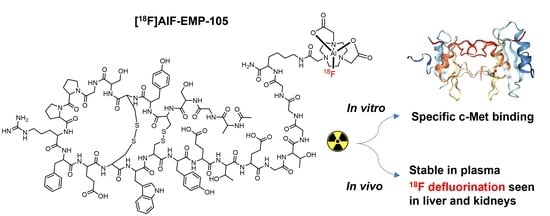
Evaluation of [18F]AlF-EMP-105 for Molecular Imaging of C-Met
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
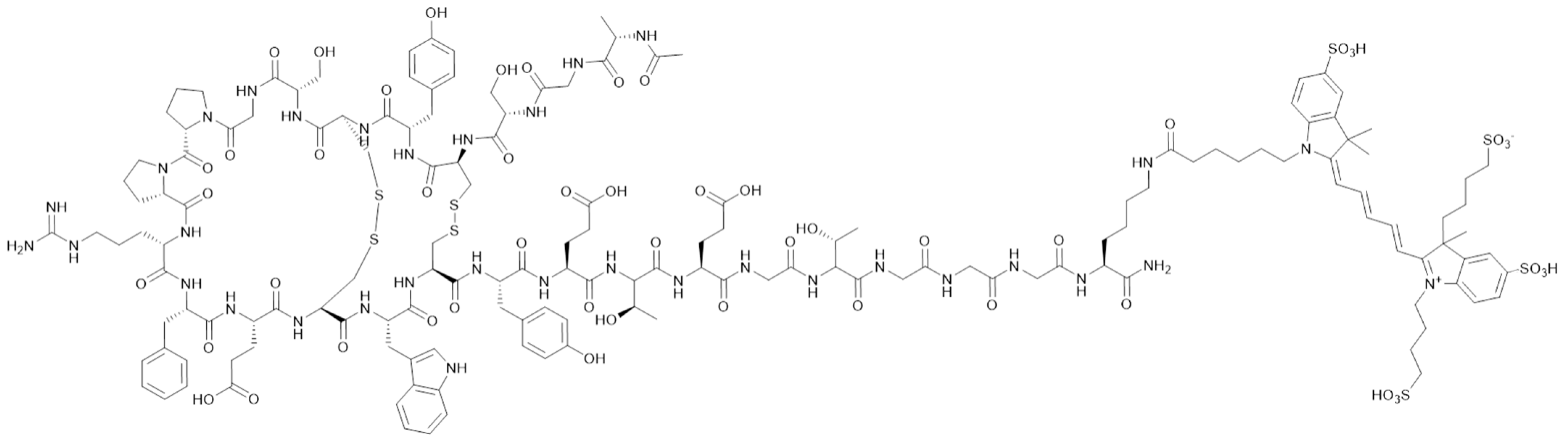
2.2. Radiosynthesis of [18F]AlF-EMP-105
2.3. Stability Tests
2.4. Cell Culture
2.5. Flow Cytometry
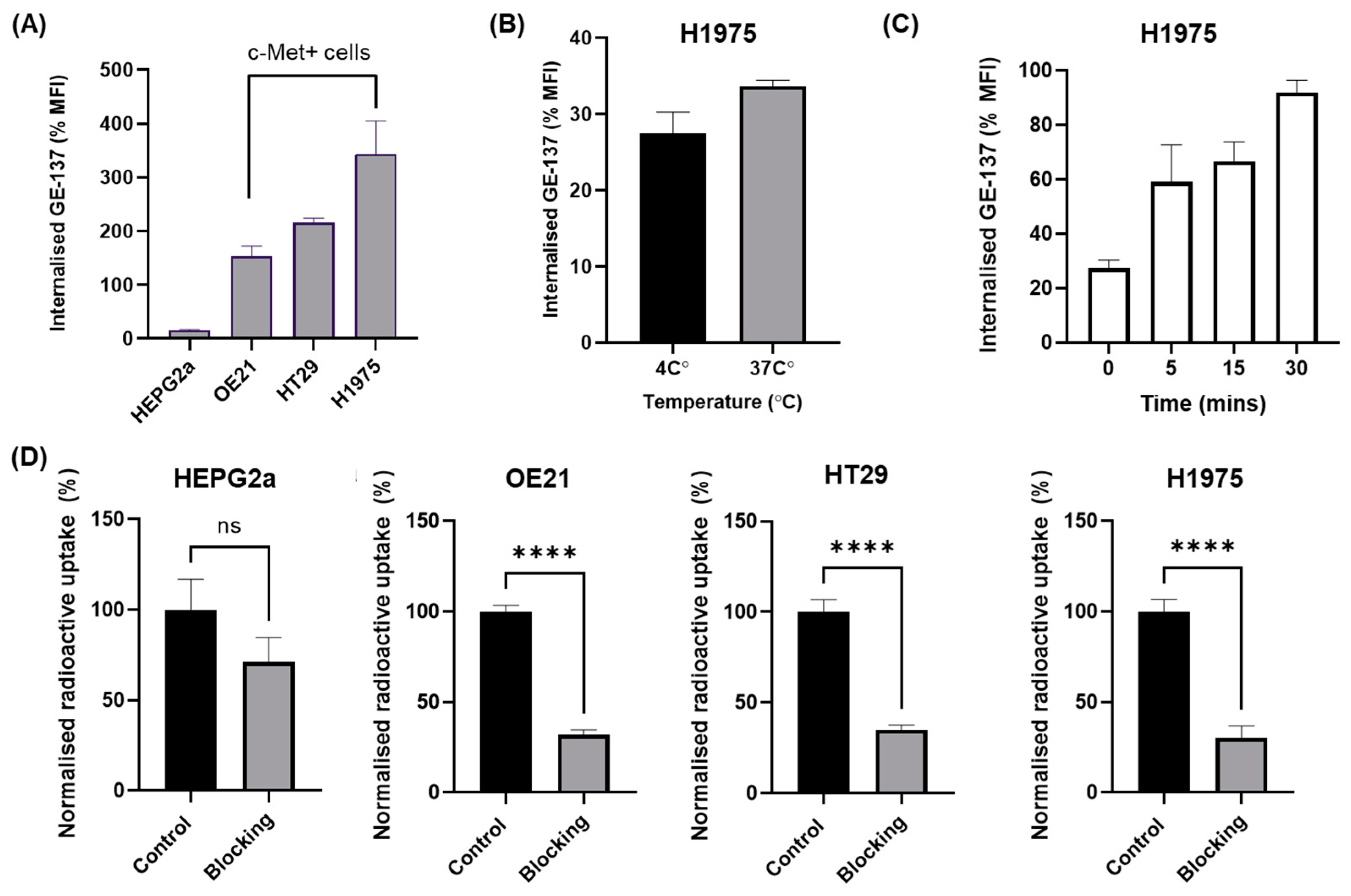
2.6. Internalisation Assay
2.7. In Vitro Uptake of [18F]AlF-EMP-105
2.8. In Vivo PET Imaging
2.9. Metabolite Analysis
2.10. Ex Vivo Biodistribution
2.11. Statistical Analysis
3. Results
3.1. Radiochemistry
3.2. In Vitro Uptake
3.3. Metabolite Analysis
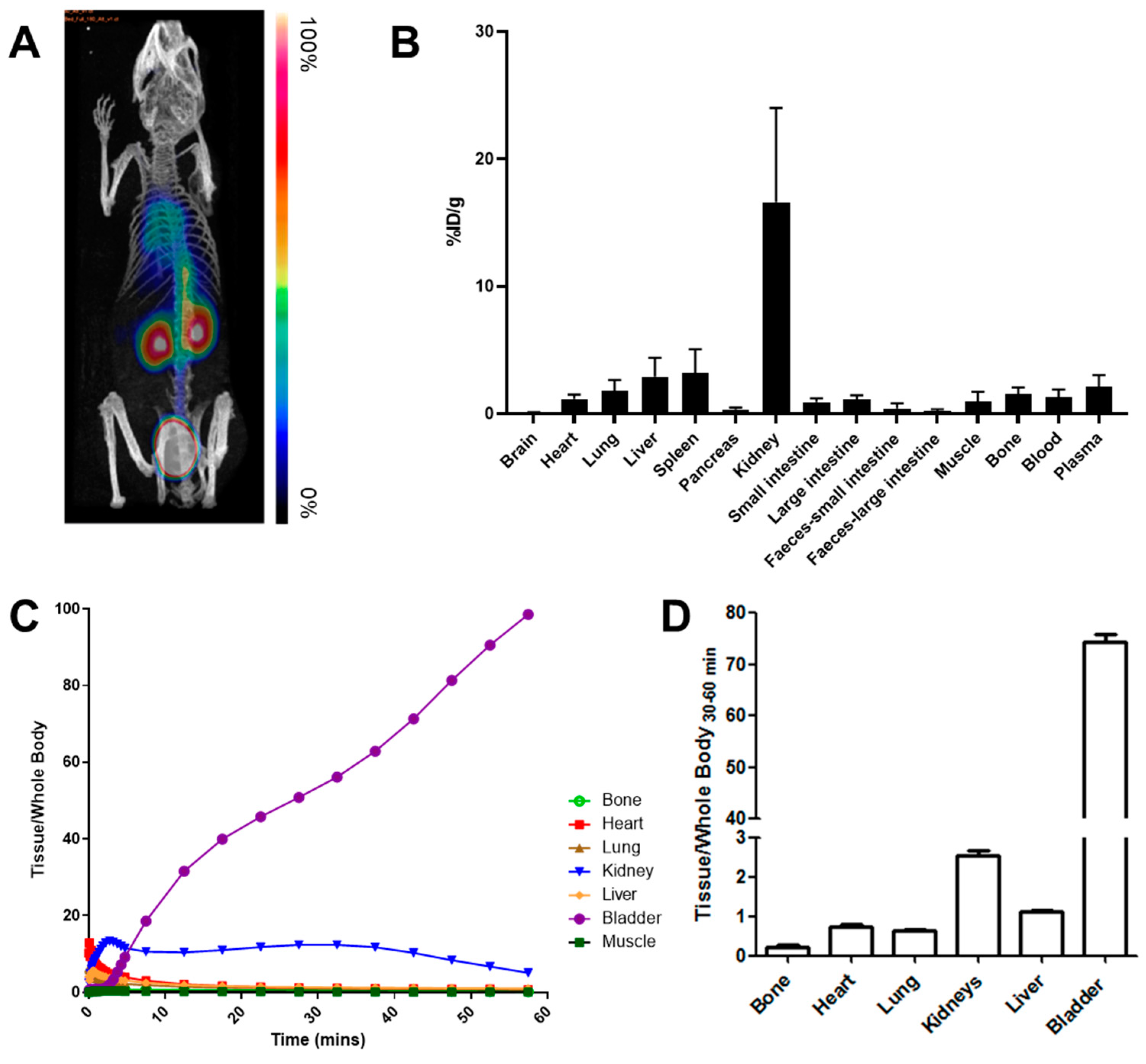
3.4. In Vivo Kinetics and Biodistribution Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Am | Molar activity |
| BCA | Bicinchoninic acid assay |
| DMSO | Dimethyl sulfoxide |
| RCC | Radiochemical conversion |
| RCP | Radiochemical purity |
| RCY | Radiochemical yield |
| MeCN | Acetonitrile |
| NODA | 1,4,7-triazacyclononane-1,4-diacetate |
| NOTA | 1,4,7-triazacyclononane-1,4,7-triacetate |
| PET | Positron emission tomography |
| RIPA | Radioimmunoprecipitation assay buffer |
| tR | Retention time |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| TFA | Trifluoroacetic acid |
References
- Floresta, G.; Abbate, V. Recent progress in the imaging of c-Met aberrant cancers with positron emission tomography. Med. Res. Rev. 2022, 42, 1588–1606. [Google Scholar] [CrossRef]
- Arulappu, A.; Battle, M.; Eisenblaetter, M.; McRobbie, G.; Khan, I.; Monypenny, J.; Weitsman, G.; Galazi, M.; Hoppmann, S.; Gazinska, P.; et al. c-Met PET Imaging Detects Early-Stage Locoregional Recurrence of Basal-Like Breast Cancer. J. Nucl. Med. 2016, 57, 765–770. [Google Scholar] [CrossRef]
- Li, W.; Zheng, H.; Xu, J.; Cao, S.; Xu, X.; Xiao, P. Imaging c-Met expression using 18F-labeled binding peptide in human cancer xenografts. PLoS ONE 2018, 13, e0199024. [Google Scholar] [CrossRef]
- Liang, H.; Wang, M. MET Oncogene in Non-Small Cell Lung Cancer: Mechanism of MET Dysregulation and Agents Targeting the HGF/c-Met Axis. OncoTargets Ther. 2020, 13, 2491–2510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.-F.; Zou, J.; Luo, Z.-H. Prognostic value of c-Met in colorectal cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 3706–3710. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, M.G.; Lesurf, R.; Petkiewicz, S.; O’Malley, F.P.; Pinnaduwage, D.; Andrulis, I.L.; Bull, S.B.; Chughtai, N.; Zuo, D.; Souleimanova, M.; et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 12903–12908. [Google Scholar] [CrossRef]
- Gelsomino, F.; Rossi, G.; Tiseo, M. MET and Small-Cell Lung Cancer. Cancers 2014, 6, 2100. [Google Scholar] [CrossRef]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Targeting HGF/c-MET Axis in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 9170. [Google Scholar] [CrossRef]
- Varkaris, A.; Corn, P.G.; Gaur, S.; Dayyani, F.; Logothetis, C.J.; Gallick, G.E. The role of HGF/c-Met signaling in prostate cancer progression and c-Met inhibitors in clinical trials. Expert Opin. Investig. Drugs 2011, 20, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Zoi, I.; Karamouzis, M.V. Current advances of targeting HGF/c-Met pathway in gastric cancer. Ann. Transl. Med. 2018, 6, 247. [Google Scholar] [CrossRef]
- Schmidt, L.; Duh, F.-M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Huntsman, D.; Resau, J.H.; Klineberg, E.; Auersperg, N. Comparison of c-met Expression in Ovarian Epithelial Tumors and Normal Epithelia of the Female Reproductive Tract by Quantitative Laser Scan Microscopy. Am. J. Pathol. 1999, 155, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Czyz, M. HGF/c-MET Signaling in Melanocytes and Melanoma. Int. J. Mol. Sci. 2018, 19, 3844. [Google Scholar] [CrossRef] [PubMed]
- Khater, A.R.; Abou-Antoun, T. Mesenchymal Epithelial Transition Factor Signaling in Pediatric Nervous System Tumors: Implications for Malignancy and Cancer Stem Cell Enrichment. Front. Cell Dev. Biol. 2021, 9, 654103. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.; Narendran, A. The hepatocyte growth factor/mesenchymal epithelial transition factor axis in high-risk pediatric solid tumors and the anti-tumor activity of targeted therapeutic agents. Front. Pediatr. 2022, 10, 910268. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Illini, O.; Fabikan, H.; Swalduz, A.; Vikström, A.; Krenbek, D.; Schumacher, M.; Dudnik, E.; Studnicka, M.; Öhman, R.; Wurm, R.; et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): A retrospective analysis from an early access program. Ther. Adv. Med. Oncol. 2022, 14, 17588359221103206. [Google Scholar] [CrossRef]
- Brazel, D.; Nagasaka, M. Spotlight on Amivantamab (JNJ-61186372) for EGFR Exon 20 Insertions Positive Non-Small Cell Lung Cancer. Lung Cancer Targets Ther. 2021, 12, 133. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.W.; Moiseenko, F.V.; Filippova, E.; Cicin, I.; Bradbury, P.A.; Daaboul, N.; Tomasini, P.; et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met–overexpressing (OE) advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2022, 40, 9016. [Google Scholar] [CrossRef]
- Lang, L.; Chen, F.; Li, Y.; Shay, C.; Yang, F.; Dan, H.; Chen, Z.G.; Saba, N.F.; Teng, Y. Adaptive c-Met-PLXDC2 Signaling Axis Mediates Cancer Stem Cell Plasticity to Confer Radioresistance-associated Aggressiveness in Head and Neck Cancer. Cancer Res. Commun. 2023, 3, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.-M.; Chang, Y.-L.; Chen, M.-S.; Wu, H.-C. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials 2011, 32, 3265–3274. [Google Scholar] [CrossRef]
- Burggraaf, J.; Kamerling, I.M.C.; Gordon, P.B.; Schrier, L.; De Kam, M.L.; Kales, A.J.; Bendiksen, R.; Indrevoll, B.; Bjerke, R.M.; Moestue, S.A.; et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat. Med. 2015, 21, 955–961. [Google Scholar] [CrossRef]
- Towner, R.A.; Smith, N.; Asano, Y.; Doblas, S.; Saunders, D.; Silasi-Mansat, R.; Lupu, F. Molecular Magnetic Resonance Imaging Approaches Used to Aid in the Understanding of the Tissue Regeneration Marker Met In Vivo: Implications for Tissue Engineering. Tissue Eng. Part A 2010, 16, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Doblas, S.; Saunders, D.; Kshirsagar, P.; Pye, Q.; Oblander, J.; Gordon, B.; Kosanke, S.; Floyd, R.A.; Towner, R.A. Phenyl-tert-butylnitrone induces tumor regression and decreases angiogenesis in a C6 rat glioma model. Free Radic. Biol. Med. 2008, 44, 63–72. [Google Scholar] [CrossRef]
- Towner, R.A.; Smith, N.; Tesiram, Y.A.; Abbott, A.; Saunders, D.; Blindauer, R.; Herlea, O.; Silasi-Mansat, R.; Lupu, F. In Vivo Detection of c-MET Expression in a Rat Hepatocarcinogenesis Model Using Molecularly Targeted Magnetic Resonance Imaging. Mol. Imaging 2007, 6, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Towner, R.A.; Smith, N.; Doblas, S.; Tesiram, Y.; Garteiser, P.; Saunders, D.; Cranford, R.; Silasi-Mansat, R.; Herlea, O.; Ivanciu, L.; et al. In vivo detection of c-Met expression in a rat C6 glioma model. J. Cell. Mol. Med. 2008, 12, 174–186. [Google Scholar] [CrossRef]
- Armstrong, G.R.; Khot, M.I.; Portal, C.; West, N.P.; Perry, S.L.; Maisey, T.I.; Tiernan, J.P.; Hughes, T.A.; Tolan, D.J.; Jayne, D.G. A novel fluorescent c-met targeted imaging agent for intra-operative colonic tumour mapping: Translation from the laboratory into a clinical trial. Surg. Oncol. 2022, 40, 101679. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, S.J.; Voskuil, F.J.; Schmidt, I.; Karrenbeld, A.; Kats-Ugurlu, G.; Meersma, G.J.; Westerhof, J.; Witjes, M.J.H.; Van Dam, G.M.; Robinson, D.J.; et al. C-Met targeted fluorescence molecular endoscopy in Barrett’s esophagus patients and identification of outcome parameters for phase-I studies. Theranostics 2020, 10, 5357–5367. [Google Scholar] [CrossRef]
- Jonker, P.K.C.; Metman, M.J.H.; Sondorp, L.H.J.; Sywak, M.S.; Gill, A.J.; Jansen, L.; Links, T.P.; van Diest, P.J.; van Ginhoven, T.M.; Löwik, C.W.G.M.; et al. Intraoperative MET-receptor targeted fluorescent imaging and spectroscopy for lymph node detection in papillary thyroid cancer: Novel diagnostic tools for more selective central lymph node compartment dissection. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3557–3570. [Google Scholar] [CrossRef]
- de Vries, H.M.; Bekers, E.; van Oosterom, M.N.; Karakullukcu, M.B.; Van Der Poel, H.G.; Van Leeuwen, F.W.B.; Brouwer, O.R. c-MET Receptor–Targeted Fluorescence on the Road to Image-Guided Surgery in Penile Squamous Cell Carcinoma Patients. J. Nucl. Med. 2021, 63, 51–56. [Google Scholar] [CrossRef]
- Mittlmeier, L.M.; Todica, A.; Gildehaus, F.-J.; Unterrainer, M.; Beyer, L.; Brendel, M.; Albert, N.L.; Ledderose, S.T.; Vettermann, F.J.; Schott, M.; et al. 68Ga-EMP-100 PET/CT—A novel ligand for visualizing c-MET expression in metastatic renal cell carcinoma—First in-human biodistribution and imaging results. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.M. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 55. [Google Scholar] [PubMed]
- Miele, E.; Spinelli, G.P.; Tomao, F.; Zullo, A.; De Marinis, F.; Pasciuti, G.; Rossi, L.; Zoratto, F.; Tomao, S. Positron Emission Tomography (PET) radiotracers in oncology–Utility of 18F-Fluoro-deoxy-glucose (FDG)-PET in the management of patients with non-small-cell lung cancer (NSCLC). J. Exp. Clin. Cancer Res. 2008, 27, 52. [Google Scholar] [CrossRef]
- Fersing, C.; Bouhlel, A.; Cantelli, C.; Garrigue, P.; Lisowski, V.; Guillet, B. A Comprehensive Review of Non-Covalent Radiofluorination Approaches Using Aluminum [18F]fluoride: Will [18F]AlF Replace 68Ga for Metal Chelate Labeling? Molecules 2019, 24, 2866. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; Allott, L. The aluminium-[18F]fluoride revolution: Simple radiochemistry with a big impact for radiolabelled biomolecules. EJNMMI Radiopharm. Chem. 2021, 6, 30. [Google Scholar] [CrossRef]
- McBride, W.J.; Sharkey, R.M.; Karacay, H.; D’Souza, C.A.; Rossi, E.A.; Laverman, P.; Chang, C.-H.; Boerman, O.C.; Goldenberg, D.M. A Novel Method of 18F Radiolabeling for PET. J. Nucl. Med. 2009, 50, 991–998. [Google Scholar] [CrossRef]
- Teh, J.H.; Braga, M.; Allott, L.; Barnes, C.; Hernández-Gil, J.; Tang, M.-X.; Aboagye, E.O.; Long, N.J. A kit-based aluminium-[18F]fluoride approach to radiolabelled microbubbles. Chem. Commun. 2021, 57, 11677–11680. [Google Scholar] [CrossRef]
- McBride, W.J.; D’Souza, C.A.; Karacay, H.; Sharkey, R.M.; Goldenberg, D.M. New Lyophilized Kit for Rapid Radiofluorination of Peptides. Bioconjugate Chem. 2012, 23, 538–547. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Boeckxstaens, L.; Dekervel, J.; Vandamme, T.; Lybaert, W.; Broeck, B.V.D.; et al. 18F-AlF-NOTA-Octreotide Outperforms68Ga-DOTATATE/NOC PET in Neuroendocrine Tumor Patients: Results from a Prospective, Multicenter Study. J. Nucl. Med. 2022, 64, 632–638. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Vassileva, V.; Braga, M.; Barnes, C.; Przystal, J.; Ashek, A.; Allott, L.; Brickute, D.; Abrahams, J.; Suwan, K.; Carcaboso, A.M.; et al. Effective Detection and Monitoring of Glioma Using [18F]FPIA PET Imaging. Biomedicines 2021, 9, 811. [Google Scholar] [CrossRef]
- McBride, W.J.; Sharkey, R.M.; Goldenberg, D.M. Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res. 2013, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu, T.; Cawthorne, C.; Serdons, K.; Pauwels, E.; Gsell, W.; Bormans, G.; Deroose, C.M.; Cleeren, F. Automated GMP compliant production of [18F]AlF-NOTA-octreotide. EJNMMI Radiopharm. Chem. 2020, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Niu, G.; Wang, Z.; Yang, X.; Kiesewetter, D.O.; Jacobson, O.; Shen, B.; Chen, X. Al[18F]NOTA-T140 Peptide for Noninvasive Visualization of CXCR4 Expression. Mol. Imaging Biol. 2016, 18, 135–142. [Google Scholar] [CrossRef] [PubMed]
- D’souza, C.A.; McBride, W.J.; Sharkey, R.M.; Todaro, L.J.; Goldenberg, D.M. High-Yielding Aqueous 18F-Labeling of Peptides via Al18F Chelation. Bioconjugate Chem. 2011, 22, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Da Pieve, C.; Allott, L.; Martins, C.D.; Vardon, A.; Ciobota, D.M.; Kramer-Marek, G.; Smith, G. Efficient [18F]AlF Radiolabeling of ZHER3:8698 Affibody Molecule for Imaging of HER3 Positive Tumors. Bioconjugate Chem. 2016, 27, 1839–1849. [Google Scholar] [CrossRef]
- McBride, W.J.; D’Souza, C.A.; Sharkey, R.M.; Goldenberg, D.M. The radiolabeling of proteins by the [18F]AlF method. Appl. Radiat. Isot. 2012, 70, 200–204. [Google Scholar] [CrossRef]
- McBride, W.J.; D’souza, C.A.; Sharkey, R.M.; Karacay, H.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Improved 18F Labeling of Peptides with a Fluoride-Aluminum-Chelate Complex. Bioconjugate Chem. 2010, 21, 1331–1340. [Google Scholar] [CrossRef]
- Chatalic, K.L.; Franssen, G.M.; van Weerden, W.M.; McBride, W.J.; Laverman, P.; de Blois, E.; Hajjaj, B.; Brunel, L.; Goldenberg, D.M.; Fehrentz, J.-A.; et al. Preclinical Comparison of Al18F- and 68Ga-Labeled Gastrin-Releasing Peptide Receptor Antagonists for PET Imaging of Prostate Cancer. J. Nucl. Med. 2014, 55, 2050–2056. [Google Scholar] [CrossRef]
- Cleeren, F.; Lecina, J.; Ahamed, M.; Raes, G.; Devoogdt, N.; Caveliers, V.; McQuade, P.; Rubins, D.J.; Li, W.; Verbruggen, A.; et al. Al18F-Labeling Of Heat-Sensitive Biomolecules for Positron Emission Tomography Imaging. Theranostics 2017, 7, 2924–2939. [Google Scholar] [CrossRef]
- Jadvar, H.; Desai, B.; Conti, P.S. Sodium 18F-Fluoride PET/CT of Bone, Joint, and Other Disorders. Semin. Nucl. Med. 2015, 45, 58–65. [Google Scholar] [CrossRef] [PubMed]
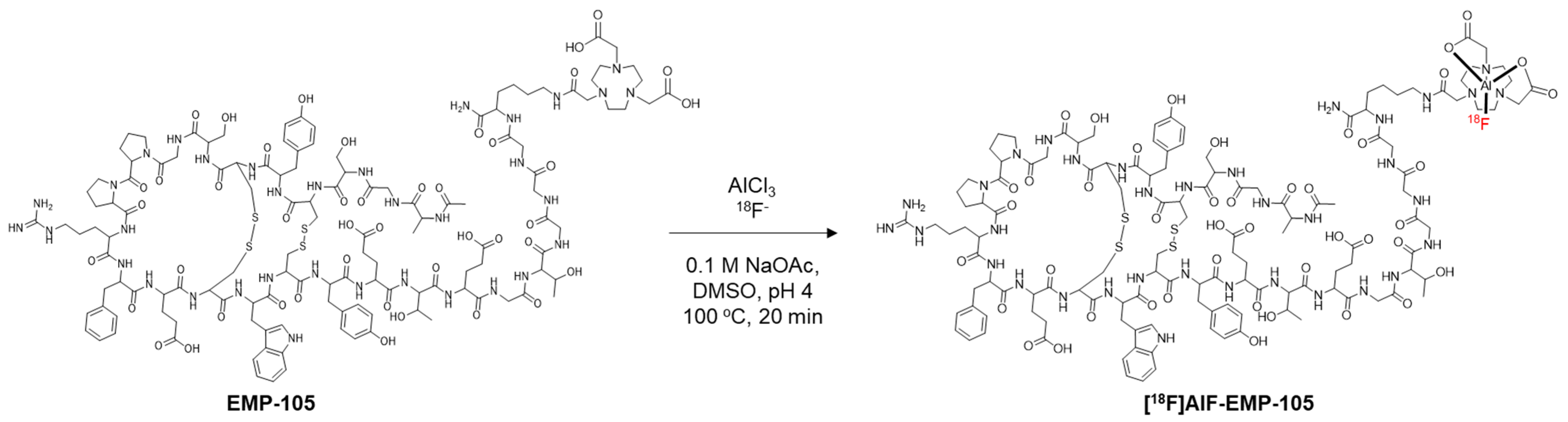

| Precursor Amount (nmol) | Radiochemical Conversion (%) a | Isolated Activity (MBq) | Radiochemical Yield (%) b | Molar Activity (GBq/µmol) |
|---|---|---|---|---|
| 20 | 30 ± 3 | 48 ± 8 | 20 ± 3 | 2.4 ± 0.4 |
| 50 | 56 ± 4 | 153 ± 20 | 46 ± 2 | 3.3 ± 0.5 |
| 100 | 58 ± 2 | 168 ± 8 | 50 ± 5 | 1.7 ± 0.1 |
| Percentage of [18F]AlF-EMP-105 Remaining (%) | Extraction Efficiency (%) | |||
|---|---|---|---|---|
| Tissue | 5 min p.i. | 30 min p.i. | 5 min p.i. | 30 min p.i. |
| Plasma | 98.3 ± 1.1 | 93.3 ± 7.4 | 50.1 ± 13.4 | 42.9 ± 25.4 |
| Kidney | 9.8 ± 12.9 | 4.3 ± 4.3 | 56.1 ± 25.9 | 47.7 ± 31.2 |
| Liver | 6.2 ± 10.8 | 0.4 ± 0.7 | 25.6 ± 11.7 | 31.9 ± 12.7 |
| Urine | - | 7.9 ± 9.2 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, J.H.; Amgheib, A.; Fu, R.; Barnes, C.; Abrahams, J.; Ashek, A.; Wang, N.; Yang, Z.; Mansoorudeen, M.; Long, N.J.; et al. Evaluation of [18F]AlF-EMP-105 for Molecular Imaging of C-Met. Pharmaceutics 2023, 15, 1915. https://doi.org/10.3390/pharmaceutics15071915
Teh JH, Amgheib A, Fu R, Barnes C, Abrahams J, Ashek A, Wang N, Yang Z, Mansoorudeen M, Long NJ, et al. Evaluation of [18F]AlF-EMP-105 for Molecular Imaging of C-Met. Pharmaceutics. 2023; 15(7):1915. https://doi.org/10.3390/pharmaceutics15071915
Chicago/Turabian StyleTeh, Jin Hui, Ala Amgheib, Ruisi Fu, Chris Barnes, Joel Abrahams, Ali Ashek, Ning Wang, Zixuan Yang, Muneera Mansoorudeen, Nicholas J. Long, and et al. 2023. "Evaluation of [18F]AlF-EMP-105 for Molecular Imaging of C-Met" Pharmaceutics 15, no. 7: 1915. https://doi.org/10.3390/pharmaceutics15071915
APA StyleTeh, J. H., Amgheib, A., Fu, R., Barnes, C., Abrahams, J., Ashek, A., Wang, N., Yang, Z., Mansoorudeen, M., Long, N. J., & Aboagye, E. O. (2023). Evaluation of [18F]AlF-EMP-105 for Molecular Imaging of C-Met. Pharmaceutics, 15(7), 1915. https://doi.org/10.3390/pharmaceutics15071915