The Long-Term Neuroprotective Effect of the Endocannabinoid 2-AG and Modulation of the SGZ’s Neurogenic Response after Neonatal Hypoxia-Ischemia
Abstract
1. Introduction
2. Results
2.1. Body and Brain Weight
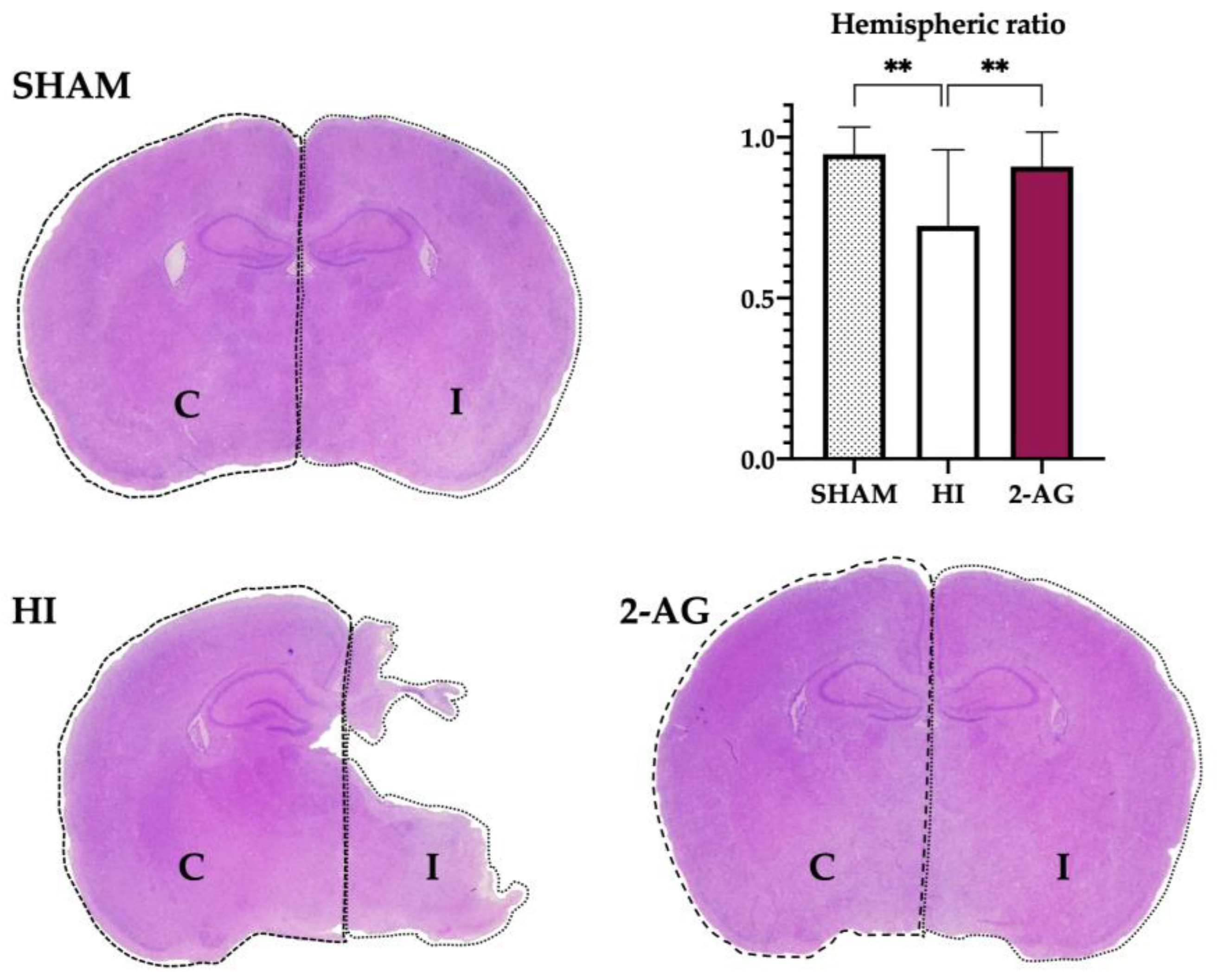
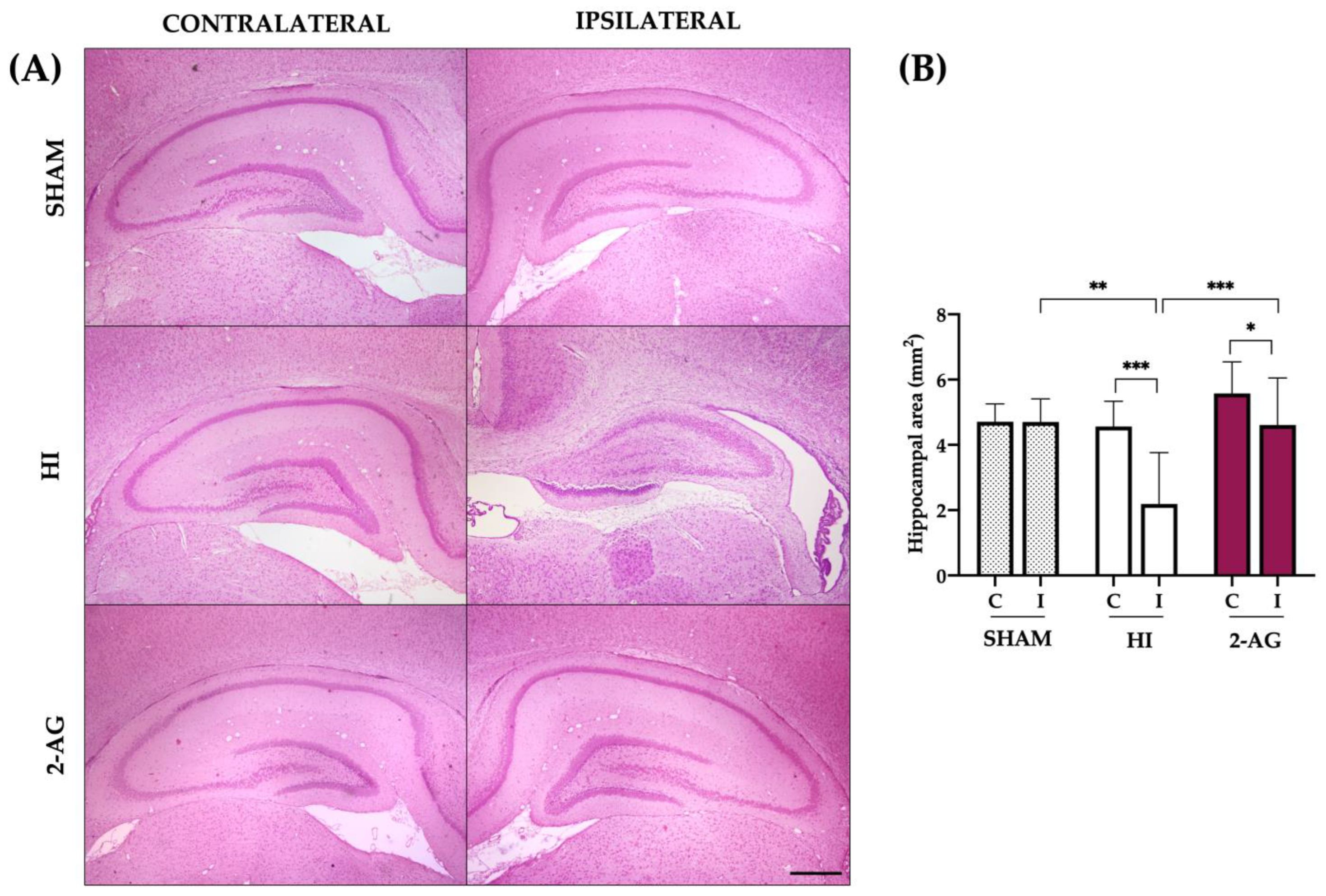
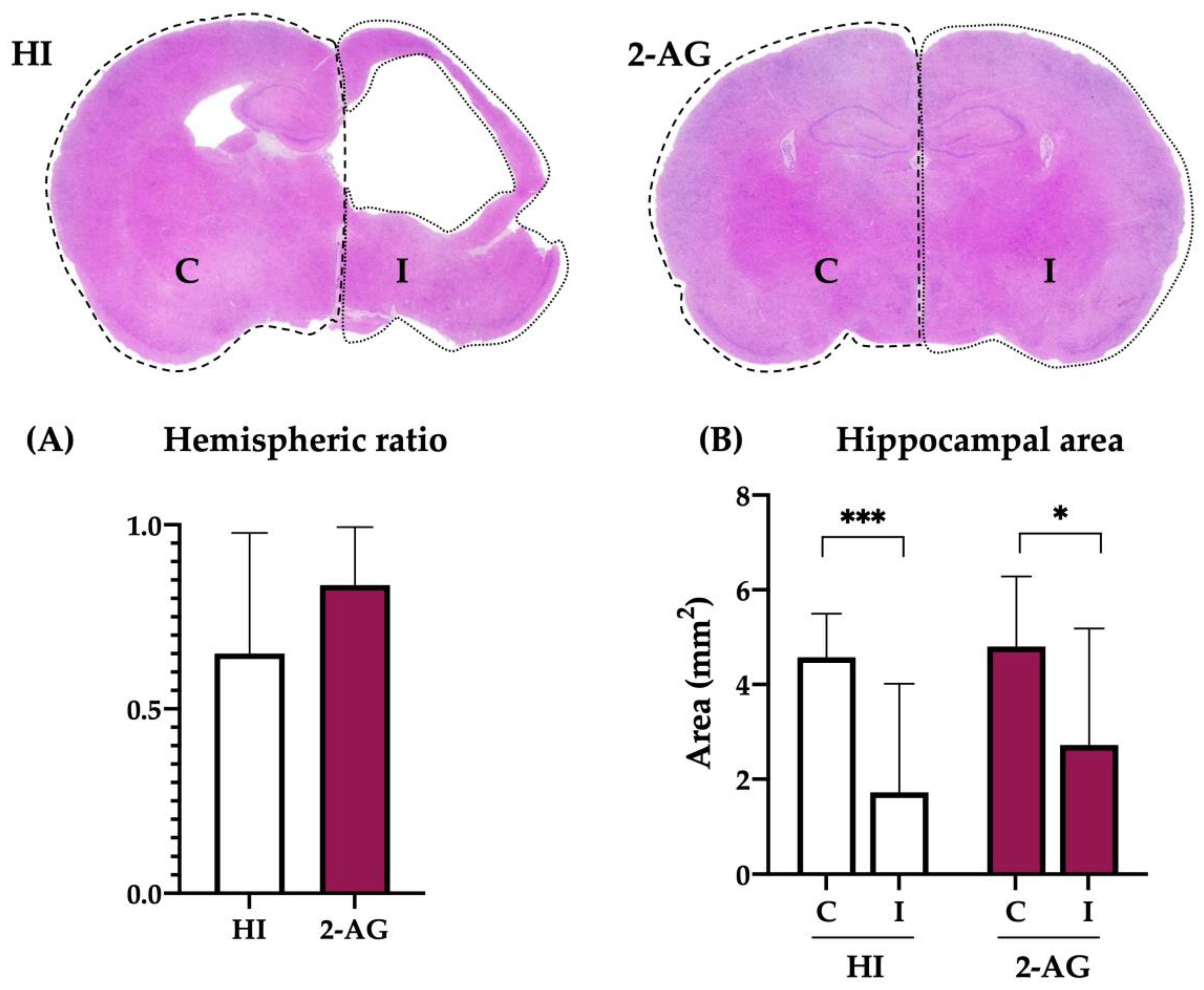
2.2. Evaluation of Hemispheric and Hippocampal Injury at P14
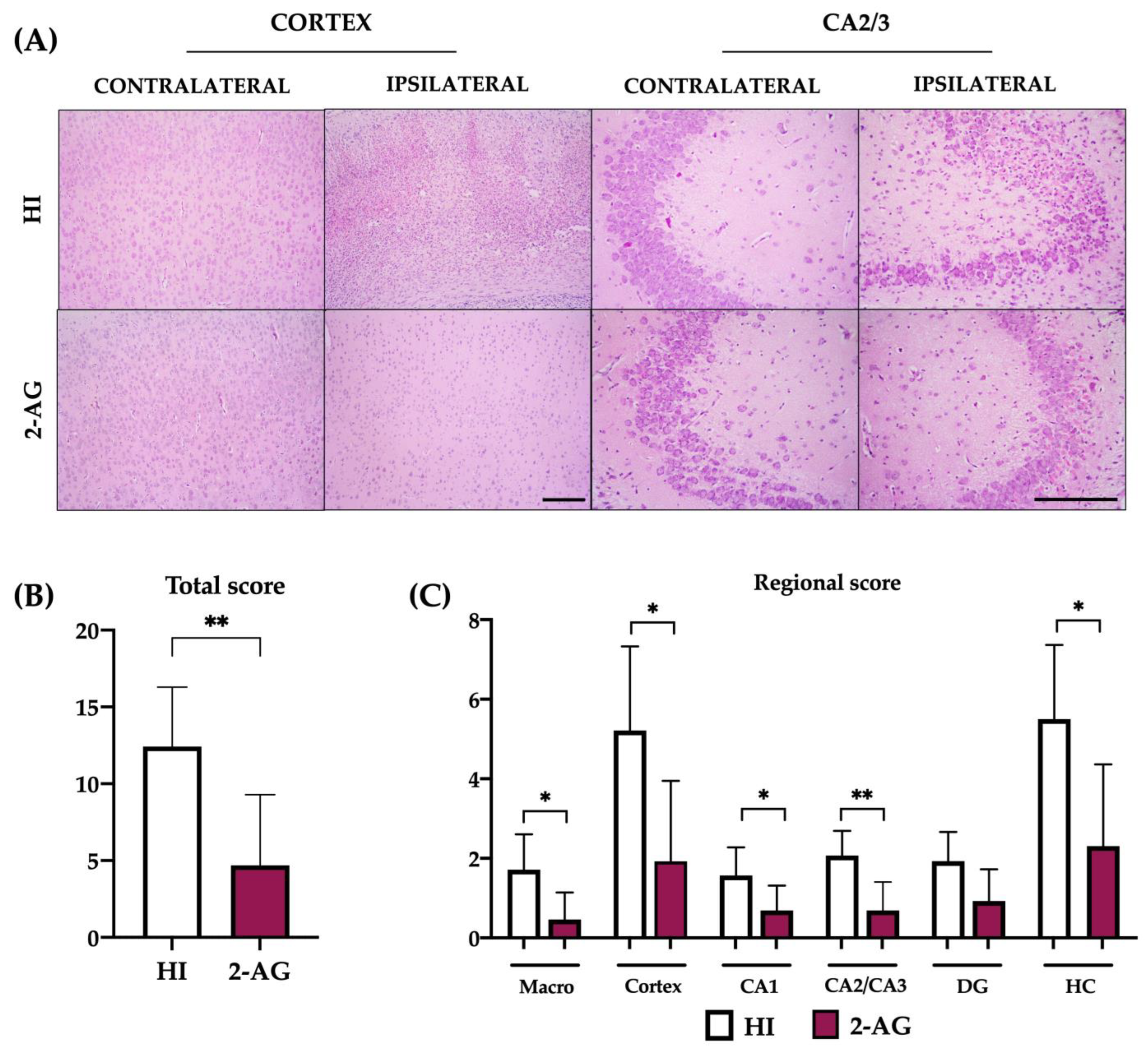
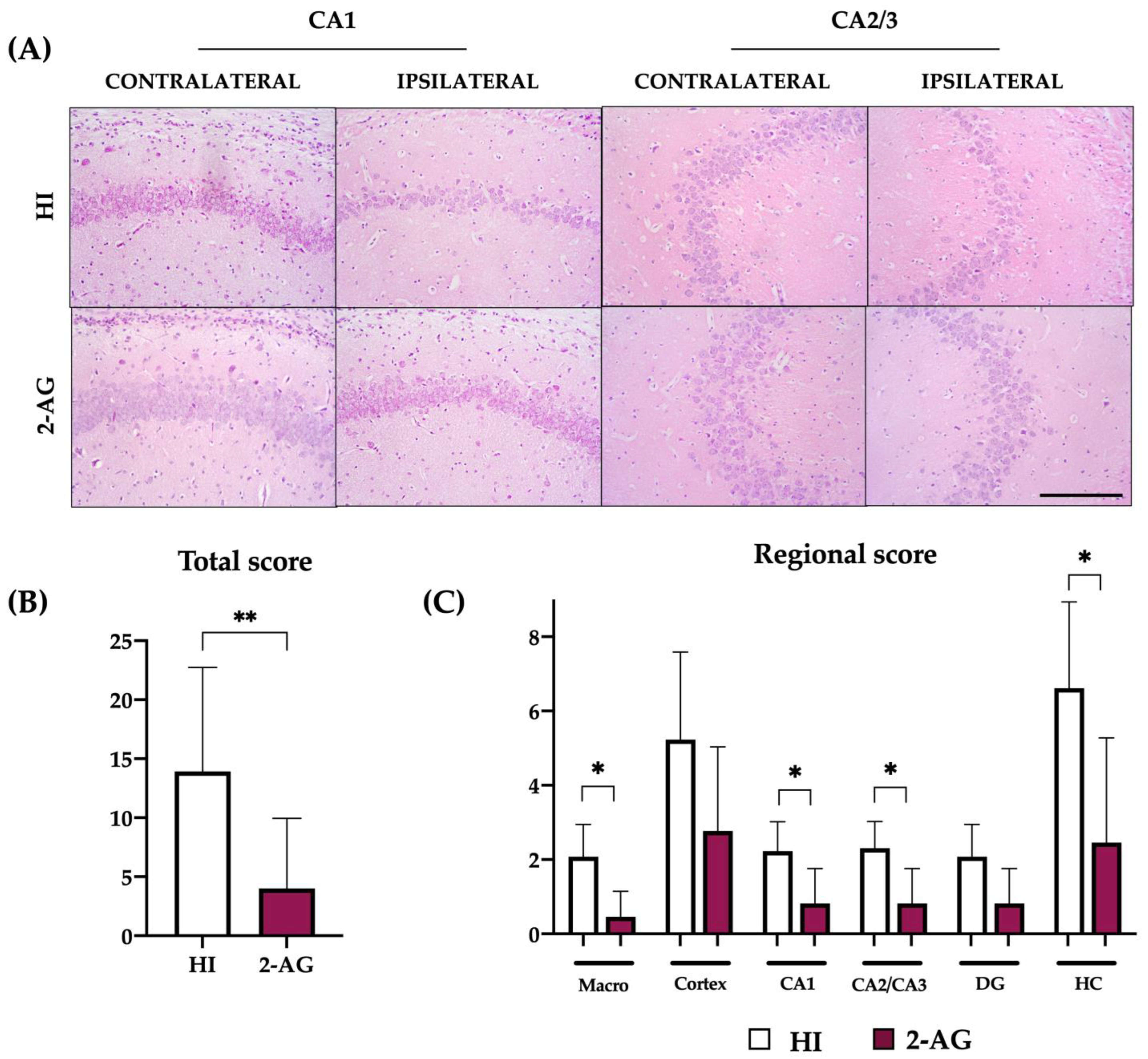
2.3. Neuropathological Score at P14
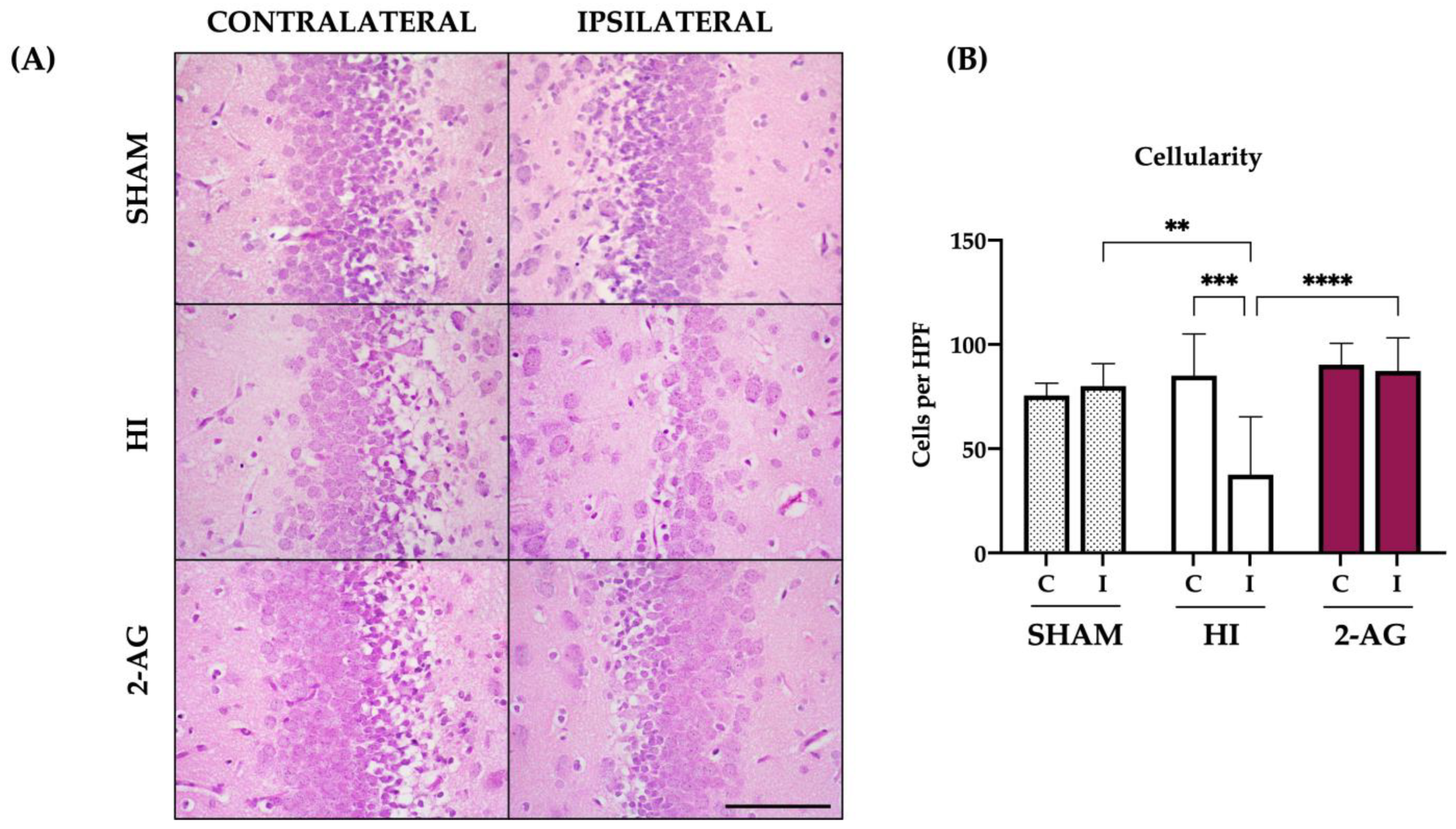
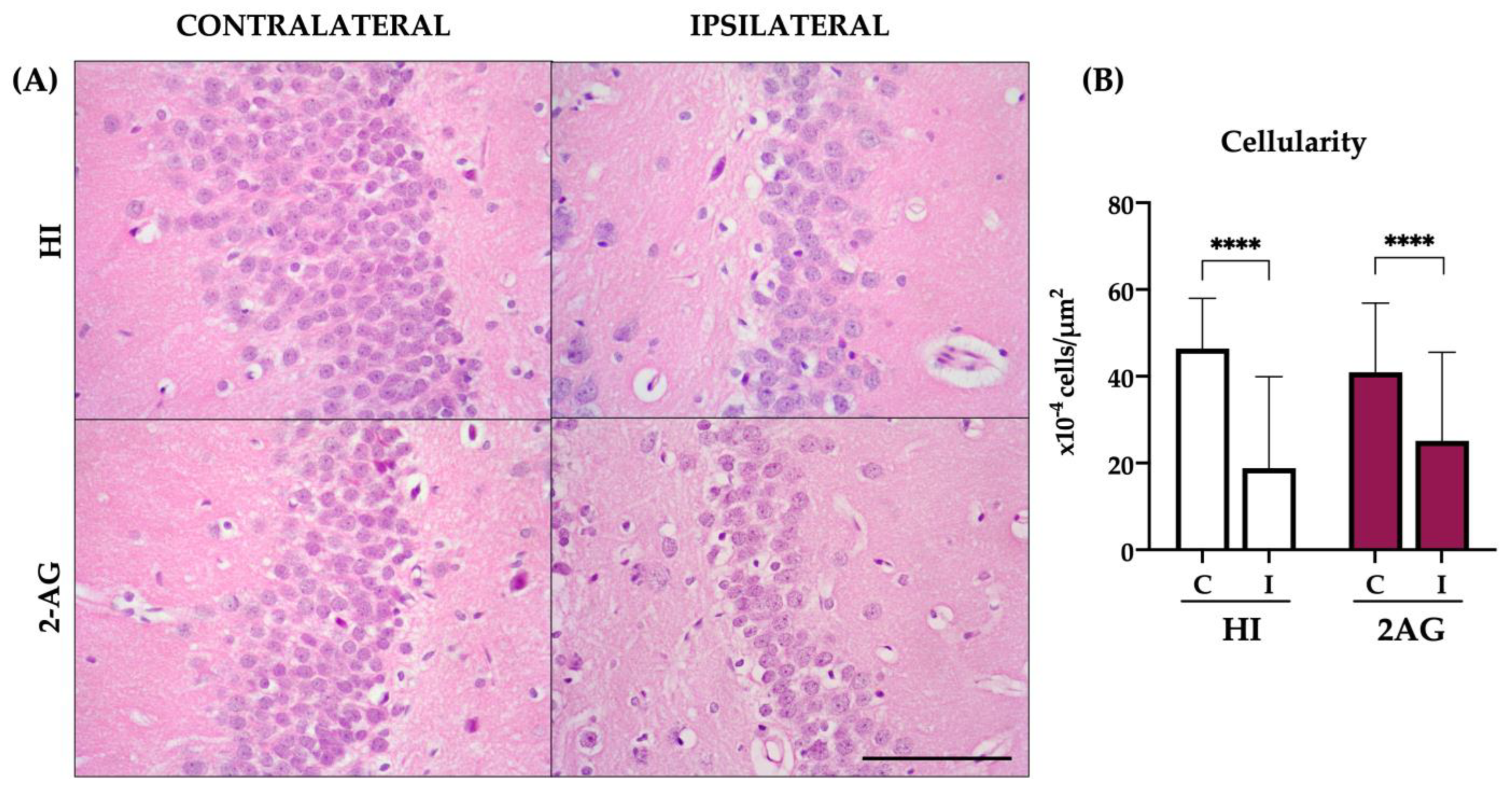
2.4. Cellularity of the DG at P14
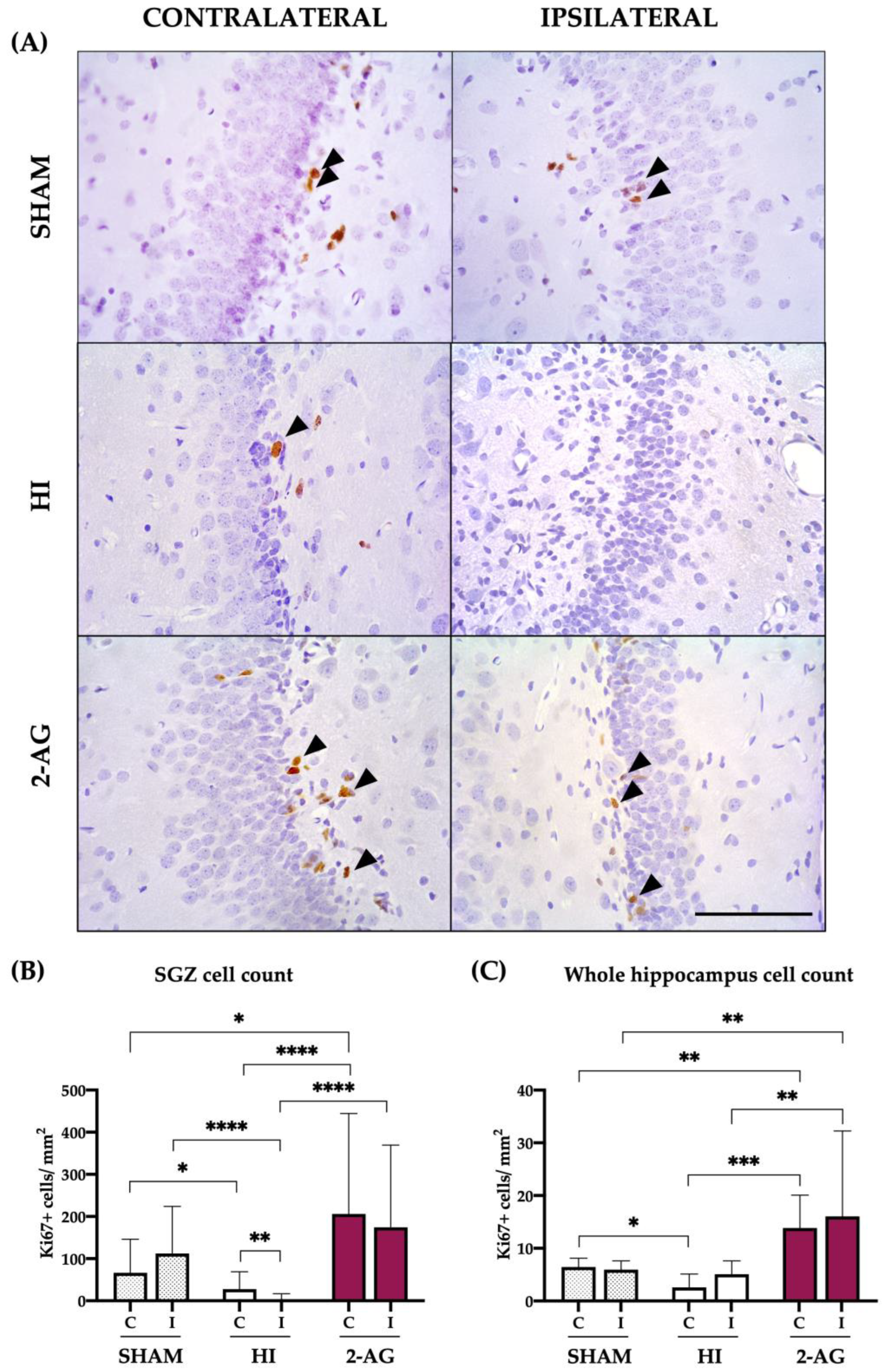
2.5. Ki67 Cell Counts in SGZ and Whole Hippocampus
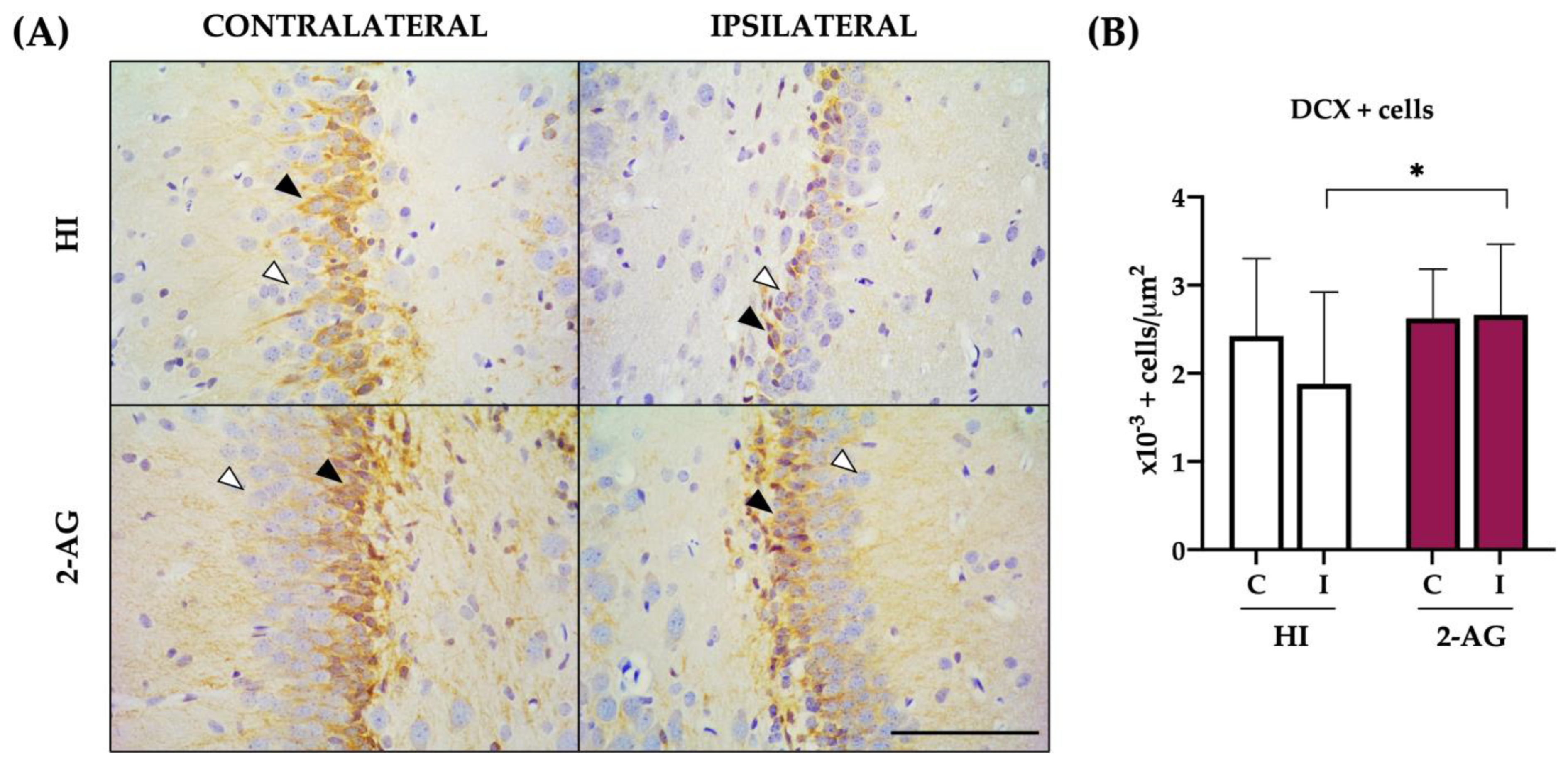
2.6. DCX Cell Counts at SGZ
2.7. Evaluation of Hemispheric and Hippocampal Injury at P90
2.8. Neuropathological Score at P90
2.9. Cellularity of the DG at P90
3. Discussion
4. Materials and Methods
4.1. Hypoxia-Ischemia
4.2. Drug Preparation
4.3. Tissue Processing
4.4. Hemispheric Ratios
4.5. Hippocampal Area
4.6. Neuropathological Score
4.7. Dentate Gyrus Cell Counts
4.8. Immunohistochemistry
4.9. Ki67 Cell Counts
4.10. DCX Cell Counts
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vannucci, R. Hypoxic-Ischemic Encephalopathy. Am. J. Perinatol. 2000, 17, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Thoresen, M. Neonatal Encephalopathy and Hypoxic–Ischemic Encephalopathy. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 217–237. ISBN 978-0-444-64029-1. [Google Scholar]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of Neonatal Encephalopathy and Hypoxic–Ischaemic Encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Zhou, K.; Bennet, L.; Thoresen, M.; Gunn, A.J. Therapeutic Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alconada, D.; Alvarez, A.; Hilario, E. Cannabinoid as a Neuroprotective Strategy in Perinatal Hypoxic-Ischemic Injury. Neurosci. Bull. 2011, 27, 275–285. [Google Scholar] [CrossRef]
- Sagredo, O.; Palazuelos, J.; Gutierrez-Rodriguez, A.; Satta, V.; Galve-Roperh, I.; Martínez-Orgado, J. Cannabinoid Signalling in the Immature Brain: Encephalopathies and Neurodevelopmental Disorders. Biochem. Pharmacol. 2018, 157, 85–96. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Moro, M.A.; Martínez-Orgado, J. Cannabinoids in Neurodegenerative Disorders and Stroke/Brain Trauma: From Preclinical Models to Clinical Applications. Neurotherapeutics 2015, 12, 793–806. [Google Scholar] [CrossRef]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and Neuroprotection in Global and Focal Cerebral Ischemia and in Neuronal Cultures. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef]
- Lara-Celador, I.; Castro-Ortega, L.; Álvarez, A.; Goñi-de-Cerio, F.; Lacalle, J.; Hilario, E. Endocannabinoids Reduce Cerebral Damage after Hypoxic–Ischemic Injury in Perinatal Rats. Brain Res. 2012, 1474, 91–99. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Alvarez, F.J.; Alvarez, A.; Mielgo, V.E.; Goñi-de-Cerio, F.; Rey-Santano, M.C.; Caballero, A.; Martinez-Orgado, J.; Hilario, E. The Cannabinoid Receptor Agonist WIN 55,212-2 Reduces the Initial Cerebral Damage after Hypoxic–ischemic Injury in Fetal Lambs. Brain Res. 2010, 1362, 150–159. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Álvarez, A.; Álvarez, F.J.; Martínez-Orgado, J.A.; Hilario, E. The Cannabinoid WIN 55212-2 Mitigates Apoptosis and Mitochondrial Dysfunction After Hypoxia Ischemia. Neurochem. Res. 2012, 37, 161–170. [Google Scholar] [CrossRef]
- Carloni, S.; Alonso-Alconada, D.; Girelli, S.; Duranti, A.; Tontini, A.; Piomelli, D.; Hilario, E.; Alvarez, A.; Balduini, W. Pretreatment with the Monoacylglycerol Lipase Inhibitor URB602 Protects from the Long-Term Consequences of Neonatal Hypoxic–ischemic Brain Injury in Rats. Pediatr. Res. 2012, 72, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Alvarez-Buylla, A. Proliferating Subventricular Zone Cells in the Adult Mammalian Forebrain Can Differentiate into Neurons and Glia. Proc. Natl. Acad. Sci. USA 1993, 90, 2074–2077. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the Adult Human Hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Kornack, D.R.; Rakic, P. Cell Proliferation without Neurogenesis in Adult Primate Neocortex. Science 2001, 294, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Asrican, B.; Paez-Gonzalez, P.; Erb, J.; Kuo, C.T. Cholinergic Circuit Control of Postnatal Neurogenesis. Neurogenesis 2016, 3, e1127310. [Google Scholar] [CrossRef]
- Benner, E.J.; Luciano, D.; Jo, R.; Abdi, K.; Paez-Gonzalez, P.; Sheng, H.; Warner, D.S.; Liu, C.; Eroglu, C.; Kuo, C.T. Protective Astrogenesis from the SVZ Niche after Injury Is Controlled by Notch Modulator Thbs4. Nature 2013, 497, 369–373. [Google Scholar] [CrossRef]
- Livneh, Y.; Adam, Y.; Mizrahi, A. Odor Processing by Adult-Born Neurons. Neuron 2014, 81, 1097–1110. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ieki, N.; Miyoshi, G.; Mochimaru, D.; Miyachi, H.; Imura, T.; Yamaguchi, M.; Fishell, G.; Mori, K.; Kageyama, R.; et al. Continuous Postnatal Neurogenesis Contributes to Formation of the Olfactory Bulb Neural Circuits and Flexible Olfactory Associative Learning. J. Neurosci. 2014, 34, 5788–5799. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Gressens, P.; Golay, X.; Robertson, N.J. Neurogenesis Is Reduced at 48 h in the Subventricular Zone Independent of Cell Death in a Piglet Model of Perinatal Hypoxia-Ischemia. Front. Pediatr. 2022, 10, 793189. [Google Scholar] [CrossRef]
- Gaffuri, A.-L.; Ladarre, D.; Lenkei, Z. Type-1 Cannabinoid Receptor Signaling in Neuronal Development. Pharmacology 2012, 90, 19–39. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Osredkar, D.; Sall, J.W.; Bickler, P.E.; Ferriero, D.M. Erythropoietin Promotes Hippocampal Neurogenesis in in Vitro Models of Neonatal Stroke. Neurobiol. Dis. 2010, 38, 259–265. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Yang, L.; Miao, X.; Lu, D.-H.; Yang, X.-Y.; Zhou, Z.-B.; Kang, W.-B.; Chen, K.-Y.; Zhou, L.-H.; et al. Neonatal Exposure to Low-Dose (1.2%) Sevoflurane Increases Rats’ Hippocampal Neurogenesis and Synaptic Plasticity in Later Life. Neurotox. Res. 2018, 34, 188–197. [Google Scholar] [CrossRef]
- Chen, K.; Lu, D.; Yang, X.; Zhou, R.; Lan, L.; Wu, Y.; Wang, C.; Xu, X.; Jiang, M.H.; Wei, M.; et al. Enhanced Hippocampal Neurogenesis Mediated by PGC-1α-Activated OXPHOS after Neonatal Low-Dose Propofol Exposure. Front. Aging Neurosci. 2022, 14, 925728. [Google Scholar] [CrossRef]
- Oddi, S.; Scipioni, L.; Maccarrone, M. Endocannabinoid System and Adult Neurogenesis: A Focused Review. Curr. Opin. Pharmacol. 2020, 50, 25–32. [Google Scholar] [CrossRef]
- Bravo-Ferrer, I.; Cuartero, M.I.; Zarruk, J.G.; Pradillo, J.M.; Hurtado, O.; Romera, V.G.; Díaz-Alonso, J.; García-Segura, J.M.; Guzmán, M.; Lizasoain, I.; et al. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome after Stroke. Stroke 2017, 48, 204–212. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Hagberg, H. Hypoxia–Ischemia in the Immature Brain. J. Exp. Biol. 2004, 207, 3149–3154. [Google Scholar] [CrossRef]
- Fernández-López, D.; Pazos, M.R.; Tolón, R.M.; Moro, M.A.; Romero, J.; Lizasoain, I.; Martínez-Orgado, J. The Cannabinoid Agonist Win55212 Reduces Brain Damage in an In Vivo Model of Hypoxic-Ischemic Encephalopathy in Newborn Rats. Pediatr. Res. 2007, 62, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, A.; Zweyer, M.; Maes, E.; Schleehuber, Y.; Doshi, H.; Sabir, H.; Bernis, M.E. Impact of Hypoxia-Ischemia on Neurogenesis and Structural and Functional Outcomes in a Mild–Moderate Neonatal Hypoxia-Ischemia Brain Injury Model. Life 2022, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Towfighi, J.; Mauger, D.; Vannucci, R.C.; Vannucci, S.J. Influence of Age on the Cerebral Lesions in an Immature Rat Model of Cerebral Hypoxia–Ischemia: A Light Microscopic Study. Dev. Brain Res. 1997, 100, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alconada, D.; Álvarez, A.; Lacalle, J.; Hilario, E. Histological Study of the Protective Effect of Melatonin on Neural Cells after Neonatal Hypoxia-Ischemia. Histol. Histopathol. 2012, 27, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Thayer, S.A. Cannabinoid Receptor Agonists Protect Cultured Rat Hippocampal Neurons from Excitotoxicity. Mol. Pharmacol. 1998, 54, 459–462. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, D.-S.; Kim, W.; Jung, H.Y.; Yu, Y.H.; Ju, Y.I.; Park, D.-K.; Hwang, I.K.; Kim, D.W.; Yoo, D.Y. Tat-Cannabinoid Receptor Interacting Protein Reduces Ischemia-Induced Neuronal Damage and Its Possible Relationship with 14-3-3η. Cells 2020, 9, 1827. [Google Scholar] [CrossRef]
- Ruiz-Contreras, H.A.; Santamaría, A.; Arellano-Mendoza, M.G.; Sánchez-Chapul, L.; Robles-Bañuelos, B.; Rangel-López, E. Modulatory Activity of the Endocannabinoid System in the Development and Proliferation of Cells in the CNS. Neurotox. Res. 2022, 40, 1690–1706. [Google Scholar] [CrossRef]
- Nakanishi, K.; Sato, Y.; Mizutani, Y.; Ito, M.; Hirakawa, A.; Higashi, Y. Rat Umbilical Cord Blood Cells Attenuate Hypoxic–Ischemic Brain Injury in Neonatal Rats. Sci. Rep. 2017, 7, 44111. [Google Scholar] [CrossRef]
- Labusek, N.; Mouloud, Y.; Köster, C.; Diesterbeck, E.; Tertel, T.; Wiek, C.; Hanenberg, H.; Horn, P.A.; Felderhoff-Müser, U.; Bendix, I.; et al. Extracellular Vesicles from Immortalized Mesenchymal Stromal Cells Protect against Neonatal Hypoxic-Ischemic Brain Injury. Inflamm. Regen. 2023, 43, 24. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Polowy, R.; Filipkowski, R.K.; Zalewska, T. Sodium Butyrate, a Histone Deacetylase Inhibitor, Exhibits Neuroprotective/Neurogenic Effects in a Rat Model of Neonatal Hypoxia-Ischemia. Mol. Neurobiol. 2017, 54, 5300–5318. [Google Scholar] [CrossRef]
- Bartley, J.; Soltau, T.; Wimborne, H.; Kim, S.; Martin-Studdard, A.; Hess, D.; Hill, W.; Waller, J.; Carroll, J. BrdU-Positive Cells in the Neonatal Mouse Hippocampus Following Hypoxic-Ischemic Brain Injury. BMC Neurosci. 2005, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Pradillo, J.M.; García-Yébenes, I.; Martínez-Orgado, J.A.; Moro, M.A.; Lizasoain, I. The Cannabinoid WIN55212-2 Promotes Neural Repair after Neonatal Hypoxia–Ischemia. Stroke 2010, 41, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Fau, S.; Renolleau, S.; Biran, V.; Charriaut-Marlangue, C.; Baud, O. Melatonin Promotes Myelination by Decreasing White Matter Inflammation after Neonatal Stroke. Pediatr. Res. 2011, 69, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Sánchez, C.; Galve-Roperh, I. Control of the Cell Survival/Death Decision by Cannabinoids. J. Mol. Med. 2001, 78, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzmán, M.; Galve-Roperh, I.; Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; et al. Non-psychoactive CB2 Cannabinoid Agonists Stimulate Neural Progenitor Proliferation. FASEB J. 2006, 20, 2405–2407. [Google Scholar] [CrossRef]
- Aguado, T.; Romero, E.; Monory, K.; Palazuelos, J.; Sendtner, M.; Marsicano, G.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. The CB1 Cannabinoid Receptor Mediates Excitotoxicity-Induced Neural Progenitor Proliferation and Neurogenesis. J. Biol. Chem. 2007, 282, 23892–23898. [Google Scholar] [CrossRef]
- Patel, S.D.; Pierce, L.; Ciardiello, A.; Hutton, A.; Paskewitz, S.; Aronowitz, E.; Voss, H.U.; Moore, H.; Vannucci, S.J. Therapeutic Hypothermia and Hypoxia–Ischemia in the Term-Equivalent Neonatal Rat: Characterization of a Translational Preclinical model. Pediatr. Res. 2015, 78, 264–271. [Google Scholar] [CrossRef]
- Kasdorf, E.; Engel, M.; Heier, L.; Perlman, J.M. Therapeutic Hypothermia in Neonates and Selective Hippocampal Injury on Diffusion-Weighted Magnetic Resonance Imaging. Pediatr. Neurol. 2014, 51, 104–108. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Lim, D.A. For the Long Run. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Chatzi, C.; Schnell, E.; Westbrook, G.L. Localized Hypoxia within the Subgranular Zone Determines the Early Survival of Newborn Hippocampal Granule Cells. eLife 2015, 4, e08722. [Google Scholar] [CrossRef]
- Mouret, A.; Gheusi, G.; Gabellec, M.-M.; de Chaumont, F.; Olivo-Marin, J.-C.; Lledo, P.-M. Learning and Survival of Newly Generated Neurons: When Time Matters. J. Neurosci. 2008, 28, 11511–11516. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, A.; Sandler, V.M.; Toni, N.; Zhao, C.; Gage, F.H. NMDA-Receptor-Mediated, Cell-Specific Integration of New Neurons in Adult Dentate Gyrus. Nature 2006, 442, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The Influence of Immaturity on Hypoxic-Ischemic Brain Damage in the Rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Zaynutdinova, D.; Ogievetsky, E.; Valeeva, G.; Mitrukhina, O.; Manent, J.-B.; Represa, A. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front. Neuroanat. 2015, 9, 161. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Nijboer, C.H.; Groenendaal, F.; Kavelaars, A.; Hagberg, H.H.; van Bel, F.; Heijnen, C.J. Gender-Specific Neuroprotection by 2-Iminobiotin after Hypoxia—Ischemia in the Neonatal Rat via a Nitric Oxide Independent Pathway. J. Cereb. Blood Flow Metab. 2007, 27, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, M.; Bagenholm, R.; Loberg, E.M.; Apricena, F.; Kjellmer, I. Posthypoxic Cooling of Neonatal Rats Provides Protection against Brain Injury. Arch. Dis. Child.-Fetal Neonatal Ed. 1996, 74, F3–F9. [Google Scholar] [CrossRef]
- Bona, E.; Hagberg, H.; Løberg, E.M.; Bågenholm, R.; Thoresen, M. Protective Effects of Moderate Hypothermia after Neonatal Hypoxia-Ischemia: Short- and Long-Term Outcome. Pediatr. Res. 1998, 43, 738–745. [Google Scholar] [CrossRef]
| Sham | HI | 2-AG | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Body weight (g) | 28.33 | 1.33 | 23.65 ** | 3.47 | 27.39 ## | 2.7 |
| Brain weight (g) | 1.19 | 0.05 | 0.93 **** | 0.11 | 1.15 ### | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldarrain, G.; Hilario, E.; Lara-Celador, I.; Chillida, M.; Catalan, A.; Álvarez-Diaz, A.Á.; Alonso-Alconada, D. The Long-Term Neuroprotective Effect of the Endocannabinoid 2-AG and Modulation of the SGZ’s Neurogenic Response after Neonatal Hypoxia-Ischemia. Pharmaceutics 2023, 15, 1667. https://doi.org/10.3390/pharmaceutics15061667
Beldarrain G, Hilario E, Lara-Celador I, Chillida M, Catalan A, Álvarez-Diaz AÁ, Alonso-Alconada D. The Long-Term Neuroprotective Effect of the Endocannabinoid 2-AG and Modulation of the SGZ’s Neurogenic Response after Neonatal Hypoxia-Ischemia. Pharmaceutics. 2023; 15(6):1667. https://doi.org/10.3390/pharmaceutics15061667
Chicago/Turabian StyleBeldarrain, Gorane, Enrique Hilario, Idoia Lara-Celador, Marc Chillida, Ana Catalan, Antonia Ángeles Álvarez-Diaz, and Daniel Alonso-Alconada. 2023. "The Long-Term Neuroprotective Effect of the Endocannabinoid 2-AG and Modulation of the SGZ’s Neurogenic Response after Neonatal Hypoxia-Ischemia" Pharmaceutics 15, no. 6: 1667. https://doi.org/10.3390/pharmaceutics15061667
APA StyleBeldarrain, G., Hilario, E., Lara-Celador, I., Chillida, M., Catalan, A., Álvarez-Diaz, A. Á., & Alonso-Alconada, D. (2023). The Long-Term Neuroprotective Effect of the Endocannabinoid 2-AG and Modulation of the SGZ’s Neurogenic Response after Neonatal Hypoxia-Ischemia. Pharmaceutics, 15(6), 1667. https://doi.org/10.3390/pharmaceutics15061667





