Abstract
β-glucan, one of the homopolysaccharides composed of D-glucose, exists widely in cereals and microorganisms and possesses various biological activities, including anti-inflammatory, antioxidant, and anti-tumor properties. More recently, there has been mounting proof that β-glucan functions as a physiologically active “biological response modulator (BRM)”, promoting dendritic cell maturation, cytokine secretion, and regulating adaptive immune responses—all of which are directly connected with β-glucan-regulated glucan receptors. This review focuses on the sources, structures, immune regulation, and receptor recognition mechanisms of β-glucan.
1. Introduction
β-glucan is a kind of polysaccharide with multiple physiological functions and is known as a “biological response regulator” because of its multiple biological functions [1]. The first defensive line of body immunity is innate immunity. In its early stage, it mainly uses phagocytic cells such as macrophages and neutrophils to engulf and kill pathogens invading the body and then further activates the adaptive immune system by secreting cytokines and chemokines. β-glucan has been found to affect several types of immune cells, including macrophages, natural killer cells, and neutrophils, resulting in various immunological effects. In recent decades, tumor immunotherapy has made extensive use of β-glucan as a natural biological effect regulator [2]. Current clinical applications of β-glucans include yeast, lentinan, Coriolus versicolor polysaccharide, mycobacterium polysaccharide, and oat. The different types of β-glucan influence the strength and immune response, depending on the source, structure, water solubility, and molecular weight [3]. The body recognizes invading pathogenic microorganisms through the pattern recognition receptor (PRR) and initiates the body’s immune response to pathogens through a series of biochemical reactions. Currently, the following β-glucan receptors have been identified: dendritic cell (DC)-associated C-type lectin-1 (Dectin-1) [4], complement receptor 3 (CR3), cluster of differentiation 11b (CD11b)/CD18, αMβ2-integrin, macrophage differentiation antigen-1 (Mac-1) [5,6], lactosylceramide (LacCer) [7], and scavenger receptors (SRs) [8]. In this review, the source and structure, immunoregulation, and receptor recognition mechanism of β-glucan are discussed, which offers fresh perspectives on the development of natural immune enhancers for anti-tumor immunotherapy.
2. β-Glucan Sources and Properties
Glucan is a homopolysaccharide of glucose as a monomer structure. It is the most common polysaccharide in nature, widely distributed in bacteria, fungi, and other plants, consisting of two types depending on the difference in the stereoisomer of the glycosidic bond as α- and β-linked glucans. The α- and β-glucans are well known to be the energy source of the body and the indigestible fibers with obvious physiological functions [9,10,11,12,13,14,15,16] mainly as α- and β-(1→4)-linked liner structures between glucose residues (amylose and cellulose), respectively. The latter one, β-glucan, has been attracting attention in recent years as an antineoplastic immunostimulant that mainly comes from yeast, barley, oats, fungi, mushrooms, and algae [17,18,19,20]. β-Glucan is also a component of the cell wall of certain pathogenic fungal (Pneumocystis carinii, Cryptococcus neoformans, Aspergillus fumigatus, Histoplasma capsulatum, Candida albicans) and fungi (Saccharomyces cerevisiae) [21,22]. The cell wall of fungi is mainly composed of polysaccharides and glycoproteins. For example, the cell wall of S. cerevisiae consists of three layers: the inner layer is insoluble β-glucan (30–35%); the middle layer is soluble β-glucan (20–22%); the outer layer is glycoprotein (30%) [23]. The β-glucan mainly exists in nature in the form of liner or branched chains of (1→2)-, (1→3)-, (1→4)-, and (1→6)-β-glucan [24,25] (Table 1, Figure 1). In addition to abundant (1→4)-β-glucan in plant cellulose, plant hemicellulose includes linear β-glucans with (1→3)-(1→4)-mixed linkages, containing tri-and tetrasaccharide (1→4)-β-glucan fragments inserted in random order. In this case, the contents of β-(1→4) and β-(1→3) linkages in the β-glucans are approximately 70% and 30%, respectively. Lichenan (Lichenin) from Icelandic moss consists of a linear (1→3)-(1→4)-mixed linkages with [→4)-β-glycosyl-(1→3)-β-glycosyl-(1→] repeating unit. Barley and oat glucans from Hordeum vulgare and Avena sativa, respectively, consist of linear (1→3)-(1→4)-mixed linkages as well. Agrobacterium tumefaciens produces a β-(1→2)-glucan structure whose function has not been reported although cyclic β-(1→2)-glucan plays an important role for plant pathogen in evading the host immune system [26,27,28,29]. Except for the large quantity of plant β-(1→4)-glucan and very rare (1→2)-β-glucan as its cyclic forms, different sources of β-glucan from algae and bacteria have certain differences in structure and function, whose glucan structures are mainly a linear (1→3)-β-glucan. Yeast produces mainly (1→3)-β-glucan containing β-(1→6)-branches. β-glucan of the fungal origin mainly contains a linear structure with a combination of β-(1→3) and β-(1→6) linkages, and cereal-derived one has a linear combination of β-(1→3)- and β-(1→4)-linkages [25].

Table 1.
Common β-glucans.
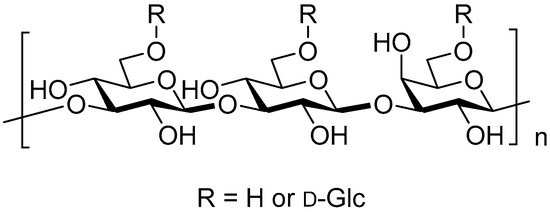
Figure 1.
Example of (1→3)-β-D–glucan.
β-glucan, mostly referred to as (1→3)-β-glucan, can be divided into the single helix, triple helix, or random helix (irregular helix) according to its three-dimensional conformation [41]. The three structural formulas can be transformed into each other (Figure 2), for example, the triple helix is opened to form a single helix structure under alkaline conditions such as NaOH [42,43]. Meanwhile, the single helix also can become an irregular helix, which is restored to a triple helix structure under heating or dialysis conditions. It is shown that the three-dimensional structure of the (1→3)-β-glucan polymers is an important determinant of receptor–ligand interactions [8]. Insoluble particulate (1→3)-β-glucan is thought to activate DCs and macrophages in rats through the Dectin-1 pathway. This activation is believed to be enhanced depending on the degree of molecular polymerization and the content of the β-glycoside bond [44]. While water-soluble (1→3)-β-glucan can bind to these cells, it does not activate them [45]. Furthermore, (1→3)-β-Glucan has a wide range of physiological functions [46], including immune system enhancement [47,48], anti-tumor [49], anti-infection [17], anti-radiation [50], metabolism regulation [51,52], anti-inflammatory [53], antioxidant [54], hypoglycemic [55], and reductions in serum lipids [56]. Additionally, it has been used as an additive in the beauty and skincare industries due to its anti-radiation, anti-aging, and free radical scavenging properties. Furthermore, (1→3)-β-glucan can help to reduce body absorption and level of cholesterol and other blood lipids in blood and has been utilized in the food and healthcare industries [57].
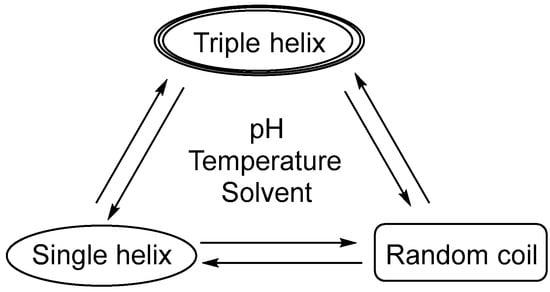
Figure 2.
Conformational change in secondary structures of (1→3)-β-glucans.
3. Immunostimulatory Properties of (1→3)-β-Glucan
As a natural barrier of the human body, the immune system has the functions of immune surveillance, defense, and regulation. It can be divided into adaptive immunity and innate immunity. The former is subdivided into cellular immunity and humoral immunity, which are respectively exerted by T lymphocytes and B lymphocytes, and the latter is mainly exerted by innate immune cells, such as monocyte macrophages and natural killer (NK) cells [58]. Therefore, modern medicine mainly evaluates immune function from four aspects: cellular immunity, humoral immunity, mononuclear macrophage phagocytosis, and NK cell activity. Carbohydrates are common surface molecules in biological systems. Due to their rich structural diversity, carbohydrate molecules play an important role in cell recognition and signal transduction, including immune recognition and activation [59,60]. Furthermore, (1→3)-β-glucan is a polysaccharide adjuvant widely existing in bacterial and fungal cell walls, which can stimulate antibacterial immune response [60]. In the 1950s, Dr. Pillemer first discovered and reported that there was a substance in the yeast cell wall that could improve immunity [61]. In later research, Diluzio’s group discovered that the immunity-boosting substance in the yeast cell wall was (1→3)-β-glucan, isolated from baker’s yeast [62,63].
Yeast (1→3)-β-glucan activates various immune cells, including macrophages and neutrophils, leading to increased production of interleukin (IL), cytokinin, and special antibodies. This comprehensive stimulation of the immune system prepares the body to better fight against diseases [64,65]. In addition, yeast (1→3)-β-glucan restores the ability of lymphocytes to produce cytokines such as IL-1 and effectively regulates immune function [66,67,68]. Many experiments have indicated that yeast (1→3)-β-glucan promotes the production of IgM antibodies, improving humoral immunity. Moreover, yeast (1→3)-β-glucan activates toll-like receptor 2 (TLR2), inducing nuclear factor (NF)- κB activation and tumor necrosis factor (TNF)-α secretion, as well as regulatory antigen-presenting cells and immune tolerance [69,70]. The yeast (1→3)-β-glucan-activated cells stimulate the host’s non-specific defense mechanism and are thus being studied for their potential in cancer, infectious disease, and wound treatment. In 2008, β-glucan extracted from S. cerevisiae’s yeast was released by the US Food and Drug Administration (FDA) as a safe food ingredient that can be added to general food. It is a very rare active immune substance, which can kill harmful viruses and maintain good immunity. Many years ago, National Aeronautics and Space Administration (NASA) listed yeast glucan as a food for astronauts to enhance their immunity.
Many researchers have not only demonstrated the regulatory effect of yeast (1→3)-β-glucan on immunity, such as induction of autoimmune arthritis or enhancement of nitric oxide (NO) synthesis, through in vitro cell experiments or in vivo experiments in mice, respectively [71,72,73], but also showed that β-glucan also has the effect of immune stimulation on zebrafish [74,75].
Wu et al. reported that the addition of (1→3)-β-glucan can also lessen the inflammatory response after lipopolysaccharide (LPS) stress [76]. Yeast (1→3)-β-glucan is also an important enhancer of mucosal immunity in the digestive tract [77]. The digestive system is the primary point of contact for many pathogens and foreign substances, and mucosal immunity in the system plays a vital role in defending against these threats. Additionally, it has also been found that (1→3)-β-glucan can improve the level of lysozyme in animal serum and the antibody titer [78]. Yeast (1→3)-β-glucan can activate neutrophils and phagocytes in gastrointestinal tissues, thereby further activating and affecting the “immune nerve endocrine” regulatory network, enhancing its anti-infection, anti-stress, and cellular adaptive protection capabilities and also enhancing macrophage-mediated tissue repair, accelerating the repair process of ulcers, and improving the repair quality [79]. Yeast β-glucan has the ability to bind with surface receptors of macrophages, neutrophils, and lymphocytes, which can affect the cellular signaling process, activate the immune activity of lymphocytes, and enable them to swiftly reach the site of infection [80]. It acts as an immune response booster and facilitates whey protein. Whey protein is a high-quality protein that contains all of the essential amino acids needed by the body. Combining with β-glucan, it can activate immune cells. While whey protein provides the building blocks necessary for these cells to function properly, they can work synergistically to enhance the immune response [81]. The latest research found that pre-treatment of mice with (1→3)-β-glucan can reduce the growth of tumors and elucidated that β-glucan transcriptomically and epigenetically rewires granulopoiesis and reprograms neutrophils towards an anti-tumor phenotype to form a long-term innate immune memory, i.e., trained immunity. Moreover, the anti-tumor effects of (1→3)-β-glucan-induced trained immunity can be transferred to recipient initial mice via bone marrow transplantation [82]. Furthermore, (1→3)-β-glucan can stimulate the innate immunity of Pagrus auratus by enhancing the respiratory burst of macrophages [83]. Besides for humans, Chang et al. also showed that the addition of (1→3)-β-glucan to the diet of shrimp enhanced the bacteriophage activity of blood cells, cell adhesion, and production of reactive oxygen species [84].
Macrophages are essential to every stage of host defense and are engaged in both innate and adaptive immune responses in case of infection. The pathogen crosses the epithelial barrier, following phagocytosis by macrophages and digestion by lysosomal enzymes, which are important processes for presenting antigens from the pathogens as phagocytic activity, and lysosomal enzymes determine the function of macrophages [85]. The secretion of cytokines (IL-1, IL-6, IL-8, IL-12, TNF-a) and inflammatory mediators (NO, hydrogen peroxide (H2O2)) are also the downstream effect of these cells. Thus, β-glucan-activated macrophage function enhances host immune defense (Figure 3) [86,87]. Furthermore, (1→3)-β-Glucan is an effective immunomodulator [88,89,90], which can enhance the anti-tumor activity of peritoneal macrophages. In vitro studies showed that the killing effect of monocytes and neutrophils on microorganisms in healthy volunteers was enhanced after taking (1→3)-β-glucan. In addition to activating macrophages, T cells, and natural killer (NK) cells, (1→3)-β-glucan also activates complement components through a selective activation pathway. When (1→3)-β-glucan is present, it can bind to complement component C3, which triggers a cascade of reactions leading to the activation of the alternative pathway. This results in the formation of the C3 convertase enzyme, which cleaves C3 into iC3b. iC3b then binds to the surface of the pathogen, marking it for destruction by immune cells [5,6]. Activation of complement components through this pathway is important because it allows for a more targeted response to pathogens, without causing excessive inflammation or tissue damage. Vaccine adjuvants have a variety of mechanisms, typically including storage effects, promoting antigen presentation, increasing the secretion of immunomodulatory cytokines to control cellular responses with T and B, stimulating innate immunity, and indirectly modulating adaptive immune responses [91]. At present, (1→3)-β-glucan is an attractive candidate for immune adjuvants and is used in a wide range of vaccine development. It can activate the immune system and induce the Th1 immune response [92,93]. The potential effects of (1→3)-β-glucan as an adjuvant on the effectiveness of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus vaccination were discussed by Alfredo’s group (Figure 3) [94].
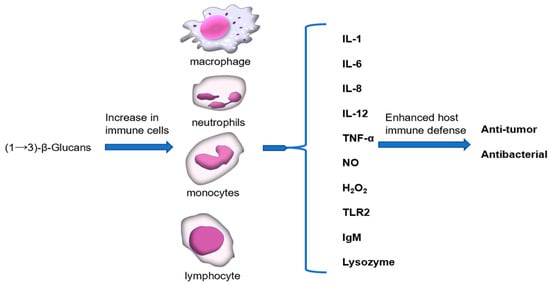
Figure 3.
Immunomodulatory action of (1→3)-β-glucans.
4. Immunoregulatory Receptor of (1→3)-β-Glucan
The innate immune response triggers the immune system through the recognition and phagocytosis by phagocytes. Pathogen-related molecular pattern substances (PAMPS) activate the adaptive immune response process by recognizing and binding to the pattern recognition receptor (PRR) on the membrane structure of phagocytes. Most cell surface immune receptors, such as TLRS, nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), and major histocompatibility complexes class I and class II (MHC-I and MHC-II), are glycoproteins. TLRs, NLRs, C-type lectin, and sialic acid-binding immunoglobulin (Ig)-like lectins (Siglecs), among other crucial receptors for immune cell activation, can identify sugar-containing ligands, including sugars expressed on the surface of many pathogenic microorganisms and cancer cells (Figure 4) [95]. As an important pathogen-associated molecular pattern (PAMP), (1→3)-β-glucan plays an immunomodulatory role mainly through three types of PRR: (1) Dectin-1 receptors; (2) CR3 [96]; (3) other receptors including scavenger receptor and LacCer [97] (Figure 4). It can be recognized by pattern-recognition receptors (PRRS) expressed on the surface of these innate immune cells, promoting the activation, maturation, and production of cytokines of immune cells, thus starting the innate immune response and regulating the subsequent adaptive immune response. Studies have shown that (1→3)-β-glucan can combine with C-type lectin Dectin-1 and CR3, promote the activation of secreted cytokines and B-cell T-cells, and enhance humoral and cellular immune responses [98]. Different sources, structures, and formulations of (1→3)-β-glucan can stimulate innate and adaptive immunity in different ways. In vitro and in vivo studies have shown that the molecular structure, molecular weight, and the number of branches are the key determinants of its immune activity [99].
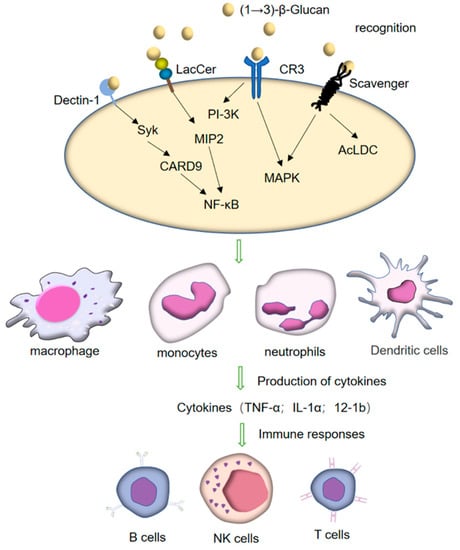
Figure 4.
Immune activation induced by (1→3)-β-glucans: (1→3)-β-glucans can act on many receptors such as Dectin-1, CR3, LacCer, and scavenger found on the immune cells (monocytes, macrophages, DCs, and neutrophils).
Antigen presenting cells (APCs) can be divided into three types: DCs, macrophages, and B cells, these three types of cells are white blood cells and originate from bone marrow tissue [96]. DC-associated C-type lectin-1 (Dectin-1) is an important receptor of (1→3)-β-glucan. Much progress has been achieved since it was first found on the surface of DCs in the study of its anti-tumor mechanism [100,101,102]. The β-glucan receptor (β-GR) is made of three components: a C-type lectin-like carbohydrate recognition domain, a short stalk, and a cytoplasmic tail with a tyrosine-based immune receptor activation motif [100]. It recognizes carbohydrates containing β-(1→3)-linked and β-(1→6)-linked glucan bonds [103]. The Dectin-1 (β-GR) receptor is not restricted to dendritic cells but is broadly expressed, with the highest surface expression on myeloid cell populations (monocyte/macrophage and neutrophil lineages). β-GR is also expressed by dendritic cells and a subset of T cells, albeit with lower surface expression levels [104]. Many studies have demonstrated that after recognition with (1→3)-β-glucan, Dectin-1 can trigger its own intracellular signal transduction through the cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM)-like motif, activating immune cells to produce a series of cellular reactions, such as phagocytosis and endocytosis of (1→3)-β-glucan, inducing respiratory burst, maturation of DCs, and producing various inflammatory cytokines TNF-α, IL-1a, IL-1b, IL-6, and chemokines, C-X-C motif chemokine ligand 2 (CXCL2), C-C motif chemokine ligand 3 (CCL3), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [105,106,107]. Recruitment of spleen tyrosine kinase (Syk), activation of caspase recruitment domain family member 9 (CARD9), activation of NF-κB, mitogen-activated protein kinases (MAPKs), and activation of nuclear factor of activated T-cells (NFAT) are all components of the Dectin-1 downstream signal transduction pathway [108]. The phagocytosis of macrophages on non-conditioned microorganisms can trigger the host’s innate immune response against infection. Cytoplasmic phospholipase A2 (cPLA2) is activated in the process of phagocytosis, releasing arachidonic acid-producing biomass, causing acute inflammation. Dectin-1 receptor can also stimulate macrophages to release arachidonic acid and cyclooxygenase 2 (COX2) expression pattern recognition receptor through pathogenic yeast and yeast cell wall. Pure particulate (1→3)-β-glucan stimulates arachidonic acid release and enhanced COX2 expression macrophage-activated lipopeptide-2 (MALP-2) [109]. Dependent on TLR2, the first result on the synergistic effect of Dectin-1 and TLR2 to activate the proinflammatory response of macrophages to mycobacterial infection has been established [110]. The Dectin-1 receptor is a PRR widely expressed in macrophages and DCs. Furthermore, (1→3)-β-glucan is specifically recognized by the Dectin-1 receptor, of which the activation can also promote Th17 cell differentiation [111,112]. Although both soluble (1→3)-β-glucan and particulate β-glucan bind to Dectin-1, the downstream signal is only triggered by the latter [113], resulting in the release of tumor necrosis factor TNF-a, a marker of Dectin-1 activation [114]. Both (1→3)-β-glucan and (1→6)-β-glucan can effectively activate the bypass pathway of complement components, leading to fungal conditioning and the recruitment of inflammatory cells. The receptor Dectin-1 can stimulate the activation of Th1, Th17, and cytotoxic T-cell responses, reverse immune tolerance, and restore the secretion of cytokines [115,116].
The complement system is a kind of activated protein with enzyme activity, which widely exists in serum, tissue fluid, or cell surface. The complement system includes more than 30 kinds of soluble proteins and membrane-binding proteins, which can be divided into three categories according to their different biological functions: complement intrinsic components, complement regulatory proteins, and complement receptors (CR). The activated complement system has a precise regulatory mechanism, and the activated complement products have biological functions, such as cell lysis, regulating phagocytosis, clearing immune complexes, and mediating inflammatory reaction [117]. CR3, also called Mac-1, CD11b/CD18, or αMβ2 lectin, belongs to the family of leukocyte adhesion receptors as an important member [118]. It is present on the surface of macrophages, NK cells, B lymphocytes, cytotoxic T cells, and neutrophils and is also expressed on activated CD8+ T cell subsets and spleen DC membranes. By facilitating contact between effector cells and target cells it enhances phagocytosis. It is a membrane glycoprotein composed of two peptide chains with two distinct domains—one specifically bound to (1→3)-β-glucan and one particularly attached to the inactivated form of C3b fragment from serum C3 (iC3b). As a (1→3)-β-glucan receptor, CR3 has one or more exogenous lectin action sites distributed on its α-methylene. When (1→3)-β-glucan binds to these sites, they transmit signals to the cell to initiate the regulation of cytotoxins and phagocytic immune responses [2,119,120]. Michalek et al. reported that the leukocytes with activated CR3 on their surface have obvious killing power against tumor cells that form immune complexes on the cells [121], Ferreira and others found that (1→3)-β-glucan binds to CR3 on the surface of macrophages. It can activate phosphatidylinositol-3 kinase (PI3K) and MAPK signal pathways, promote the generation of inflammatory factors, and play its role in immune regulation [122]. The latest research shows that blocking CR3 can significantly lower the endocytosis of (1→3)-β-glucan by neutrophils and inhibit the production of (1→3)-β-glucan-induced reactive oxygen species [123]. By binding with CR3 on macrophages or NK cells, (1→3)-β-glucan continuously triggers the cytotoxicity of cells against iC3b tumor tissues and enhances the phagocytosis of macrophages and NK cells.
Other receptors include scavenger receptor, LacCer, etc. In the late 1970s, Goldstein and others first reported the binding site of acetylated low-density lipoprotein (AcLDL) on macrophages, which could mediate the uptake and degradation of AcLDL. The scavenger receptor (SR) is a glycoprotein that mainly exists on the surface of the macrophage membrane, can specifically bind and ingest oxidized low-density lipoprotein (ox-LDL), and has the function of binding with multiple ligands. It is a glycoprotein that induces the activation of urokinase-type plasminogen and the generation of inflammatory cytokines. NO is a crucial effector molecule for macrophage activation. Fucose and (1→3)-β-glucan were discovered to combine with scavenger receptor to play an immunomodulatory role [124]. Scavenger receptors play two roles in the process of the immune response: first, as pattern recognition receptors in the immune system to clear foreign bodies by recognizing specific pathogen-related molecular patterns; second, to clean up apoptotic nuclear fragments in vivo by identifying damage-related molecular patterns [125]; LacCer (CDw17) is a glycosphingolipid found in the plasma membrane of multiple kinds of cells. It was identified as (1→3)-β-glucan receptor through biochemical analysis of the interaction between β-glucan and isolated human leukocyte membrane components [126]. It has been demonstrated that the binding of (1→3)-β-glucan with this receptor can activate macrophage inflammatory protein (MIP)-2 and NF-κB, enhancing the oxidative burst and antibacterial function of neutrophils. However, the mechanism by which this occurs is yet unclear.
5. Clinical Applications of (1→3)-β-Glucans
(1→3)-β-Glucan has gained significant attention in recent years due to its potential health benefits. Numerous studies have been conducted for a long time to investigate the impact of (1→3)-β-glucan on various health conditions and have found many promising results. In Table 2, the clinical research on (1→3)-β-glucan and its immunomodulatory effects are summarized. Gudej et al. evaluated the efficacy of oat (1→3)-β-glucans in treating gastritis and found that oat β-glucans improved the quality of life for patients with gastritis, suggesting that it can be a potential natural treatment for gastritis and related gastrointestinal disorders [127]. Patients with high-risk neuroblastoma who had previously experienced disease progression showed strong antibody responses when treated with the GD2/GD3 vaccine in combination with (1→3)-β-glucan [128]. Medeiros et al. first studied to investigate the impact of S. cerevisiae (1→3)-β-glucan on venous ulcer healing in humans, and it could serve as a natural biological response modifier for wound healing [129]. Supplementing with 3 g/day of oat (1→3)-β-glucan can effectively reduce low density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and non-high density lipoprotein cholesterol (HDL-C) levels in individuals with mild hypercholesterolemia over the medium term [130]. It was suggested that adding insoluble (1→3)-β-glucan from Pleurotus ostreatus to the diet may help regulate the exercise-induced alterations in NKCA observed in highly trained athletes [131]. Administration of yeast (1→3)-/(1→6)-β-glucan on a daily basis may provide protection against upper respiratory tract infections (URTIs) and shorten the duration of URTI symptoms in older individuals upon infections [132]. Furthermore, (1→3)-β-glucan has the potential to enhance serum IL-12 levels, shorten mechanical ventilation duration, and decrease organ failure in critically ill patients with multiple trauma [133]. Lehne et al. reported about soluble barley (1→3)-β-glucan (SBG) and observed an increase in the concentration of immunoglobulin (IG) A in saliva [134]. The preparation of yeast (1→3)-β-glucan increased the body’s ability to protect against invading pathogens [135]. Lee et al. and Carpenter et al. found that (1→3)-β-glucan has the potential to stimulate protective immunity without enhancing inflammation and modify immune responses after a strenuous exercise session, respectively [136,137]. However, while all (1→3)-β-glucans share a similar structure, the biological differences between (1→3)-β-glucans from different sources exist due to their differences in molecular weight, solubility, and purity as well as contents in other types of β-glucans with various branching patterns shown in Table 1. As shown in Table 2, (1→3)-β-glucans from different sources exhibit varying biological activities and clinical outcomes. Understanding these differences is crucial when evaluating the potential health benefits of β-glucans from different sources.

Table 2.
Clinical applications on (1→3)-β-glucans.
6. Conclusions
Among various β-glucans shown in this review, β-glucans possessing (1→3)-β-glucan backbone are a new, safe, and effective bioregulator. They exert a wide range of immunological activities, including activating macrophages, DCs, and monocytes, inducing the synthesis of NO, regulating cell signal transmission related to immune response, minimizing the harm caused by ionizing radiation to the body’s immune system, and promoting the synthesis of IG. By binding pentatricopeptide repeat (PPR) of various immune cells, a series of cascade responses of immune defense is triggered, modifying both innate and adaptive immune responses, such as the key role of a group of (1→3)-β-glucans in regulating DC function. Further in-depth studies are required to understand the relationship between its various sources, complicated structure, and broad biological function. At present, the (1→3)-β-glucans have been widely employed in daily life as well as in the fields of medicine and biology. For instance, oral administration of (1→3)-β-glucans has been reported to lower levels of lipid in blood, regulate immune responses, and exhibit anti-tumor properties. They are also used as pharmaceutical and cosmetic ingredients, and so on. It is not expected to take much time, but it would show vast application prospects and huge application value. Regulating the human body’s immune response with (1→3)-β-glucans is promising to help improve the body’s immune status and provide new avenues for clinical anti-tumor, anti-infection, and anti-inflammatory treatment. With ongoing research into the immunological function and corresponding mechanism of action of the (1→3)-β-glucans, the functionally further evaluated (1→3)-β-glucans are expected to be employed more and more frequently.
Author Contributions
X.Z., G.W. and F.L. contributed equally. F.D., X.Z. and G.W. contributed significantly to the design and wrote the initial manuscript draft. S.F., S.Z., A.I., A.G.T. and M.S. provided critical input on the interpretation of results. They also played a key role in revising the manuscript for publication. H.C. and F.D. conceptualized and directed the project and finalized the manuscript draft. All authors contributed to discussions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported partly by the National Natural Science Foundation of China (No. 22277150 to H.C.; No. 22007106 to Q.L.), the Fundamental Research Funds for the Central Universities (No. 22lglj10 to H.C.; No. 22qntd4601 to F.D.), the Province Natural Science Fund of Guangdong (No. 2022A1515011109 to H.C.; No. 2021A1515010189 to F.D.), and Shenzhen Science and Technology Program (JCYJ20220530144415035 to L.F.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Yukishige Ito from Osaka University, Japan, Katsunori Tanaka from Tokyo Institute of Technology, Japan, and Xue-wei Liu from Nanyang Technological University, Singapore, for their valuable discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demleitner, S.; Kraus, J.; Franz, G. Synthesis and antitumour activity of derivatives of curdlan and lichenan branched at C-6. Carbohydr. Res. 1992, 226, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Větvička, V.C.; Xia, Y.; Coxon, A.; Carroll, M.C.; Mayadas, T.N.; Ross, G.D. β-Glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18). J. Immunol. 1999, 163, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Batbayar, S.; Lee, D.-H.; Kim, H.-W. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular interactions of β-(1→3)-glucans with their receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Masuda, H.; Kaga, N.; Nakayama, H.; Matsumoto, R.; Iwahara, C.; Yoshizaki, F.; Tamaki, Y.; Kobayashi, T.; Hayakawa, T. Properties and functions of lactosylceramide from mouse neutrophils. Glycobiology 2015, 25, 655–668. [Google Scholar] [CrossRef]
- PrabhuDas, M.; Bowdish, D.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; Means, T.K.; Moestrup, S.K. Standardizing scavenger receptor nomenclature. J. Immunol. 2014, 192, 1997–2006. [Google Scholar] [CrossRef]
- Mueller, A.; Raptis, J.; Rice, P.J.; Kalbfleisch, J.H.; Stout, R.D.; Ensley, H.E.; Browder, W.; Williams, D.L. The influence of glucan polymer structure and solution conformation on binding to (1→3)-β-D-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef]
- Read, S.M.; Currie, G.; Bacic, A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 1996, 281, 187–201. [Google Scholar] [CrossRef]
- Ding, F.; Ishiwata, A.; Ito, Y. Recent advances of the stereoselective bimodal glycosylations for the synthesis of various glucans. In Studies in Natural Products Chemistry (Bioactive Natural Products); Elsevier: Amsterdam, The Netherlands, 2022; Volume 74, pp. 1–40. [Google Scholar] [CrossRef]
- Ding, F.; Ishiwata, A.; Zhou, S.; Zhong, X.; Ito, Y. Unified strategy toward sterecontrolled assembly of various glucans based on bimodal glycosyl donors. J. Org. Chem. 2020, 85, 5536–5558. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, A.; Tanaka, K.; Ao, J.; Ding, F.; Ito, Y. Recent advances in stereoselective 1.2-cis-O-glycosylations. Front. Chem. 2022, 10, 972429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhong, X.; Guo, A.; Xiao, Q.; Ao, J.; Wanmeng Zhu, W.; Cai, H.; Ishiwata, A.; Ito, Y.; Liu, X.-W.; et al. ZnI2-directed stereocontrolled α-glucosylation. Org. Lett. 2021, 23, 6841–6845. [Google Scholar] [CrossRef]
- Błaszczyk, K.; Wilczak, J.; Harasym, J.; Gudej, S.; Suchecka, D.; Królikowski, T.; Lange, E.; Gromadzka-Ostrowska, J. Impact of low and high molecular weight oat beta-glucan on oxidative stress and antioxidant defense in spleen of rats with LPS induced enteritis. Food Hydrocoll. 2015, 51, 272–280. [Google Scholar] [CrossRef]
- Bacon, J.; Farmer, V.; Jones, D.; Taylor, I.F. The glucan components of the cell wall of baker’s yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem. J. 1969, 114, 557–567. [Google Scholar] [CrossRef]
- Wakshull, E.; Brunke-Reese, D.; Lindermuth, J.; Fisette, L.; Nathans, R.S.; Crowley, J.J.; Tufts, J.C.; Zimmerman, J.; Mackin, W.; Adams, D.S. PGG-Glucan, a soluble β-(1, 3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-κB-like factor in human PMN: Evidence for a glycosphingolipid β-(1, 3)-glucan receptor. Immunopharmacology 1999, 41, 89–107. [Google Scholar] [CrossRef]
- De la Cruz, J.; Pintor-Toro, J.A.; Benitez, T.; LLobell, A. Purification and characterization of an endo-beta-1, 6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J. Bacteriol. 1995, 177, 1864–1871. [Google Scholar] [CrossRef]
- Karácsonyi, Š.; Kuniak, Ľ. Polysaccharides of Pleurotus ostreatus: Isolation and structure of pleuran, an alkali-insoluble β-D-glucan. Carbohydr. Polym. 1994, 24, 107–111. [Google Scholar] [CrossRef]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar]
- Perlin, A.; Suzuki, S. The structure of lichenin: Selective enzymolysis studies. Can. J. Chem. 1962, 40, 50–56. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, Y.; Shi, L.; Yao, J.; Li, J.; Ma, F.; Ding, K. A novel water-soluble β-D-glucan isolated from the spores of Ganoderma lucidum. Carbohydr. Res. 2012, 353, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.-F.; Zhen, Y.; Ruan, L.; Fang, J.-N. Purification, characterization, and modification of T lymphocyte-stimulating polysaccharide from spores of Ganoderma lucidum. Chem. Pharm. Bull. 2002, 50, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, L.; Ding, K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 2019, 141, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Allendorf, D.J.; Brandley, B. Yeast whole glucan particle (WGP) β-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opin. Biol. Ther. 2005, 5, 691–702. [Google Scholar] [CrossRef]
- Guidolin, L.S.; Arce-Gorvel, V.; Ciocchini, A.E.; Comerci, D.J.; Gorvel, J.P. Cyclic β-glucans at the bacteria–host cells interphase: One sugar ring to rule them all. Cell. Microbiol. 2018, 20, e12850. [Google Scholar] [CrossRef]
- Arellano-Reynoso, B.; Lapaque, N.; Salcedo, S.; Briones, G.; Ciocchini, A.E.; Ugalde, R.; Moreno, E.; Moriyón, I.; Gorvel, J.-P. Cyclic β-1,2-glucan is a brucella virulence factor required for intracellular survival. Nat. Immunol. 2005, 6, 618–625. [Google Scholar] [CrossRef]
- Bertin, C.; Pau-Roblot, C.; Courtois, J.; Manso-Silván, L.; Tardy, F.; Poumarat, F.; Citti, C.; Sirand-Pugnet, P.; Gaurivaud, P.; Thiaucourt, F. Highly Dynamic Genomic Loci Drive the Synthesis of Two Types of Capsular or Secreted Polysaccharides within the Mycoplasma mycoides Cluster. Appl. Environ. Microbiol. 2015, 81, 676–687. [Google Scholar] [CrossRef]
- Sedzicki, J.; Ni, D.; Lehmann, F.; Wu, N.; Zenobi, R.; Jung, S.; Stahlberg, H.; Dehio, C. Mechanism of cyclic β-glucan export by ABC transporter Cgt of Brucella. Nat. Struct. Mol. Biol. 2022, 29, 1170–1177. [Google Scholar] [CrossRef]
- Fisher, M.; Yang, L.-X. Anticancer effects and mechanisms of polysaccharide-K (PSK): Implications of cancer immunotherapy. Anticancer Res. 2002, 22, 1737–1754. [Google Scholar]
- Albersheim, P.; Darvill, A.; Augur, C.; Cheong, J.J.; Eberhard, S.; Hahn, M.G.; Marfa, V.; Mohnen, D.; O’Neill, M.A. Oligosaccharins: Oligosaccharide regulatory molecules. Acc. Chem. Res. 1992, 25, 77–83. [Google Scholar] [CrossRef]
- Saito, H.; Misaki, A.; Harada, T. A comparison of the structure of curdlan and pachyman. Agric. Biol. Chem. 1968, 32, 1261–1269. [Google Scholar] [CrossRef]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.; Wouters, I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Ohno, N.; Yadomae, T. Intravenously administered (1→3)-β-D-glucan, SSG, obtained from Sclerotinia sclerotiorum IFO 9395 augments murine peritoneal macrophage functions in vivo. Chem. Pharm. Bull. 1992, 40, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Bae, A.-H.; Lee, S.-W.; Ikeda, M.; Sano, M.; Shinkai, S.; Sakurai, K. Rod-like architecture and helicity of the poly (C)/schizophyllan complex observed by AFM and SEM. Carbohydr. Res. 2004, 339, 251–258. [Google Scholar] [CrossRef]
- Iorio, E.; Torosantucci, A.; Bromuro, C.; Chiani, P.; Ferretti, A.; Giannini, M.; Cassone, A.; Podo, F. Candida albicans cell wall comprises a branched β-d-(1→6)-glucan with β-d-(1→3)-side chains. Carbohydr. Res. 2008, 343, 1050–1061. [Google Scholar] [CrossRef]
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Beattie, A.; Hirst, E.; Percival, E. Studies on the metabolism of the Chrysophyceae. Comparative structural investigations on leucosin (chrysolaminarin) separated from diatoms and laminarin from the brown algae. Biochem. J. 1961, 79, 531. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Immune-enhancing effects of Maitake (Grifola frondosa) and Shiitake (Lentinula edodes) extracts. Ann. Transl. Med. 2014, 2, 14. [Google Scholar] [CrossRef]
- Di Carlo, F.J.; Fiore, J.V. On the composition of zymosan. Science 1958, 127, 756–757. [Google Scholar] [CrossRef]
- Young, S.-H.; Jacobs, R.R. Sodium hydroxide-induced conformational change in schizophyllan detected by the fluorescence dye, aniline blue. Carbohydr. Res. 1998, 310, 91–99. [Google Scholar] [CrossRef]
- Yadomae, T. Structure and biological activities of fungal beta-1, 3-glucans. Yakugaku Zasshi 2000, 120, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Zhang, L. Thermally induced conformation transition of triple-helical lentinan in NaCl aqueous solution. J. Phys. Chem. B 2008, 112, 10343–10351. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Agarwal, S.; Ram, S.; Rice, P.A.; Specht, C.A.; Levitz, S.M. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J. Immunol. 2012, 189, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef]
- Di Luzio, N.R. Update on the immunomodulating activities of glucans. In Springer Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 1985; pp. 387–400. [Google Scholar]
- Wang, W.-J.; Wu, Y.-S.; Chen, S.; Liu, C.-F.; Chen, S.-N. Mushroom β-glucan may immunomodulate the tumor-associated macrophages in the Lewis lung carcinoma. BioMed Res. Int. 2015, 2015, 604385. [Google Scholar] [CrossRef]
- Tian, X.; Tian, J.; Tang, X.; Rui, K.; Zhang, Y.; Ma, J.; Wang, Y.; Xu, H.; Lu, L.; Wang, S. Particulate β-glucan regulates the immunosuppression of granulocytic myeloid-derived suppressor cells by inhibiting NFIA expression. Oncoimmunology 2015, 4, e1038687. [Google Scholar] [CrossRef]
- Miura, T.; Ohno, N.; Miura, N.; Adachi, Y.; Shimada, S.; Yadomae, T. Antigen-specific response of murine immune system toward a yeast β-glucan preparation, zymosan. FEMS Immunol. Med. Microbiol. 1999, 24, 131–139. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- El Khoury, D.; Cuda, C.; Luhovyy, B.; Anderson, G. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R. Effects of low molecular weight yeast β-glucan on antioxidant and immunological activities in mice. Int. J. Mol. 2015, 16, 21575–21590. [Google Scholar] [CrossRef] [PubMed]
- Karumuthil-Melethil, S.; Gudi, R.; Johnson, B.M.; Perez, N.; Vasu, C. Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J. Immunol. 2014, 193, 3308–3321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Pillemer, L.; Blum, L.; Lepow, I.H.; Ross, O.A.; Todd, E.W.; Wardlaw, A.C. The properdin system and immunity: I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science 1954, 120, 279–285. [Google Scholar] [CrossRef]
- Mahla, R.S.; Reddy, M.C.; Prasad, D.V.R.; Kumar, H. Sweeten PAMPs: Role of sugar complexed PAMPs in innate immunity and vaccine biology. Front. Immunol. 2013, 4, 248. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011, 2, 115. [Google Scholar] [CrossRef]
- Di Luzio, N.; McNamee, R.; Williams, D.; Gilbert, K.; Spanjers, M. Glucan induced inhibition of tumor growth and enhancement of survival in a variety of transplantable and spontaneous murine tumor models. In Macrophages and Lymphocytes; Springer: Berlin/Heidelberg, Germany, 1980; pp. 269–290. [Google Scholar]
- Di Luzio, N.R. Immunopharmacology of glucan: A broad spectrum enhancer of host defense mechanisms. Trends Pharmacol. Sci. 1983, 4, 344–347. [Google Scholar] [CrossRef]
- Demir, G.; Klein, H.; Mandel-Molinas, N.; Tuzuner, N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int. Immunopharmacol. 2007, 7, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Mueller, A.; Browder, W. Glucan-based macrophage stimulators. Clin. Immunother. 1996, 5, 392–399. [Google Scholar] [CrossRef]
- Salama, S.F. Beta-glucan ameliorates gamma-rays induced oxidative injury in male swiss albino rats. Pak. J. Zool. 2011, 43, 933–939. [Google Scholar]
- Iraz, M.; Iraz, M.; Eşrefoğlu, M.; Aydin, M.Ş. Protective effect of\beta-glucan on acute lung injury induced bylipopolysaccharide in rats. Turk. J. Med. Sci. 2015, 45, 261–267. [Google Scholar] [CrossRef]
- Barton, C.; Vigor, K.; Scott, R.; Jones, P.; Lentfer, H.; Bax, H.J.; Josephs, D.H.; Karagiannis, S.N.; Spicer, J.F. Beta-glucan contamination of pharmaceutical products: How much should we accept? Cancer Immunol. Immun. 2016, 65, 1289–1301. [Google Scholar] [CrossRef]
- Sato, M.; Sano, H.; Iwaki, D.; Kudo, K.; Konishi, M.; Takahashi, H.; Takahashi, T.; Imaizumi, H.; Asai, Y.; Kuroki, Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003, 171, 417–425. [Google Scholar] [CrossRef]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Sakaguchi, N.; Kobayashi, K.; Brown, G.D.; Tagami, T.; Sakihama, T.; Hirota, K.; Tanaka, S.; Nomura, T.; Miki, I. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 2005, 201, 949–960. [Google Scholar] [CrossRef]
- Ohno, N.; Egawa, Y.; Hashimoto, T.; Adachi, Y.; Yadomae, T. Effect of β-glucans on the nitric oxide synthesis by peritoneal macrophage in mice. Biol. Pharm. Bull. 1996, 19, 608–612. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ohno, N.; Adachi, Y.; Yadomae, T. Enhanced production of inducible nitric oxide synthase by β-glucans in mice. FEMS Immunol. Med. Microbiol. 1997, 19, 131–135. [Google Scholar] [CrossRef]
- Medina-Gali, R.M.; del Mar Ortega-Villaizan, M.; Mercado, L.; Novoa, B.; Coll, J.; Perez, L. Beta-glucan enhances the response to SVCV infection in zebrafish. Dev. Comp. Immunol. 2018, 84, 307–314. [Google Scholar] [CrossRef]
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Q.; Wang, R.; Qin, L.; Peng, X.; Hu, L.; Liu, Y.; Fang, Z.; Lin, Y.; Xu, S.; et al. Effects of dietary β-glucan supplementation on growth performance and immunological and metabolic parameters of weaned pigs administered with Escherichia coli lipopolysaccharide. Food Funct. 2018, 9, 3338–3343. [Google Scholar] [CrossRef] [PubMed]
- Effects of Bread Yeast Cell Wall Beta-Glucans on Mice with Loperamide-Induced Constipation. J. Med. Food 2019, 22, 1009–1021. [CrossRef] [PubMed]
- Cao, Y.; Sun, Y.; Zou, S.; Duan, B.; Sun, M.; Xu, X. Yeast β-Glucan Suppresses the Chronic Inflammation and Improves the Microenvironment in Adipose Tissues of ob/ob Mice. J. Agric. Food Chem. 2018, 66, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Fan, H.; Yao, M.; Yang, S.; Han, J. Oral administration of yeast β-glucan ameliorates inflammation and intestinal barrier in dextran sodium sulfate-induced acute colitis. J. Funct. Foods 2017, 35, 115–126. [Google Scholar] [CrossRef]
- Pelizon, A.C.; Kaneno, R.; Soares, A.; Meira, D.A.; Sartori, A. Immunomodulatory activities associated with β-glucan derived from Saccharomyces cerevisiae. Physiol. Res. 2005, 54, 557–564. [Google Scholar] [CrossRef]
- Hellinga, A.H.; Tsallis, T.; Eshuis, T.; Triantis, V.; Ulfman, L.H.; van Neerven, R.J.J. In Vitro Induction of Trained Innate Immunity by bIgG and Whey Protein Extracts. Int. J. Mol. Sci. 2020, 21, 9077. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef]
- Cook, M.T.; Hayball, P.J.; Hutchinson, W.; Nowak, B.; Hayball, J.D. The efficacy of a commercial β-glucan preparation, EcoActiva™, on stimulating respiratory burst activity of head-kidney macrophages from pink snapper (Pagrus auratus), Sparidae. Fish Shellfish Immunol. 2001, 11, 661–672. [Google Scholar] [CrossRef]
- Muthusamy, G.; Joardar, S.N.; Samanta, I.; Isore, D.P.; Roy, B.; Maiti, K. β-glucan from edible mushroom (Pleurotus florida) enhances mucosal immunity in poultry. Adv. Anim. Vet. Sci. 2013, 1, 116–119. [Google Scholar]
- Kwon, K.H.; Kim, K.I.; Jun, W.J.; Shin, D.H.; Cho, H.Y.; Hong, B.S. In Vitro and in Vivo Effects of Macrophage-Stimulatory Polysaccharide from Leaves of Perilla frutescens var crispa. Biol. Pharm. Bull. 2002, 25, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Tanaka, H.; Kinoshita, A.; Oikawa, S.; Osawa, M.; Yadomae, T. Effect of orally administered β-glucan on macrophage function in mice. Int. J. Immunopharmacol. 1990, 12, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Ross, P.; Farrell, M.P. The Road to Structurally Defined β-Glucans. Chem. Rec. 2021, 21, 3178–3193. [Google Scholar]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Chauhan, N.; Tiwari, S.; Iype, T.; Jain, U. An overview of adjuvants utilized in prophylactic vaccine formulation as immunomodulators. Expert Rev. Vaccines 2017, 16, 491–502. [Google Scholar] [CrossRef]
- Petrovsky, N.; Cooper, P.D. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. [Google Scholar] [CrossRef]
- Hu, X.; Liu, R.; Zhu, N. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology 2013, 218, 1524–1528. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Caballero-García, A.; Roche, E.; Noriega, D.C. β-Glucans Could Be Adjuvants for SARS-CoV-2 Virus Vaccines (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 12636. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Van Kooyk, Y.; Cobb, B.A. Glycobiology of immune responses. Ann. N. Y. Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef]
- Hamilos, D.L. Antigen presenting cells. Immunol. Res. 1989, 8, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.M.; Gani, A.; Mir, S.A.; Masoodi, F.A.; Khanday, F.A. β-Glucan: A dual regulator of apoptosis and cell proliferation. Int. J. Biol. Macromol. 2021, 182, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Barreto-Bergter, E.; Figueiredo, R.T. Fungal glycans and the innate immune recognition. Front. Cell. Infect. Microbiol. 2014, 4, 145. [Google Scholar] [CrossRef]
- Ariizumi, K.; Shen, G.-L.; Shikano, S.; Xu, S.; Ritter, R.; Kumamoto, T.; Edelbaum, D.; Morita, A.; Bergstresser, P.R.; Takashima, A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 2000, 275, 20157–20167. [Google Scholar] [CrossRef]
- Ikeda, Y.; Adachi, Y.; Ishii, T.; Tamura, H.; Aketagawa, J.; Tanaka, S.; Ohno, N. Blocking Effect of Anti-Dectin-1 Antibodies on the Anti-tumor Activity of 1, 3-β-Glucan and the Binding of Dectin-1 to 1, 3-β-Glucan. Biol. Pharm. Bull. 2007, 30, 1384–1389. [Google Scholar] [CrossRef]
- Willment, J.A.; Gordon, S.; Brown, G.D. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 2001, 276, 43818–43823. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Plato, A.; Willment, J.A.; Brown, G.D. C-type lectin-like receptors of the dectin-1 cluster: Ligands and signaling pathways. Int. Rev. Immunol. 2013, 32, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, Y. The biological role of dectin-1 in immune response. Int. Rev. Immunol. 2007, 26, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Hoving, J.C.; Wilson, G.J.; Brown, G.D. Signalling C-type lectin receptors, microbial recognition and immunity. Cell. Microbiol. 2014, 16, 185–194. [Google Scholar] [CrossRef]
- Suram, S.; Brown, G.D.; Ghosh, M.; Gordon, S.; Loper, R.; Taylor, P.R.; Akira, S.; Uematsu, S.; Williams, D.L.; Leslie, C.C. Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the β-glucan receptor. J. Biol. Chem. 2006, 281, 5506–5514. [Google Scholar] [CrossRef]
- Yadav, M.; Schorey, J.S. The β-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 2006, 108, 3168–3175. [Google Scholar] [CrossRef]
- Dedloff, M.R.; Effler, C.S.; Holban, A.M.; Gestal, M.C. Use of biopolymers in mucosally-administered vaccinations for respiratory disease. Materials 2019, 12, 2445. [Google Scholar] [CrossRef]
- De Smet, R.; Demoor, T.; Verschuere, S.; Dullaers, M.; Ostroff, G.R.; Leclercq, G.; Allais, L.; Pilette, C.; Dierendonck, M.; De Geest, B.G. β-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control. Release 2013, 172, 671–678. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.; Magee, A.S.; Danielson, M.E. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef]
- Hoffman, O.; Standing, J.; Limper, A. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J. Immunol. 1993, 150, 3932–3940. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Igyártó, B.Z.; Gerami-Nejad, M.; Kumamoto, Y.; Mohammed, J.; Jarrett, E.; Drummond, R.A.; Zurawski, S.M.; Zurawski, G.; Berman, J. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 2015, 42, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Giovannini, G.; De Luca, A.; D’angelo, C.; Casagrande, A.; Iannitti, R.G.; Ricci, G.; Cunha, C.; Romani, L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell. Mol. Immunol. 2012, 9, 276–286. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Thornton, B.P.; Vĕtvicka, V.; Pitman, M.; Goldman, R.C.; Ross, G.D. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 1996, 156, 1235–1246. [Google Scholar] [CrossRef]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anti-Cancer Agents Med. (Former. Curr. Med. Chem.—Anti-Cancer Agents) 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Le Cabec, V.; Carréno, S.; Moisand, A.; Bordier, C.; Maridonneau-Parini, I. Complement receptor 3 (CD11b/CD18) mediates type I and type II phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J. Immunol. 2002, 169, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Michalek, M.; Melican, D.; Brunke-Reese, D.; Langevin, M.; Lemerise, K.; Galbraith, W.; Patchen, M.; Mackin, W. Activation of rat macrophages by Betafectin PGG-glucan requires cross-linking of membrane receptors distinct from complement receptor three (CR3). J. Leukoc. Biol. 1998, 64, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Baert, K.; Sonck, E.; Goddeeris, B.M.; Devriendt, B.; Cox, E. Cell type-specific differences in β-glucan recognition and signalling in porcine innate immune cells. Dev. Comp. Immunol. 2015, 48, 192–203. [Google Scholar] [CrossRef]
- Peiser, L.; Mukhopadhyay, S.; Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 2002, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Means, T.K. Fungal pathogen recognition by scavenger receptors in nematodes and mammals. Virulence 2010, 1, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A novel carbohydrate-glycosphingolipid interaction between a β-(1–3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998, 273, 22014–22020. [Google Scholar] [CrossRef] [PubMed]
- Gudej, S.; Filip, R.; Harasym, J.; Wilczak, J.; Dziendzikowska, K.; Oczkowski, M.; Jałosińska, M.; Juszczak, M.; Lange, E.; Gromadzka-Ostrowska, J. Clinical outcomes after oat beta-glucans dietary treatment in gastritis patients. Nutrients 2021, 13, 2791. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.Y.; Cheung, N.V.; Modak, S.; Mauguen, A.; Feng, Y.; Basu, E.; Roberts, S.S.; Ragupathi, G.; Kushner, B.H. Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients with High-Risk Neuroblastoma with Prior Disease Progression. J. Clin. Oncol. 2021, 39, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.D.V.; Cordeiro, S.L.; Cavalcanti, J.E.C.; Melchuna, K.M.; Lima, A.; Filho, I.A.; Medeiros, A.C.; Rocha, K.B.F.; Oliveira, E.M.; Faria, E.D.B.; et al. Effects of purified Saccharomyces cerevisiae (1→3)-β-glucan on venous ulcer healing. Int. J. Mol. Sci. 2012, 13, 8142–8158. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Strocchi, E.; Grandi, E.; Rizzoli, E.; Poli, A.; Marangoni, F.; Borghi, C. A randomized Placebo-Controlled Clinical Trial to Evaluate the Medium-Term Effects of Oat Fibers on Human Health: The Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients 2020, 12, 686. [Google Scholar] [CrossRef]
- Bobovčák, M.; Kuniaková, R.; Gabriž, J.; Majtán, J. Effect of Pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl. Physiol. Nutr. Metab. 2010, 35, 755–762. [Google Scholar] [CrossRef]
- Fuller, R.; Moore, M.V.; Lewith, G.; Stuart, B.L.; Ormiston, R.V.; Fisk, H.L.; Noakes, P.S.; Calder, P.C. Yeast-derived β-1,3/1,6 glucan, upper respiratory tract infection and innate immunity in older adults. Nutrition 2017, 39–40, 30–35. [Google Scholar] [CrossRef]
- Fazilaty, Z.; Chenari, H.; Shariatpanahi, Z.V. Effect of ß-glucan on serum levels of IL-12, hs-CRP, and clinical outcomes in multiple-trauma patients: A prospective randomized study. Ulus. Travma Acil Cerrahi Derg. 2018, 24, 287–293. [Google Scholar]
- Lehne, G.; Haneberg, B.; Gaustad, P.; Johansen, P.W.; Preus, H.; Abrahamsen, T.G. Oral administration of a new soluble branched beta-1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin. Exp. Immunol. 2006, 143, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Auinger, A.; Riede, L.; Bothe, G.; Busch, R.; Gruenwald, J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body’s defence against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur. J. Nutr. 2013, 52, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Kim, Y.S.; Lee, Y.J.; Ahn, H.Y.; Kim, M.; Kim, M.; Cho, M.J.; Cho, Y.; Lee, J.H. Effect of Immune-Enhancing Enteral Nutrition Enriched with or without Beta-Glucan on Immunomodulation in Critically Ill Patients. Nutrients 2016, 8, 336. [Google Scholar] [CrossRef]
- Carpenter, K.C.; Breslin, W.L.; Davidson, T.; Adams, A.; McFarlin, B.K. Baker’s yeast β-glucan supplementation increases monocytes and cytokines post-exercise: Implications for infection risk? Br. J. Nutr. 2013, 109, 478–486. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).