RETRACTED: An Innovative Tool for Evidence-Based, Personalized Treatment Trials in Mucopolysaccharidosis
Abstract
1. Introduction
2. Materials and Methods
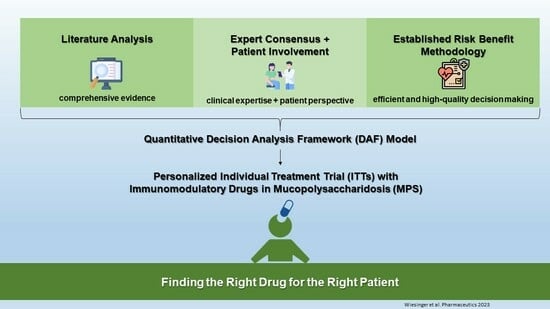
2.1. Three-Step Development Process
2.2. DAF Framing
2.3. DAF—Data Collection and Processing
2.4. DAF—Subjective Data and RBA
2.5. Integration of Patient Characteristics to Allow Personalizability
3. Results
3.1. Beneficial and Adverse Effects of the Most Relevant Immunomodulatory Drugs
3.2. Drug Selection for Defined Phenotypic Groups
3.3. Value Tree
3.4. Weighing Potential Risks and Benefits
- -
- Best-case scenario: 60%
- -
- Worst-case scenario: 40%
3.5. Assessing the Chance of Improvement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verheyen, S.; Blatterer, J.; Speicher, M.R.; Bhavani, G.S.; Boons, G.J.; Ilse, M.B.; Andrae, D.; Sproß, J.; Vaz, F.M.; Kircher, S.G.; et al. Novel subtype of mucopolysaccharidosis caused by arylsulfatase K (ARSK) deficiency. J. Med. Genet. 2022, 59, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.; Tomatsu, S.C.; Tomatsu, S.; Khan, S.A. Epidemiology of Mucopolysaccharidoses Update. Diagnostics 2021, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of lysosomal storage disorders. JAMA 1999, 281, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.C.; Kaiser-Kupfer, M.I.; Muenzer, J.; Ludwig, I.H.; Zasloff, M.A.; Mercer, P.A. Electroretinographic findings in the mucopolysaccharidoses. Ophthalmology 1986, 93, 1612–1616. [Google Scholar] [CrossRef]
- Poorthuis, B.J.; Wevers, R.A.; Kleijer, W.J.; Groener, J.E.; de Jong, J.G.; van Weely, S.; Niezen-Koning, K.E.; van Diggelen, O.P. The frequency of lysosomal storage diseases in The Netherlands. Hum. Genet. 1999, 105, 151–156. [Google Scholar] [CrossRef]
- Muenzer, J. Mucopolysaccharidoses. Adv. Pediatr. 1986, 33, 269–302. [Google Scholar]
- Wiesinger, A.-M.; Bigger, B.; Giugliani, R.; Scarpa, M.; Moser, T.; Lampe, C.; Kampmann, C.; Lagler, F.B. The Inflammation in the Cytopathology of Patients with Mucopolysaccharidoses-Immunomodulatory Drugs as an Approach to Therapy. Front. Pharmacol. 2022, 13, 863667. [Google Scholar] [CrossRef]
- Archewith, D.; Langford-Smith, K.J.; Bigger, B.W.; Fildes, J.E. Mucopolysaccharide diseases: A complex interplay between neuroinflammation, microglial activation and adaptive immunity. J. Inherit. Metab. Dis. 2014, 37, 1–12. [Google Scholar] [CrossRef]
- Parker, H.; Bigger, B.W. The role of innate immunity in mucopolysaccharide diseases. J. Neurochem. 2019, 148, 639–651. [Google Scholar] [CrossRef]
- Mandolfo, O.; Parker, H.; Bigger, B. Innate Immunity in Mucopolysaccharide Diseases. Int. J. Mol. Sci. 2022, 23, 1999. [Google Scholar] [CrossRef]
- Monticelli, M.; Liguori, L.; Allocca, M.; Bosso, A.; Andreotti, G.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Mele, B.H. Drug Repositioning for Fabry Disease: Acetylsalicylic Acid Potentiates the Stabilization of Lysosomal Alpha-Galactosidase by Pharmacological Chaperones. Int. J. Mol. Sci. 2022, 23, 5105. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, W.; Simeonov, A. Drug discovery and development for rare genetic disorders. Am. J. Med. Genet. A 2017, 173, 2307–2322. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, M.; Mele, B.H.; Allocca, M.; Liguori, L.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Andreotti, G. Curcumin Has Beneficial Effects on Lysosomal Alpha-Galactosidase: Potential Implications for the Cure of Fabry Disease. Int. J. Mol. Sci. 2023, 24, 1095. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Platt, F.M. Emptying the stores: Lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 2018, 17, 133–150. [Google Scholar] [CrossRef]
- Heemstra, H.E.; van Weely, S.; Büller, H.A.; Leufkens, H.G.; de Vrueh, R.L. Translation of rare disease research into orphan drug development: Disease matters. Drug Discov. Today 2009, 14, 1166–1173. [Google Scholar] [CrossRef]
- Kakkis, E.D. Enzyme replacement therapy for the mucopolysaccharide storage disorders. Expert Opin. Investig. Drugs 2002, 11, 675–685. [Google Scholar] [CrossRef]
- Muenzer, J.; Wraith, J.E.; Clarke, L.A. Mucopolysaccharidosis I: Management and treatment guidelines. Pediatrics 2009, 123, 19–29. [Google Scholar] [CrossRef]
- Fung, A.; Yue, X.; Wigle, P.R.; Guo, J.J. Off-label medication use in rare pediatric diseases in the United States. Intractable Rare Dis. Res. 2021, 10, 238–245. [Google Scholar] [CrossRef]
- Vohra, S.; Shamseer, L.; Sampson, M.; Bukutu, C.; Schmid, C.H.; Tate, R.; Nikles, J.; Zucker, D.R.; Kravitz, R.; Guyatt, G.; et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 Statement. BMJ 2015, 350, h1738. [Google Scholar] [CrossRef] [PubMed]
- Wolters, T.L.C.; Van Vlijmen, J. N-of-1 trials: The one and only. Ned. Tijdschr. Geneeskd. 2021, 165, D6149. [Google Scholar] [PubMed]
- Wiesinger, A.M.; Strobl, H.; Lagler, F.B. Individual Treatment Trials-Do Experts Know and Use This Option to Improve the Treatability of Mucopolysaccharidosis? Pharmaceuticals 2023, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- van der Zanden, T.M.; Mooij, M.G.; Vet, N.J.; Neubert, A.; Rascher, W.; Lagler, F.B.; Male, C.; Grytli, H.; Halvorsen, T.; de Hoog, M.; et al. Benefit-Risk Assessment of Off-Label Drug Use in Children: The Bravo Framework. Clin. Pharmacol. Ther. 2021, 110, 952–965. [Google Scholar] [CrossRef]
- Nixon, R.; Dierig, C.; Mt-Isa, S.; Stöckert, I.; Tong, T.; Kuhls, S.; Hodgson, G.; Pears, J.; Waddingham, E.; Hockley, K.; et al. A case study using the PrOACT-URL and BRAT frameworks for structured benefit risk assessment. Biom. J. 2016, 58, 8–27. [Google Scholar] [CrossRef]
- de Ruijter, J.; Valstar, M.J.; Narajczyk, M.; Wegrzyn, G.; Kulik, W.; Ijlst, L.; Wagemans, T.; van der Wal, W.M.; Wijburg, F.A. Genistein in Sanfilippo disease: A randomized controlled crossover trial. Ann. Neurol. 2012, 71, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Rust, S.; Langford-Smith, K.; Weisberg, D.; Canal, M.; Breen, C.; Hepburn, M.; Tylee, K.; Vaz, F.M.; Vail, A.; et al. High dose genistein in Sanfilippo syndrome: A randomised controlled trial. J. Inherit. Metab. Dis. 2021, 44, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- EMA. Proposal for a Framework to Support Not-For-Profit Organisations and Academia (Institutions and Individuals) in Drug Repurposing; European Medicines Agency—EMA: Amsterdam, The Netherlands, 2019; p. 12. [Google Scholar]
- EMA. Benefit-Risk Methodology Project; Work Package 3 Report: Field Tests; European Medicines Agency—EMA: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Meunier, B.; Rico, A.; Seguier, J.; Boutiere, C.; Ebbo, M.; Harle, J.R.; Schleinitz, N.; Pelletier, J. Life-threatening autoimmune warm hemolytic anemia following treatment for multiple sclerosis with alemtuzumab. Mult. Scler. 2018, 24, 811–813. [Google Scholar] [CrossRef] [PubMed]
- di Ioia, M.; Farina, D.; di Tommaso, V.; Travaglini, D.; Pietrolongo, E.; Onofrj, M.; de Luca, G. Simultaneous early-onset severe autoimmune hemolytic anemia and albuminuria during alemtuzumab treatment for multiple sclerosis. Mult. Scler. 2018, 24, 813–815. [Google Scholar] [CrossRef]
- Ferraro, D.; Camera, V.; Vitetta, F.; Zennaro, M.; Ciolli, L.; Nichelli, P.F.; Sola, P. Acute coronary syndrome associated with alemtuzumab infusion in multiple sclerosis. Neurology 2018, 90, 852–854. [Google Scholar] [CrossRef]
- Liou, A.A.; Skiver, B.M.; Yates, E.; Persad, P.; Meyer, D.; Farland, A.M.; Rocco, M.V. Acute Thrombotic Microangiopathy and Cortical Necrosis Following Administration of Alemtuzumab: A Case Report. Am. J. Kidney Dis. 2019, 73, 615–619. [Google Scholar] [CrossRef]
- Whiteside, D.; Barth, S.; Datta, A.; Trip, S.A. Pneumonitis secondary to alemtuzumab in a patient with multiple sclerosis—A non-infectious cause of breathlessness. Mult. Scler. Relat. Disord. 2018, 22, 139–140. [Google Scholar] [CrossRef] [PubMed]
- El Sankari, S.; Dahlqvist, G.; Monino, L.; van Pesch, V. Auto-immune hepatitis in a patient with multiple sclerosis treated with alemtuzumab. Acta Neurol. Belg. 2018, 118, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Giarola, B.; Massey, J.; Barnett, Y.; Rodrigues, M.; Sutton, I. Autoimmune encephalitis following alemtuzumab treatment of multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 31–33. [Google Scholar] [CrossRef]
- Midaglia, L.; Gratacòs, M.; Caronna, E.; Raguer, N.; Sastre-Garriga, J.; Montalban, X.; Tintoré, M. Myasthenia gravis following alemtuzumab therapy for multiple sclerosis. Neurology 2018, 91, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papadimitriou, D.; Anastasiou, A.I.; Siakantaris, M. Neutropenia with fatal outcome in a multiple sclerosis patient 23 days after alemtuzumab infusion. Mult. Scler. Relat. Disord. 2018, 23, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.M.; Zeid, N.A.; Alam, U.; Caress, J.B. Lambert-Eaton myasthenic syndrome associated with alemtuzumab administration. Mult. Scler. Relat. Disord. 2019, 27, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Lapucci, C.; Gualandi, F.; Mikulska, M.; Palmeri, S.; Mancardi, G.; Uccelli, A.; Laroni, A. Serum sickness (Like Reaction) in a patient treated with alemtuzumab for multiple sclerosis: A case report. Mult. Scler. Relat. Disord. 2018, 26, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Ringelstein, M.; Lepka, K.; Schaller, J.; Quack, H.; Hartung, H.P.; Aktas, O.; Albrecht, P. Acute sarcoidosis in a multiple sclerosis patient after alemtuzumab treatment. Mult. Scler. 2018, 24, 1776–1778. [Google Scholar] [CrossRef]
- Willis, M.D.; Hope-Gill, B.; Flood-Page, P.; Joseph, F.; Needham, E.; Jones, J.; Coles, A.; Robertson, N.P. Sarcoidosis following alemtuzumab treatment for multiple sclerosis. Mult. Scler. 2018, 24, 1779–1782. [Google Scholar] [CrossRef]
- Ruck, T.; Pfeuffer, S.; Schulte-Mecklenbeck, A.; Gross, C.C.; Lindner, M.; Metze, D.; Ehrchen, J.; Sondermann, W.; Pul, R.; Kleinschnitz, C.; et al. Vitiligo after alemtuzumab treatment: Secondary autoimmunity is not all about B cells. Neurology 2018, 91, e2233–e2237. [Google Scholar] [CrossRef]
- Alcalá, C.; Pzére-Miralles, F.; Gascón, F.; Evole, M.; Estutia, M.; Gil-Perotín, S.; Casanova, B. Recurrent and universal alopecia areata following alemtuzumab treatment in multiple sclerosis: A secondary autoimmune disease. Mult. Scler. Relat. Disord. 2019, 27, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Myro, A.Z.; Bjerke, G.; Zarnovicky, S.; Holmøy, T. Diffuse alveolar hemorrhage during alemtuzumab infusion in a patient with multiple sclerosis: A case report. BMC Pharmacol. Toxicol. 2018, 19, 75. [Google Scholar] [CrossRef]
- Pisa, M.; Della Valle, P.; Coluccia, A.; Martinelli, V.; Comi, G.; D’Angelo, A.; Moiola, L. Acquired haemophilia A as a secondary autoimmune disease after alemtuzumab treatment in multiple sclerosis: A case report. Mult. Scler. Relat. Disord. 2019, 27, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Aouad, P.; Yiannikas, C.; Fernando, S.L.; Parratt, J. A case of autoimmune myositis after treatment with alemtuzumab for multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318819012. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Wagner, B.; Celius, E.G. Two cases of diabetes mellitus type 1 after alemtuzumab treatment for multiple sclerosis: Another probable secondary autoimmune disease. J. Neurol. 2019, 266, 1270–1271. [Google Scholar] [CrossRef]
- Gerevini, S.; Capra, R.; Bertoli, D.; Sottini, A.; Imberti, L. Immune profiling of a patient with alemtuzumab-associated progressive multifocal leukoencephalopathy. Mult. Scler. 2019, 25, 1196–1201. [Google Scholar] [CrossRef]
- Polgreen, L.E.; Kunin-Batson, A.; Rudser, K.; Vehe, R.K.; Utz, J.J.; Whitley, C.B.; Dickson, P. Pilot study of the safety and effect of adalimumab on pain, physical function, and musculoskeletal disease in mucopolysaccharidosis types I and II. Mol. Genet. Metab. Rep. 2017, 10, 75–80. [Google Scholar] [CrossRef]
- Polgreen, L.; Chen, A.; O’Neill, C.; Luzzi, A.; Iacovino, M.; Eisengart, J. Open-label clinical trial of anakinra in mucopolysaccharidosis type III: Interim analysis. Mol. Genet. Metab. 2020, 132, S87–S88. [Google Scholar] [CrossRef]
- Polgreen, L.; O’Neill, C.; Chen, A.; Eisengart, J. Phase I/II clinical trial of anakinra in Sanfilippo syndrome: Outcomes from 8 weeks of palliative treatment. Mol. Genet. Metab. 2022, 135, S100. [Google Scholar] [CrossRef]
- Moser, T.; Ziemssen, T.; Sellner, J. Real-world evidence for cladribine tablets in multiple sclerosis: Further insights into efficacy and safety. Wien Med. Wochenschr. 2022, 172, 365–372. [Google Scholar] [CrossRef]
- Leist, T.P.; Weissert, R. Cladribine: Mode of action and implications for treatment of multiple sclerosis. Clin. Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Hoepner, L.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Cladribine Alters Immune Cell Surface Molecules for Adhesion and Costimulation: Further Insights to the Mode of Action in Multiple Sclerosis. Cells 2021, 10, 3116. [Google Scholar] [CrossRef]
- Sjöström, E.O.; Culot, M.; Leickt, L.; Åstrand, M.; Nordling, E.; Gosselet, F.; Kaiser, C. Transport study of interleukin-1 inhibitors using a human in vitro model of the blood-brain barrier. Brain Behav. Immun. Health 2021, 16, 100307. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Ellison, S.M.; Holley, R.J.; O’Leary, C.; Liao, A.; Asadi, J.; Glover, E.; Ghosh, A.; Jones, S.; Wilkinson, F.L.; et al. Haematopoietic stem cell gene therapy with IL-1Ra rescues cognitive loss in mucopolysaccharidosis IIIA. EMBO Mol. Med. 2020, 12, e11185. [Google Scholar] [CrossRef]
- Olcum, M.; Tastan, B.; Kiser, C.; Genc, S.; Genc, K. Microglial NLRP3 inflammasome activation in multiple sclerosis. Adv. Protein Chem. Struct. Biol. 2020, 119, 247–308. [Google Scholar] [CrossRef]
- Polgreen, L.E.; Vehe, R.K.; Rudser, K.; Kunin-Batson, A.; Utz, J.J.; Dickson, P.; Shapiro, E.; Whitley, C.B. Elevated TNF-α is associated with pain and physical disability in mucopolysaccharidosis types I, II, and VI. Mol. Genet. Metab. 2016, 117, 427–430. [Google Scholar] [CrossRef]
- Eliyahu, E.; Wolfson, T.; Ge, Y.; Jepsen, K.J.; Schuchman, E.H.; Simonaro, C.M. Anti-TNF-alpha therapy enhances the effects of enzyme replacement therapy in rats with mucopolysaccharidosis type VI. PLoS ONE 2011, 6, e22447. [Google Scholar] [CrossRef]
- Simonaro, C.M.; Ge, Y.; Eliyahu, E.; He, X.; Jepsen, K.J.; Schuchman, E.H. Involvement of the Toll-like receptor 4 pathway and use of TNF-alpha antagonists for treatment of the mucopolysaccharidoses. Proc. Natl. Acad. Sci. USA 2010, 107, 222–227. [Google Scholar] [CrossRef]
- de Oliveira, P.G.; Baldo, G.; Mayer, F.Q.; Martinelli, B.; Meurer, L.; Giugliani, R.; Matte, U.; Xavier, R.M. Characterization of joint disease in mucopolysaccharidosis type I mice. Int. J. Exp. Pathol. 2013, 94, 305–311. [Google Scholar] [CrossRef]
- PhRMA. The PhRMA BRAT Framework for Benefit-Risk Assessment. In User’s Guide to the Process; PhRMA: Washington, DC, USA, 2011. [Google Scholar]
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020, 179, 839–847. [Google Scholar] [CrossRef]
- Castaneda, C.; Nalley, K.; Mannion, C.; Bhattacharyya, P.; Blake, P.; Pecora, A.; Goy, A.; Suh, K.S. Clinical decision support systems for improving diagnostic accuracy and achieving precision medicine. J. Clin. Bioinform. 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Juhaeri, J. Benefit-risk evaluation: The past, present and future. Ther. Adv. Drug Saf. 2019, 10, 2042098619871180. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E. The therapeutic importance of understanding mechanisms of neuronal cell death in neurodegenerative disease. Mol. Neurodegener. 2009, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Jones, S.J. Drug repositioning for personalized medicine. Genome Med. 2012, 4, 27. [Google Scholar] [CrossRef]
- Bobe, J.R.; De Freitas, J.K.; Glicksberg, B.S. Exploring the Potential for Collaborative Use of an App-Based Platform for n-of-1 Trials Among Healthcare Professionals That Treat Patients with Insomnia. Front. Psychiatry 2020, 11, 530995. [Google Scholar] [CrossRef]
- Mt-Isa, S.; Hallgreen, C.E.; Wang, N.; Callréus, T.; Genov, G.; Hirsch, I.; Hobbiger, S.F.; Hockley, K.S.; Luciani, D.; Phillips, L.D.; et al. Balancing benefit and risk of medicines: A systematic review and classification of available methodologies. Pharmacoepidemiol. Drug Saf. 2014, 23, 667–678. [Google Scholar] [CrossRef]
- Vasilev, F.; Sukhomyasova, A.; Otomo, T. Mucopolysaccharidosis-Plus Syndrome. Int. J. Mol. Sci. 2020, 21, 421. [Google Scholar] [CrossRef]
- Kiykim, E.; Barut, K.; Cansever, M.S.; Zeybek, C.A.; Zubarioglu, T.; Aydin, A.; Kasapcopur, O. Screening Mucopolysaccharidosis Type IX in Patients with Juvenile Idiopathic Arthritis. JIMD Rep. 2016, 25, 21–24. [Google Scholar]




| Problem | Define the unmet medical need. Is there neurocognitive involvement? As a general rule, the decision problem is defined as whether intended off-label use is rational based on the available scientific evidence complemented with expert opinion and clinical practice, preferably in a multidisciplinary group of MPS clinicians and experts. |
| Alternatives | What are the alternative treatment options (label and off-label), and why are they unsuitable? |
| Objectives: Efficacy, Safety | What do you need to know before you can decide on the immunomodulatory drug repurposing use? The efficacy of off-label use in the intended population is established or is plausible based on extrapolation from other populations. Risks are acceptable after mitigation measures have been installed. Appropriate dosing to attain efficacy in the intended population is known. What clinical parameters and cut-offs define sufficient efficacy and unacceptable risk? |
| Consequences | Summary of information on what you needed to know and identification of benefits and risks.The consequences provide an explicit overview of what you need to know and specify the identified benefits and risks as a result of the objectives. |
| Trade Offs | Assess the balance between benefits and risks. |
| Uncertainty | Recognize what you do not know for sure and how it affects the benefit–risk balance. Report the uncertainty associated with the favorable and unfavorable effects. Reports on the level of evidence indicate the extent to which one can be confident that off-label use will do more good than harm. The assessment should review the quality of the studies, the consistency of the results across the studies, and the applicability to the population of interest (“directness”). Consider how the balance between favorable and unfavorable effects is affected by uncertainty. If the evidence is weak, why are the benefits and risks assumed to be acceptable for this population? |
| Risk Tolerance | Complement the balance with a transparent consensus and expert opinion. Judge the relative importance of the decision-maker’s risk attitude for immunomodulatory drug repurposing. How does the risk tolerance of team members affect the balance? |
| Linked Decisions | Reflect on the impact of the decision on future decisions or on its consistency with previous decisions. The outcome of the RBA triggers subsequent decisions and recommended actions (informed consent, dissemination of knowledge). |
| Drug | PRO | CON | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MPS Clinical Data | MPS Pre-Clinical Data | Pediatric Data beyond MPS | Malignancy * | Infection | Low CNS Bioavailability | Renal Impair-ment | Hepatic Impair-ment | Cardiac Involve-ment | Invasive | |
| adalimumab | X MPS I n = 1, MPS II n = 1 | X | X | X 3.2/100 PY | X | X | X (sc) | |||
| abatacept | X MPS I ** | X | X | X 1.3/100 PY | X | X (iv/sc) | ||||
| anakinra | X MPS III n = 7 | X | X 5.4/100 PY | X | X | X (sc) | ||||
| cladribine | X | (X) | X 0.9/100 PY | X | X | X | ||||
| Mobility | QoL | Behavior | Cogn/Comm | ROM | ||
|---|---|---|---|---|---|---|
| Value of Importance | 33% | 66% | 80% | 90% | 33% | |
| Chance of Improvement | ||||||
| Anakinra | ||||||
| Drug | Expert consensus | 5% | 80% | 80% | 40% | 5% |
| Polgreen 2022, NCT04018755 | 60% | 60% | 60% | |||
| Schnaberg 2020, NCT03265132 | 90% | 90% | 70% | |||
| mean | 48% | 77% | 70% | 50% | 38% | |
| Placebo | Polgreen 2022, NCT04018755 | 20% | 5% | 5% | 5% | |
| Schnaberg 2020, NCT03265132 | 20% | 20% | 20% | |||
| mean | 20% | 13% | 5% | 5% | 20% | |
| Adalimumab | ||||||
| Drug | Expert consensus | 80% | 40% | 20% | 20% | 80% |
| Polgreen 2017, PMID: 28119823 | 40% | 30% | 50% | 90% | ||
| Burgos-Vargas 2015, PMID: 26223543, NCT01166282 | 70% | 90% | ||||
| mean | 63% | 53% | 35% | 20% | 85% | |
| Placebo | Polgreen 2017, PMID: 28119823 | 5% | 5% | 5% | 5% | 20% |
| Burgos-Vargas 2015, PMID: 26223543, NCT01166282 | 40% | 40% | ||||
| mean | 23% | 23% | 5% | 5% | 20% | |
| Abatacept | ||||||
| Drug | Expert consensus | 60% | 60% | 5% | 5% | 60% |
| Ruperto 2008, PMID: 18632147, PMID: 20597110, NCT00095173 | 60% | 40% | 50% | |||
| Lovell 2015, PMID: 26097215 | 80% | 5% | 5% | |||
| mean | 67% | 50% | 5% | 5% | 55% | |
| Placebo | Ruperto 2008, PMID: 18632147, PMID: 20597110, NCT00095173 | 20% | 20% | 20% | ||
| Lovell 2015, PMID: 26097215 | 20% | 5% | 5% | |||
| mean | 20% | 20% | 5% | 5% | 20% | |
| Cladribine | ||||||
| Drug | Expert consensus | 60% | 60% | 60% | 40% | 60% |
| Dhall 2008, PMID: 17455311 | 90% | 70% | 70% | 90% | ||
| Stine 2004, PMID: 15170896 | 90% | 90% | 90% | 90% | ||
| Giovannoni 2010, PMID: 20089960 | 30% | 80% | 80% | 80% | 30% | |
| mean | 68% | 70% | 75% | 70% | 68% | |
| Placebo | Giovannoni 2010, PMID: 20089960 | 20% | 20% | 5% | 5% | 20% |
| mean | 20% | 20% | 5% | 5% | 20% |
| ITT without DAF | DAF-Based ITT | |
|---|---|---|
| pre-appraised from 2270 publications L by expert E and patient/parent P consensus | ||
| Identification of best drugs | from primary literature | 4 top candidates identified from 18 selected publications out of 2270 L by expert consensus E |
| Assessment of putative beneficial and adverse treatment effects | from primary literature | quantitatively pre-appraised for all candidates L,E |
| Estimation of putative effect size and probability | from primary literature | quasi-quantitative consensus L,E for 3 phenotypic groups |
| Identification of patient factors, which predispose for beneficial/adverse response | single expert opinion | quasi-quantitative consensus L,E for 3 phenotypic groups |
| Discussion with peer and/or interdisciplinary/interprofessional experts (e.g., scientist, pharmacist etc.) | dependent on personal network | expert consensus for all assessments L,E |
| Assessment of patient/parent values | individual | sentinel P plus individual patient perspective |
| Weighing of pros and cons | based on clinical experience | expert and sentinel patient consensus E,P |
| Informed consent/board and/or payers approval | individual preparation | use of prepared literature appraisal for justification |
| Treatment and assessment plan | based on clinical experience | expert and sentinel patient consensus E,P to be individualized |
| Learning from ITT experience | single center experience possibly publication of case report | integration into and availability to public by DAF and mutual publications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiesinger, A.-M.; Bigger, B.; Giugliani, R.; Lampe, C.; Scarpa, M.; Moser, T.; Kampmann, C.; Zimmermann, G.; Lagler, F.B. RETRACTED: An Innovative Tool for Evidence-Based, Personalized Treatment Trials in Mucopolysaccharidosis. Pharmaceutics 2023, 15, 1565. https://doi.org/10.3390/pharmaceutics15051565
Wiesinger A-M, Bigger B, Giugliani R, Lampe C, Scarpa M, Moser T, Kampmann C, Zimmermann G, Lagler FB. RETRACTED: An Innovative Tool for Evidence-Based, Personalized Treatment Trials in Mucopolysaccharidosis. Pharmaceutics. 2023; 15(5):1565. https://doi.org/10.3390/pharmaceutics15051565
Chicago/Turabian StyleWiesinger, Anna-Maria, Brian Bigger, Roberto Giugliani, Christina Lampe, Maurizio Scarpa, Tobias Moser, Christoph Kampmann, Georg Zimmermann, and Florian B. Lagler. 2023. "RETRACTED: An Innovative Tool for Evidence-Based, Personalized Treatment Trials in Mucopolysaccharidosis" Pharmaceutics 15, no. 5: 1565. https://doi.org/10.3390/pharmaceutics15051565
APA StyleWiesinger, A.-M., Bigger, B., Giugliani, R., Lampe, C., Scarpa, M., Moser, T., Kampmann, C., Zimmermann, G., & Lagler, F. B. (2023). RETRACTED: An Innovative Tool for Evidence-Based, Personalized Treatment Trials in Mucopolysaccharidosis. Pharmaceutics, 15(5), 1565. https://doi.org/10.3390/pharmaceutics15051565