The Nanostructured Self-Assembly and Thermoresponsiveness in Water of Amphiphilic Copolymers Carrying Oligoethylene Glycol and Polysiloxane Side Chains
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
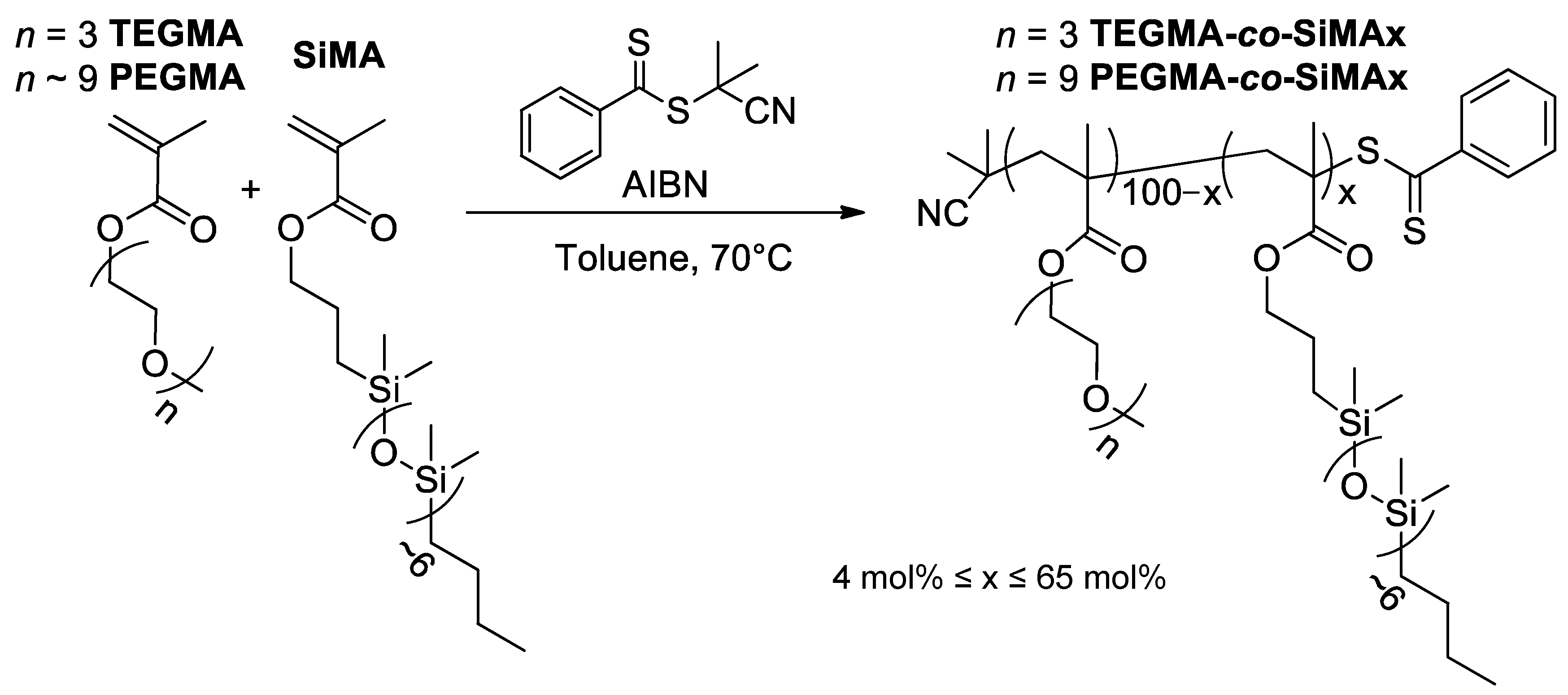
2.2. Synthesis of Copolymers PEGMA-co-SiMAx
2.3. Synthesis of Copolymers TEGMAx-co-SiMAy
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of PEGMA-co-SiMAx and TEGMA-co-SiMAx Copolymers
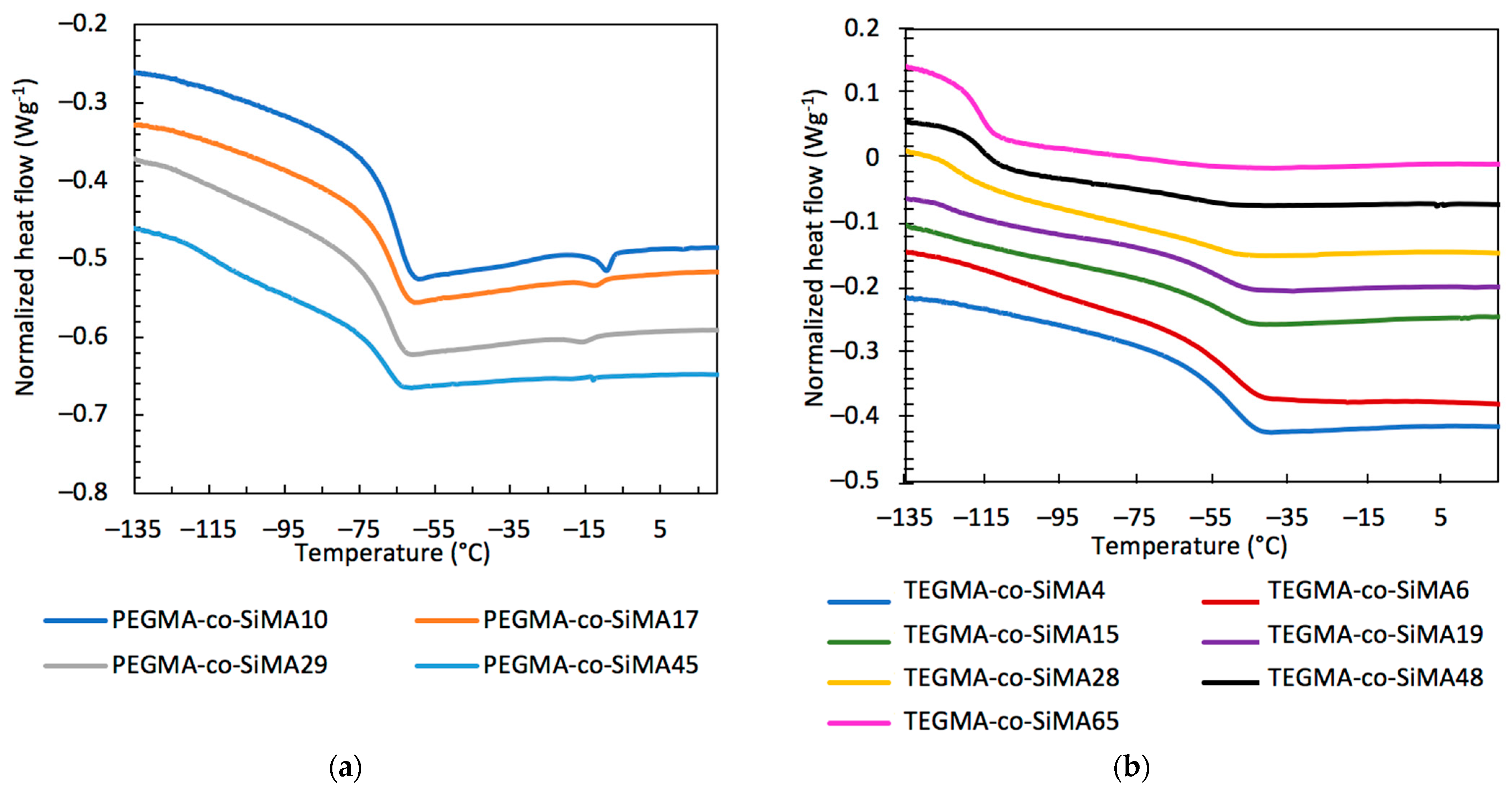
3.2. Differential Scanning Calorimetry
3.3. Self-Assembly in Solution
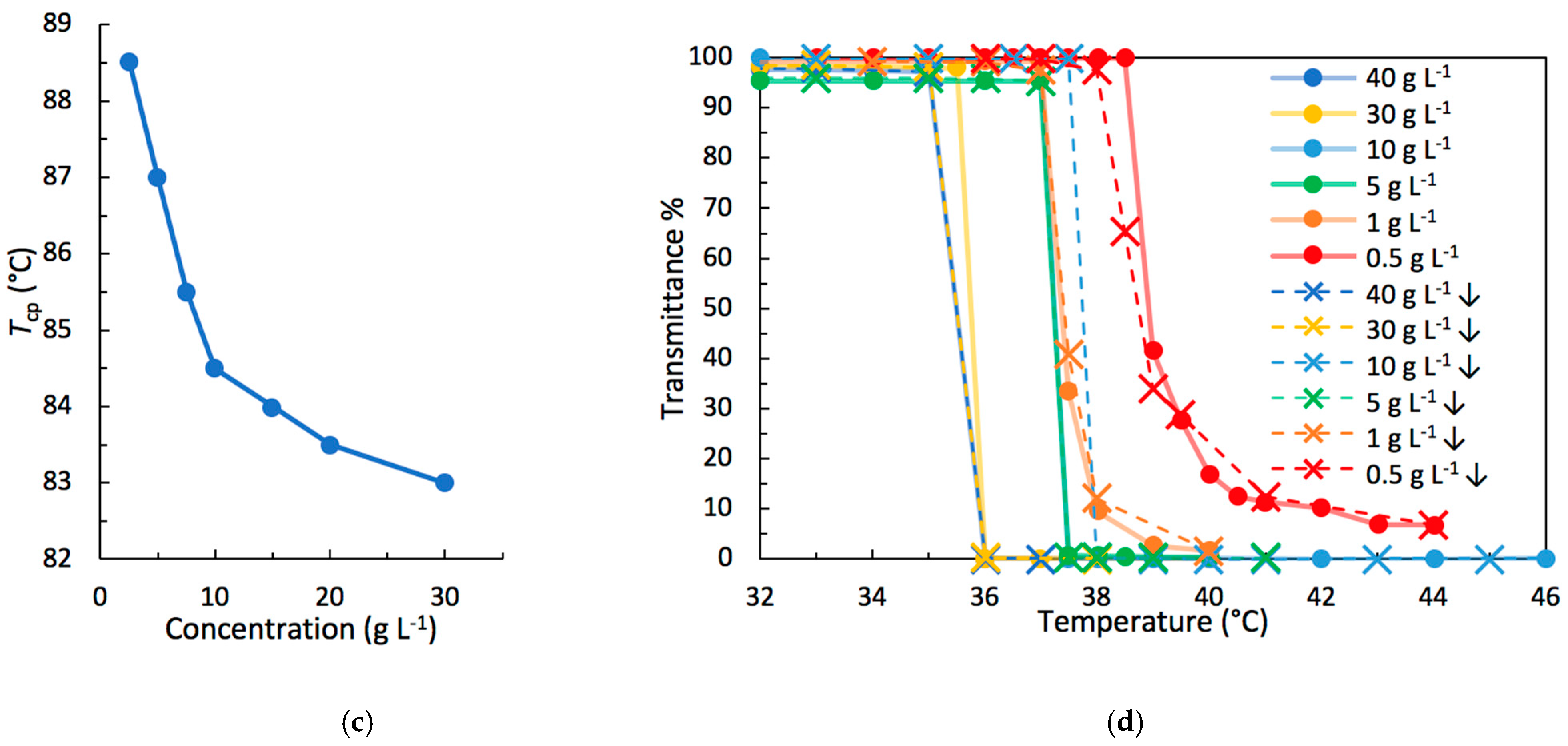
3.3.1. Light Transmittance Measurements
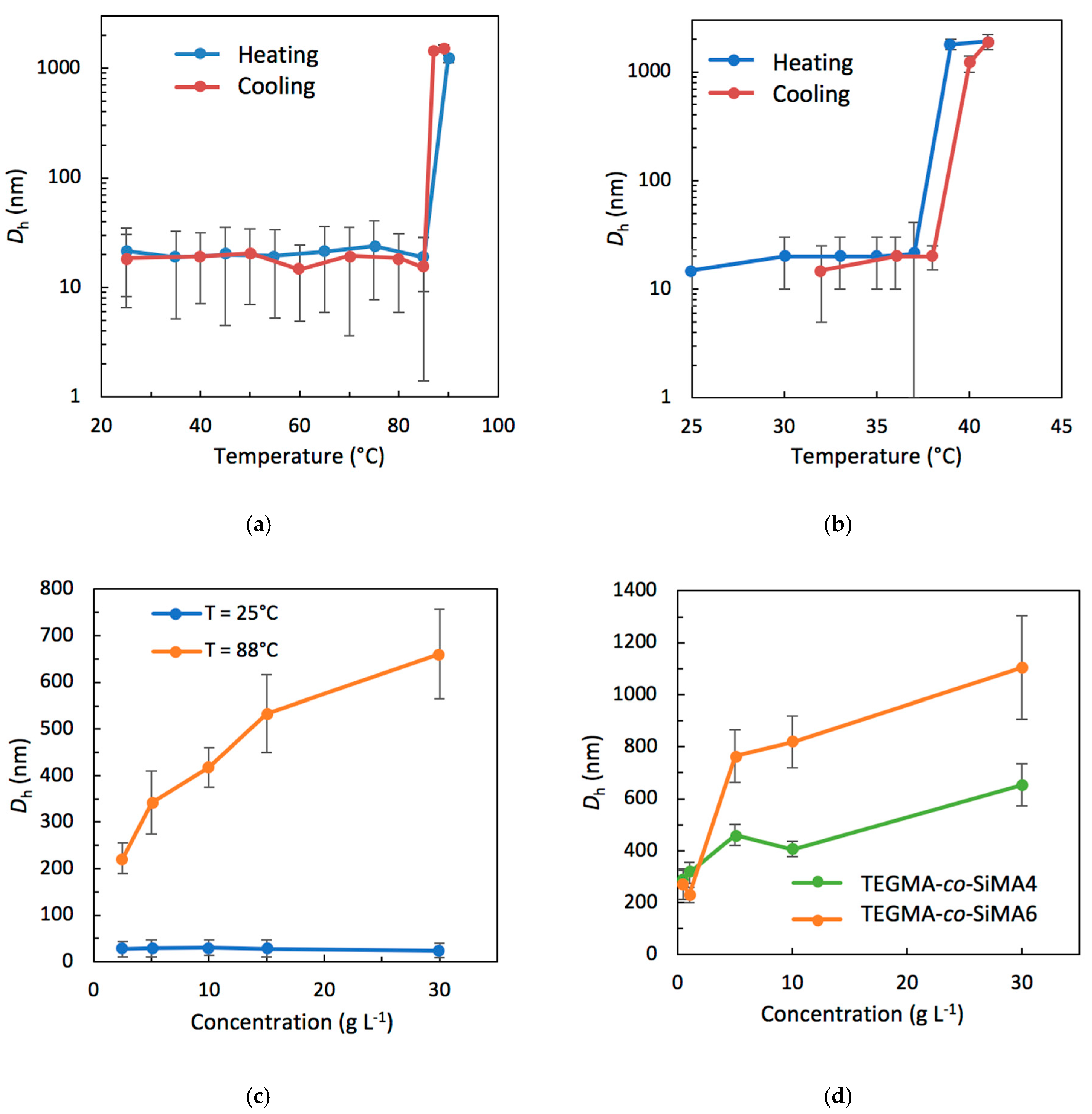
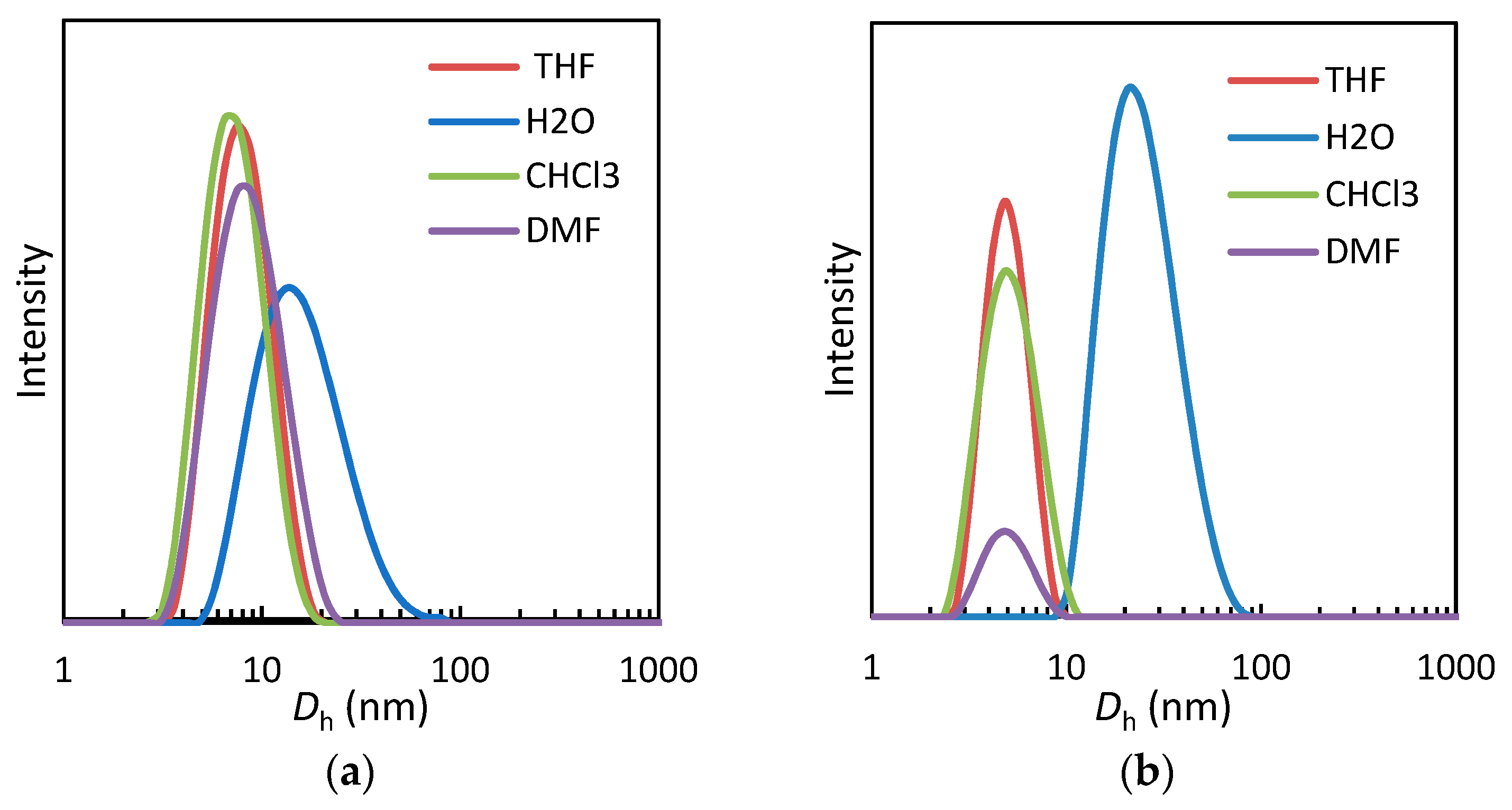
3.3.2. Dynamic Light Scattering
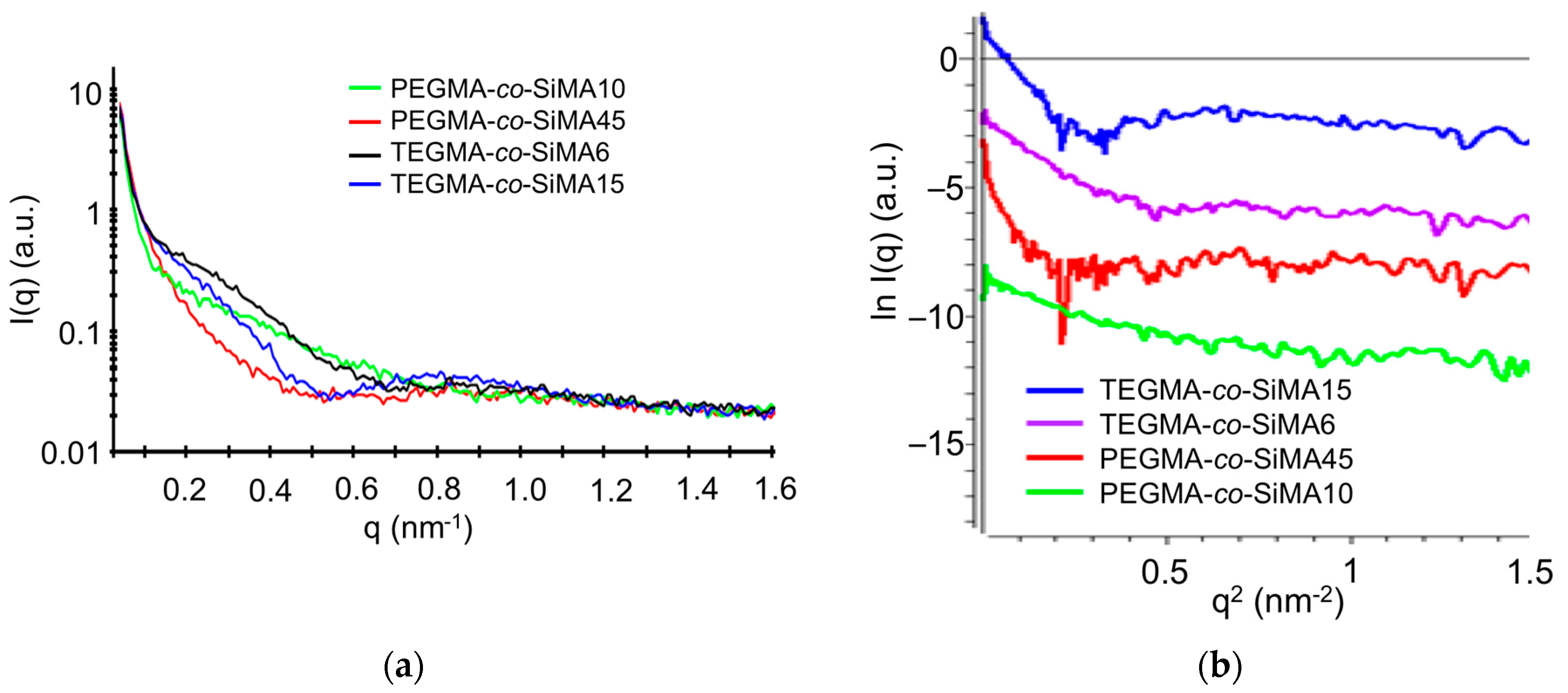
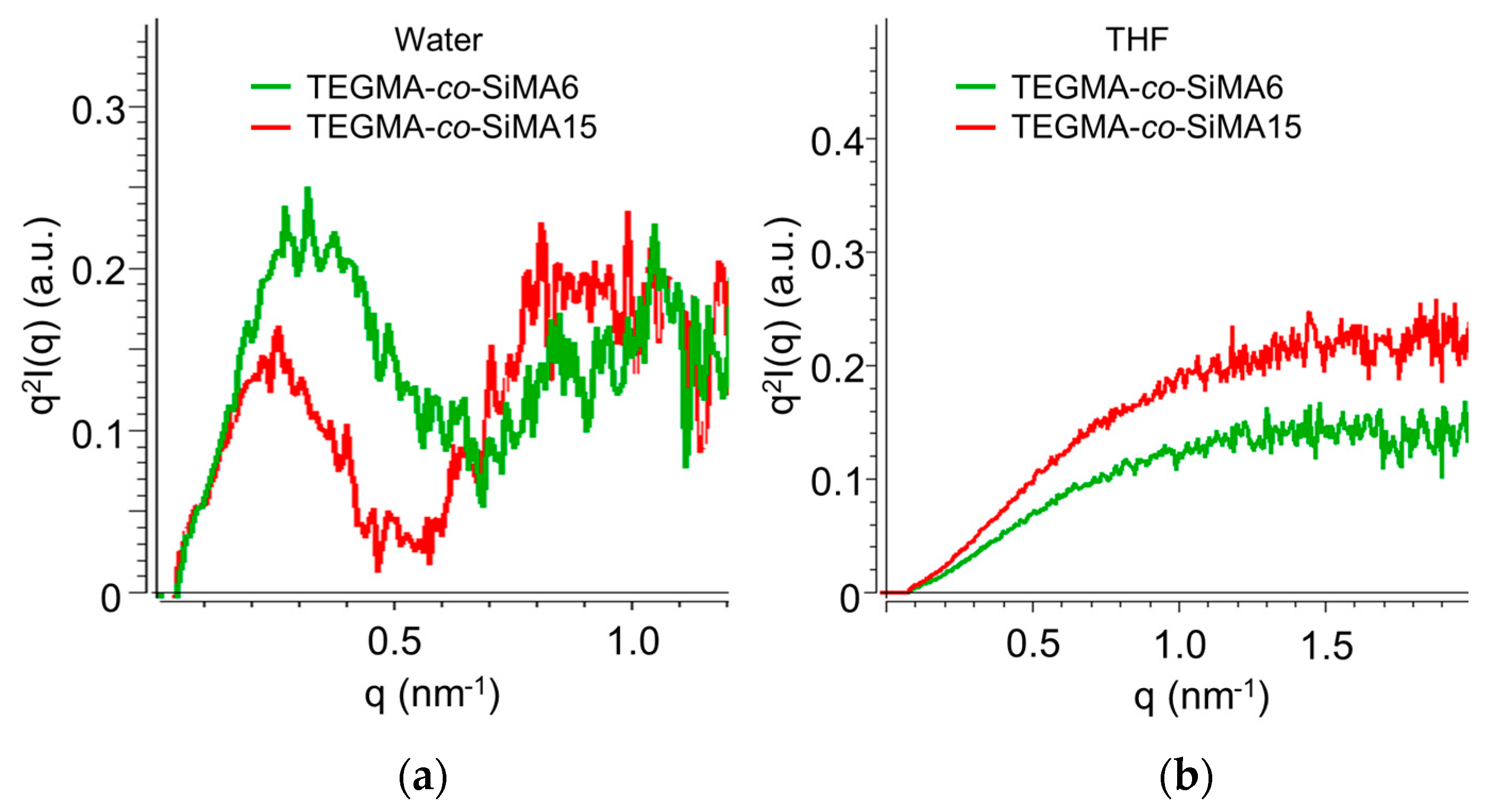
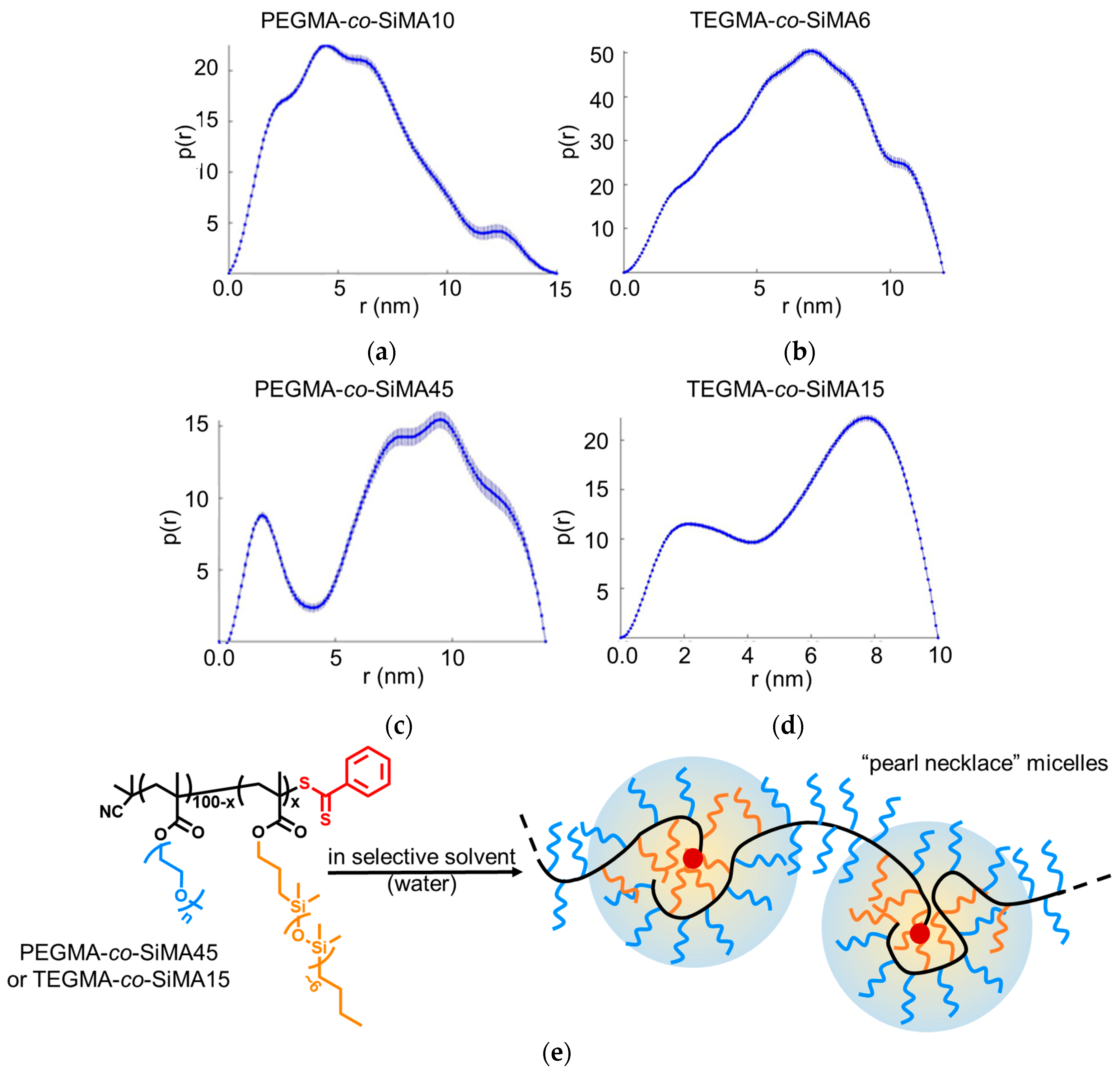
3.3.3. Small-Angle X-ray Scattering
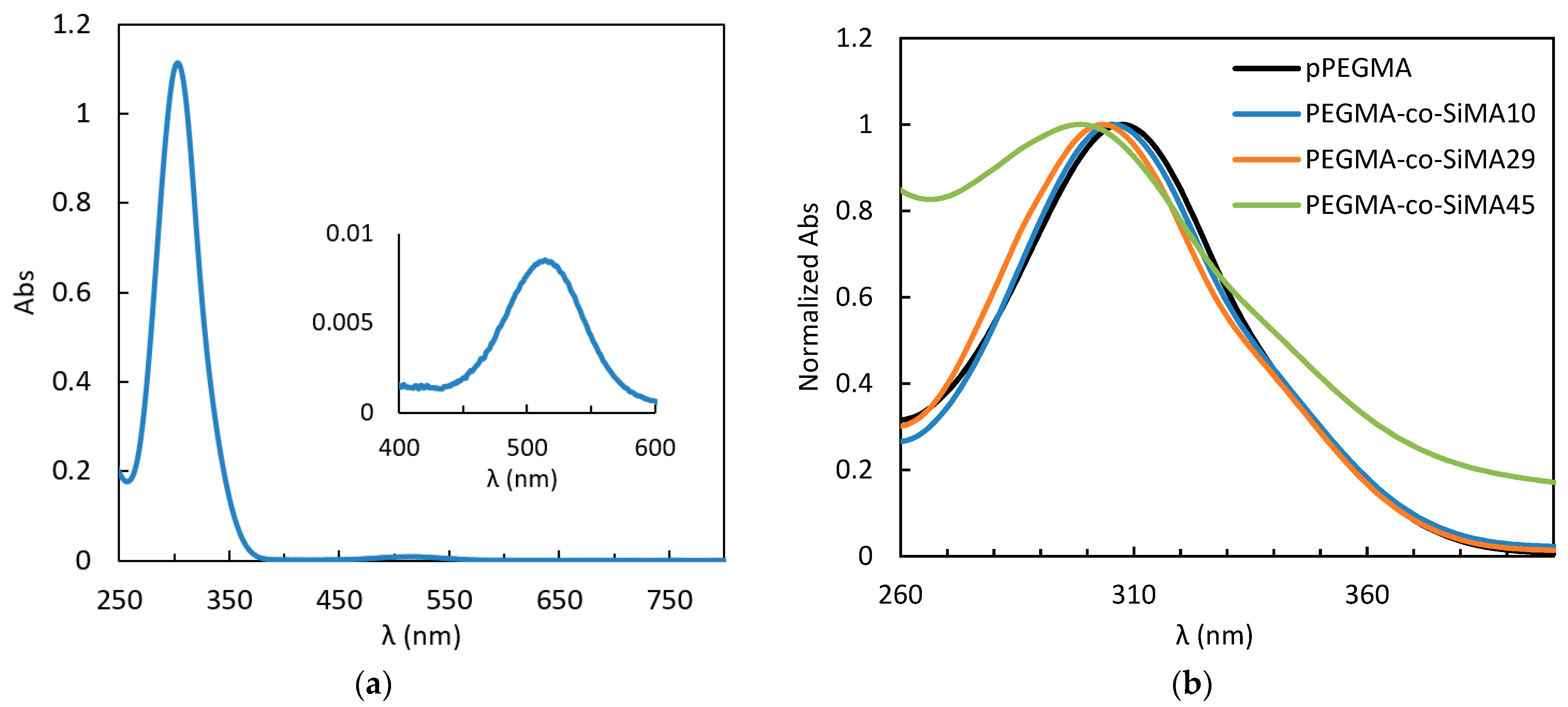
3.3.4. Solvatochromism of the RAFT CTA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Hall, A.; Eres, M.; Hemmatian, Z.; Qiao, B.; Zhou, Y.; Ruan, Z.; Couse, A.D.; Heller, W.T.; Huang, H.; et al. Single-Chain Heteropolymers Transport Protons Selectively and Rapidly. Nature 2020, 577, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Raghupathi, K.; Song, C.; Prasad, P.; Thayumanavan, S. Self-Assembly of Random Copolymers. Chem. Commun. 2014, 50, 13417–13432. [Google Scholar] [CrossRef] [PubMed]
- Guazzelli, E.; Masotti, E.; Calosi, M.; Kriechbaum, M.; Uhlig, F.; Galli, G.; Martinelli, E. Single-Chain Folding and Self-Assembling of Amphiphilic Polyethyleneglycol-Modified Fluorinated Styrene Homopolymers in Water Solution. Polymer 2021, 231, 124107. [Google Scholar] [CrossRef]
- Li, Q.; Constantinou, A.P.; Georgiou, T.K. A Library of Thermoresponsive PEG -based Methacrylate Homopolymers: How Do the Molar Mass and Number of Ethylene Glycol Groups Affect the Cloud Point? J. Polym. Sci. 2021, 59, 230–239. [Google Scholar] [CrossRef]
- Pelosi, C.; Guazzelli, E.; Calosi, M.; Bernazzani, L.; Tiné, M.R.; Duce, C.; Martinelli, E. Investigation of the LCST-Thermoresponsive Behavior of Novel Oligo(Ethylene Glycol)-Modified Pentafluorostyrene Homopolymers. Appl. Sci. 2021, 11, 2711. [Google Scholar] [CrossRef]
- Kimura, Y.; Terashima, T. Morphology Transition of Amphiphilic Homopolymer Self-Assemblies in Water Triggered by Pendant Design and Chain Length. Eur. Polym. J. 2020, 139, 110001. [Google Scholar] [CrossRef]
- Takahashi, R.; Doi, K.; Fujii, S.; Sakurai, K. Flower Necklaces of Controllable Length Formed From N -(2-Hydroxypropyl) Methacrylamide-Based Amphiphilic Statistical Copolymers. Langmuir 2020, 36, 11556–11563. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Kawata, Y.; Ishihara, K.; Yusa, S. Synthesis of Amphiphilic Statistical Copolymers Bearing Methoxyethyl and Phosphorylcholine Groups and Their Self-Association Behavior in Water. Polymers 2020, 12, 1808. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Yin, Q.; Du, K.; Zhang, T.; Yin, Q. Multi-Responsive Hollow Nanospheres Self-Assembly by Amphiphilic Random Copolymer and Azobenzene. Polymer 2019, 175, 235–242. [Google Scholar] [CrossRef]
- Pires-Oliveira, R.; Tang, J.; Percebom, A.M.; Petzhold, C.L.; Tam, K.C.; Loh, W. Effect of Molecular Architecture and Composition on the Aggregation Pathways of POEGMA Random Copolymers in Water. Langmuir 2020, 36, 15018–15029. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Tanaka, K.; Ouchi, M.; Terashima, T. Amphiphilic Random-Block Copolymer Micelles in Water: Precise and Dynamic Self-Assembly Controlled by Random Copolymer Association. Macromolecules 2022, 55, 178–189. [Google Scholar] [CrossRef]
- Upadhya, R.; Murthy, N.S.; Hoop, C.L.; Kosuri, S.; Nanda, V.; Kohn, J.; Baum, J.; Gormley, A.J. PET-RAFT and SAXS: High Throughput Tools To Study Compactness and Flexibility of Single-Chain Polymer Nanoparticles. Macromolecules 2019, 52, 8295–8304. [Google Scholar] [CrossRef]
- Terashima, T.; Sugita, T.; Fukae, K.; Sawamoto, M. Synthesis and Single-Chain Folding of Amphiphilic Random Copolymers in Water. Macromolecules 2014, 47, 589–600. [Google Scholar] [CrossRef]
- Ko, J.H.; Bhattacharya, A.; Terashima, T.; Sawamoto, M.; Maynard, H.D. Amphiphilic Fluorous Random Copolymer Self-assembly for Encapsulation of a Fluorinated Agrochemical. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 352–359. [Google Scholar] [CrossRef]
- Guazzelli, E.; Masotti, E.; Kriechbaum, M.; Uhlig, F.; Galli, G.; Martinelli, E. Thermoresponsive Reversible Unimer Micelles of Amphiphilic Fluorinated Copolymers. Macromol. Chem. Phys. 2023, 224, 2200360. [Google Scholar] [CrossRef]
- Blazquez-Martín, A.; Verde-Sesto, E.; Moreno, A.J.; Arbe, A.; Colmenero, J.; Pomposo, J.A. Advances in the Multi-Orthogonal Folding of Single Polymer Chains into Single-Chain Nanoparticles. Polymers 2021, 13, 293. [Google Scholar] [CrossRef]
- Nam, J.; Kwon, S.; Yu, Y.-G.; Seo, H.-B.; Lee, J.-S.; Lee, W.B.; Kim, Y.; Seo, M. Folding of Sequence-Controlled Graft Copolymers to Subdomain-Defined Single-Chain Nanoparticles. Macromolecules 2021, 54, 8829–8838. [Google Scholar] [CrossRef]
- Nitti, A.; Carfora, R.; Assanelli, G.; Notari, M.; Pasini, D. Single-Chain Polymer Nanoparticles for Addressing Morphologies and Functions at the Nanoscale: A Review. ACS Appl. Nano Mater. 2022, 5, 13985–13997. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, Z. Progress in Polymer Single-Chain Based Hybrid Nanoparticles. Prog. Polym. Sci. 2022, 133, 101593. [Google Scholar] [CrossRef]
- Verde-Sesto, E.; Arbe, A.; Moreno, A.J.; Cangialosi, D.; Alegría, A.; Colmenero, J.; Pomposo, J.A. Single-Chain Nanoparticles: Opportunities Provided by Internal and External Confinement. Mater. Horiz. 2020, 7, 2292–2313. [Google Scholar] [CrossRef]
- Hirai, Y.; Terashima, T.; Takenaka, M.; Sawamoto, M. Precision Self-Assembly of Amphiphilic Random Copolymers into Uniform and Self-Sorting Nanocompartments in Water. Macromolecules 2016, 49, 5084–5091. [Google Scholar] [CrossRef]
- Balafouti, A.; Pispas, S. P(OEGMA-co-LMA) Hyperbranched Amphiphilic Copolymers as Self-assembled Nanocarriers. J. Polym. Sci. 2022, 60, 1931–1943. [Google Scholar] [CrossRef]
- Kimura, Y.; Terashima, T.; Sawamoto, M. Self-Assembly of Amphiphilic Random Copolyacrylamides into Uniform and Necklace Micelles in Water. Macromol. Chem. Phys. 2017, 218, 1700230. [Google Scholar] [CrossRef]
- Shin, M.; Kim, H.; Park, G.; Park, J.; Ahn, H.; Yoon, D.K.; Lee, E.; Seo, M. Bilayer-Folded Lamellar Mesophase Induced by Random Polymer Sequence. Nat. Commun. 2022, 13, 2433. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M. Self-Assembly and Morphology Control of New L-Glutamic Acid-Based Amphiphilic Random Copolymers: Giant Vesicles, Vesicles, Spheres, and Honeycomb Film. Langmuir 2011, 27, 12844–12850. [Google Scholar] [CrossRef]
- Biglione, C.; Neumann-Tran, T.M.P.; Kanwal, S.; Klinger, D. Amphiphilic Micro- and Nanogels: Combining Properties from Internal Hydrogel Networks, Solid Particles, and Micellar Aggregates. J. Polym. Sci. 2021, 59, 2665–2703. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Wang, F.; Tang, J.; Liu, H.; Yu, G.; Zou, Y. Self-Assembled Polymeric Micelles as Amphiphilic Particulate Emulsifiers for Controllable Pickering Emulsions. Mater. Chem. Front. 2019, 3, 356–364. [Google Scholar] [CrossRef]
- Jennings, J.; Webster-Aikman, R.R.; Ward-O’Brien, N.; Xie, A.; Beattie, D.L.; Deane, O.J.; Armes, S.P.; Ryan, A.J. Hydrocarbon-Based Statistical Copolymers Outperform Block Copolymers for Stabilization of Ethanol–Water Foams. ACS Appl. Mater. Interfaces 2022, 14, 39548–39559. [Google Scholar] [CrossRef]
- Veiga, N.; Diesendruck, Y.; Peer, D. Targeted Nanomedicine: Lessons Learned and Future Directions. J. Control. Release 2023, 355, 446–457. [Google Scholar] [CrossRef]
- Mane, S.R.; Sathyan, A.; Shunmugam, R. Biomedical Applications of PH-Responsive Amphiphilic Polymer Nanoassemblies. ACS Appl. Nano Mater. 2020, 3, 2104–2117. [Google Scholar] [CrossRef]
- Tomara, M.; Selianitis, D.; Pispas, S. Dual-Responsive Amphiphilic P(DMAEMA-Co-LMA-Co-OEGMA) Terpolymer Nano-Assemblies in Aqueous Media. Nanomaterials 2022, 12, 3791. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.G.; Mete, S.; Chaudhury, S.S.; Sar, P.; Ksendzov, E.; Mukhopadhyay, C.D.; Kostjuk, S.V.; De, P. Self-Assembly of Amphiphilic Copolymers with Sequence-Controlled Alternating Hydrophilic–Hydrophobic Pendant Side Chains. ACS Appl. Polym. Mater. 2020, 2, 2035–2045. [Google Scholar] [CrossRef]
- Sadeghi, I.; Asatekin, A. Spontaneous Self-Assembly and Micellization of Random Copolymers in Organic Solvents. Macromol. Chem. Phys. 2017, 218, 1700226. [Google Scholar] [CrossRef]
- Xiao, X.; Cai, H.; Huang, Q.; Wang, B.; Wang, X.; Luo, Q.; Li, Y.; Zhang, H.; Gong, Q.; Ma, X.; et al. Polymeric Dual-Modal Imaging Nanoprobe with Two-Photon Aggregation-Induced Emission for Fluorescence Imaging and Gadolinium-Chelation for Magnetic Resonance Imaging. Bioact. Mater. 2023, 19, 538–549. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zhao, L.; Yan, D.; Li, X.; Gao, Q.; Zheng, J.; Zhou, S.; Lai, S.; Feng, Y.; et al. Multifunctional AIE Nanosphere-Based “Nanobomb” for Trimodal Imaging-Guided Photothermal/Photodynamic/Pharmacological Therapy of Drug-Resistant Bacterial Infections. ACS Nano 2023, 17, 4601–4618. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Wang, W.; Ma, Z.; Chi, Z.; Liu, S. A Cationic Amphiphilic AIE Polymer for Mitochondrial Targeting and Imaging. Pharmaceutics 2022, 15, 103. [Google Scholar] [CrossRef]
- Uddin, M.A.; Yu, H.; Wang, L.; Liu, J.; Mehmood, S.; Amin, B.U.; Haq, F.; Liang, R.; Shen, D.; Ni, Z. Multi-Stimuli-Responsive Performance and Morphological Changes of Radical-Functionalized Self-Assembled Micellar Nanoaggregates and Their Multi-Triggered Drug Release. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126807. [Google Scholar] [CrossRef]
- Uddin, M.A.; Yu, H.; Wang, L.; Naveed, K.-R.; Amin, B.U.; Mehmood, S.; Haq, F.; Nazir, A.; Lin, T.; Chen, X.; et al. Multiple-Stimuli-Responsiveness and Conformational Inversion of Smart Supramolecular Nanoparticles Assembled from Spin Labeled Amphiphilic Random Copolymers. J. Colloid Interface Sci. 2021, 585, 237–249. [Google Scholar] [CrossRef]
- Calosi, M.; Guazzelli, E.; Braccini, S.; Lessi, M.; Bellina, F.; Galli, G.; Martinelli, E. Self-Assembled Amphiphilic Fluorinated Random Copolymers for the Encapsulation and Release of the Hydrophobic Combretastatin A-4 Drug. Polymers 2022, 14, 774. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive Polymer Nanocarriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Terashima, T.; Sawamoto, M. Multimode Self-Folding Polymers via Reversible and Thermoresponsive Self-Assembly of Amphiphilic/Fluorous Random Copolymers. Macromolecules 2016, 49, 4534–4543. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of Sub-100 Nm Polymeric Micelles in Poorly Permeable Tumours Depends on Size. Nat. Nanotech. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.K.; Alpar, H.O. Potential Use of Nanoparticles for Transcutaneous Vaccine Delivery: Effect of Particle Size and Charge. Int. J. Pharm. 2004, 275, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Mojsiewicz-Pieńkowska, K. Review of Current Pharmaceutical Applications of Polysiloxanes (Silicones). In Handbook of Polymers for Pharmaceutical Technologies; Thakur, V.K., Thakur, M.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 363–381. ISBN 978-1-119-04141-2. [Google Scholar]
- Bai, L.; Yan, H.; Bai, T.; Feng, Y.; Zhao, Y.; Ji, Y.; Feng, W.; Lu, T.; Nie, Y. High Fluorescent Hyperbranched Polysiloxane Containing β-Cyclodextrin for Cell Imaging and Drug Delivery. Biomacromolecules 2019, 20, 4230–4240. [Google Scholar] [CrossRef]
- Blanco, I. Polysiloxanes in Theranostics and Drug Delivery: A Review. Polymers 2018, 10, 755. [Google Scholar] [CrossRef]
- Racles, C.; Cazacu, M.; Zaltariov, M.; Iacob, M.; Butnaru, M. Siloxane-Based Compounds with Tailored Surface Properties for Health and Environment. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 972–977. [Google Scholar] [CrossRef]
- Guazzelli, E.; Martinelli, E.; Galli, G.; Cupellini, L.; Jurinovich, S.; Mennucci, B. Single-Chain Self-Folding in an Amphiphilic Copolymer: An Integrated Experimental and Computational Study. Polymer 2019, 161, 33–40. [Google Scholar] [CrossRef]
- Bradford, K.G.E.; Petit, L.M.; Whitfield, R.; Anastasaki, A.; Barner-Kowollik, C.; Konkolewicz, D. Ubiquitous Nature of Rate Retardation in Reversible Addition–Fragmentation Chain Transfer Polymerization. J. Am. Chem. Soc. 2021, 143, 17769–17777. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Buback, M.; Charleux, B.; Coote, M.L.; Drache, M.; Fukuda, T.; Goto, A.; Klumperman, B.; Lowe, A.B.; Mcleary, J.B.; et al. Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization. I. The Current Situation. J. Polym. Sci. A Polym. Chem. 2006, 44, 5809–5831. [Google Scholar] [CrossRef]
- Koda, Y.; Terashima, T.; Sawamoto, M.; Maynard, H.D. Amphiphilic/Fluorous Random Copolymers as a New Class of Non-Cytotoxic Polymeric Materials for Protein Conjugation. Polym. Chem. 2015, 6, 240–247. [Google Scholar] [CrossRef]
- Zuppardi, F.; Malinconico, M.; D’Agosto, F.; D’Ayala, G.G.; Cerruti, P. Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water. Nanomaterials 2020, 10, 1779. [Google Scholar] [CrossRef]
- Faggio, N.; Zuppardi, F.; Staiano, C.; Poggetto, G.D.; d’Ayala, G.G.; Cerruti, P. Removal of Anionic and Cationic Dyes from Aqueous Solution Using Thermo- and PH-Responsive Amphiphilic Copolymers. J. Water Process Eng. 2022, 49, 103107. [Google Scholar] [CrossRef]
- Martinelli, E.; Annunziata, L.; Guazzelli, E.; Pucci, A.; Biver, T.; Galli, G. The Temperature-Responsive Nanoassemblies of Amphiphilic Random Copolymers Carrying Poly(Siloxane) and Poly(Oxyethylene) Pendant Chains. Macromol. Chem. Phys. 2018, 219, 1800082. [Google Scholar] [CrossRef]
- Martini, F.; Guazzelli, E.; Martinelli, E.; Borsacchi, S.; Geppi, M.; Galli, G. Molecular Dynamics of Amphiphilic Random Copolymers in the Bulk: A 1 H and 19 F NMR Relaxometry Study. Macromol. Chem. Phys. 2019, 220, 1900177. [Google Scholar] [CrossRef]
- Guazzelli, E.; Perondi, F.; Criscitiello, F.; Pretti, C.; Oliva, M.; Casu, V.; Maniero, F.; Gazzera, L.; Galli, G.; Martinelli, E. New Amphiphilic Copolymers for PDMS-Based Nanocomposite Films with Long-Term Marine Antifouling Performance. J. Mater. Chem. B 2020, 8, 9764–9776. [Google Scholar] [CrossRef]
- Szweda, D.; Szweda, R.; Dworak, A.; Trzebicka, B. Thermoresponsive Poly[Oligo(Ethylene Glycol) Methacrylate]s and Their Bioconjugates—Synthesis and Solution Behavior. Polimery 2017, 62, 298–310. [Google Scholar] [CrossRef]
- Domenici, F.; Guazzelli, E.; Masotti, E.; Mahmoudi, N.; Gabrielli, S.; Telling, M.T.F.; Martinelli, E.; Galli, G.; Paradossi, G. Understanding the Temperature-Responsive Self-Assemblies of Amphiphilic Random Copolymers by SANS in D2O Solution. Macromol. Chem. Phys. 2021, 222, 2000447. [Google Scholar] [CrossRef]
- Glatter, O. A New Method for the Evaluation of Small-Angle Scattering Data. J. Appl. Crystallogr. 1977, 10, 415–421. [Google Scholar] [CrossRef]
- Brosey, C.A.; Tainer, J.A. Evolving SAXS Versatility: Solution X-Ray Scattering for Macromolecular Architecture, Functional Landscapes, and Integrative Structural Biology. Curr. Opin. Struct. Biol. 2019, 58, 197–213. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Kiselev, M.A. Structural Characterization of Biomaterials by Means of Small Angle X-Rays and Neutron Scattering (SAXS and SANS), and Light Scattering Experiments. Molecules 2020, 25, 5624. [Google Scholar] [CrossRef]
- Halacheva, S.; Price, G.J.; Garamus, V.M. Effects of Temperature and Polymer Composition upon the Aqueous Solution Properties of Comblike Linear Poly(Ethylene Imine)/Poly(2-Ethyl-2-Oxazoline)-Based Polymers. Macromolecules 2011, 44, 7394–7404. [Google Scholar] [CrossRef]
- Franklin, J.M.; Surampudi, L.N.; Ashbaugh, H.S.; Pozzo, D.C. Numerical Validation of IFT in the Analysis of Protein–Surfactant Complexes with SAXS and SANS. Langmuir 2012, 28, 12593–12600. [Google Scholar] [CrossRef] [PubMed]
- Ospinal-Jiménez, M.; Pozzo, D.C. Structural Analysis of Protein Complexes with Sodium Alkyl Sulfates by Small-Angle Scattering and Polyacrylamide Gel Electrophoresis. Langmuir 2011, 27, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, K.; Takahashi, R.; Terao, K.; Sato, T. Local and Global Conformations of Flower Micelles and Flower Necklaces Formed by an Amphiphilic Alternating Copolymer in Aqueous Solution. Polym. J. 2016, 48, 863–867. [Google Scholar] [CrossRef]
- Morishima, K.; Terao, K.; Sato, T. Structural Analysis of Hydrophobe-Uptake Micelle of an Amphiphilic Alternating Copolymer in Aqueous Solution. Langmuir 2016, 32, 7875–7881. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Hashidzume, A.; Sato, T. Unicore−Multicore Transition of the Micelle Formed by an Amphiphilic Alternating Copolymer in Aqueous Media by Changing Molecular Weight. Macromolecules 2011, 44, 2970–2977. [Google Scholar] [CrossRef]


| Copolymer | Conversion (a) (%) | SiMA (mol%) | SiMA (wt%) | Mn(b) (g mol–1) | Mn(c) (g mol–1) | Đ (c) | Water Solubility |
|---|---|---|---|---|---|---|---|
| PEGMA-co-SiMA10 | 97 | 10 | 14 | 20,300 | 17,300 | 1.30 | Yes |
| PEGMA-co-SiMA17 | 96 | 17 | 23 | 23,500 | 21,000 | 1.29 | Yes |
| PEGMA-co-SiMA29 | 94 | 29 | 37 | 31,500 | 17,600 | 1.59 | Yes |
| PEGMA-co-SiMA45 | 74 | 45 | 54 | 29,000 | 15,600 | 1.60 | Yes |
| TEGMA-co-SiMA4 | 96 | 4 | 12 | 7700 | 17,100 | 1.17 | Yes |
| TEGMA-co-SiMA6 | 97 | 6 | 17 | 8500 | 16,100 | 1.16 | Yes |
| TEGMA-co-SiMA15 | 94 | 15 | 34 | 8000 | 20,000 | 1.22 | Yes |
| TEGMA-co-SiMA19 | 89 | 19 | 41 | 8700 | 21,400 | 1.17 | Yes |
| TEGMA-co-SiMA28 | 90 | 28 | 53 | 8800 | 20,400 | 1.13 | No |
| TEGMA-co-SiMA48 | 80 | 48 | 73 | 12,200 | 26,000 | 1.33 | No |
| TEGMA-co-SiMA65 | 78 | 65 | 85 | 8600 | 22,500 | 1.30 | No |
| Copolymer | Tg (a) (°C) | ΔCp (a) (J (gK)−1) | Tg (b) (°C) | ΔCp (b) (J (gK)−1) | Tm (b) (°C) | ΔHm (b) (J g−1) |
|---|---|---|---|---|---|---|
| pPEGMA(c) | −63 | 1.44 | ||||
| PEGMA-co-SiMA10 | n.d. (d) | −64 | 1.20 | −9 | −0.64 | |
| PEGMA-co-SiMA17 | n.d. (d) | −66 | 0.86 | −12 | −0.23 | |
| PEGMA-co-SiMA29 | n.d. (d) | −67 | 0.78 | −15 | −0.26 | |
| PEGMA-co-SiMA45 | −120 | 0.23 | −68 | 0.45 | −18 | −0.06 |
| pTEGMA (c) | −48 | 0.60 | ||||
| TEGMA-co-SiMA4 | n.d. (d) | −50 | 0.63 | |||
| TEGMA-co-SiMA6 | n.d. (d) | −49 | 0.59 | |||
| TEGMA-co-SiMA15 | −122 | 0.09 | −53 | 0.34 | ||
| TEGMA-co-SiMA19 | −124 | 0.19 | −52 | 0.32 | ||
| TEGMA-co-SiMA28 | −123 | 0.29 | −53 | 0.21 | ||
| TEGMA-co-SiMA48 | −115 | 0.38 | −58 | 0.11 | ||
| TEGMA-co-SiMA65 | −115 | 0.55 | n.d. (d) | |||
| pSiMA (c) | −107 | 0.95 |
| Copolymer | Concentration (g L−1) | Tcp (°C) |
|---|---|---|
| PEGMA-co-SiMA10 | 2.5 | 88.5 |
| 5.0 | 87.0 | |
| 7.5 | 85.5 | |
| 10 | 84.5 | |
| 15 | 84.0 | |
| 20 | 83.5 | |
| 30 | 83.0 | |
| PEGMA-co-SiMA17 | 10 | 83.0 |
| PEGMA-co-SiMA29 | 10 | 79.0 |
| PEGMA-co-SiMA45 | 10 | 73.0 |
| TEGMA-co-SiMA4 | 10 | 41.0 |
| TEGMA-co-SiMA6 | 0.5 | 38.9 |
| 1.0 | 37.3 | |
| 5.0 | 37.3 | |
| 10 | 37.2 | |
| 30 | 35.8 | |
| 40 | 35.5 | |
| TEGMA-co-SiMA15 | 0.5 | 33.0 |
| 1.0 | 31.0 | |
| 5.0 | 28.5 | |
| 10 | 28.4 | |
| 40 | 28.3 | |
| TEGMA-co-SiMA19 | 0.5 | 33.0 |
| 1.0 | 32.0 | |
| 5.0 | 29.5 | |
| 10 | 26.5 |
| Copolymer | Dh (H2O) (nm) | Dh (CHCl3) (nm) | Dh (DMF) (nm) | Dh (THF) (nm) | |
|---|---|---|---|---|---|
| 25 °C | T > Tcp | 25 °C | 25 °C | 25 °C | |
| PEGMA-co-SiMA10 | 17 ± 9 | 1200 ± 100 (a) | 7 ± 3 | 8 ± 3 | 8 ± 3 |
| PEGMA-co-SiMA29 | 30 ± 20 | 420 ± 40 (a) | 11 ± 4 | 10 ± 4 | 9 ± 3 |
| PEGMA-co-SiMA45 | 70 ± 40 | 100 ± 10 (a) | 10 ± 5 | 15 ± 6 | 9 ± 4 |
| TEGMA-co-SiMA4 | 13 ± 4 | 310 ± 40 (b) | 5 ± 2 | 6 ± 1 | 5 ± 1 |
| TEGMA-co-SiMA6 | 16 ± 4 | 230 ± 30 (b) | 8 ± 4 | 7 ± 3 | 11 ± 6 |
| TEGMA-co-SiMA15 | 24 ± 9 | 140 ± 30 (b) | 5 ± 1 | 5 ± 1 | 5 ± 1 |
| TEGMA-co-SiMA19 | 37 ± 9 | 170 ± 60 (b) | 5 ± 2 | 6 ± 2 | 5 ± 1 |
| Copolymer | Solvent | Aggregation | Rg (nm) |
|---|---|---|---|
| PEGMA-co-SiMA10 | H2O | Aggregates | 5.0 ± 0.3 |
| THF | Random coil | 2.30 ± 0.03 | |
| PEGMA-co-SiMA45 | H2O | Aggregates | 6.1 ± 0.1 |
| THF | Random coil | 2.01 ± 0.07 | |
| TRIGMA-co-SiMA6 | H2O | Aggregates | 6.3 ± 0.3 |
| THF | Random coil | 2.26 ± 0.05 | |
| TRIGMA-co-SiMA15 | H2O | Aggregates | 7.1 ± 0.6 |
| THF | Random coil | 2.31 ± 0.04 |
| CTA in Solvent | λmax (nm) | Ɛr 20 °C | Copolymer in Water | λmax (nm) |
|---|---|---|---|---|
| H2O | 308 | 81.1 | pPEGMA | 307 |
| DMSO | 307 | 46.7 | PEGMA-co-SiMA10 | 305 |
| Diglyme | 304 | 7.3 | PEGMA-co-SiMA29 | 303 |
| CH2Cl2 | 303 | 9.1 | PEGMA-co-SiMA45 | 299 |
| THF | 303 | 7.5 | ||
| CHCl3 | 303 | 4.8 | TEGMA-co-SiMA4 | 305 |
| n-hexane | 299 | 1.9 | TEGMA-co-SiMA6 | 304 |
| PDMS | 298 | 2.6 | TEGMA-co-SiMA15 | 303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guazzelli, E.; Pisano, G.; Turriani, M.; Biver, T.; Kriechbaum, M.; Uhlig, F.; Galli, G.; Martinelli, E. The Nanostructured Self-Assembly and Thermoresponsiveness in Water of Amphiphilic Copolymers Carrying Oligoethylene Glycol and Polysiloxane Side Chains. Pharmaceutics 2023, 15, 1703. https://doi.org/10.3390/pharmaceutics15061703
Guazzelli E, Pisano G, Turriani M, Biver T, Kriechbaum M, Uhlig F, Galli G, Martinelli E. The Nanostructured Self-Assembly and Thermoresponsiveness in Water of Amphiphilic Copolymers Carrying Oligoethylene Glycol and Polysiloxane Side Chains. Pharmaceutics. 2023; 15(6):1703. https://doi.org/10.3390/pharmaceutics15061703
Chicago/Turabian StyleGuazzelli, Elisa, Giuseppe Pisano, Marco Turriani, Tarita Biver, Manfred Kriechbaum, Frank Uhlig, Giancarlo Galli, and Elisa Martinelli. 2023. "The Nanostructured Self-Assembly and Thermoresponsiveness in Water of Amphiphilic Copolymers Carrying Oligoethylene Glycol and Polysiloxane Side Chains" Pharmaceutics 15, no. 6: 1703. https://doi.org/10.3390/pharmaceutics15061703
APA StyleGuazzelli, E., Pisano, G., Turriani, M., Biver, T., Kriechbaum, M., Uhlig, F., Galli, G., & Martinelli, E. (2023). The Nanostructured Self-Assembly and Thermoresponsiveness in Water of Amphiphilic Copolymers Carrying Oligoethylene Glycol and Polysiloxane Side Chains. Pharmaceutics, 15(6), 1703. https://doi.org/10.3390/pharmaceutics15061703











