Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Cytotoxicity, Proliferation and Apoptosis Assays
2.3. esMT/MesT
2.4. Pluripotency Exit
2.5. Gastruloid Formation Assay
2.6. RNA Extraction and Real Time PCR
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
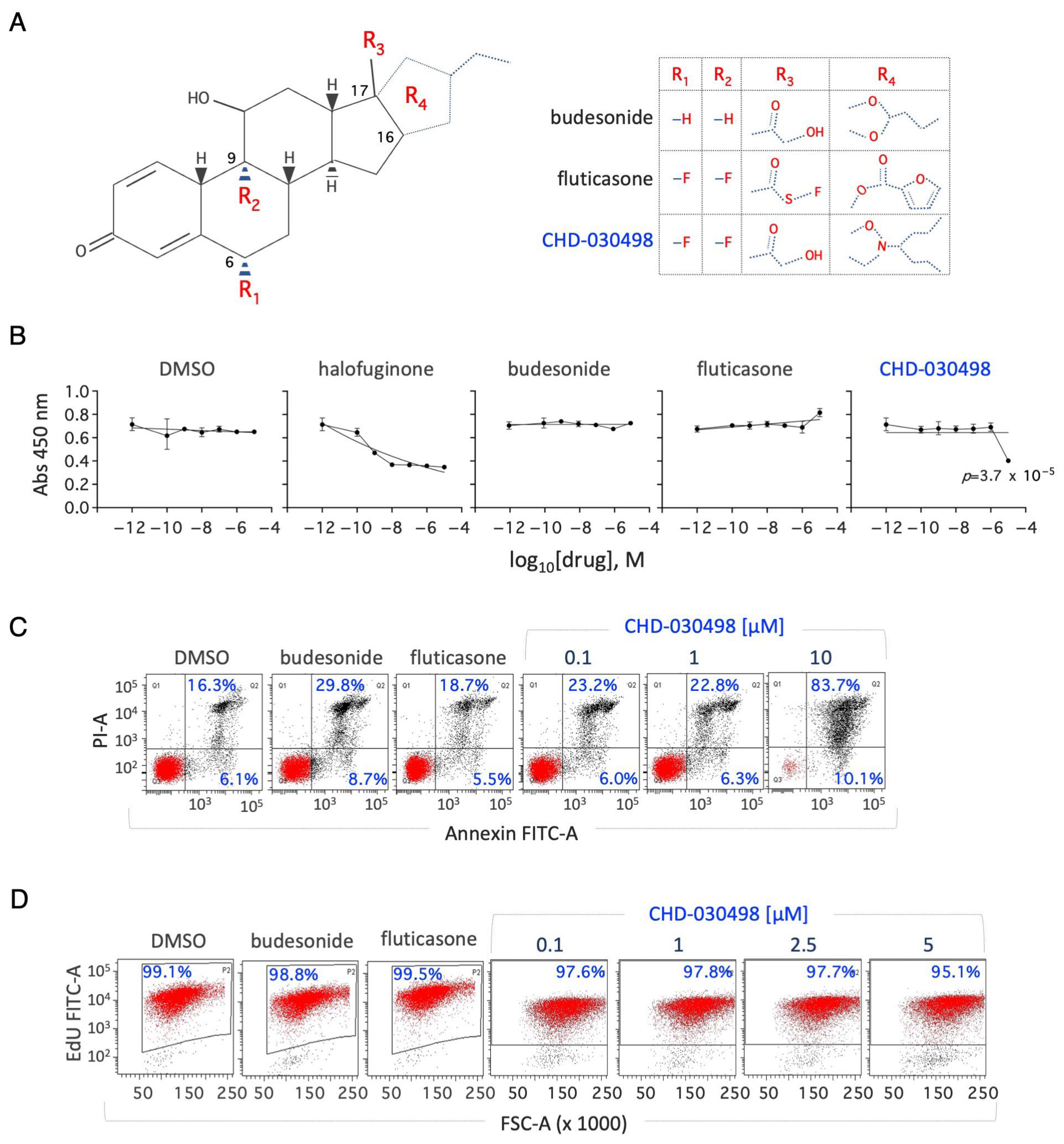
3.1. Effect of Budesonide-Analogue Glucocorticoids (BA-GCs) on ESC Proliferation
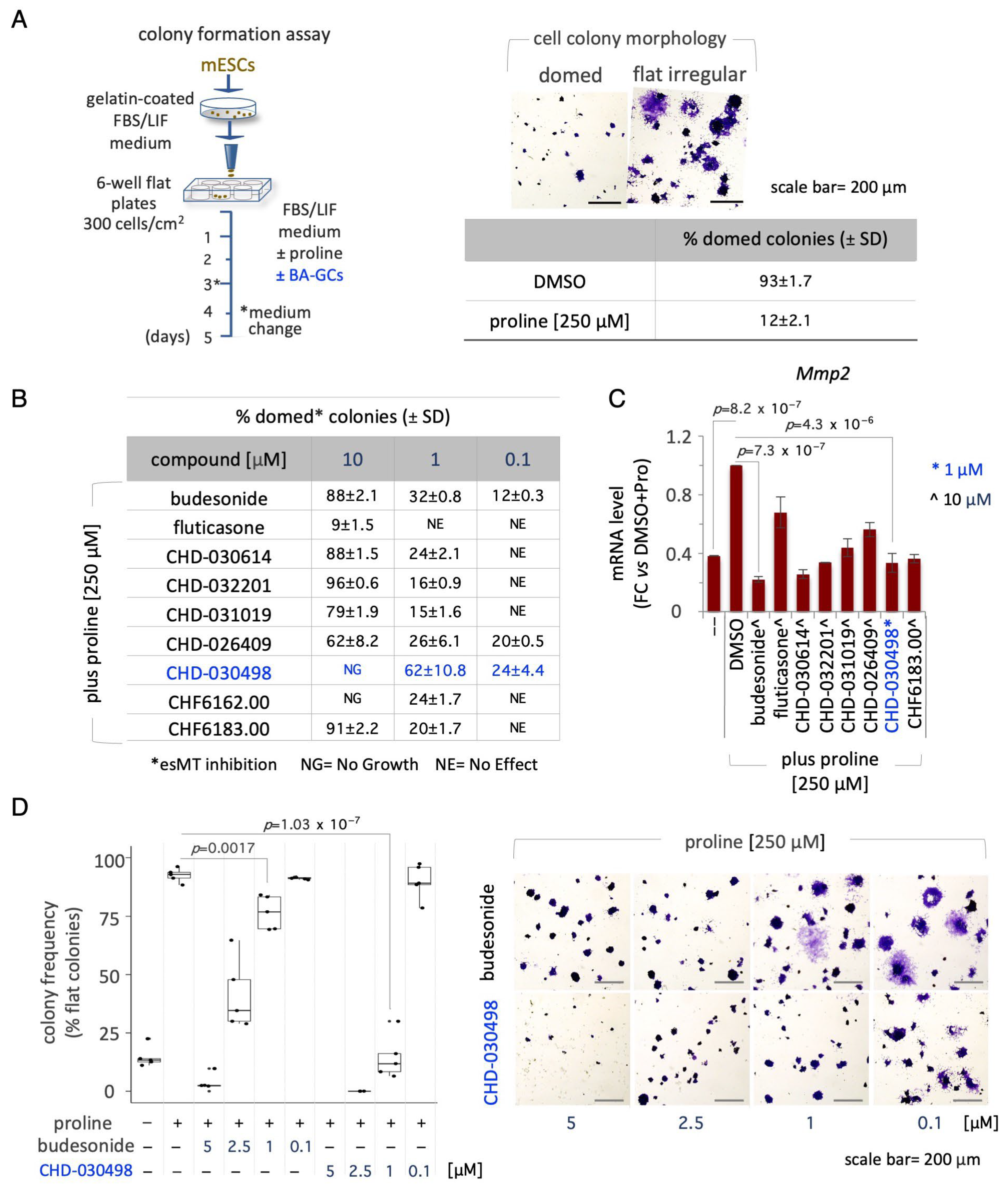
3.2. BA-GCs Prevent Embryonic Stem Cell-to-Mesenchymal Transition (esMT) Induction
3.3. EsMT Inhibitory Activity of BA-GCs Is Independent of the Glucocorticoid Receptor (GR)
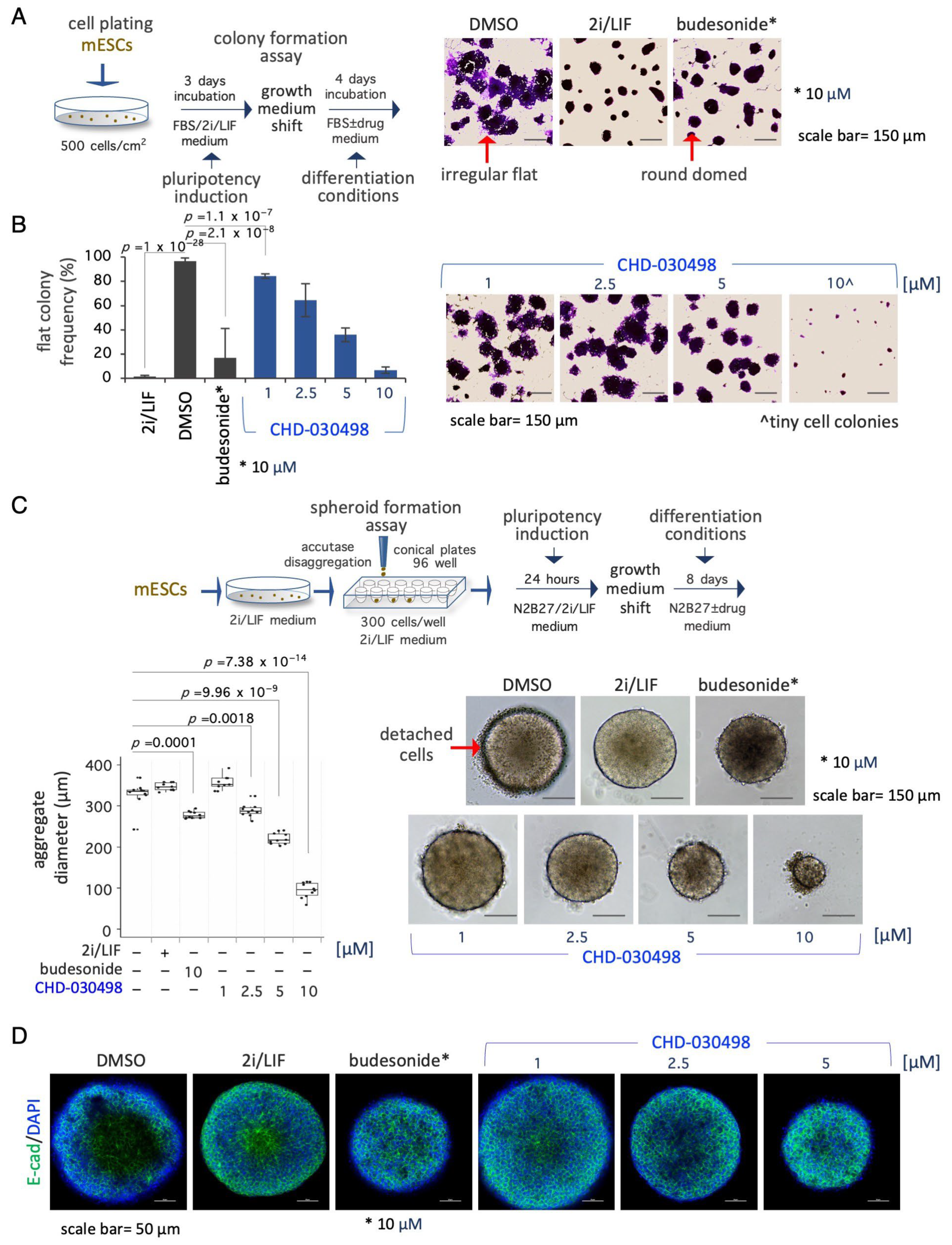
3.4. BA-GCs Counteract Exit from Pluripotency in Cell Colonies and Spheroids
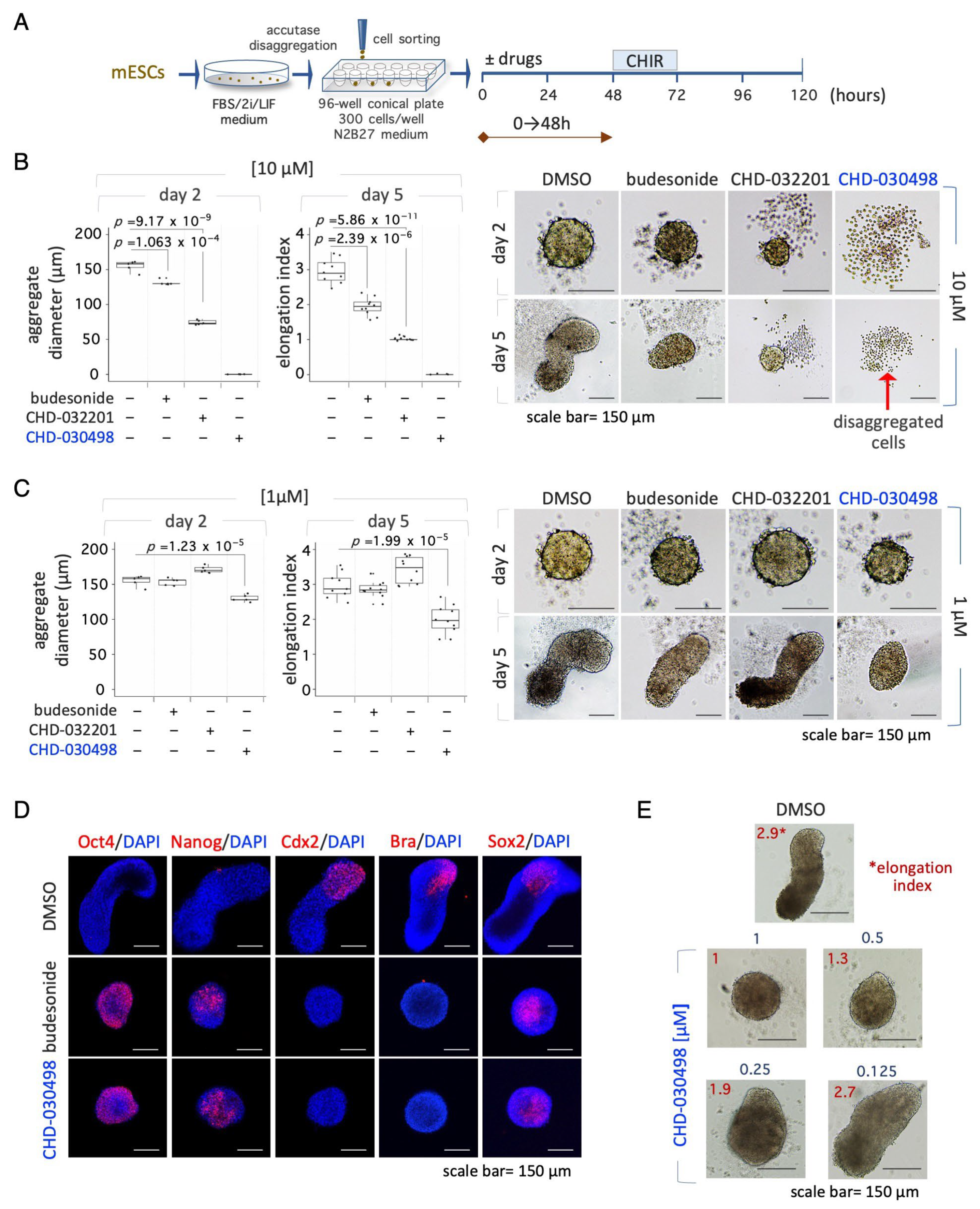
3.5. BA-GCs Prevent Symmetry Breaking and Gastruloid Elongation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, T.; Wang, X.; Liu, D. Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration. Pharmaceutics 2022, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, F.; Guardiola, O.; Scagliola, A.; Andolfi, G.; Esposito, F.; Serrano, A.; Perdiguero, E.; Brunelli, S.; Muñoz-Cánoves, P.; Minchiotti, G. Cripto shapes macrophage plasticity and restricts EndMT in injured and diseased skeletal muscle. EMBO Rep. 2020, 21, e49075. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, O.; Andolfi, G.; Tirone, M.; Iavarone, F.; Brunelli, S.; Minchiotti, G. Induction of Acute Skeletal Muscle Regeneration by Cardiotoxin Injection. J. Vis. Exp. 2017, 119, e54515. [Google Scholar]
- Prezioso, C.; Iaconis, S.; Andolfi, G.; Zentilin, L.; Iavarone, F.; Guardiola, O.; Minchiotti, G. Conditional Cripto overexpression in satellite cells promotes myogenic commitment and enhances early regeneration. Front. Cell Dev. Biol. 2015, 3, 31. [Google Scholar] [CrossRef]
- Martins-Lima, C.; Chianese, U.; Benedetti, R.; Altucci, L.; Jerónimo, C.; Correia, M.P. Tumor microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game? Front. Mol. Biosci. 2022, 9, 1070383. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Horta, C.A.; Yang, J. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics. Semin. Cancer Biol. 2023, 88, 46–66. [Google Scholar] [CrossRef]
- Yu, L.; Wei, Y.; Duan, J.; Schmitz, D.A.; Sakurai, M.; Wang, L.; Wang, K.; Zhao, S.; Hon, G.C.; Wu, J. Blastocyst-like structures generated from human pluripotent stem cells. Nature 2021, 591, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Tlili, S.; Perrin, P.; Lowndes, M.; Peradziryi, H.; Brickman, J.M.; Arias, A.M.; Lenne, P.F. Cell-state transitions and collective cell movement generate an endoderm-like region in gastruloids. Elife 2022, 11, e59371. [Google Scholar] [CrossRef] [PubMed]
- Busada, J.T.; Cidlowski, J.A. Mechanisms of Glucocorticoid Action During Development. Curr. Top. Dev. Biol. 2017, 125, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Cermola, F.; Amoroso, F.; Saracino, F.; Ibello, E.; De Cesare, D.; Fico, A.; Cobellis, G.; Scalera, E.; Casiraghi, C.; D’aniello, C.; et al. Stabilization of cell-cell adhesions prevents symmetry breaking and locks in pluripotency in 3D gastruloids. Stem Cell Rep. 2022, 17, 2548–2564. [Google Scholar] [CrossRef]
- D’Aniello, C.; Cermola, F.; Palamidessi, A.; Wanderlingh, L.G.; Gagliardi, M.; Migliaccio, A.; Varrone, F.; Casalino, L.; Matarazzo, M.R.; De Cesare, D.; et al. Collagen Prolyl Hydroxylation–Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Res. 2019, 79, 3235–3250. [Google Scholar] [CrossRef]
- Cermola, F.; Patriarca, E.J.; Minchiotti, G. Generation of Gastruloids from Epiblast-Like Cells. In Epiblast Stem Cells: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 197–204. [Google Scholar] [CrossRef]
- D’Aniello, C.; Fico, A.; Casalino, L.; Guardiola, O.; Di Napoli, G.; Cermola, F.; De Cesare, D.; Tate, R.; Cobellis, G.; Patriarca, E.J.; et al. A novel autoregulatory loop between the Gcn2-Atf4 pathway and (L)-Proline [corrected] metabolism controls stem cell identity. Cell Death Differ. 2015, 22, 1094–1105. [Google Scholar] [CrossRef]
- Comes, S.; Gagliardi, M.; Laprano, N.; Fico, A.; Cimmino, A.; Palamidessi, A.; De Cesare, D.; De Falco, S.; Angelini, C.; Scita, G.; et al. L-Proline Induces a Mesenchymal-like Invasive Program in Embryonic Stem Cells by Remodeling H3K9 and H3K36 Methylation. Stem Cell Rep. 2013, 1, 307–321. [Google Scholar] [CrossRef]
- Cermola, F.; D’Aniello, C.; Tatè, R.; De Cesare, D.; Martinez-Arias, A.; Minchiotti, G.; Patriarca, E.J. Gastruloid Development Competence Discriminates Different States of Pluripotency. Stem Cell Rep. 2021, 16, 354–369. [Google Scholar] [CrossRef]
- Baillie-Johnson, P.; Van den Brink, S.C.; Balayo, T.; Turner, D.A.; Arias, A.M. Generation of Aggregates of Mouse Embryonic Stem Cells that Show Symmetry Breaking, Polarization and Emergent Collective Behaviour In Vitro. J. Vis. Exp. 2015, 105, e53252. [Google Scholar]
- Gade, E.J.; Thomsen, S.F.; Lindenberg, S.; Kyvik, K.O.; Lieberoth, S.; Backer, V. Asthma affects time to pregnancy and fertility: A register-based twin study. Eur. Respir. J. 2014, 43, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.V.; Ali, Z.; Malchau, S.S.; Blafoss, J.; Pinborg, A.; Ulrik, C.S. Fertility treatment among women with asthma: A case–control study of 3689 women with live births. Eur. Respir. J. 2019, 53, 1800597. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| N-Cad | AGCGCAGTCTTACCGAAGG | TCGCTGCTTTCATACTGAACTTT |
| Mmp2 | TTCCCTAAGCTCATCGCAGACT | CACGCTCTTGAGACTTTGGTTCT |
| T | GAACCTCGGATTCACATCGT | TTCTTTGGCATCAAGGAAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoroso, F.; Ibello, E.; Saracino, F.; Cermola, F.; Ponticelli, G.; Scalera, E.; Ricci, F.; Villetti, G.; Cobellis, G.; Minchiotti, G.; et al. Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development. Pharmaceutics 2023, 15, 1897. https://doi.org/10.3390/pharmaceutics15071897
Amoroso F, Ibello E, Saracino F, Cermola F, Ponticelli G, Scalera E, Ricci F, Villetti G, Cobellis G, Minchiotti G, et al. Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development. Pharmaceutics. 2023; 15(7):1897. https://doi.org/10.3390/pharmaceutics15071897
Chicago/Turabian StyleAmoroso, Filomena, Eduardo Ibello, Federica Saracino, Federica Cermola, Giovanna Ponticelli, Enrica Scalera, Francesca Ricci, Gino Villetti, Gilda Cobellis, Gabriella Minchiotti, and et al. 2023. "Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development" Pharmaceutics 15, no. 7: 1897. https://doi.org/10.3390/pharmaceutics15071897
APA StyleAmoroso, F., Ibello, E., Saracino, F., Cermola, F., Ponticelli, G., Scalera, E., Ricci, F., Villetti, G., Cobellis, G., Minchiotti, G., Patriarca, E. J., De Cesare, D., & D’Aniello, C. (2023). Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development. Pharmaceutics, 15(7), 1897. https://doi.org/10.3390/pharmaceutics15071897







