Efficacy and Safety of Low-Intensity Pulsed Ultrasound-Induced Blood–Retinal Barrier Opening in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
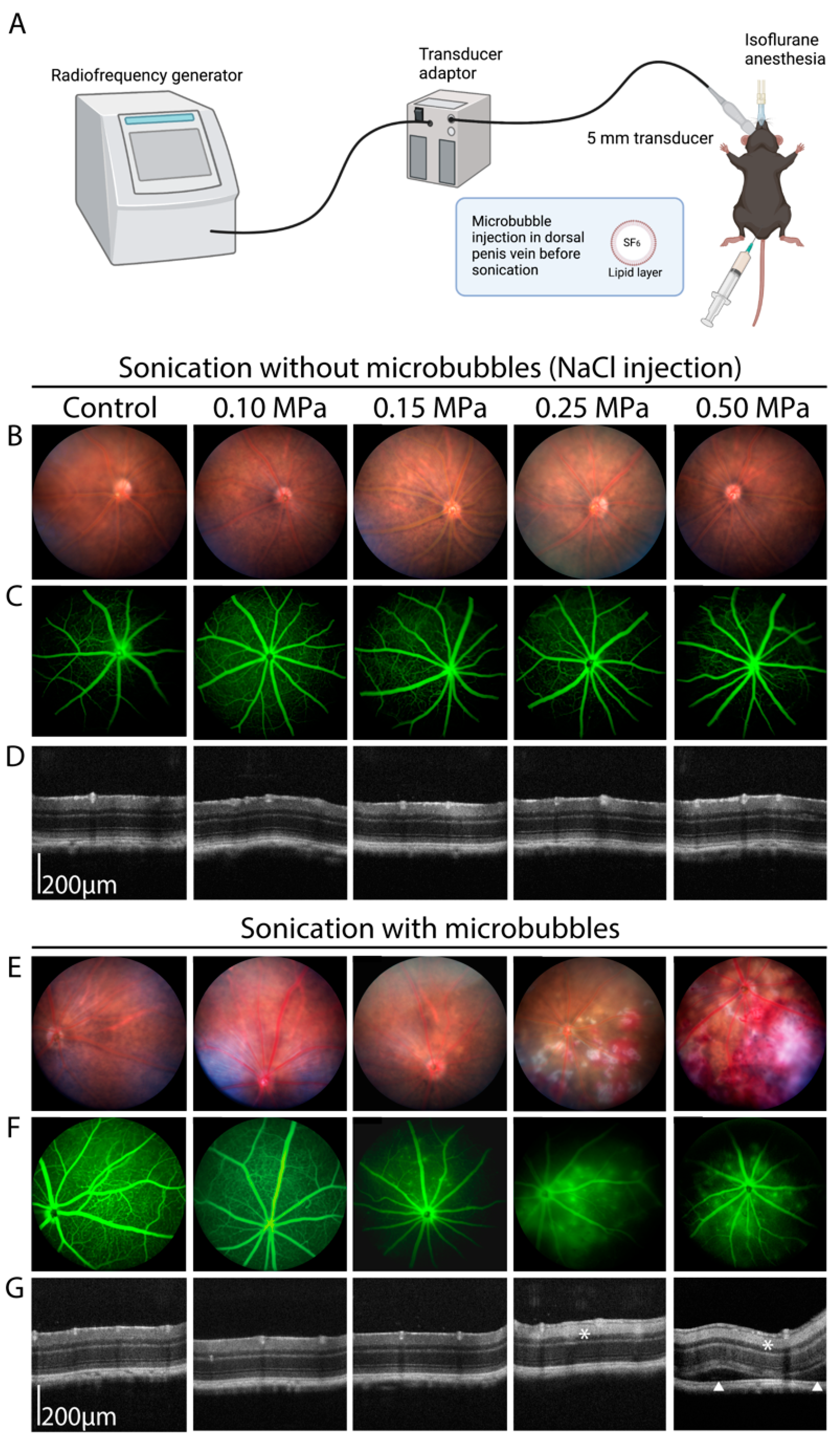
2.2. Ultrasound Sonication
2.3. Fundus Photography, Angiography and Optical Coherence Tomography (OCT) Imaging
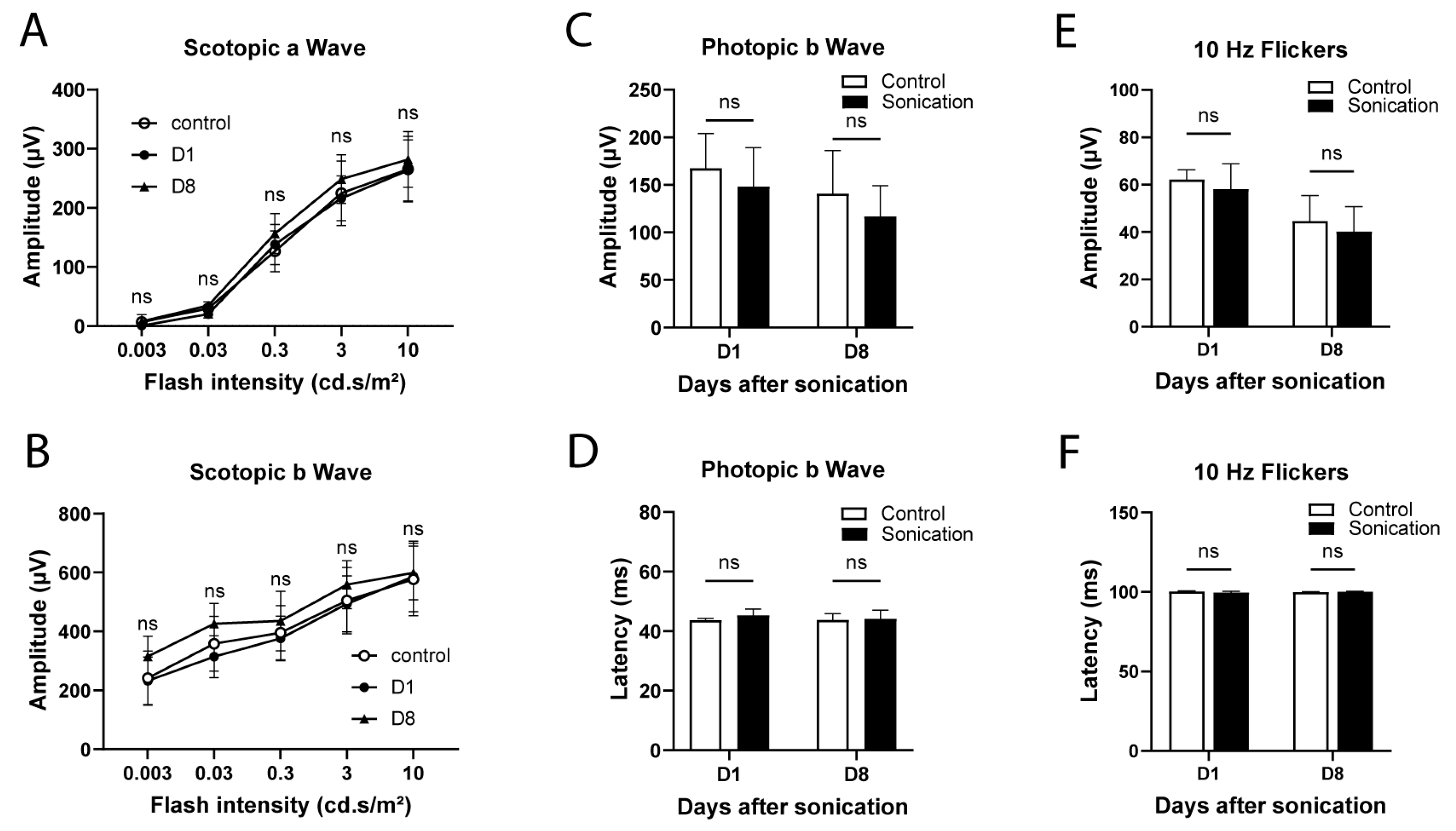
2.4. Fundus Electroretinography (ERG)
2.5. Immunohistochemistry
2.6. Reverse Transcription and Quantitative Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
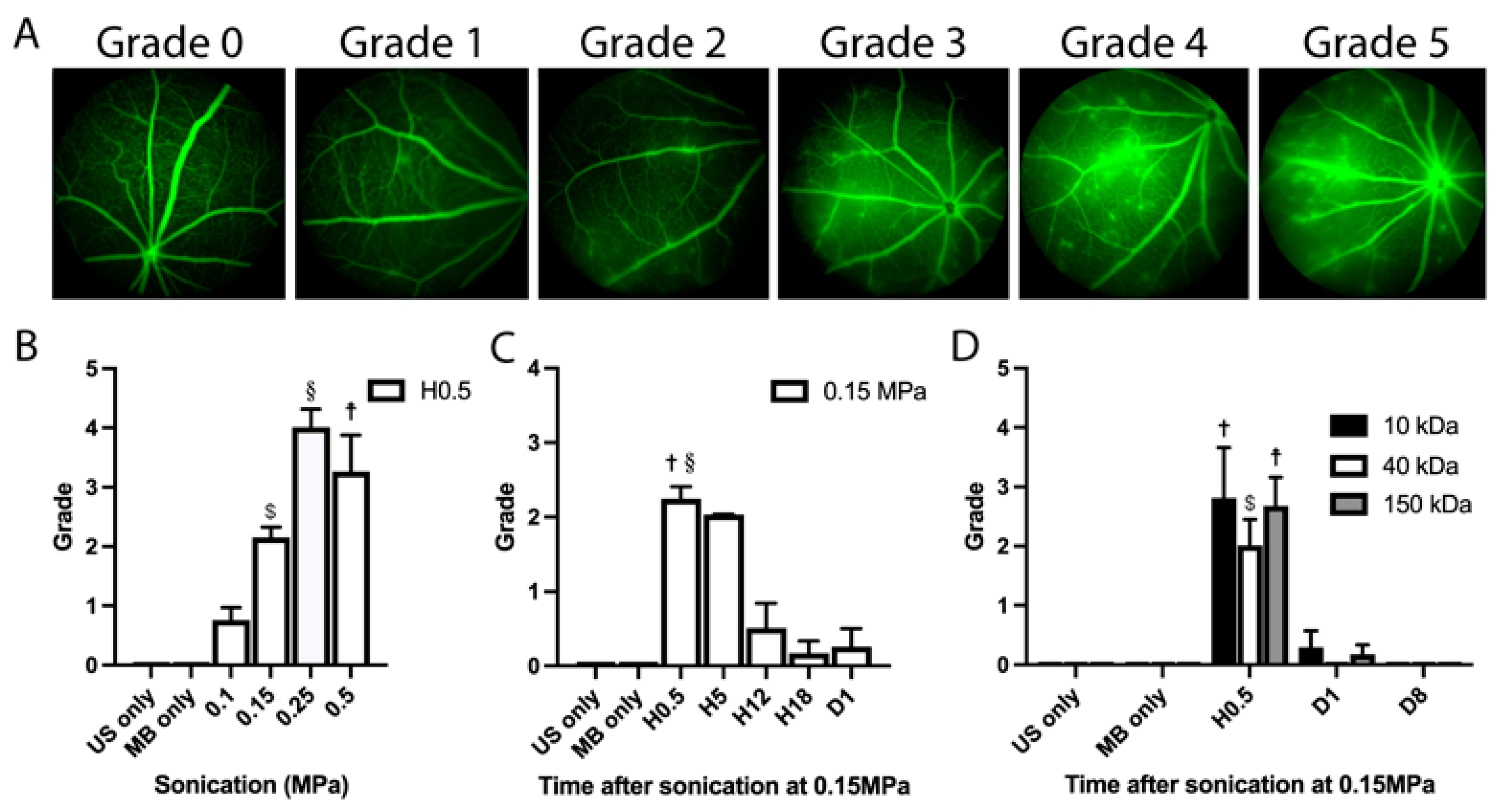
3.1. Determination of the Optimal Ultrasound Acoustic Pressure
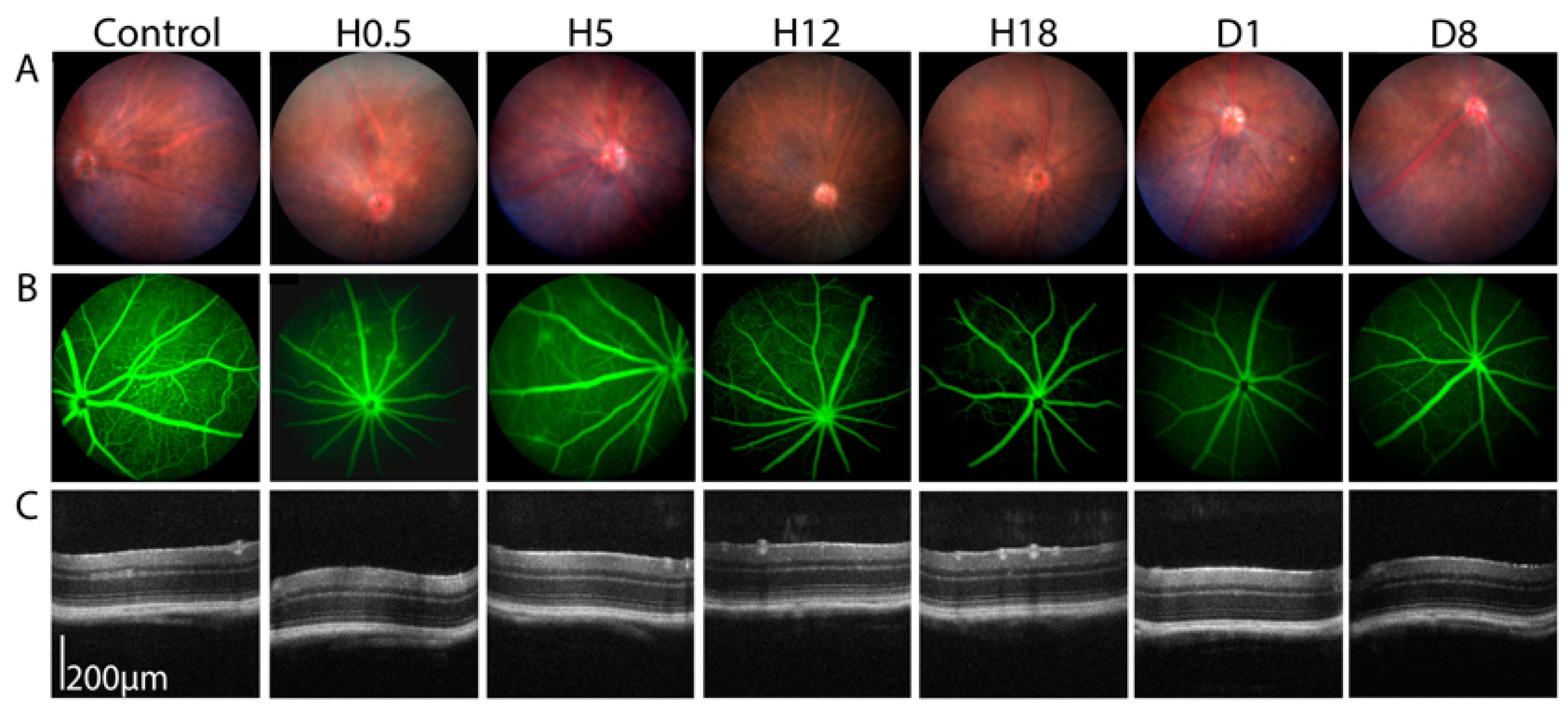
3.2. Angiographic Leakage Quantification
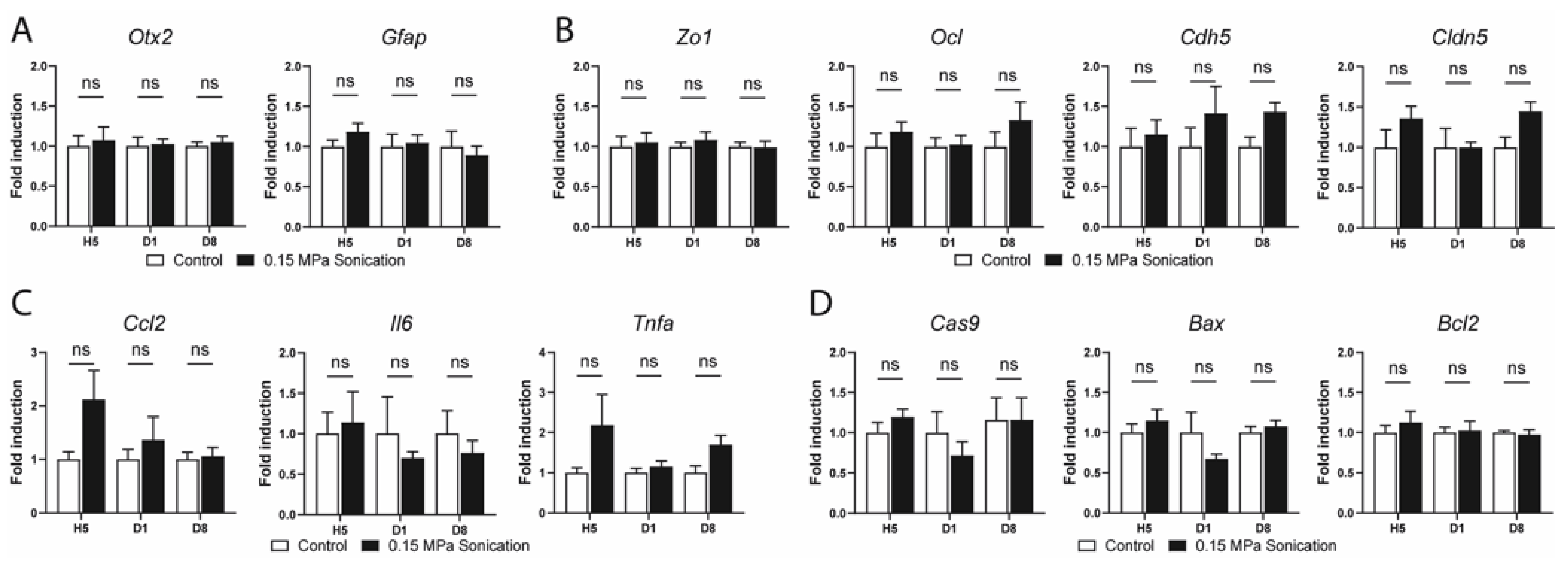
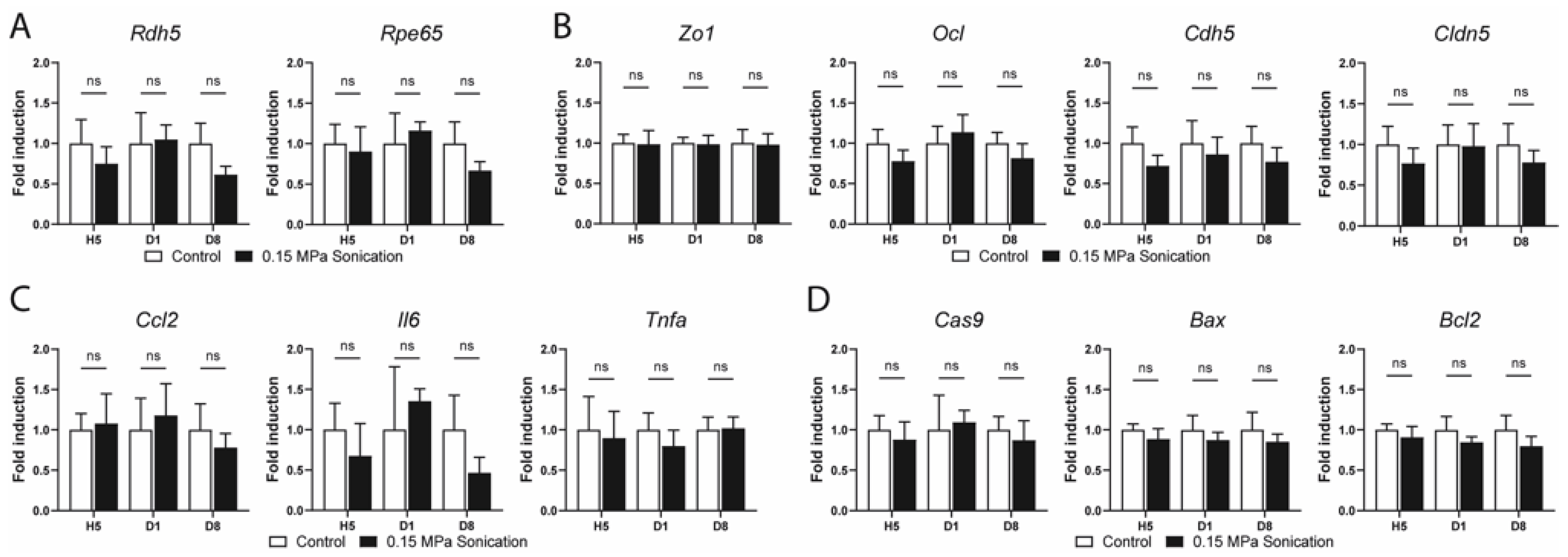
3.3. Safety Analysis
4. Discussion
4.1. Optimization of Sonication Parameters
4.2. Duration of BRB Disruption, Efficacy of Sonication and the Size of Leaked Molecules
4.3. Safety
4.4. Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Runkle, E.A.; Antonetti, D.A. The Blood-Retinal Barrier: Structure and Functional Significance. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2011; Volume 686, pp. 133–148. [Google Scholar]
- Kooiman, K.; Vos, H.J.; Versluis, M.; De Jong, N. Acoustic Behavior of Microbubbles and Implications for Drug Delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med. Biol. 2008, 34, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Goldwirt, L.; Canney, M.; Horodyckid, C.; Poupon, J.; Mourah, S.; Vignot, A.; Chapelon, J.Y.; Carpentier, A. Enhanced Brain Distribution of Carboplatin in a Primate Model after Blood-Brain Barrier Disruption Using an Implantable Ultrasound Device. Cancer Chemother. Pharmacol. 2016, 77, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved Anti-Tumor Effect of Liposomal Doxorubicin After Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated Blood–Brain Barrier Opening with an Implantable Ultrasound Device for Delivery of Albumin-Bound Paclitaxel in Patients with Recurrent Glioblastoma: A Phase 1 Trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef]
- Hirokawa, T.; Karshafian, R.; Pavlin, C.J.; Burns, P.N. Insonation of the Eye in the Presence of Microbubbles Preliminary Study of the Duration and Degree of Vascular Bioeffects—Work in Progress. J. Ultrasound Med. 2007, 26, 731–738. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Akula, J.D.; McDannold, N.J. Targeted and Reversible Blood-Retinal Barrier Disruption via Focused Ultrasound and Microbubbles. PLoS ONE 2012, 7, e42754. [Google Scholar] [CrossRef]
- Touahri, Y.; Dixit, R.; Kofoed, R.H.; Miloska, K.; Park, E.J.; Raeisossadati, R.; Markham-Coultes, K.; David, L.A.; Rijal, H.; Zhao, J.; et al. Focused Ultrasound as a Novel Strategy for Noninvasive Gene Delivery to Retinal Müller Glia. Theranostics 2020, 10, 2982–2999. [Google Scholar] [CrossRef]
- Rousou, C.; van Kronenburg, N.; Sonnen, A.F.P.; van Dijk, M.; Moonen, C.; Storm, G.; Mastrobattista, E.; Deckers, R. Microbubble-Assisted Ultrasound for Drug Delivery to the Retina in an Ex Vivo Eye Model. Pharmaceutics 2023, 15, 1220. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical Trial of Blood-Brain Barrier Disruption by Pulsed Ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, C.H.; Mouravieff, M. Reliable and Simple Method of Intravenous Injection into the Laboratory Rat. J. Pharm. Sci. 1973, 62, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, A.; Lin, C.M.; Shanmugam, S.; Lindner, H.M.; Abcouwer, S.F.; Antonetti, D.A. Ischemia-Reperfusion Injury Induces Occludin Phosphorylation/Ubiquitination and Retinal Vascular Permeability in a VEGFR-2-Dependent Manner. J. Cereb. Blood Flow Metab. 2014, 34, 522–531. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Nitta, C.F.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef]
- Tugal-Tutkun, I.; Herbort, C.P.; Khairallah, M.; Allegri, P.; Biziorek, B.; Bodaghi, B.; Bouchenaki, N.; Cimino, L.; Fardeau, C.; Gupta, A.; et al. Scoring of Dual Fluorescein and ICG Inflammatory Angiographic Signs for the Grading of Posterior Segment Inflammation (Dual Fluorescein and ICG Angiographic Scoring System for Uveitis). Int. Ophthalmol. 2010, 30, 539–552. [Google Scholar] [CrossRef]
- Koike, C.; Nishida, A.; Ueno, S.; Saito, H.; Sanuki, R.; Sato, S.; Furukawa, A.; Aizawa, S.; Matsuo, I.; Suzuki, N.; et al. Functional Roles of Otx2 Transcription Factor in Postnatal Mouse Retinal Development. Mol. Cell. Biol. 2007, 27, 8318–8329. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J. Chapter 7 Astrocytes in Mammalian Retina. Prog. Retin. Res. 1988, 7, 209–231. [Google Scholar] [CrossRef]
- Sarthy, P.V.; Fu, M.; Huang, J. Developmental Expression of the Glial Fibrillary Acidic Protein (GFAP) Gene in the Mouse Retina. Cell. Mol. Neurobiol. 1991, 11, 623–637. [Google Scholar] [CrossRef]
- Lhor, M.; Salesse, C. Retinol Dehydrogenases: Membrane-Bound Enzymes for the Visual Function1. Biochem. Cell Biol. 2014, 92, 510–523. [Google Scholar] [CrossRef]
- Redmond, T.M.; Yu, S.; Lee, E.; Bok, D.; Hamasaki, D.; Chen, N.; Goletz, P.; Ma, J.-X.; Crouch, R.K.; Pfeifer, K. Rpe65 Is Necessary for Production of 11-Cis-Vitamin A in the Retinal Visual Cycle. Nat. Genet. 1998, 20, 344–351. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Goldwirt, L.; Fernandez, C.; Adam, C.; Piquet, J.; Autret, G.; Clément, O.; Lafon, C.; Chapelon, J.Y.; et al. Opening of the Blood-Brain Barrier with an Unfocused Ultrasound Device in Rabbits. J. Neurosurg. 2013, 119, 887–898. [Google Scholar] [CrossRef]
- Dréan, A.; Lemaire, N.; Bouchoux, G.; Goldwirt, L.; Canney, M.; Goli, L.; Bouzidi, A.; Schmitt, C.; Guehennec, J.; Verreault, M.; et al. Temporary Blood–Brain Barrier Disruption by Low Intensity Pulsed Ultrasound Increases Carboplatin Delivery and Efficacy in Preclinical Models of Glioblastoma. J. Neurooncol. 2019, 144, 33–41. [Google Scholar] [CrossRef]
- Montero, A.S.; Bielle, F.; Goldwirt, L.; Lalot, A.; Bouchoux, G.; Canney, M.; Belin, F.; Beccaria, K.; Pradat, P.F.; Salachas, F.; et al. Ultrasound-Induced Blood–Spinal Cord Barrier Opening in Rabbits. Ultrasound Med. Biol. 2019, 45, 2417–2426. [Google Scholar] [CrossRef]
- McDannold, N.; Zhang, Y.; Vykhodtseva, N. Blood-Brain Barrier Disruption and Vascular Damage Induced by Ultrasound Bursts Combined with Microbubbles Can Be Influenced by Choice of Anesthesia Protocol. Ultrasound Med. Biol. 2011, 37, 1259–1270. [Google Scholar] [CrossRef]
- Augustin, S.; Lam, M.; Lavalette, S.; Verschueren, A.; Blond, F.; Forster, V.; Przegralek, L.; He, Z.; Lewandowski, D.; Bemelmans, A.P.; et al. Melanophages Give Rise to Hyperreflective Foci in AMD, a Disease-Progression Marker. J. Neuroinflamm. 2023, 20, 28. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.C.; Lentacker, I. Opening Doors with Ultrasound and Microbubbles: Beating Biological Barriers to Promote Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Guo, X.; Tu, J.; Zhang, D. Mechanisms Underlying Sonoporation: Interaction between Microbubbles and Cells. Ultrason. Sonochem. 2020, 67, 105096. [Google Scholar] [CrossRef] [PubMed]
- Snipstad, S.; Sulheim, E.; de Lange Davies, C.; Moonen, C.; Storm, G.; Kiessling, F.; Schmid, R.; Lammers, T. Sonopermeation to Improve Drug Delivery to Tumors: From Fundamental Understanding to Clinical Translation. Expert Opin. Drug Deliv. 2018, 15, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Wang, P.; Liu, Y.; Zhang, Z.; Xue, Y. Mechanism of Low-Frequency Ultrasound in Opening Blood-Tumor Barrier by Tight Junction. J. Mol. Neurosci. 2011, 43, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Kremers, J.; Tanimoto, N. Measuring Retinal Function in the Mouse. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1753, pp. 27–40. [Google Scholar]
- Ahmed, M.H.; Hernández-Verdin, I.; Quissac, E.; Lemaire, N.; Guerin, C.; Guyonnet, L.; Zahr, N.; Mouton, L.; Santin, M.; Petiet, A.; et al. Low-Intensity Pulsed Ultrasound-Mediated Blood-Brain Barrier Opening Increases Anti-Programmed Death-Ligand 1 Delivery and Efficacy in Gl261 Mouse Model. Pharmaceutics 2023, 15, 455. [Google Scholar] [CrossRef]
- Horodyckid, C.; Canney, M.; Vignot, A.; Boisgard, R.; Drier, A.; Huberfeld, G.; François, C.; Prigent, A.; Santin, M.D.; Adam, C.; et al. Safe Long-Term Repeated Disruption of the Blood-Brain Barrier Using an Implantable Ultrasound Device: A Multiparametric Study in a Primate Model. J. Neurosurg. 2017, 126, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Cassoux, N.; Lumbroso, L.; Levy-Gabriel, C.; Aerts, I.; Doz, F.; Desjardins, L. Retinoblastoma: Update on Current Management. Asia-Pac. J. Ophthalmol. 2017, 6, 290–295. [Google Scholar]
- Mendelsohn, M.E.; Abramson, D.H.; Madden, T.; Tong, W.; Tran, H.T.; Dunkel, I.J. Intraocular Concentrations of Chemotherapeutic Agents After Systemic or Local Administration. Arch. Ophthalmol. 1998, 116, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourdin, A.; Ortoli, M.; Karadayi, R.; Przegralek, L.; Sennlaub, F.; Bodaghi, B.; Guillonneau, X.; Carpentier, A.; Touhami, S. Efficacy and Safety of Low-Intensity Pulsed Ultrasound-Induced Blood–Retinal Barrier Opening in Mice. Pharmaceutics 2023, 15, 1896. https://doi.org/10.3390/pharmaceutics15071896
Bourdin A, Ortoli M, Karadayi R, Przegralek L, Sennlaub F, Bodaghi B, Guillonneau X, Carpentier A, Touhami S. Efficacy and Safety of Low-Intensity Pulsed Ultrasound-Induced Blood–Retinal Barrier Opening in Mice. Pharmaceutics. 2023; 15(7):1896. https://doi.org/10.3390/pharmaceutics15071896
Chicago/Turabian StyleBourdin, Alexandre, Manon Ortoli, Remi Karadayi, Lauriane Przegralek, Florian Sennlaub, Bahram Bodaghi, Xavier Guillonneau, Alexandre Carpentier, and Sara Touhami. 2023. "Efficacy and Safety of Low-Intensity Pulsed Ultrasound-Induced Blood–Retinal Barrier Opening in Mice" Pharmaceutics 15, no. 7: 1896. https://doi.org/10.3390/pharmaceutics15071896
APA StyleBourdin, A., Ortoli, M., Karadayi, R., Przegralek, L., Sennlaub, F., Bodaghi, B., Guillonneau, X., Carpentier, A., & Touhami, S. (2023). Efficacy and Safety of Low-Intensity Pulsed Ultrasound-Induced Blood–Retinal Barrier Opening in Mice. Pharmaceutics, 15(7), 1896. https://doi.org/10.3390/pharmaceutics15071896







