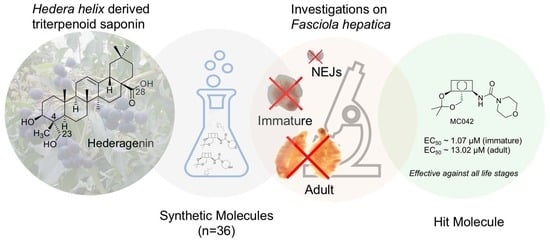
Modified Hederagenin Derivatives Demonstrate Ex Vivo Anthelmintic Activity against Fasciola hepatica
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
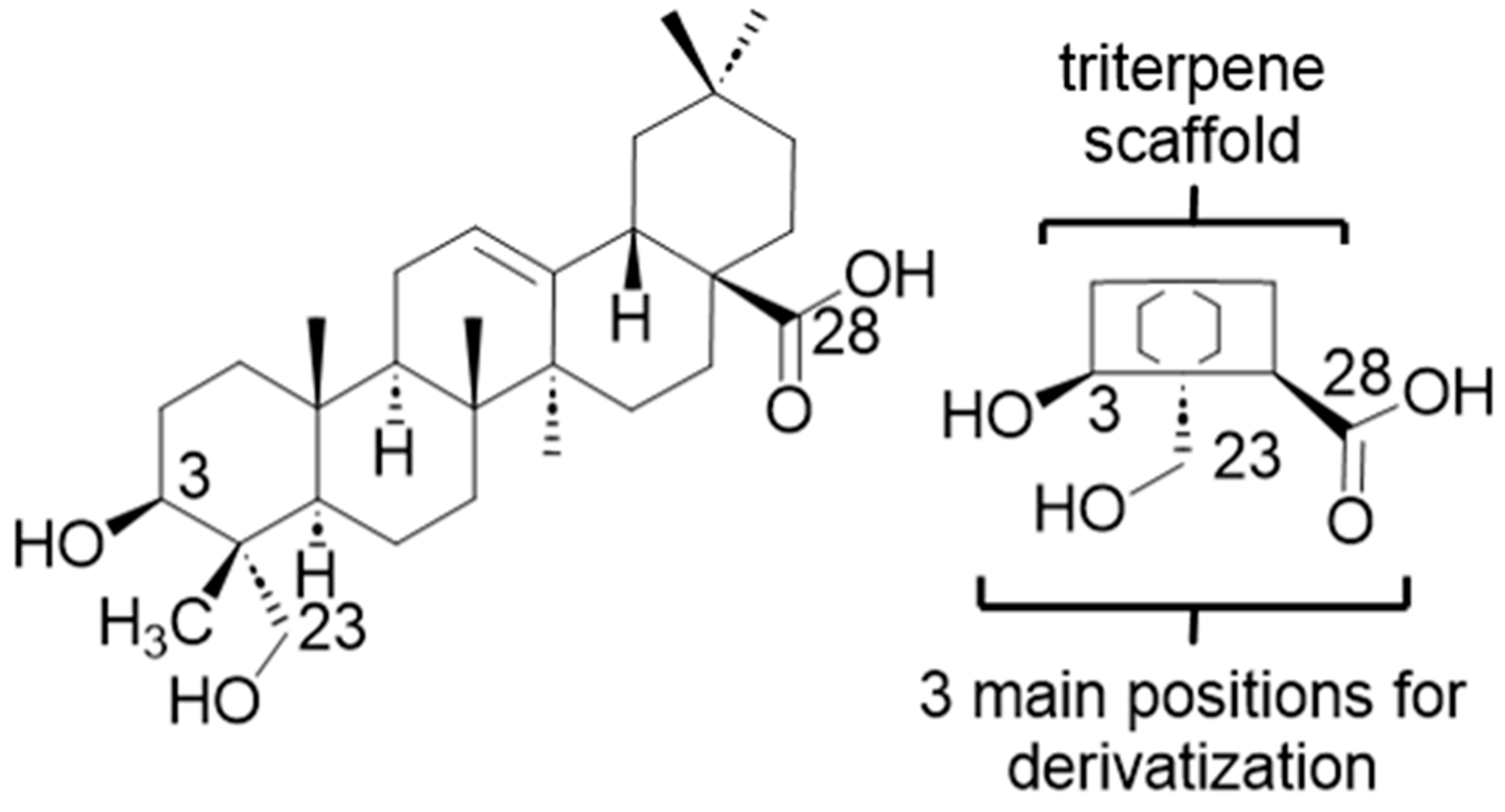
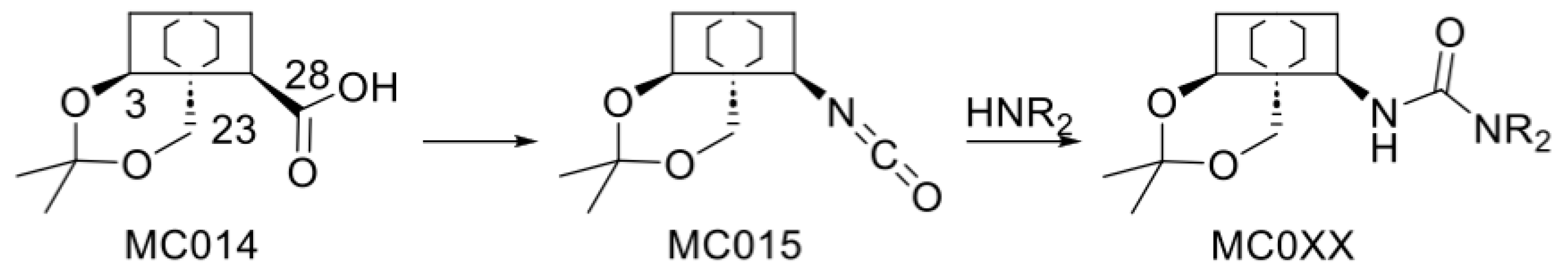
2.2. Modified Plant Saponins from H. helix
2.3. Parasites and Dose Preparation
2.4. Ex Vivo Assays on Life Stages of F. hepatica (Italian or Wild Strain) Parasites
2.4.1. Effect against Newly Excysted Juveniles (Italian Strain)
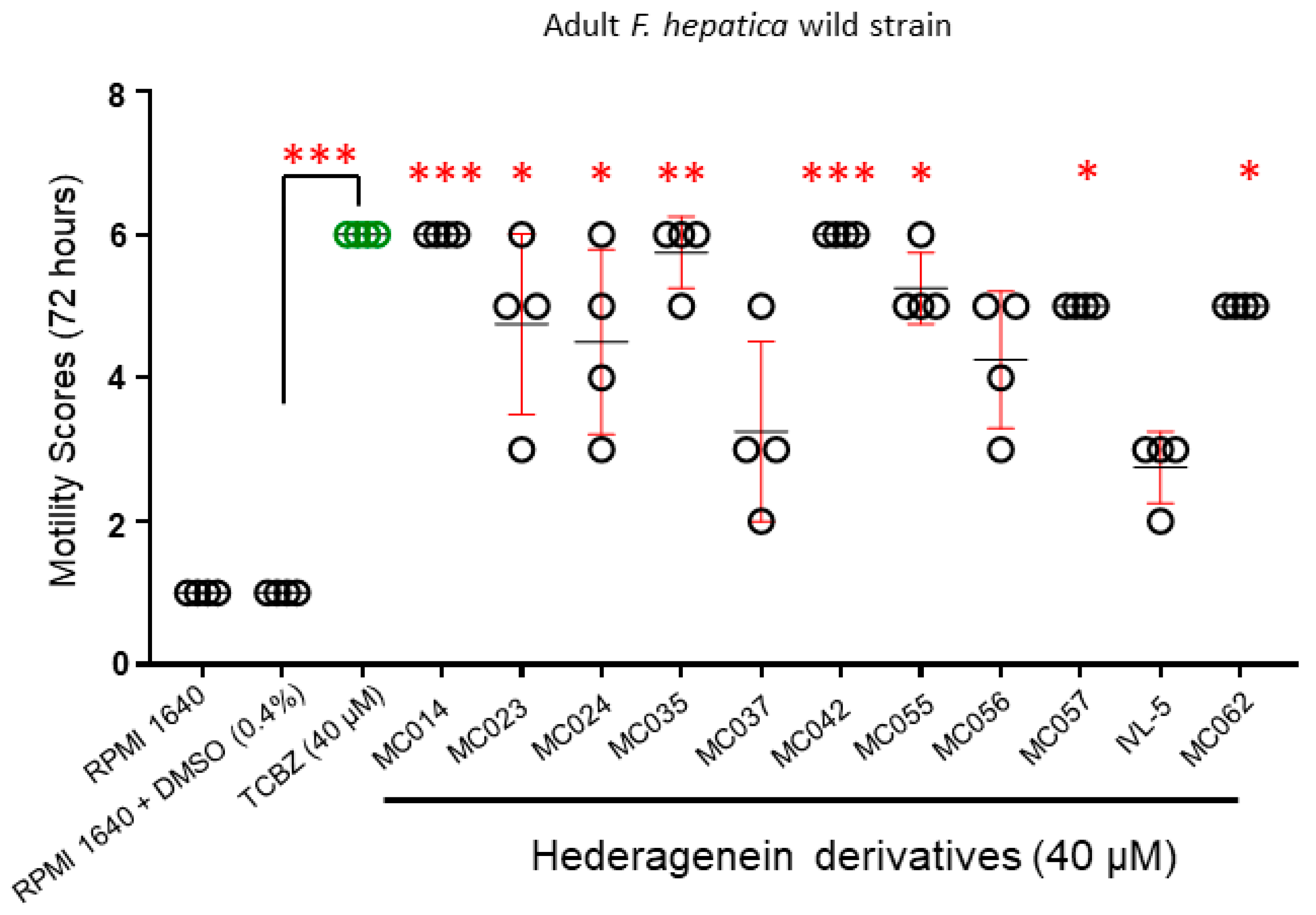
2.4.2. Activity against Adult Liver Flukes (Wild Strain)
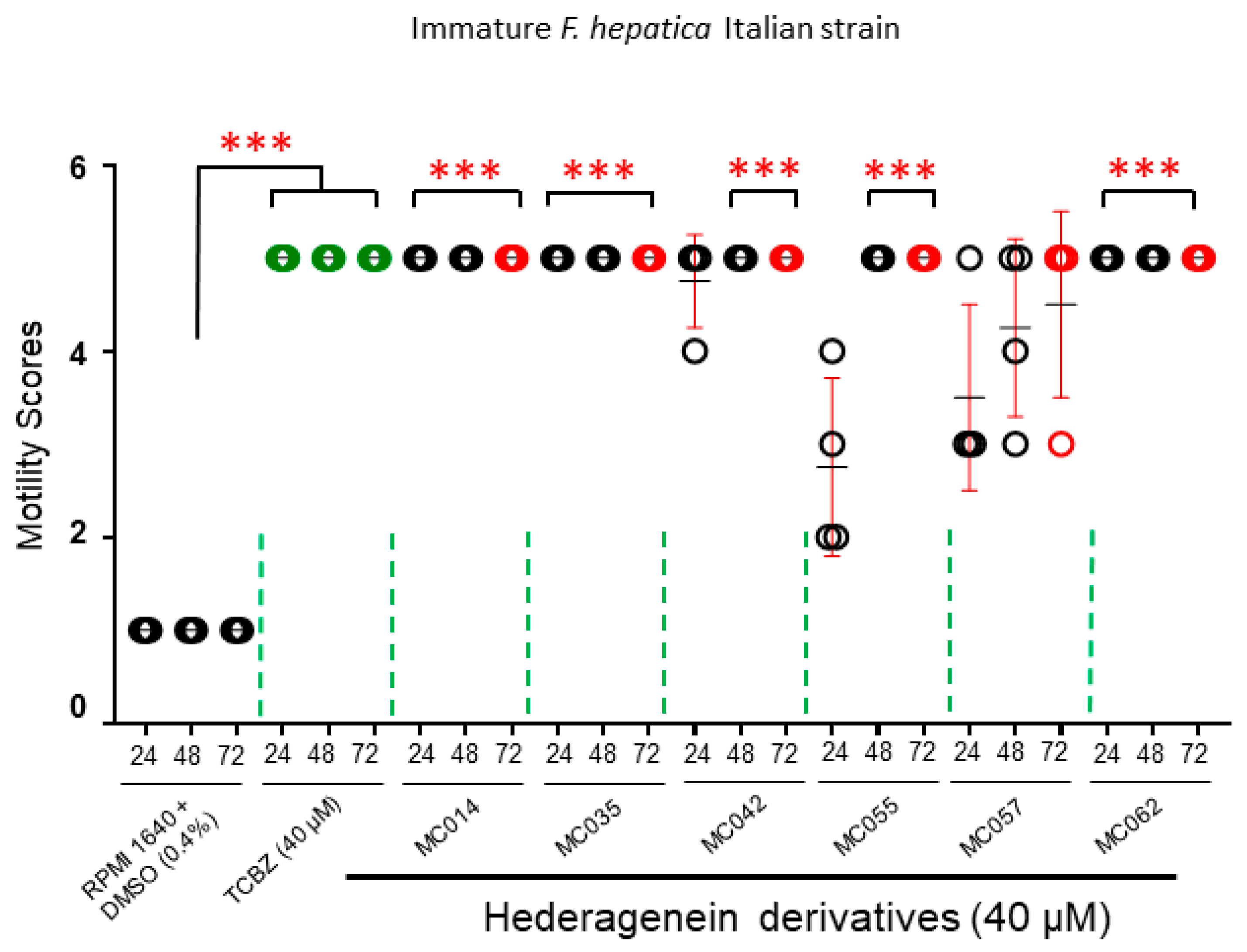
2.4.3. Activity against Immature Liver Flukes (Italian Strain)
2.4.4. Dose Response Studies on Adult Liver Flukes (Italian Strain)
2.5. MTT Assay on MDBK Cells
2.6. Statistics
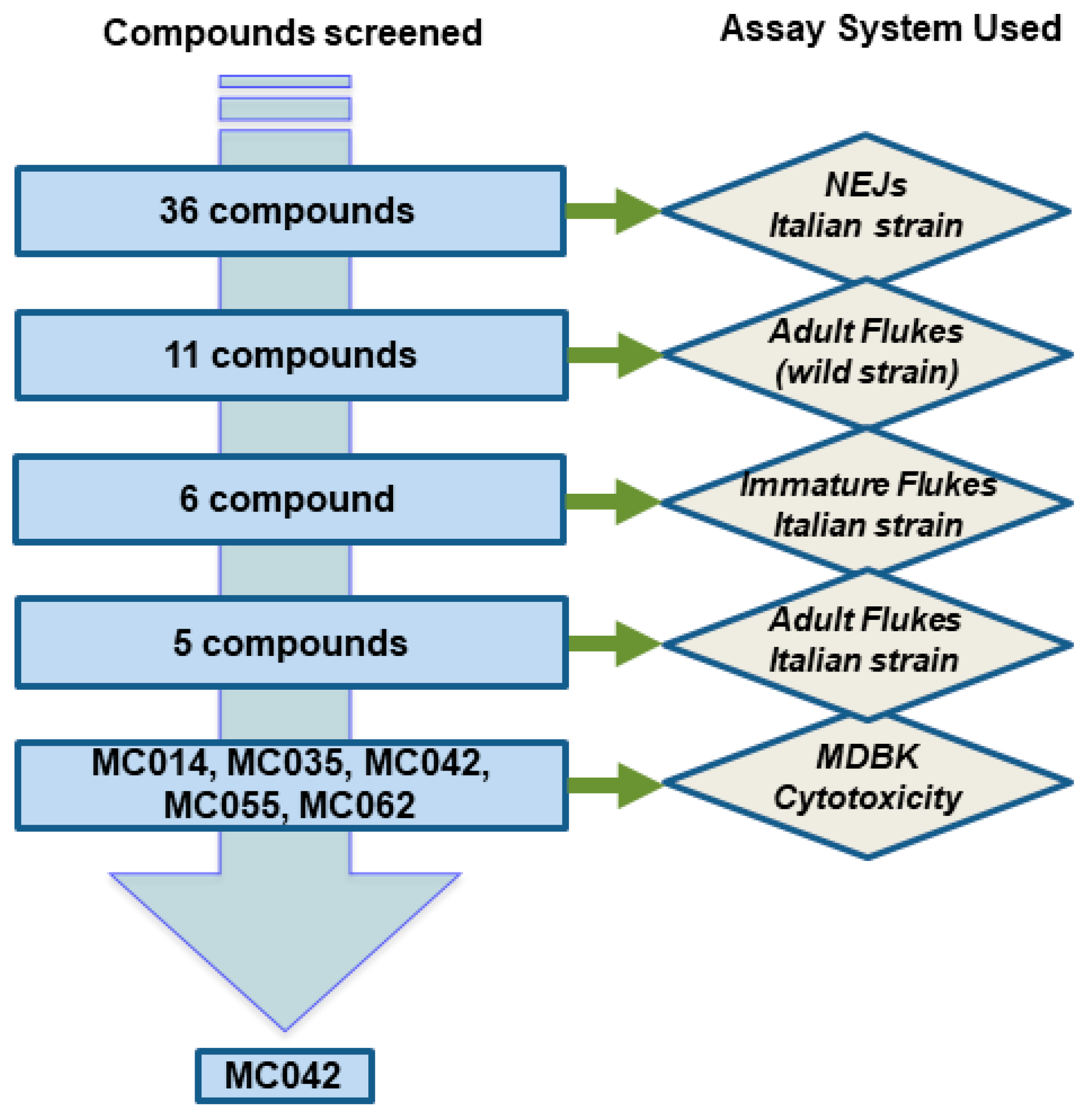
3. Results
4. Discussion
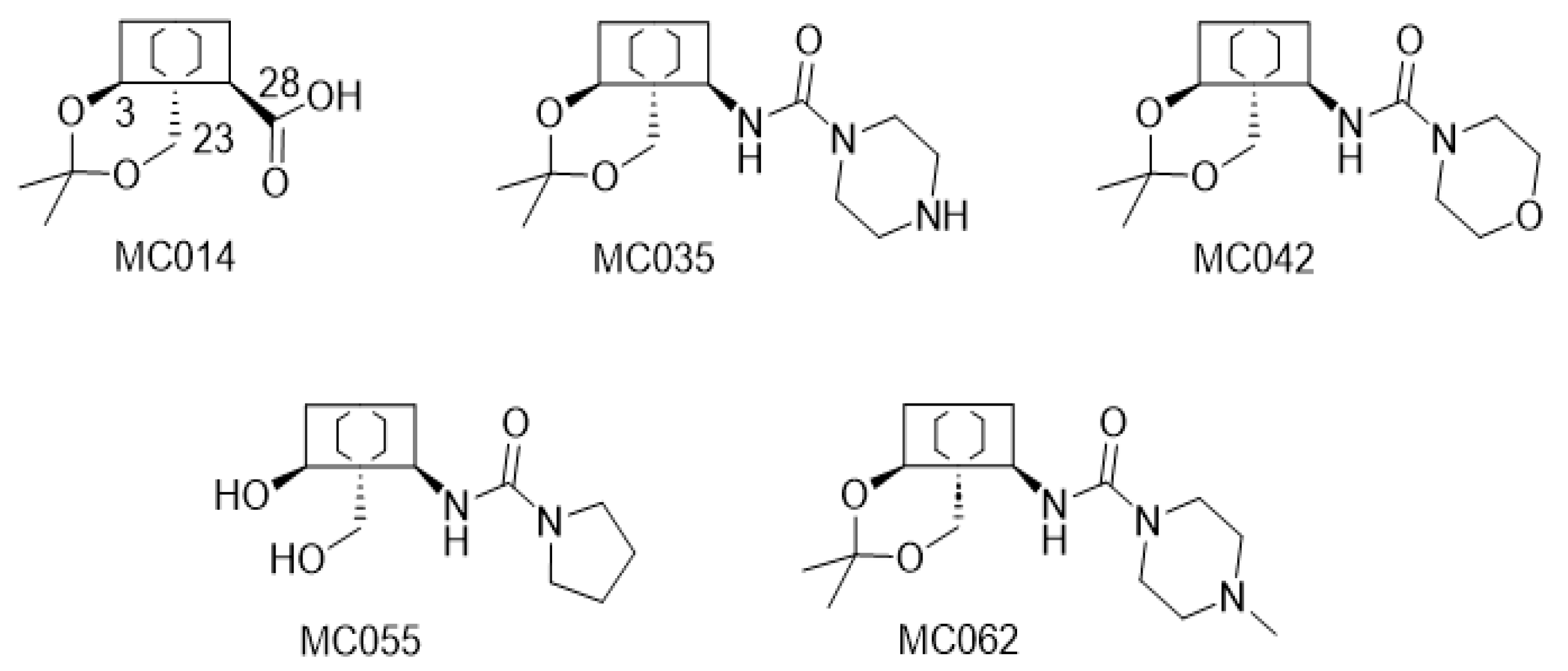
4.1. The Compounds Evaluated
4.2. Effect of Compounds on Different F. hepatica Life Stages
4.3. Structure-Activity Relationship Investigations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, L.S.; Janovy, J.; Schmidt, G.D. Foundations of Parasitology, 6th ed.; McGraw-Hill Education: Boston, MA, USA, 2000. [Google Scholar]
- Rojo-Vazquez, F.A.; Meana, A.; Valcarcel, F.; Martinez-Valladares, M. Update on trematode infections in sheep. Vet. Parasitol. 2012, 189, 15–38. [Google Scholar] [CrossRef]
- Alemneh, T. An Introductory to Fasciolosis. Concepts Dairy Vet. Sci. 2019, 2, 190–194. [Google Scholar] [CrossRef]
- McNulty, S.N.; Tort, J.F.; Rinaldi, G.; Fischer, K.; Rosa, B.A.; Smircich, P.; Fontenla, S.; Choi, Y.-J.; Tyagi, R.; Hallsworth-Pepin, K.; et al. Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia endobacteria related to the agents of potomac horse and human sennetsu fevers. PLoS Genet. 2017, 13, e1006537. [Google Scholar] [CrossRef] [PubMed]
- Piedrafita, D.; Spithill, T.W.; Smith, R.E.; Raadsma, H.W. Improving animal and human health through understanding liver fluke immunology. Parasite Immunol. 2010, 32, 572–581. [Google Scholar] [CrossRef]
- Fox, N.J.; White, P.C.; McClean, C.J.; Marion, G.; Evans, A.; Hutchings, M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS ONE 2011, 6, e16126. [Google Scholar] [CrossRef]
- Winkelhagen, A.J.S.; Mank, T.; de Vries, P.J.; Soetekouw, R. Apparent triclabendazole-resistant human Fasciola hepatica infection, the Netherlands. Emerg. Infect. Dis. 2012, 18, 1028–1029. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Barratt, A.; Fox, N.J.; Ahmadi, B.V.; Hutchings, M.R. Financial Impacts of Liver Fluke on Livestock Farms Under Climate Change—A Farm Level Assessment. Front. Vet. Sci. 2020, 7, 564795. [Google Scholar] [CrossRef]
- Mazeri, S.; Rydevik, G.; Handel, I.; Bronsvoort, B.M.D.; Sargison, N. Estimation of the impact of Fasciola hepatica infection on time taken for UK beef cattle to reach slaughter weight. Sci. Rep. 2017, 7, 7319. [Google Scholar] [CrossRef]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing drugs for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef]
- Barrera, B.; Otero, J.A.; Egido, E.; Prieto, J.G.; Seelig, A.; Álvarez, A.I.; Merino, G. The anthelmintic triclabendazole and its metabolites inhibit the membrane transporter ABCG2/BCRP. Antimicrob. Agents Chemother. 2012, 56, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.P.; Fairweather, I.; Trudgett, A.; Hoey, E.; McConville, M.; Meaney, M.; Robinson, M.; McFerran, N.; Ryan, L.; Lanusse, C.; et al. Understanding triclabendazole resistance. Exp. Mol. Pathol. 2007, 82, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Zadoks, R.; Skuce, P.; Sargison, N. Confirmation of triclabendazole resistance in liver fluke in the UK. Vet. Rec. 2012, 171, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.M.; Elliott, T.P.; Beddoe, T.; Anderson, G.; Skuce, P.; Spithill, T.W. Current Threat of Triclabendazole Resistance in Fasciola hepatica. Trends Parasitol. 2016, 32, 458–469. [Google Scholar] [CrossRef]
- Anderson, O.; Beckett, J.; Briggs, C.C.; Natrass, L.A.; Cranston, C.F.; Wilkinson, E.J.; Owen, J.H.; Mir Williams, R.; Loukaidis, A.; Bouillon, M.E.; et al. An investigation of the antileishmanial properties of semi-synthetic saponins. RSC Med. Chem. 2020, 11, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.G.; Bouzada, M.L.M.; Fabri, R.L.; Matos, M.d.O.; Moreira, F.O.; Scio, E.; Coimbra, E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007, 111, 396–402. [Google Scholar] [CrossRef]
- Edwards, J.; Brown, M.; Peak, E.; Bartholomew, B.; Nash, R.J.; Hoffmann, K.F. The diterpenoid 7-keto-sempervirol, derived from Lycium chinense, displays anthelmintic activity against both Schistosoma mansoni and Fasciola hepatica. PLoS Negl. Trop. Dis. 2015, 9, e0003604. [Google Scholar] [CrossRef]
- Majester-Savornin, B.; Elias, R.; Diaz-Lanza, A.M.; Balansard, G.; Gasquet, M.; Delmas, F. Saponins of the ivy plant, Hedera helix, and their Leishmanicidic activity. Planta Med. 1991, 57, 260–262. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Chávez-Fumagalli, M.A.; Valadares, D.G.; Franca, J.R.; Lage, P.S.; Duarte, M.C.; Andrade, P.H.R.; Martins, V.T.; Costa, L.E.; Arruda, A.L.A.; et al. Antileishmanial activity and cytotoxicity of Brazilian plants. Exp. Parasitol. 2014, 143, 60–68. [Google Scholar] [CrossRef]
- Chakroborty, A.; Pritchard, D.; Bouillon, M.E.; Cervi, A.; Cookson, A.; Wild, C.; Fenn, C.; Payne, J.; Holdsworth, P.; Capner, C.; et al. Flukicidal effects of abietane diterpenoid derived analogues against the food borne pathogen Fasciola hepatica. Vet. Parasitol. 2022, 309, 109766. [Google Scholar] [CrossRef]
- Crusco, A.; Bordoni, C.; Chakroborty, A.; Whatley, K.C.L.; Whiteland, H.; Westwell, A.D.; Hoffmann, K.F. Design, synthesis and anthelmintic activity of 7-keto-sempervirol analogues. Eur. J. Med. Chem. 2018, 152, 87–100. [Google Scholar] [CrossRef]
- Whiteland, H.L.; Chakroborty, A.; Forde-Thomas, J.E.; Crusco, A.; Cookson, A.; Hollinshead, J.; Fenn, C.A.; Bartholomew, B.; Holdsworth, P.A.; Fisher, M.; et al. An Abies procera-derived tetracyclic triterpene containing a steroid-like nucleus core and a lactone side chain attenuates in vitro survival of both Fasciola hepatica and Schistosoma mansoni. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 465–474. [Google Scholar] [CrossRef]
- Faizal, A.; Geelen, D. Saponins and their role in biological processes in plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Lorent, J.; Le Duff, C.S.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, α- and δ-Hederin. J. Biol. Chem. 2013, 288, 14000–14017. [Google Scholar] [CrossRef]
- Bun, S.-S.; Elias, R.; Baghdikian, B.; Ciccolini, J.; Ollivier, E.; Balansard, G. α -hederin potentiates 5-FU antitumor activity in human colon adenocarcinoma cells. Phytother. Res. 2008, 22, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Danloy, S.; Quetin-Leclercq, J.; Coucke, P.; De Pauw-Gillet, M.C.; Elias, R.; Balansard, G.; Angenot, L.; Bassleer, R. Effects of alpha-hederin, a saponin extracted from Hedera helix, on cells cultured in vitro. Planta Med. 1994, 60, 45–49. [Google Scholar] [CrossRef]
- Hooshyar, H.; Talari, S.; Feyzi, F. Therapeutic effect of Hedera helix alcoholic extract against Cutaneous Leishmaniasis caused by Leishmania major in Balb/C mice. Jundishapur J. Microbiol. 2014, 7, e9432. [Google Scholar] [CrossRef]
- Quetin-Leclercq, J.; Elias, R.; Balansard, G.; Bassleer, R.; Angenot, L. Cytotoxic Activity of Some Triterpenoid Saponins. Planta Med. 1992, 58, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Yeo, S.G.; Hong, E.H.; Lee, B.R.; Kim, J.W.; Kim, J.H.; Jeong, H.G.; Kwon, Y.S.; Kim, H.P.; Lee, S.; et al. Antiviral activity of hederasaponin B from Hedera helix against enterovirus 71 subgenotypes C3 and C4a. Biomol. Ther. 2014, 22, 41–46. [Google Scholar] [CrossRef]
- Julien, J.; Gasquet, M.; Maillard, C.; Balansard, G.; Timon-David, P. Extracts of the Ivy Plant, Hedera helix, and their anthelminthic activity on liver flukes. Planta Med. 1985, 51, 205–208. [Google Scholar] [CrossRef]
- Rinaldi, L.; Biggeri, A.; Musella, V.; de Waal, T.; Hertzberg, H.; Mavrot, F.; Torgerson, P.R.; Selemetas, N.; Coll, T.; Bosco, A.; et al. Sheep and Fasciola hepatica in Europe: The GLOWORM experience. Geospat. Health 2015, 9, 309–317. [Google Scholar] [CrossRef]
- Bouillon, M.E.; Bertocco, K.; Bischoff, L.; Buri, M.; Davies, L.R.; Wilkinson, E.J.; Lahmann, M. Synthesis of Anemoclemosides A and B, two saponins isolated from Anemoclema glaucifolium. Eur. J. Org. Chem. 2020, 48, 7470–7473. [Google Scholar] [CrossRef]
- Al-dulayymi, J.R.; Baird, M.; Bouillon, M.E.; Duval, S.; Ramos Morales, E.; Newbold, C.J.; Preskett, D.; Braganka, R.; Strawson, S.W.; Wehrli, C.; et al. New Bis Esters of Ivy Sapogenins for Ruminants. Patent No. WO/2016/193309, 8 December 2016. [Google Scholar]
- Singh, S.K.; Tripathi, V.J.; Singh, R.H. 3β,24-Dihydroxyolean-12-en-28-oic acid, a pentacyclic triterpene acid from Lantana indica. Phytochemistry 1990, 29, 3360–3362. [Google Scholar] [CrossRef]
- Caldwell, J.C. Alterations in cell proliferation, cell death, or nutrient supply. In Tumour Site Concordance and Mechanisms of Carcinogenesis; IARC Scientific Publications: Lyon, France, 2021; pp. 117–128. [Google Scholar]
- Ramdani, D.; Yuniarti, E.; Jayanegara, A.; Chaudhry, A.S. Roles of essential oils, polyphenols, and saponins of medicinal plants as natural additives and anthelmintics in ruminant diets: A systematic review. Animals 2023, 13, 767. [Google Scholar] [CrossRef]
- Ali, N.; Shah, S.W.A.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium Stocksianum boiss. BMC Complement. Altern. Med. 2011, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, M.; Tava, A.; Mancini, S.; Tedesco, D.; Perrucci, S. In vitro anthelmintic activity of saponins from Medicago spp. against sheep gastrointestinal mematodes. Molecules 2020, 25, 242. [Google Scholar] [CrossRef]
- Maestrini, M.; Tava, A.; Mancini, S.; Salari, F.; Perrucci, S. In vitro anthelmintic activity of saponins derived from Medicago spp. plants against donkey gastrointestinal nematodes. Vet. Sci. 2019, 6, 35. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, J.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X. Anthelmintic efficacy of natural saponins against Gyrodactylus kobayashii in goldfish (Carassius auratus) and their 3D-QSAR analysis. Parasitol. Res. 2021, 120, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.; Lins, L.; Domenech, Ò.; Quetin-Leclercq, J.; Brasseur, R.; Mingeot-Leclercq, M.P. Domain formation and permeabilization induced by the saponin α-hederin and its aglycone hederagenin in a cholesterol-containing bilayer. Langmuir 2014, 30, 4556–4569. [Google Scholar] [CrossRef]
- Gao, Y.; He, C.; Bi, W.; Wu, G.; Altman, E. Bioassay guided fractionation identified hederagenin as a major cytotoxic agent from Cyclocarya paliurus Leaves. Planta Med. 2016, 82, 171–179. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, T.; Xue, M.; Chen, J.; Feng, L.; Du, R.; Feng, Y. Current knowledge and development of hederagenin as a promising medicinal agent: A comprehensive review. RSC Adv. 2018, 8, 24188–24202. [Google Scholar] [CrossRef]
- Upegui Zapata, Y.A.; Echeverri, F.; Quiñones, W.; Torres, F.; Nacher, M.; Rivas, L.I.; Meira, C.D.S.; Gedamu, L.; Escobar, G.; Archbold, R.; et al. Mode of action of a formulation containing hydrazones and saponins against leishmania spp. Role in mitochondria, proteases and reinfection process. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, Y.; Jørgensen, L.V.G.; Strobel, B.W.; Hansen, H.C.B.; Cedergreen, N. Where does the toxicity come from in saponin extract? Chemosphere 2018, 204, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.; Zhou, J.Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.F.; Yuan, J.R.; Zhao, B.J.; Feng, L.; Jia, X.B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014, 14, 412. [Google Scholar] [CrossRef] [PubMed]
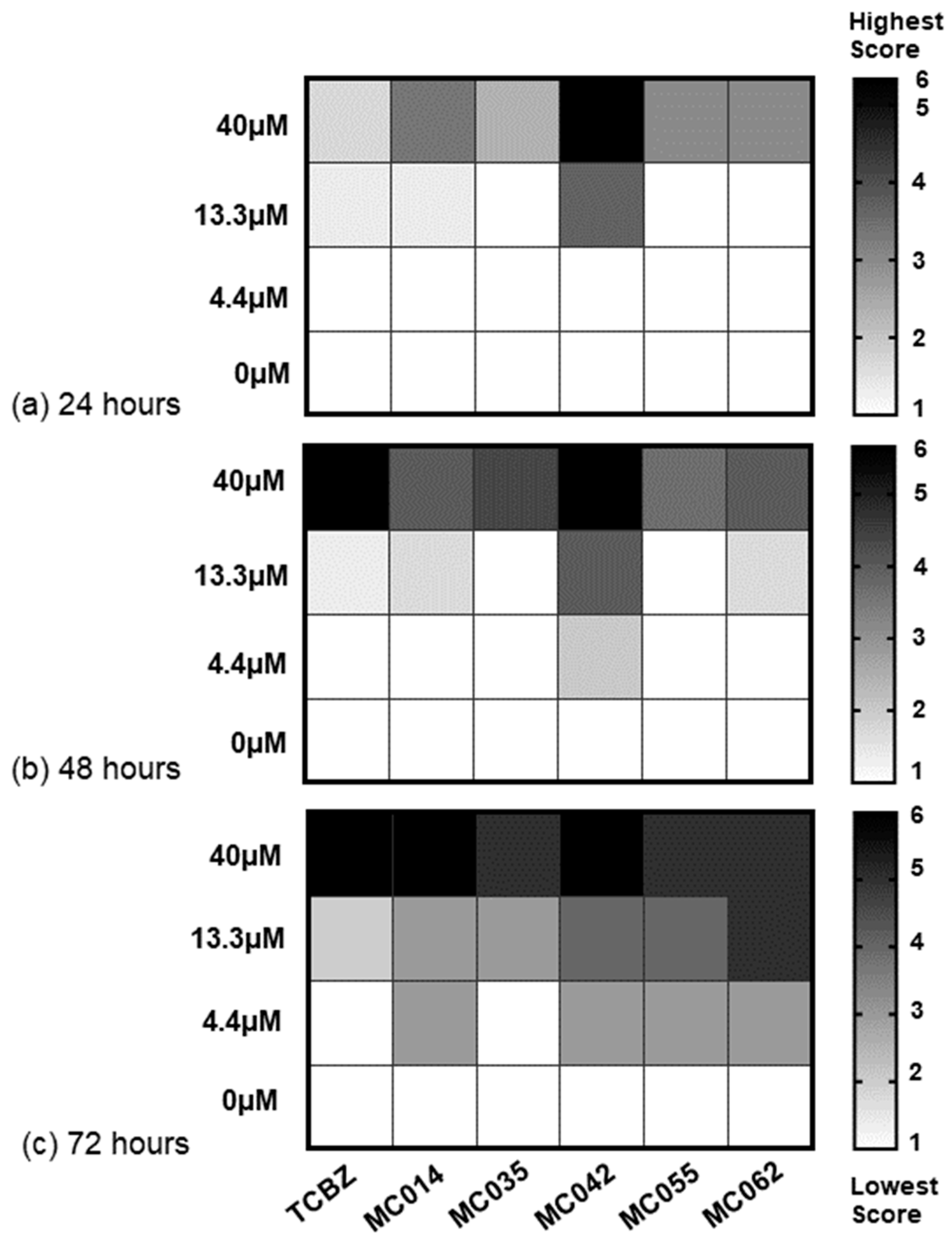
| Compound | Biological Material and Parameters Assessed | EC50 (Parasites) and CC50 (Cells) Values b (µM) with 95% Lower and Higher Confidence Intervals (CI) Indicated | Selectivity Indices (SI) | |
|---|---|---|---|---|
| MC014 | Adult | Motility | 13.46 (7.07; 23.62) | Not determined |
| MDBK | Cytotoxicity | Undefined c | ||
| MC035 | Adult | Motility | Not determined d | Not determined |
| MDBK | Cytotoxicity | 79.84 (46.45; 198.9) | ||
| MC042 a | Immature | Motility | 1.07 (0.07; 3.04) | 44.37 |
| Adult | Motility | 13.02 e | 3.64 | |
| MDBK | Cytotoxicity | 47.48 (36.41; 65.29) | ||
| MC055 | Adult | Motility | Not determined d | Not determined |
| MDBK | Cytotoxicity | 34.70 (29.93; 40.11) | ||
| MC062 | Adult | Motility | Not determined d | Not determined |
| MDBK | Cytotoxicity | 13.65 (11.59; 16.05) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakroborty, A.; Pritchard, D.R.; Bouillon, M.E.; Cervi, A.; Kraehenbuehl, R.; Wild, C.; Fenn, C.; Holdsworth, P.; Capner, C.; Padalino, G.; et al. Modified Hederagenin Derivatives Demonstrate Ex Vivo Anthelmintic Activity against Fasciola hepatica. Pharmaceutics 2023, 15, 1869. https://doi.org/10.3390/pharmaceutics15071869
Chakroborty A, Pritchard DR, Bouillon ME, Cervi A, Kraehenbuehl R, Wild C, Fenn C, Holdsworth P, Capner C, Padalino G, et al. Modified Hederagenin Derivatives Demonstrate Ex Vivo Anthelmintic Activity against Fasciola hepatica. Pharmaceutics. 2023; 15(7):1869. https://doi.org/10.3390/pharmaceutics15071869
Chicago/Turabian StyleChakroborty, Anand, Deiniol R. Pritchard, Marc E. Bouillon, Anna Cervi, Rolf Kraehenbuehl, Charlotte Wild, Caroline Fenn, Peter Holdsworth, Colin Capner, Gilda Padalino, and et al. 2023. "Modified Hederagenin Derivatives Demonstrate Ex Vivo Anthelmintic Activity against Fasciola hepatica" Pharmaceutics 15, no. 7: 1869. https://doi.org/10.3390/pharmaceutics15071869
APA StyleChakroborty, A., Pritchard, D. R., Bouillon, M. E., Cervi, A., Kraehenbuehl, R., Wild, C., Fenn, C., Holdsworth, P., Capner, C., Padalino, G., Forde-Thomas, J. E., Payne, J., Smith, B. G., Fisher, M., Lahmann, M., Baird, M. S., & Hoffmann, K. F. (2023). Modified Hederagenin Derivatives Demonstrate Ex Vivo Anthelmintic Activity against Fasciola hepatica. Pharmaceutics, 15(7), 1869. https://doi.org/10.3390/pharmaceutics15071869