Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment
Abstract
1. Introduction
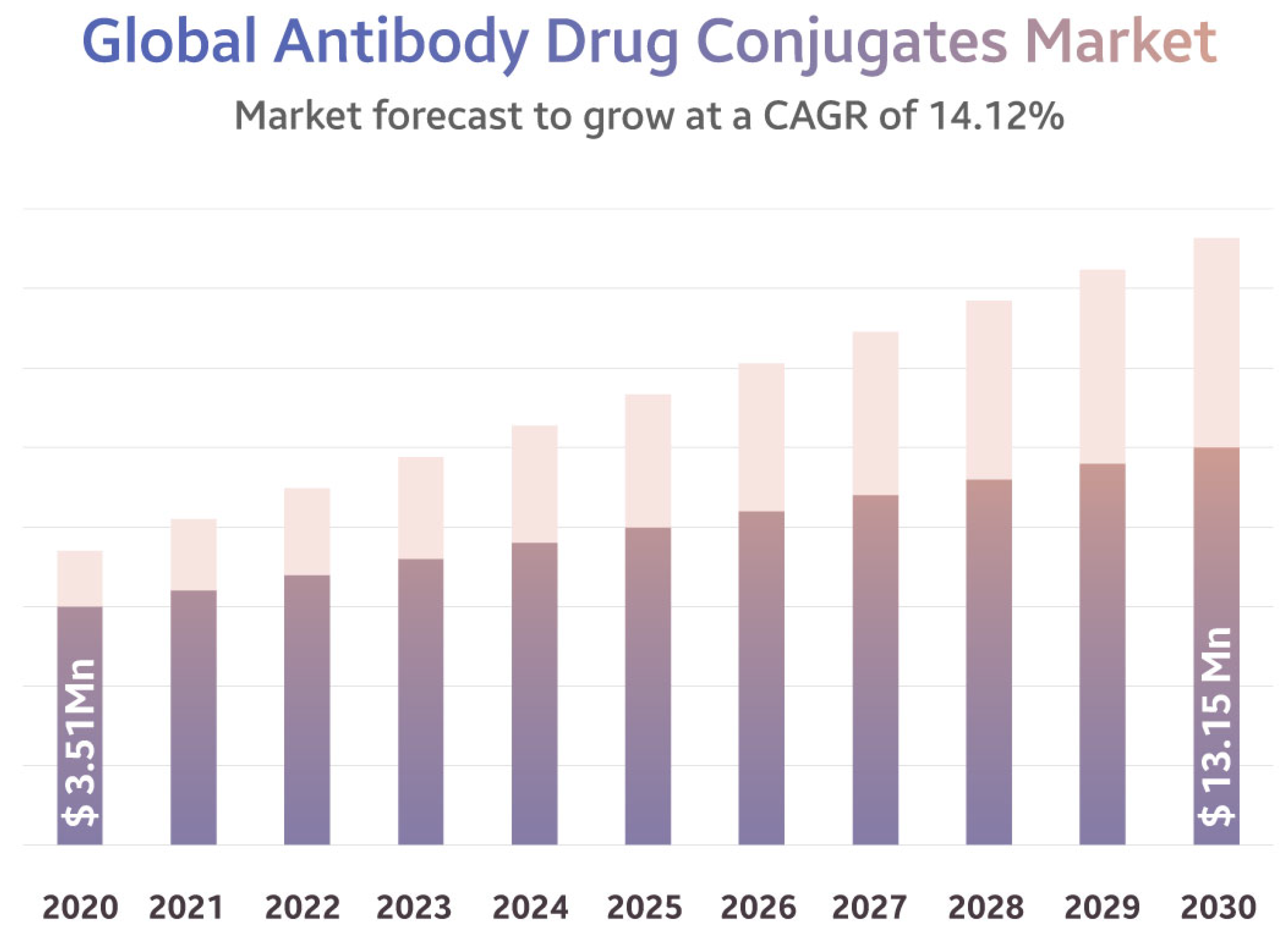
2. The Market of ADCs
3. Nanotechnology in Medical Biotechnology
3.1. Nanoparticle-Based Drug Delivery
3.2. Gene Therapy
3.3. Photodynamic Therapy
3.4. Immunotherapy
3.5. Tissue Engineering
3.6. Diagnostic Imaging
4. R&D of ADC Technologies
- mAb selection: For targeting, biologics, such as mAbs, fragments, and other backbones (e.g., single-chain variable fragment (scFv), affibody, Pentarin, and antibody–cytokine fusion proteins), are used to target HER2 or other antigens. Generally, lysines with free amines are more common than cysteines with disulfides and are not evenly distributed in the antibody [56,57]. However, Genentech is currently testing modified antibodies with engineered cysteines. Antibody mimetics are also the focus of recent research due to their importance. The site specificity/efficacy of the composed drug can be improved by adding a new building block as an antibody mimetic, e.g., single domain antibody [58], nanobody [59], or affibody [60].
- Toxins: There are numerous cytotoxic drugs that can be used as payloads, such as Maytansine (DM1, DM4), Auristatine (MMAE), SN-38, Doxorubicin, and Duocarmycin analogues [61].
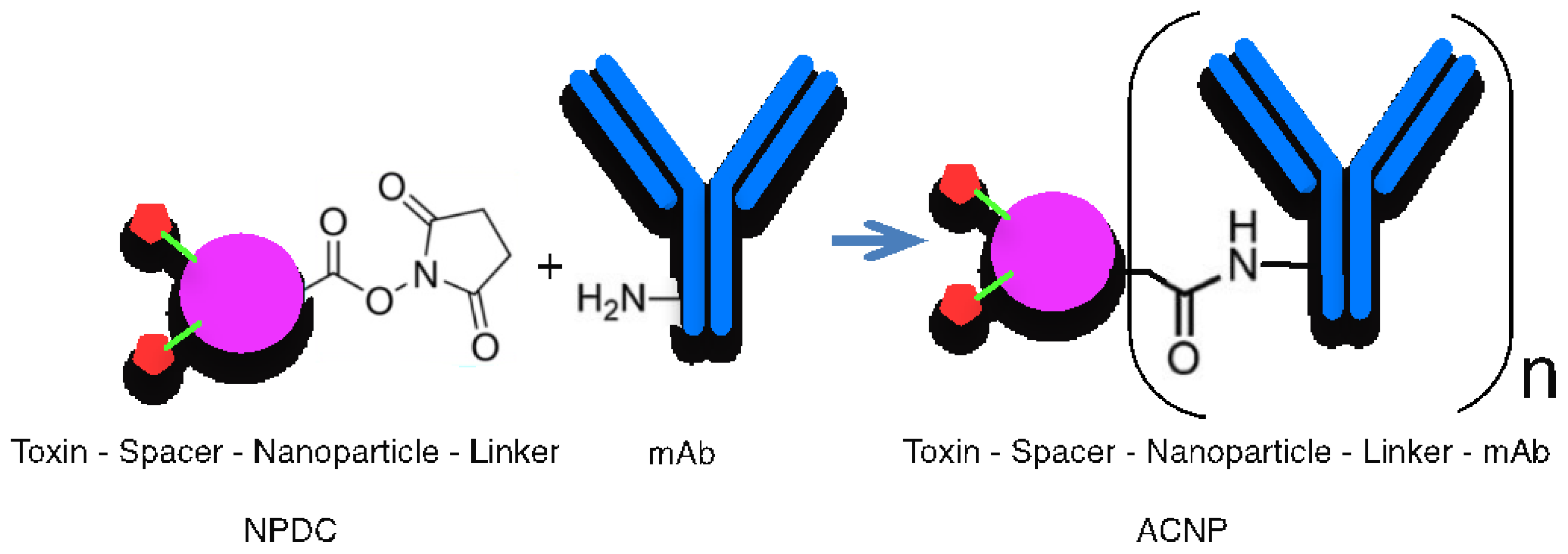
- Linker selection: Linkers have a crucial role in the ADC and ACNP constructions, respectively. This part of the construction is responsible for the stability of cargo. The linker must be stable during the circulation in the bloodstream to avoid the leakage of drug molecules. A class of linkers is designed according to bio-orthogonal chemistry [62], which allows cleavage in the microenvironment of cancer cells [63]. Linkers can be either cleavable or non-cleavable, with various types of cleavable linkers, such as chemically labile linkers and enzyme-cleavable linkers (e.g., pH-sensitive linkers, disulfide linkers, peptide linkers, β-glucuronide linkers, and aldehyde tags [64]). Examples of linker platforms include the ImmunoGen Platform, Val-Cit, Disulphide, and Hydrazon [65]. For the nondegradable linkers, the connection of the cytotoxic and the antibody is non-sensitive to proteolytic degradation [66].
5. Challenges and Business Risks in R&D of ADCs
5.1. Risks for R&D, Limits, and Failures
- Unreliable published data;
- Biopharmaceutical issues, such as suboptimal pharmacokinetics;
- Poorly predictive preclinical models used in discovery research and preclinical testing;
- The concept of target-based drug discovery, which involves complex target selection, competition for proprietary targets, and the validation process;
- Complexities of clinical trials, particularly in treating chronic diseases, along with increasing demands from regulatory authorities and payers.
- Complex Manufacturing: ADCs are complex molecules that require precise conjugation of the antibody and the cytotoxic agent. The manufacturing process can be challenging and time-consuming, and any variability in the manufacturing process can affect the quality and efficacy of the final product.
- Regulatory Challenges: ADCs are subject to strict regulatory oversight, and the approval process can be lengthy and expensive. Regulators require extensive data on the safety and efficacy of ADCs, including data on the pharmacokinetics, pharmacodynamics, and toxicology of the drug.
- Resistance: As with any cancer therapy, the development of resistance is a significant challenge for ADCs. Cancer cells can develop resistance to the antibody, the cytotoxic agent, or both, rendering the ADC ineffective.
- Intellectual Property: ADC development involves complex intellectual property issues, including patenting of the antibody, the linker, and the cytotoxic agent. Companies must navigate these issues carefully to avoid infringement and protect their intellectual property.
- Cost: Developing ADCs can be extremely expensive, with high costs associated with manufacturing, clinical trials, and regulatory approval. There is also significant competition in the market, which can drive down prices and limit profitability.
- Specialized multidisciplinary expertise is required, with a group of mixed academia and regulatory experts pooling their knowledge of quality, safety, and kinetics to support evaluation and formulate guidelines [83].
- Close cooperation with other scientific committees (such as the Scientific Committee on Emerging and Newly Identified Health Risks and the European Food Safety Authority), networks (such as the Nanotechnology Knowledge Base and the European Technology Platform for Nanomedicine), and the European Commission [83].
- International cooperation, with EMA chairing an international expert group that includes the US FDA, Japan MHLW, Health Canada, and TGA Australia.
5.2. Case Studies
5.3. New Forms for R&D
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Antibody–Drug Conjugate |
| mAb | Monoclonal Antibody |
| HER | Human Epidermal Growth Factor |
| TZM | Trastuzumab |
| CAGR | Compound Annual Growth Rate |
| ADCC | Antibody-Dependent Cell-Mediated Cytotoxicity |
| CDC | Complement-Dependent Cytotoxicity |
| MRI | Magnetic Resonance Imaging |
| CT | Computer Tomography |
| ACNP | Antibody-Conjugated Nanoparticle |
| NPDC | Nanoparticle–Drug Conjugate |
| PDUFA | Prescription Drug User Fee Act |
| ROI | Return of Investment |
| NME | New Molecular Entity |
| M&A | Mergers and Acquisitions |
| PBF | Project-Based Firm |
| PNO | Project Network Organization |
References
- Fernald, K.D.S.; Pennings, H.P.G.; Bosch, J.F.V.D.; Commandeur, H.R.; Claassen, E. The moderating role of absorptive capacity and the differential effects of acquisitions and alliances on Big Pharma firms’ innovation performance. PLoS ONE 2017, 12, e0172488. [Google Scholar] [CrossRef]
- Bayer ADC Fails Pivotal Mesothelioma Trial. Available online: https://www.fiercebiotech.com/biotech/bayer-adc-fails-pivotal-mesothelioma-trial (accessed on 24 July 2017).
- Pfizer Invests $43 Billion to Battle Cancer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-invests-43-billion-battle-cancer (accessed on 13 March 2023).
- With $43B Buyout, Pfizer Sees Cancer Specialist Seagen as a ‘Goose’ Laying ‘Golden Eggs’. Available online: https://www.fiercepharma.com/pharma/43b-buyout-pfizer-sees-seagen-its-golden-goose (accessed on 13 March 2023).
- After Dose De-Escalation, Death Drives Magenta to Pause Antibody-Drug Conjugate Leukemia Trial. Available online: https://www.fiercebiotech.com/biotech/after-dose-de-escalation-death-drives-magenta-pause-antibody-drug-conjugate-leukemia-trial (accessed on 26 January 2023).
- Zolot, R.S.; Basu, S.; Million, R.P. Antibody–drug conjugates. Nat. Rev. Drug Discov. 2013, 12, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Kapinos, K.A.; Hu, E.; Trivedi, J.; Geethakumari, P.R.; Kansagra, A. Cost-Effectiveness Analysis of CAR T-Cell Therapies vs Antibody Drug Conjugates for Patients with Advanced Multiple Myeloma. Cancer Control. 2023, 30, 10732748221142945. [Google Scholar] [CrossRef]
- Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef]
- Wu, M.; Huang, W.; Yang, N.; Liu, Y. Learn from antibody–drug conjugates: Consideration in the future construction of peptide-drug conjugates for cancer therapy. Exp. Hematol. Oncol. 2022, 11, 93. [Google Scholar] [CrossRef]
- Espelin, C.W.; Leonard, S.C.; Geretti, E.; Wickham, T.J.; Hendriks, B.S. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res. 2016, 76, 1517–1527. [Google Scholar] [CrossRef]
- Hu, X.; Kwon, N.; Yan, K.; Sedgwick, A.C.; Chen, G.; He, X.; James, T.D.; Yoon, J. Bio-Conjugated Advanced Materials for Targeted Disease Theranostics. Adv. Funct. Mater. 2020, 30, 1907906. [Google Scholar] [CrossRef]
- Rodallec, A.; Franco, C.; Robert, S.; Sicard, G.; Giacometti, S.; Lacarelle, B.; Bouquet, F.; Savina, A.; Lacroix, R.; Dignat-George, F.; et al. Prototyping Trastuzumab Docetaxel Immunoliposomes with a New FCM-Based Method to Quantify Optimal Antibody Density on Nanoparticles. Sci. Rep. 2020, 10, 4147. [Google Scholar] [CrossRef] [PubMed]
- Matusewicz, L.; Filip-Psurska, B.; Psurski, M.; Tabaczar, S.; Podkalicka, J.; Wietrzyk, J.; Ziółkowski, P.; Czogalla, A.; Sikorski, A.F. EGFR-targeted immunoliposomes as a selective delivery system of simvastatin, with potential use in treatment of triple-negative breast cancers. Int. J. Pharm. 2019, 569, 118605. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6018. [Google Scholar] [CrossRef] [PubMed]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting immunoliposomes to EGFR-positive glioblastoma. ESMO Open 2022, 7, 100365. [Google Scholar] [CrossRef]
- Mamot, C.; Wicki, A.; Hasler-Strub, U.; Riniker, S.; Li, Q.; Holer, L.; Bärtschi, D.; Zaman, K.; von Moos, R.; Dedes, K.J.; et al. A multicenter phase II trial of anti-EGFR-immunoliposomes loaded with doxorubicin in patients with advanced triple negative breast cancer. Sci. Rep. 2023, 13, 3705. [Google Scholar] [CrossRef]
- Kumar, A.; Lale, S.V.; Alex, M.A.; Choudhary, V.; Koul, V. Folic Acid and Trastuzumab Conjugated Redox Responsive Random Multiblock Copolymeric Nanocarriers for Breast Cancer Therapy: In-Vitro and in-Vivo Studies. Colloids Surf. B Biointerfaces 2017, 149, 369–378. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-Conjugated Paclitaxel Loaded PCL-PEG Worm-like Nanocrystal Micelles for the Combinatorial Treatment of HER2-Positive Breast Cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef] [PubMed]
- Kolahkaj, F.F.; Derakhshandeh, K.; Khaleseh, F.; Azandaryani, A.H.; Mansouri, K.; Khazaei, M. Active Targeting Carrier for Breast Cancer Treatment: Monoclonal Antibody Conjugated Epirubicin Loaded Nanoparticle. J. Drug Deliv. Sci. Technol. 2019, 53, 101136. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/Cisplatin Co-Loaded Hyaluronic Acid/Chitosan-Based Nanoparticles for in Vitro Synergistic Combination Chemotherapy of Breast Cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ling, Z.; Zhou, Z.; He, J.; Ran, H.; Wang, Z.; Zhang, Q.; Song, W.; Zhang, Y.; Luo, J. Herceptin-Decorated Paclitaxel-Loaded Poly(Lactide-Co-Glycolide) Nanobubbles: Ultrasound-Facilitated Release and Targeted Accumulation in Breast Cancers. Pharm. Dev. Technol. 2020, 25, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, G.A.; Pedersen, J.S.; Briscoe, W.H. Dendrimer nanofluids in the concentrated regime: From polymer melts to soft spheres. Langmuir 2015, 31, 3333–3342. [Google Scholar] [CrossRef]
- Choi, J.-H.; Gu, H.-J.; Park, K.-H.; Hwang, D.-S.; Kim, G.-C. Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines 2022, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.S.; Khalid, F.; Khan, M.T.; Samra, Z.Q.; Muhammad, S.; Zhang, Y.-J.; Mou, K. A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery. Molecules 2022, 27, 261. [Google Scholar] [CrossRef]
- Wilcock, P.; Webster, R.M. The breast cancer drug market. Nat. Rev. Drug Discov. 2021, 20, 339–340. [Google Scholar] [CrossRef]
- Antibody Drug Conjugate Market, a $13.15 billion Industry by 2030 with a CAGR of 14.12%. Available online: https://www.globenewswire.com/en/news-release/2022/06/21/2465821/0/en/Antibody-Drug-Conjugate-Market-a-13-15-billion-Industry-by-2030-with-a-CAGR-of-14-12.html (accessed on 21 June 2022).
- Pazo, C.D.; Nawaz, K.; Webster, R.M. The oncology market for antibody–drug conjugates. Nat. Rev. Drug Discov. 2021, 20, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Antibody-Drug Conjugates (ADCs) List Approved by FDA (2000–2023). Available online: https://axispharm.com/antibody-drug-conjugatesadcs-list-approved-by-fda2000-2022/ (accessed on 27 December 2022).
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Abbasi, M.; Moaddeli, M.R.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics 2022, 6, 400–423. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamada, Y.; Sato, Y.; Khalil, I.A.; Harashima, H. Innovative nanotechnologies for enhancing nucleic acids/gene therapy: Controlling intracellular trafficking to targeted biodistribution. Biomaterials 2019, 218, 119329. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Pan, M.; Yu, Y.; Sun, A.; Shi, K.; Qu, Y.; Qian, Z. Application of nanotechnology for enhancing photodynamic therapy via ameliorating, neglecting, or exploiting tumor hypoxia. View 2020, 1, e6. [Google Scholar] [CrossRef]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Akkın, S.; Varan, G.; Bilensoy, E. A Review on Cancer Immunotherapy and Applications of Nanotechnology to Chemoimmunotherapy of Different Cancers. Molecules 2021, 26, 3382. [Google Scholar] [CrossRef]
- Deng, C.; Xu, C.; Zhou, Q.; Cheng, Y. Advances of nanotechnology in osteochondral regeneration. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1576. [Google Scholar] [CrossRef]
- Kumar, R.; Aadil, K.R.; Ranjan, S.; Kumar, V.B. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101617. [Google Scholar] [CrossRef]
- Körhegyi, Z.; Rózsa, D.; Hajdu, I.; Bodnár, M.; Kertész, I.; Kerekes, K.; Kun, S.; Kollár, J.; Varga, J.; Garai, I.; et al. Synthesis of 68Ga-Labeled Biopolymer-based Nanoparticle Imaging Agents for Positron-emission Tomography. Anticancer Res. 2019, 39, 2415–2427. [Google Scholar] [CrossRef]
- Szabó, J.P.; Csige, K.; Kálmán-Szabó, I.; Arató, V.; Opposits, G.; Jószai, I.; Kertész, I.; Képes, Z.; Méhes, G.; Fenyvesi, F.; et al. In vivo assessment of tumor targeting potential of 68Ga-labelled randomly methylated beta-cyclodextrin (RAMEB) and 2-hydroxypropyl-β-cyclodextrin (HPβCD) using positron emission tomography. Int. J. Pharm. 2023, 630, 122462. [Google Scholar] [CrossRef]
- Hajdu, I.; Trencsényi, G.; Bodnár, M.; Emri, M.; Bánfalvi, G.; Sikula, J.; Márián, T.; Kollár, J.; Vámosi, G.; Borbély, J. Tumor-specific Localization of Self-assembled Nanoparticle PET/MR Modalities. Anticancer Res. 2014, 34, 49–59. [Google Scholar] [PubMed]
- Pallares, R.M.; Mottaghy, F.M.; Schulz, V.; Kiessling, F.; Lammers, T. Nanoparticle diagnostics and theranostics in the clinic. J. Nucl. Med. 2022, 63, 1802–1808. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent development of gold nanoparticles as contrast agents for cancer diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Nanomedicine Market Size, Share & Trends Analysis Report by Product (Therapeutics, Diagnostics), by Application (Oncology, Infectious Diseases), by Region, and Segment Forecasts, 2021–2028. Report ID: 978-1-68038-942-5. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/nanomedicine-market (accessed on 13 March 2023).
- Marques, M.R.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Bremer-Hoffmann, S.; Wacker, M.G. Nanomedicines—Tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151–152, 23–43. [Google Scholar] [CrossRef]
- Whaby, M.; Khan, I.; O’Bryan, J.P. Chapter Eight—Targeting The “Undruggable” RAS with Biologics. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2022; Volume 153, pp. 237–266. ISBN 9780128244852. ISSN 0065-230X. [Google Scholar] [CrossRef]
- Singh, K.; Canakci, M.; Kanjilal, P.; Williams, N.; Shanthalingam, S.; Osborne, B.A.; Thayumanavan, S. Evaluation of Cellular Targeting by Fab′ vs Full-Length Antibodies in Antibody–Nanoparticle Conjugates (ANCs) Using CD4 T-cells. Bioconjugate Chem. 2022, 33, 486–495. [Google Scholar] [CrossRef]
- Johnston, M.C.; Scott, C.J. Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy. Drug Discov. Today Technol. 2018, 30, 63–69. [Google Scholar] [CrossRef]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Gharibi, T.; Mardaneh, J.; Farhadihosseinabadi, B.; Larki, P.; Faghfourian, B.; Sepehr, K.S.; Abbaszadeh-Goudarzi, K.; Johari, B.; et al. Antibody–drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J. Cell. Physiol. 2018, 234, 5628–5642. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kong, T.W.S.; Khoo, J.Y.X.; Loh, T.-P. Recent developments in chemical conjugation strategies targeting native amino acids in proteins and their applications in antibody–drug conjugates. Chem. Sci. 2021, 12, 13613–13647. [Google Scholar] [CrossRef]
- Oltolina, F.; Colangelo, D.; Miletto, I.; Clemente, N.; Miola, M.; Verné, E.; Prat, M.; Follenzi, A. Tumor Targeting by Monoclonal Antibody Functionalized Magnetic Nanoparticles. Nanomaterials 2019, 9, 1575. [Google Scholar] [CrossRef]
- Żelechowska-Matysiak, K.; Wawrowicz, K.; Wierzbicki, M.; Budlewski, T.; Bilewicz, A.; Majkowska-Pilip, A. Doxorubicin- and Trastuzumab-Modified Gold Nanoparticles as Potential Multimodal Agents for Targeted Therapy of HER2+ Cancers. Molecules 2023, 28, 2451. [Google Scholar] [CrossRef]
- Zumaya, A.L.V.; Rimpelová, S.; Štějdířová, M.; Ulbrich, P.; Vilčáková, J.; Hassouna, F. Antibody Conjugated PLGA Nanocarriers and Superparmagnetic Nanoparticles for Targeted Delivery of Oxaliplatin to Cells from Colorectal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Hasan, M.; Laws, M.; Jin, P.; Rahman, K.M. Factors influencing the choice of monoclonal antibodies for antibody–drug conjugates. Drug Discov. Today 2021, 27, 354–361. [Google Scholar] [CrossRef]
- Nessler, I.; Khera, E.; Vance, S.; Kopp, A.; Qiu, Q.; Keating, T.A.; Abu-Yousif, A.O.; Sandal, T.; Legg, J.; Thompson, L.; et al. Increased Tumor Penetration of Single-Domain Antibody–Drug Conjugates Improves In Vivo Efficacy in Prostate Cancer Models. Cancer Res. 2020, 80, 1268–1278. [Google Scholar] [CrossRef]
- Salvador, J.-P.; Vilaplana, L.; Marco, M.-P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.Å.; Löfblom, J. Affibody Molecules in Biotechno-logical and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload diversification: A key step in the development of antibody–drug conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.C.B.; Kim, Y.C.; Bañas, S.; Barfield, R.M.; Drake, P.M.; Rupniewski, I.; Haskins, W.E.; Rabuka, D. Antibody-drug conjugate library prepared by scanning insertion of the aldehyde tag into IgG1 constant regions. mAbs 2018, 10, 1182–1189. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody–drug conjugates (ADCs) for cancer therapy. Cancer Cell Int. 2022, 22, 255. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2016, 9, 33–46. [Google Scholar] [CrossRef]
- Csikós, Z.; Fazekas, E.; Rózsa, D.; Borbély, J.; Kerekes, K. Crosslinked poly-γ-glutamic acid based nanosystem for drug delivery. J. Drug Deliv. Sci. Technol. 2018, 48, 478–489. [Google Scholar] [CrossRef]
- Csikós, Z.; Kerekes, K.; Fazekas, E.; Kun, S.; Borbély, J. Biopolymer based nanosystem for doxorubicin targeted delivery. Am. J. Cancer Res. 2017, 7, 715–726. [Google Scholar]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics 2023, 15, 216. [Google Scholar] [CrossRef]
- Aptamer Group and PinotBio Extend Collaboration for the Development of Optimer-Drug Conjugates. Available online: https://aptamergroup.com/aptamer-group-and-pinotbio-extend-collaboration-for-the-development-of-optimer-drug-conjugates/ (accessed on 23 May 2022).
- Aptamer Group and PinotBio Extend Collaboration. Available online: https://markets.ft.com/data/announce/detail?dockey=1323-15463214-6M6DQKFL242JNP55NP7RKRB7S6 (accessed on 23 May 2022).
- ADC Therapeutics Announces Results from Pivotal Phase 2 Clinical Trial of Camidanlumab Tesirine (Cami) in Relapsed or Refractory Hodgkin Lymphoma. Available online: https://ir.adctherapeutics.com/press-releases/press-release-details/2022/ADC-Therapeutics-Announces-Results-from-Pivotal-Phase-2-Clinical-Trial-of-Camidanlumab-Tesirine-Cami-in-Relapsed-or-Refractory-Hodgkin-Lymphoma/default.aspx (accessed on 10 June 2022).
- Spirea Raises £2.4M in Funding. Available online: https://www.finsmes.com/2022/06/spirea-raises-2-4m-in-funding.html (accessed on 14 June 2022).
- FDA Grants Regular Approval to Fam-Trastuzumab Deruxtecan-Nxki for Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-fam-trastuzumab-deruxtecan-nxki-breast-cancer (accessed on 5 November 2022).
- Burden, N.; Aschberger, K.; Chaudhry, Q.; Clift, M.J.D.; Fowler, P.; Johnston, H.; Landsiedel, R.; Rowland, J.; Stone, V.; Doak, S.H. Aligning nanotoxicology with the 3Rs: What is needed to realise the short, medium and long-term opportunities? Regul. Toxicol. Pharmacol. 2017, 91, 257–266. [Google Scholar] [CrossRef]
- Hogle, L.F. Concepts of Risk in Nanomedicine Research. J. Law Med. Ethics 2012, 40, 809–822. [Google Scholar] [CrossRef]
- Accomasso, L.; Cristallini, C.; Giachino, C. Risk assessment and risk minimization in nanomedicine: A need for predictive, alternative, and 3Rs strategies. Front. Pharmacol. 2018, 9, 228. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Gassmann, O.; Hinder, M. Changing R & D models in research-based pharmaceutical companies. J. Transl. Med. 2016, 14, 105. [Google Scholar] [CrossRef]
- Domonkos, D.; Hronszki, I. Risk Assessment of Innovations in The Biopharmaceutical Industry/Book 2; IntechOpen: Rijeka, Croatia, 2012; pp. 85–104. [Google Scholar]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody–Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Weiner, G.J. Picking the Optimal Target for Antibody-Drug Conjugates. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, e103–e107. [Google Scholar] [CrossRef] [PubMed]
- Haubenreisser, S. EMA perspective on the development of Nanomedicines. In Proceedings of the PQRI Nanotechnology Workshop, Washington, DC, USA, 14–15 January 2014. [Google Scholar]
- Pepić, I.; Hafner, A.; Lovrić, J.; Lakos, G.P. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef]
- Hardison, S. Oncology Dealmaking in 2020. Biopharm. Deal. 2021, B5. Available online: https://www.nature.com/articles/d43747-021-00024-y (accessed on 27 December 2022).
- Sambandan, P.; Raja, H.B. Open Innovation in Pharmaceutical Industry, a Case Study of Eli Lilly: How Do Big Pharmas Implement OI?—A Critical Analysis of the Current OI Practices through a Case Study. Master’s Thesis, Department of Industrial Economics and Management (INDEK), KTH School of Industrial Engineering and Management (ITM), Stockholm, Sweden, 2015. [Google Scholar]
- Manning, S. The rise of project network organizations: Building core teams and flexible partner pools for interorganizational projects. Res. Policy 2017, 46, 1399–1415. [Google Scholar] [CrossRef]
- Tong, J.T.W.; Harris, P.W.R.; Brimble, M.A.; Kavianinia, I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lim, A.; Tremblay, M.S. Next Horizons: ADCs Beyond Oncology. In Innovations for Next-Generation Antibody-Drug Conjugates; Damelin, M., Ed.; Humana Press: Cham, Switzerland, 2018; pp. 321–347. [Google Scholar] [CrossRef]

| ADC Drug | Maker | Disease Indication | Payload/Payload Class | Target | mAb | Linker | Approval Year |
|---|---|---|---|---|---|---|---|
| Mirvetuximab soravtansine | ImmunoGen | Platinum-resistant ovarian cancer | Maytansinoid DM4 | FRα | IgG1 | / | 2022 |
| Tisotumab vedotin-tftv | Seagen Inc | Recurrent or metastatic cervical cancer | MMAE/auristatin | Tissue factor | IgG1 | Enzyme-cleavable | 2021 |
| Loncastuximab tesirine-lpyl | ADC Therapeutics | Large B-cell lymphoma | SG3199/PBD dimer | CD19 | IgG1 | Enzyme-cleavable | 2021 |
| Belantamab mafodotin-blmf | GlaxoSmithKline (GSK) | Adult patients with relapsed or refractory multiple myeloma | MMAF/auristatin | BCMA | IgG1 | Non-cleavable | 2020, withdrawn on 22 November, 2022 |
| Sacituzumab govitecan | Immunomedics | Adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for patients with relapsed or refractory metastatic disease | SN-38/camptothecin | TROP2 | IgG1 | Acid-cleavable | 2020 |
| Trastuzumab deruxtecan | AstraZeneca/Daiichi Sankyo | Adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens | DXd/camptothecin | HER2 | IgG1 | Enzyme-cleavable | 2019 |
| Enfortumab vedotin | Astellas/Seagen Genetics | Adult patients with locally advanced or metastatic urothelial cancer who have received a PD-1 or PD-L1 inhibitor and a Pt-containing therapy | MMAE/auristatin | Nectin4 | IgG1 | Enzyme-cleavable | 2019 |
| Polatuzumab vedotin-piiq | Genentech, Roche | Relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) | MMAE/auristatin | CD79 | IgG1 | Enzyme-cleavable | 2019 |
| Moxetumomab pasudotox | Astrazeneca | Adults with relapsed or refractory hairy cell leukemia (HCL) | PE38 (Pseudotox) | CD22 | IgG1 | Cleavable | 2018 |
| Inotuzumab ozogamicin | Pfizer/Wyeth | Relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia | Ozogamicin/calicheamicin | CD22 | IgG4 | Acid-cleavable | 2017 |
| Trastuzumab emtansine | Genentech, Roche | HER2-positive metastatic breast cancer (mBC) following treatment with trastuzumab and a maytansinoid | DM1/maytansinoid | HER2 | IgG1 | Nnon-cleavable | 2013 |
| Brentuximab vedotin | Seagen Genetics, Millennium/Takeda | Relapsed HL and relapsed sALCL | MMAE/auristatin | CD30 | IgG1 | Enzyme-cleavable | 2011 |
| Gemtuzumab ozogamicin | Pfizer/Wyeth | Relapsed acute myelogenous leukemia (AML) | Ozogamicin/calicheamicin | CD33 | IgG4 | Acid-cleavable | 2017; 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, B.; Borbély, J. Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment. Pharmaceutics 2023, 15, 1761. https://doi.org/10.3390/pharmaceutics15061761
Kiss B, Borbély J. Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment. Pharmaceutics. 2023; 15(6):1761. https://doi.org/10.3390/pharmaceutics15061761
Chicago/Turabian StyleKiss, Balázs, and János Borbély. 2023. "Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment" Pharmaceutics 15, no. 6: 1761. https://doi.org/10.3390/pharmaceutics15061761
APA StyleKiss, B., & Borbély, J. (2023). Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment. Pharmaceutics, 15(6), 1761. https://doi.org/10.3390/pharmaceutics15061761







