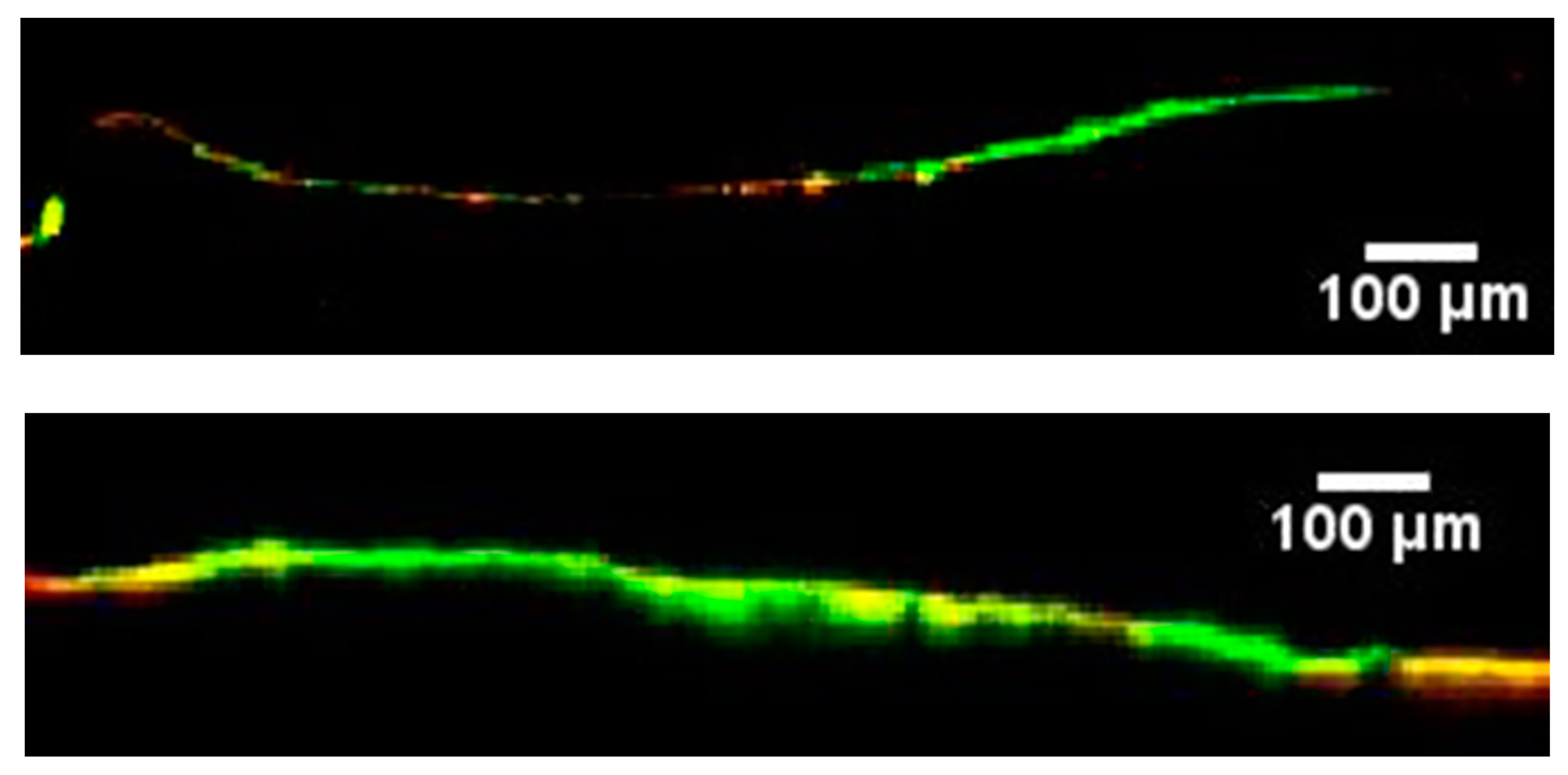
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damiani, G.; Eggenhöffner, R.; Pigatto, P.D.M.; Bragazzi, N.L. Nanotechnology Meets Atopic Dermatitis: Current Solutions, Challenges and Future Prospects. Insights and Implications from a Systematic Review of the Literature. Bioact. Mater. 2019, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. In Management of Atopic Dermatitis: Methods and Challenges; Fortson, E.A., Feldman, S.R., Strowd, L.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–37. ISBN 978-3-319-64804-0. [Google Scholar]
- Abdel-Mottaleb, M.M.A. Nanoparticles for Treatment of Atopic Dermatitis. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–175. ISBN 978-0-12-802926-8. [Google Scholar]
- Han, H.; Roan, F.; Ziegler, S.F. The Atopic March: Current Insights into Skin Barrier Dysfunction and Epithelial Cell-Derived Cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine Strategies for Targeting Skin Inflammation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of Care for the Management of Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Zhang, K.; Li, Z.; Zhang, H.-Y.; Guo, T.; Li, Y.-Y.; Zhao, J.-H.; Feng, N.-P. DOC-LS, a new liposome for dermal delivery, and its endocytosis by HaCaT and CCC-ESF-1 cells. IET Nanobiotechnol. 2018, 12, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Abuelella, K.E.; Abd-Allah, H.; Soliman, S.M.; Abdel-Mottaleb, M.M.A. Skin Targeting by Chitosan/Hyaluronate Hybrid Nanoparticles for the Management of Irritant Contact Dermatitis: In Vivo Therapeutic Efficiency in Mouse-Ear Dermatitis Model. Int. J. Biol. Macromol. 2023, 232, 123458. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Abd-Allah, H.; El-Gogary, R.I.; Nasr, M. Versatile Hyaluronic Acid Nanoparticles for Improved Drug Delivery. In Drug Delivery Aspects; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. ISBN 978-0-12-821222-6. [Google Scholar]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tacrolimus-Loaded Chitosan Nanoparticles for Enhanced Skin Deposition and Management of Plaque Psoriasis. Carbohydr. Polym. 2021, 268, 118238. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Moulari, B.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Nanoparticles Enhance Therapeutic Outcome in Inflamed Skin Therapy. Eur. J. Pharm. Biopharm. 2012, 82, 151–157. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Moulari, B.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Surface-Charge-Dependent Nanoparticles Accumulation in Inflamed Skin. J. Pharm. Sci. 2012, 101, 4231–4239. [Google Scholar] [CrossRef]
- Try, C.; Moulari, B.; Béduneau, A.; Fantini, O.; Pin, D.; Pellequer, Y.; Lamprecht, A. Size Dependent Skin Penetration of Nanoparticles in Murine and Porcine Dermatitis Models. Eur. J. Pharm. Biopharm. 2016, 100, 101–108. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Abdel-Mottaleb, M.M.A.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. A Nanoparticle-Based Approach to Improve the Outcome of Cancer Active Immunotherapy with Lipopolysaccharides. Drug Deliv. 2018, 25, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Abdel-Mottaleb, M.M.A.; El-assal, M.; Sammour, O. Novel Biocompatible Essential Oil-Based Lipid Nanocapsules with Antifungal Properties. J. Drug Deliv. Sci. Technol. 2020, 56, 101605. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Lamprecht, A. In Vivo Skin Penetration of Macromolecules in Irritant Contact Dermatitis. Int. J. Pharm. 2016, 515, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.; Guy, R.; Fessi, H. Skin Penetration and Distribution of Polymeric Nanoparticles. J. Control Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Arima, K.; Ohta, S.; Takagi, A.; Shiraishi, H.; Masuoka, M.; Ontsuka, K.; Suto, H.; Suzuki, S.; Yamamoto, K.; Ogawa, M.; et al. Periostin Contributes to Epidermal Hyperplasia in Psoriasis Common to Atopic Dermatitis. Allergol. Int. 2015, 64, 41–48. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Neumann, D.; Lamprecht, A. Lipid Nanocapsules for Dermal Application: A Comparative Study of Lipid-Based versus Polymer-Based Nanocarriers. Eur. J. Pharm. Biopharm. 2011, 79, 36–42. [Google Scholar] [CrossRef]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight Junction Defects in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef]
- Gruber, R.; Börnchen, C.; Rose, K.; Daubmann, A.; Volksdorf, T.; Wladykowski, E.; Vidal-y-Sy, S.; Peters, E.M.; Danso, M.; Bouwstra, J.A.; et al. Diverse Regulation of Claudin-1 and Claudin-4 in Atopic Dermatitis. Am. J. Pathol. 2015, 185, 2777–2789. [Google Scholar] [CrossRef]
- Janssens, M.; van Smeden, J.; Puppels, G.J.; Lavrijsen, A.P.M.; Caspers, P.J.; Bouwstra, J.A. Lipid to Protein Ratio Plays an Important Role in the Skin Barrier Function in Patients with Atopic Eczema. Br. J. Dermatol. 2014, 170, 1248–1255. [Google Scholar] [CrossRef]
- Batista, D.I.S.; Perez, L.; Orfali, R.L.; Zaniboni, M.C.; Samorano, L.P.; Pereira, N.V.; Sotto, M.N.; Ishizaki, A.S.; Oliveira, L.M.S.; Sato, M.N.; et al. Profile of Skin Barrier Proteins (Filaggrin, Claudins 1 and 4) and Th1/Th2/Th17 Cytokines in Adults with Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1091–1095. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A.; Kawasaki, H.; Yoshida, K.; Ishii, K.; Furuse, M.; Amagai, M. Epidermal Tight Junction Barrier Function Is Altered by Skin Inflammation, but Not by Filaggrin-Deficient Stratum Corneum. J. Dermatol. Sci. 2015, 77, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, S.; Makris, M.; Vakirlis, E.; Gregoriou, S. The Role of Tight Junctions in Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2023, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Yuki, T.; Tobiishi, M.; Kusaka-Kikushima, A.; Ota, Y.; Tokura, Y. Impaired Tight Junctions in Atopic Dermatitis Skin and in a Skin-Equivalent Model Treated with Interleukin-17. PLoS ONE 2016, 11, e0161759. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.A.; Lamprecht, A. Polymeric Nano (and Micro) Particles as Carriers for Enhanced Skin Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, I.H., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2016; pp. 187–199. ISBN 978-3-662-47862-2. [Google Scholar]
| Sex (M/F) | Average Age (Years) Median [min–max] | |
|---|---|---|
| Healthy volunteers | 5/5 | 31 [21–42] |
| Atopic dermatitis Patients | 2/4 | 26.5 [21–30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Try, C.; Abdel-Mottaleb, M.M.A.; Béduneau, A.; Moulari, B.; Pazart, L.; Vidal, C.; Brunotte, G.; Castelain, F.; Lamprecht, A.; Humbert, P.; et al. Polymeric Nanoparticles’ Accumulation in Atopic Dermatitis: Clinical Comparison between Healthy, Non-Lesional, and Lesional Skin. Pharmaceutics 2023, 15, 1927. https://doi.org/10.3390/pharmaceutics15071927
Try C, Abdel-Mottaleb MMA, Béduneau A, Moulari B, Pazart L, Vidal C, Brunotte G, Castelain F, Lamprecht A, Humbert P, et al. Polymeric Nanoparticles’ Accumulation in Atopic Dermatitis: Clinical Comparison between Healthy, Non-Lesional, and Lesional Skin. Pharmaceutics. 2023; 15(7):1927. https://doi.org/10.3390/pharmaceutics15071927
Chicago/Turabian StyleTry, Céline, Mona M. A. Abdel-Mottaleb, Arnaud Béduneau, Brice Moulari, Lionel Pazart, Chrystelle Vidal, Gaëlle Brunotte, Florence Castelain, Alf Lamprecht, Philippe Humbert, and et al. 2023. "Polymeric Nanoparticles’ Accumulation in Atopic Dermatitis: Clinical Comparison between Healthy, Non-Lesional, and Lesional Skin" Pharmaceutics 15, no. 7: 1927. https://doi.org/10.3390/pharmaceutics15071927
APA StyleTry, C., Abdel-Mottaleb, M. M. A., Béduneau, A., Moulari, B., Pazart, L., Vidal, C., Brunotte, G., Castelain, F., Lamprecht, A., Humbert, P., & Pellequer, Y. (2023). Polymeric Nanoparticles’ Accumulation in Atopic Dermatitis: Clinical Comparison between Healthy, Non-Lesional, and Lesional Skin. Pharmaceutics, 15(7), 1927. https://doi.org/10.3390/pharmaceutics15071927







