Dry Powder Inhalation for Lung Delivery in Cystic Fibrosis
Abstract
1. Introduction
1.1. Respiratory Tract
1.2. Cystic Fibrosis
1.3. Understanding Dry Powder Inhalers
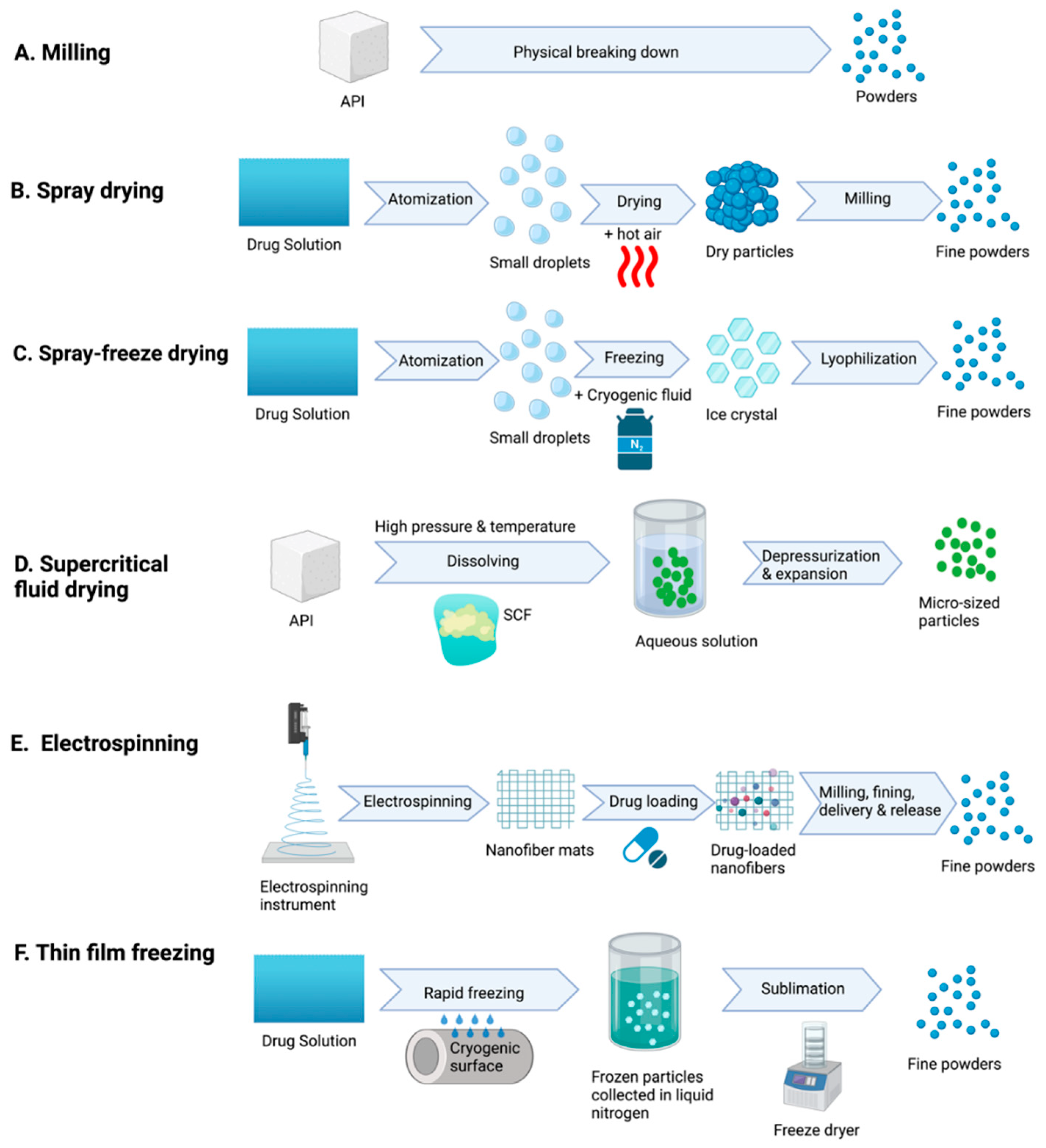
1.4. Physicochemical Properties of Dry Powder
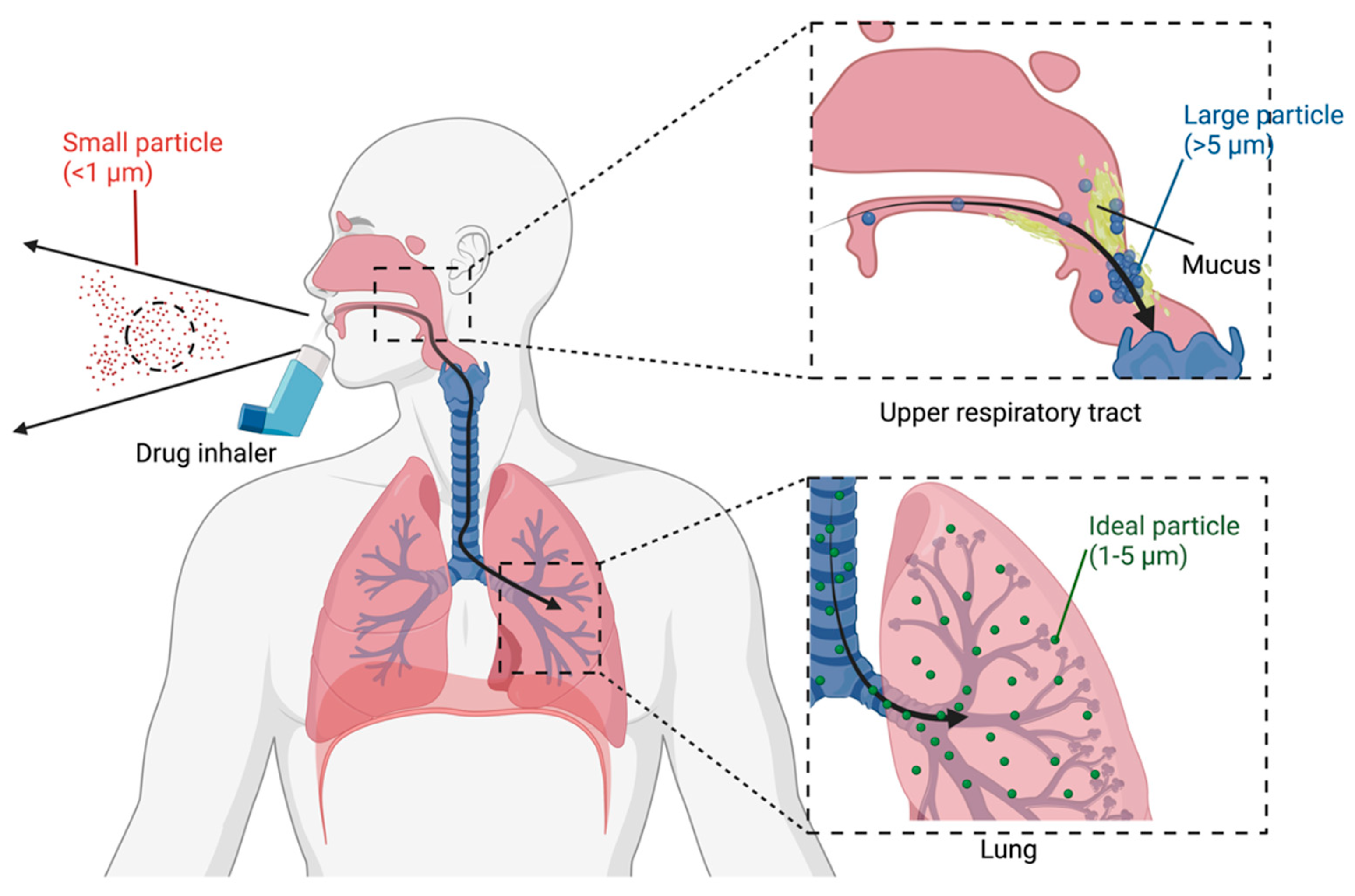
1.4.1. Size
1.4.2. Shape and Surface Morphology
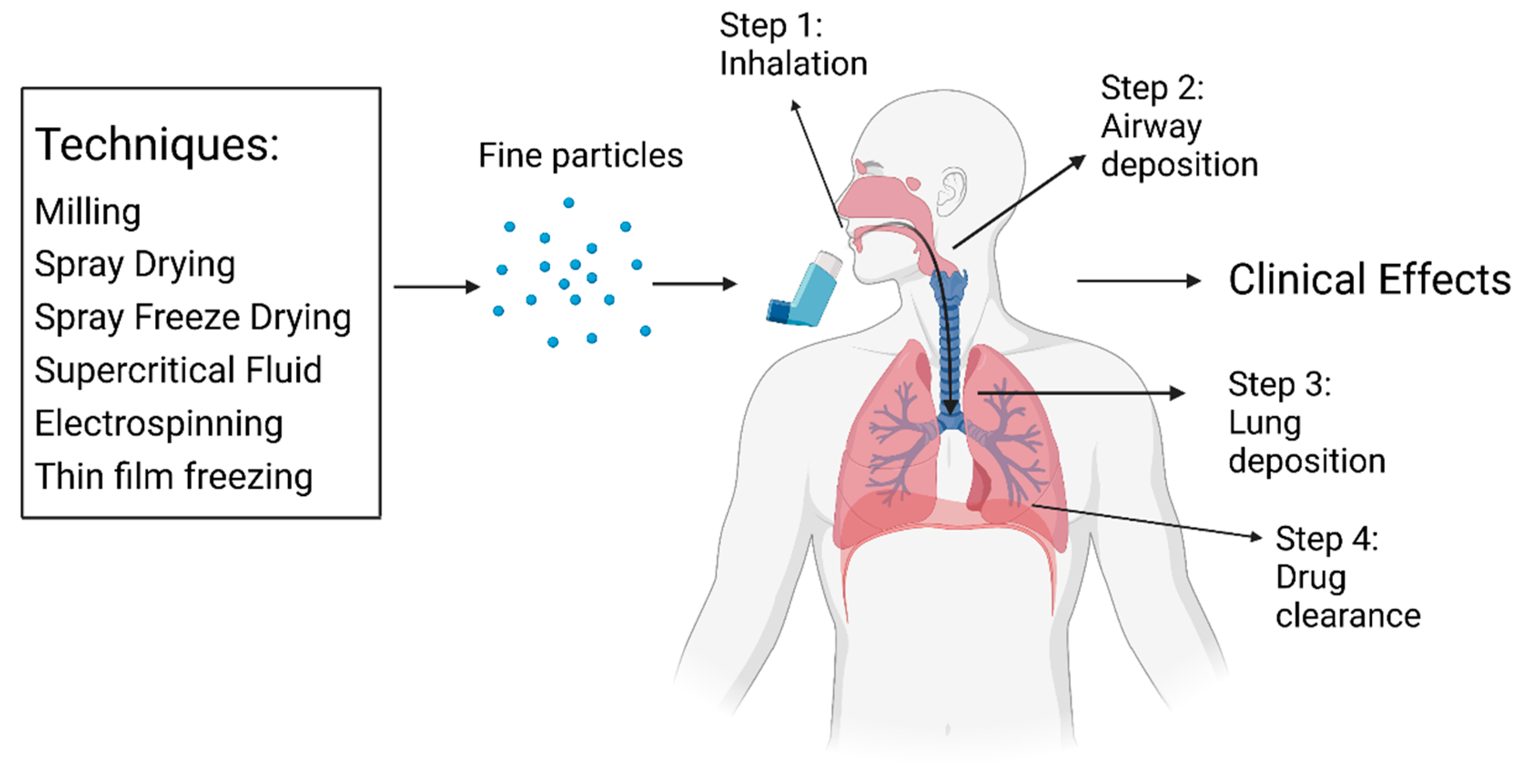
2. Drug Administration
2.1. Administration and Clearance
2.2. Advantages and Limitations
3. Factors Affecting Clinical Efficacy
4. Bacterial Infection in CF
5. Cystic Fibrosis Treatments
5.1. CFTR Modulators
5.2. Antibiotics
5.3. Anti-Inflammatories
6. Dry Powder Inhalers in CF Therapy
6.1. Mucus Thinners
6.2. Bronchodilators
6.3. Antibiotics
7. Case Studies of the Effectiveness of DPIs on CF
8. Dry Powder Inhalers in the Pipeline
9. Opportunity for Inhalable CFTR Modulators
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| DPI | Dry powder inhaler |
| ENaC | Epithelium sodium channel |
| FEV1 | Forced expiratory volume per second |
| IDP | Inhalable dry powder |
| LRT | Lower respiratory tract |
| P. aeruginosa | Pseudomonas Aeruginosa |
| SABA | short-acting beta-agonists |
| URT | Upper respiratory tract |
References
- Paranjpe, M.; Muller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Weibel, E.R. Morphometry of the human lung: The state of the art after two decades. Bull. Eur. Physiopathol. Respir. 1979, 15, 999–1013. [Google Scholar]
- Chaurasiya, B.; Zhao, Y.Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Danjo, K. Application of supercritical fluid to preparation of powders of high-molecular weight drugs for inhalation. Adv. Drug. Deliv. Rev. 2008, 60, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J. Aerosol Med. Pulm. Drug. Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef]
- Ye, Y.; Ma, Y.; Zhu, J. The future of dry powder inhaled therapy: Promising or discouraging for systemic disorders? Int. J. Pharm. 2022, 614, 121457. [Google Scholar] [CrossRef]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic fibrosis. Nat. Rev. Dis. Prim. 2015, 1, 15010. [Google Scholar] [CrossRef]
- Keown, K.; Brown, R.; Doherty, D.F.; Houston, C.; McKelvey, M.C.; Creane, S.; Linden, D.; McAuley, D.F.; Kidney, J.C.; Weldon, S.; et al. Airway Inflammation and Host Responses in the Era of CFTR Modulators. Int. J. Mol. Sci. 2020, 21, 6379. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Chow, M.Y.T.; Khanal, D.; Chen, D.; Chan, H.K. Dry powder pharmaceutical biologics for inhalation therapy. Adv. Drug. Deliv. Rev. 2021, 172, 64–79. [Google Scholar] [CrossRef]
- Komase, Y.; Asako, A.; Kobayashi, A.; Sharma, R. Ease-of-use preference for the ELLIPTA(R) dry powder inhaler over a commonly used single-dose capsule dry powder inhaler by inhalation device-naive Japanese volunteers aged 40 years or older. Int. J. Chron. Obstr. Pulm. Dis. 2014, 9, 1365–1375. [Google Scholar] [CrossRef]
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for dry powder drug delivery to the lung. AAPS PharmSciTech 2015, 16, 479–490. [Google Scholar] [CrossRef]
- Steinkamp, G. Trockenpulverinhalation bei Mukoviszidose. Pneumologie 2014, 68, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Katona, G.; Csoka, I.; Ambrus, R. Physicochemical stability and aerosolization performance of dry powder inhalation system containing ciprofloxacin hydrochloride. J. Pharm. Biomed. Anal. 2018, 148, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Courrier, H.M.; Butz, N.; Vandamme, T.F. Pulmonary drug delivery systems: Recent developments and prospects. Crit. Rev. Ther. Drug. Carr. Syst. 2002, 19, 425–498. [Google Scholar] [CrossRef]
- Islam, N.; Gladki, E. Dry powder inhalers (DPIs)--a review of device reliability and innovation. Int. J. Pharm. 2008, 360, 1–11. [Google Scholar] [CrossRef]
- Chew, N.Y.; Chan, H.K. The role of particle properties in pharmaceutical powder inhalation formulations. J. Aerosol Med. 2002, 15, 325–330. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R.W. Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties. AAPS PharmSciTech 2009, 10, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.M.; Martin, G.P.; Marriott, C.; Pritchard, J. The influence of carrier morphology on drug delivery by dry powder inhalers. Int. J. Pharm. 2000, 200, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef]
- Chew, N.Y.; Bagster, D.F.; Chan, H.K. Effect of particle size, air flow and inhaler device on the aerosolisation of disodium cromoglycate powders. Int. J. Pharm. 2000, 206, 75–83. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O'Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Leon Gradon, T.R.S. Formation of particles for dry powder inhalers. Adv. Powder Technol. 2014, 25, 43–55. [Google Scholar] [CrossRef]
- Shoyele, S.A.; Cawthorne, S. Particle engineering techniques for inhaled biopharmaceuticals. Adv. Drug. Deliv. Rev. 2006, 58, 1009–1029. [Google Scholar] [CrossRef]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yamazoe, E.; Tahara, K. Dry Powder Inhalers for Proteins Using Cryo-Milled Electrospun Polyvinyl Alcohol Nanofiber Mats. Molecules 2022, 27, 5158. [Google Scholar] [CrossRef]
- Praphawatvet, T.; Cui, Z.; Williams, R.O. Pharmaceutical dry powders of small molecules prepared by thin-film freezing and their applications–A focus on the physical and aerosol properties of the powders. Int. J. Pharm. 2022, 629, 122357. [Google Scholar] [CrossRef]
- Wang, J.L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Williams Iii, R.O.; David Charles Smyth, H.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; Kole, E.B.; Kapare, H.S.; Chandankar, S.M.; Shinde, P.J.; Boisa, G.S.; Salgaonkar, S.S.; Giram, P.S.; More, M.P.; Kolimi, P.; et al. Progress on Thin Film Freezing Technology for Dry Powder Inhalation Formulations. Pharmaceutics 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Ganga Srinivasan, A.S. Advancements In Dry Powder Inhaler. Asian J. Pharm. Clin. Res. 2017, 10, 8–12. [Google Scholar] [CrossRef]
- Levy, M.L.; Carroll, W.; Izquierdo Alonso, J.L.; Keller, C.; Lavorini, F.; Lehtimaki, L. Understanding Dry Powder Inhalers: Key Technical and Patient Preference Attributes. Adv. Ther. 2019, 36, 2547–2557. [Google Scholar] [CrossRef]
- Crompton, G.K. Dry powder inhalers: Advantages and limitations. J. Aerosol Med. 1991, 4, 151–156. [Google Scholar] [CrossRef]
- Bilton, D.; Robinson, P.; Cooper, P.; Gallagher, C.G.; Kolbe, J.; Fox, H.; Jaques, A.; Charlton, B.; Investigators, C.F.S. Inhaled dry powder mannitol in cystic fibrosis: An efficacy and safety study. Eur. Respir. J. 2011, 38, 1071–1080. [Google Scholar] [CrossRef]
- Westerik, J.A.M.; Carter, V.; Chrystyn, H.; Burden, A.; Thompson, S.L.; Ryan, D.; Gruffydd-Jones, K.; Haughney, J.; Roche, N.; Lavorini, F.; et al. Characteristics of patients making serious inhaler errors with a dry powder inhaler and association with asthma-related events in a primary care setting. J. Asthma 2016, 53, 321–329. [Google Scholar] [CrossRef]
- Guenette, E.; Barrett, A.; Kraus, D.; Brody, R.; Harding, L.; Magee, G. Understanding the effect of lactose particle size on the properties of DPI formulations using experimental design. Int. J. Pharm. 2009, 380, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Du, J.; Smyth, H.D. Evaluation of granulated lactose as a carrier for DPI formulations 1: Effect of granule size. AAPS PharmSciTech 2014, 15, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.; Kaialy, W.; Chen, Q.; Commandeur, D.; Nokhodchi, A. Agglomerated novel spray-dried lactose-leucine tailored as a carrier to enhance the aerosolization performance of salbutamol sulfate from DPI formulations. Drug. Deliv. Transl. Res. 2018, 8, 1769–1780. [Google Scholar] [CrossRef]
- Frijlink, H.W.; De Boer, A.H. Dry powder inhalers for pulmonary drug delivery. Expert Opin. Drug. Deliv. 2004, 1, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Garg, T.; Rath, G.; Goyal, A.K. Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit. Rev. Ther. Drug. Carr. Syst. 2014, 31, 495–530. [Google Scholar] [CrossRef]
- Vanderbist, F.; Wery, B.; Baran, D.; Van Gansbeke, B.; Schoutens, A.; Moes, A.J. Deposition of nacystelyn from a dry powder inhaler in healthy volunteers and cystic fibrosis patients. Drug. Dev. Ind. Pharm. 2001, 27, 205–212. [Google Scholar] [CrossRef]
- Sandler, N.; Holländer, J.; Långström, D.; Santtila, P.; Saukkonen, A.; Torvinen, S. Evaluation of inhaler handling-errors, inhaler perception and preference with Spiromax, Easyhaler and Turbuhaler devices among healthy Finnish volunteers: A single site, single visit crossover study (Finhaler). BMJ Open. Respir. Res. 2016, 3, e000119. [Google Scholar] [CrossRef]
- Janson, C.; Loof, T.; Telg, G.; Stratelis, G.; Nilsson, F. Difference in resistance to humidity between commonly used dry powder inhalers: An in vitro study. NPJ Prim. Care Respir. Med. 2016, 26, 16053. [Google Scholar] [CrossRef]
- Haikarainen, J.; Selroos, O.; Löytänä, T.; Metsärinne, S.; Happonen, A.; Rytilä, P. Budesonide/Formoterol Easyhaler®: Performance Under Simulated Real-Life Conditions. Pulm. Ther. 2017, 3, 125–138. [Google Scholar] [CrossRef]
- Ghosh, S.; Ohar, J.A.; Drummond, M.B. Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: Implications for Dry Powder Inhalers. J. Aerosol Med. Pulm. Drug. Deliv. 2017, 30, 381–387. [Google Scholar] [CrossRef]
- Azouz, W.; Chetcuti, P.; Hosker, H.S.; Saralaya, D.; Stephenson, J.; Chrystyn, H. The inhalation characteristics of patients when they use different dry powder inhalers. J. Aerosol Med. Pulm. Drug. Deliv. 2015, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F. Inhaled drug delivery in the hands of the patient. J. Aerosol Med. Pulm. Drug. Deliv. 2014, 27, 414–418. [Google Scholar] [CrossRef]
- Melani, A.S.; Bonavia, M.; Cilenti, V.; Cinti, C.; Lodi, M.; Martucci, P.; Serra, M.; Scichilone, N.; Sestini, P.; Aliani, M.; et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir. Med. 2011, 105, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.M.; Armstrong, D.S.; Carzino, R.; Carlin, J.B.; Olinsky, A.; Robertson, C.F.; Grimwood, K. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J. Pediatr. 2001, 138, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Allobawi, R.; Ghelani, D.P.; Schneider-Futschik, E.K. Metabolomic Description of Ivacaftor Elevating Polymyxin B Mediated Antibacterial Activity in Cystic Fibrosis Pseudomonas aeruginosa. ACS Pharmacol. Transl. Sci. 2020, 3, 433–443. [Google Scholar] [CrossRef]
- Sibley, C.D.; Rabin, H.; Surette, M.G. Cystic fibrosis: A polymicrobial infectious disease. Future Microbiol. 2006, 1, 53–61. [Google Scholar] [CrossRef]
- Schneider, E.K.; Azad, M.A.; Han, M.L.; Tony Zhou, Q.; Wang, J.; Huang, J.X.; Cooper, M.A.; Doi, Y.; Baker, M.A.; Bergen, P.J.; et al. An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect. Dis. 2016, 2, 478–488. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Cohen-Cymberknoh, M.; Shoseyov, D.; Kerem, E. Managing cystic fibrosis: Strategies that increase life expectancy and improve quality of life. Am. J. Respir. Crit. Care Med. 2011, 183, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Futschik, E.K.; Paulin, O.K.A.; Hoyer, D.; Roberts, K.D.; Ziogas, J.; Baker, M.A.; Karas, J.; Li, J.; Velkov, T. Sputum Active Polymyxin Lipopeptides: Activity against Cystic Fibrosis Pseudomonas aeruginosa Isolates and Their Interactions with Sputum Biomolecules. ACS Infect. Dis. 2018, 4, 646–655. [Google Scholar] [CrossRef]
- Harwood, K.H.; McQuade, R.M.; Jarnicki, A.; Schneider-Futschik, E.K. Anti-Inflammatory Influences of Cystic Fibrosis Transmembrane Conductance Regulator Drugs on Lung Inflammation in Cystic Fibrosis. Int. J. Mol. Sci. 2021, 22, 7606. [Google Scholar] [CrossRef]
- Harwood, K.H.; McQuade, R.M.; Jarnicki, A.; Schneider-Futschik, E.K. Ivacaftor Alters Macrophage and Lymphocyte Infiltration in the Lungs Following Lipopolysaccharide Exposure. ACS Pharmacol. Transl. Sci. 2022, 5, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; White, R.; Tobin, P. Keep them breathing: Cystic fibrosis pathophysiology, diagnosis, and treatment. JAAPA 2017, 30, 23–27. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2019, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.K.; Reyes-Ortega, F.; Li, J.; Velkov, T. Can Cystic Fibrosis Patients Finally Catch a Breath With Lumacaftor/Ivacaftor? Clin. Pharmacol. Ther. 2017, 101, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.T.; Flume, P.A. Cystic Fibrosis: Translating Molecular Mechanisms into Effective Therapies. Ann. Am. Thorac. Soc. 2018, 15, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Heltshe, S.L.; Gonska, T.; Donaldson, S.H.; Borowitz, D.; Gelfond, D.; Sagel, S.D.; Khan, U.; Mayer-Hamblett, N.; Van Dalfsen, J.M.; et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, D.P.; Schneider-Futschik, E.K. Emerging Cystic Fibrosis Transmembrane Conductance Regulator Modulators as New Drugs for Cystic Fibrosis: A Portrait of in Vitro Pharmacology and Clinical Translation. ACS Pharmacol. Transl. Sci. 2020, 3, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Hayes, E.; Keenan, J.; Henry, M.; Meleady, P.; Molloy, K.; Jundi, B.; Bergin, D.A.; McCarthy, C.; McElvaney, O.J.; et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood 2014, 124, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; McColley, S.A.; Rietschel, E.; Li, X.; Bell, S.C.; Konstan, M.W.; Marigowda, G.; Waltz, D.; Boyle, M.P. Lumacaftor/Ivacaftor Treatment of Patients with Cystic Fibrosis Heterozygous for F508del-CFTR. Ann. Am. Thorac. Soc. 2017, 14, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef]
- Ridley, K.; Condren, M. Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy. J. Pediatr. Pharmacol. Ther. 2020, 25, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Drevinek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Mogayzel, P.J.J.; Naureckas, E.T.; Robinson, K.A.; Mueller, G.; Hadjiliadis, D.; Hoag, J.B.; Lubsch, L.; Hazle, L.; Sabadosa, K.; Marshall, B.; et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 2013, 187, 680–689. [Google Scholar] [CrossRef]
- Radtke, T.; Nevitt, S.J.; Hebestreit, H.; Kriemler, S. Physical exercise training for cystic fibrosis. Cochrane Database Syst. Rev. 2017, 11, CD002768. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.F.; Konstan, M.W.; Elborn, J.S. Antibiotic and anti-inflammatory therapies for cystic fibrosis. Cold Spring Harb. Perspect. Med. 2013, 3, a009779. [Google Scholar] [CrossRef]
- van Koningsbruggen-Rietschel, S.; Davies, J.C.; Pressler, T.; Fischer, R.; MacGregor, G.; Donaldson, S.H.; Smerud, K.; Meland, N.; Mortensen, J.; Fosbol, M.O.; et al. Inhaled dry powder alginate oligosaccharide in cystic fibrosis: A randomised, double-blind, placebo-controlled, crossover phase 2b study. ERJ Open. Res. 2020, 6, 00132-2020. [Google Scholar] [CrossRef] [PubMed]
- Hordvik, N.L.; Sammut, P.H.; Judy, C.G.; Colombo, J.L. Effects of standard and high doses of salmeterol on lung function of hospitalized patients with cystic fibrosis. Pediatr. Pulmonol. 1999, 27, 43–53. [Google Scholar] [CrossRef]
- de Pablo, E.; Fernández-García, R.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Nebulised antibiotherapy: Conventional versus nanotechnology-based approaches, is targeting at a nano scale a difficult subject? Ann. Transl. Med. 2017, 5, 448. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Geller, D.E. Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: A review. Med. Devices Evid. Res. 2011, 4, 179–188. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa-Carrillo, D.; Suárez-Cuartín, G.; Golpe, R.; Máiz Carro, L.; Martinez-Garcia, M.A. Inhaled Colistimethate Sodium in the Management of Patients with Bronchiectasis Infected by Pseudomonas aeruginosa: A Narrative Review of Current Evidence. Infect. Drug. Resist. 2022, 15, 7271–7292. [Google Scholar] [CrossRef] [PubMed]
- Dorkin, H.L.; Staab, D.; Operschall, E.; Alder, J.; Criollo, M. Ciprofloxacin DPI: A randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis. BMJ Open. Respir. Res. 2015, 2, e000100. [Google Scholar] [CrossRef]
- Henke, M.O.; Ratjen, F. Mucolytics in cystic fibrosis. Paediatr. Respir. Rev. 2007, 8, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Montgomery, M. Dornase alfa for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 9, CD001127. [Google Scholar] [CrossRef]
- Wark, P.; McDonald, V.M. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 9, CD001506. [Google Scholar] [CrossRef]
- Hsu, E.; Bajaj, T. Beta 2 Agonists; StatPearls: Treasure Island, FL, USA, 2022.
- Retsch-Bogart, G.Z.; Quittner, A.L.; Gibson, R.L.; Oermann, C.M.; McCoy, K.S.; Montgomery, A.B.; Cooper, P.J. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest 2009, 135, 1223–1232. [Google Scholar] [CrossRef]
- Schuster, A.; Haliburn, C.; Doring, G.; Goldman, M.H.; Freedom Study, G. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax 2013, 68, 344–350. [Google Scholar] [CrossRef]
- Westerman, E.M.; De Boer, A.H.; Le Brun, P.P.; Touw, D.J.; Roldaan, A.C.; Frijlink, H.W.; Heijerman, H.G. Dry powder inhalation of colistin in cystic fibrosis patients: A single dose pilot study. J. Cyst. Fibros. 2007, 6, 284–292. [Google Scholar] [CrossRef]
- O'Sullivan, M.E.; Poitevin, F.; Sierra, R.G.; Gati, C.; Dao, E.H.; Rao, Y.; Aksit, F.; Ciftci, H.; Corsepius, N.; Greenhouse, R.; et al. Aminoglycoside ribosome interactions reveal novel conformational states at ambient temperature. Nucleic Acids Res. 2018, 46, 9793–9804. [Google Scholar] [CrossRef]
- Kotra, L.P.; Haddad, J.; Mobashery, S. Aminoglycosides: Perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 2000, 44, 3249–3256. [Google Scholar] [CrossRef]
- Sabnis, A.; Hagart, K.L.H.; Klöckner, A.; Becce, M.; Evans, L.E.; Furniss, R.C.D.; Mavridou, D.A.I.; Murphy, R.; Stevens, M.M.; Davies, J.C.; et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. eLife 2021, 10, e65836. [Google Scholar] [CrossRef] [PubMed]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef]
- Stass, H.; Nagelschmitz, J.; Willmann, S.; Delesen, H.; Gupta, A.; Baumann, S. Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: A phase I study. Clin. Drug. Investig. 2013, 33, 419–427. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Haarman, E.; Hull, J.; Lands, L.C.; Moeller, A.; Munck, A.; Riethmuller, J.; Tiddens, H.; Volpi, S.; Leadbetter, J.; et al. Inhaled dry powder mannitol in children with cystic fibrosis: A randomised efficacy and safety trial. J. Cyst. Fibros. 2017, 16, 380–387. [Google Scholar] [CrossRef] [PubMed]
- (CHMP) CfMPfHU. Guideline on the Clinical Development of Medicinal Products for the Treatment of Cystic Fibrosis; European Medicines Agency: London, UK, 2009. [Google Scholar]
- Galeva, I.; Konstan, M.W.; Higgins, M.; Angyalosi, G.; Brockhaus, F.; Piggott, S.; Thomas, K.; Chuchalin, A.G. Tobramycin inhalation powder manufactured by improved process in cystic fibrosis: The randomized EDIT trial. Curr. Med. Res. Opin. 2013, 29, 947–956. [Google Scholar] [CrossRef]
- Konstan, M.W.; Flume, P.A.; Kappler, M.; Chiron, R.; Higgins, M.; Brockhaus, F.; Zhang, J.; Angyalosi, G.; He, E.; Geller, D.E. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J. Cyst. Fibros. 2011, 10, 54–61. [Google Scholar] [CrossRef]
- Tappenden, P.; Harnan, S.; Uttley, L.; Mildred, M.; Walshaw, M.; Taylor, C.; Brownlee, K. The cost effectiveness of dry powder antibiotics for the treatment of Pseudomonas aeruginosa in patients with cystic fibrosis. Pharmacoeconomics 2014, 32, 159–172. [Google Scholar] [CrossRef]
- Stass, H.; Weimann, B.; Nagelschmitz, J.; Rolinck-Werninghaus, C.; Staab, D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: A phase I, randomized, dose-escalation study. Clin. Ther. 2013, 35, 1571–1581. [Google Scholar] [CrossRef]
- Aitken, M.L.; Bellon, G.; De Boeck, K.; Flume, P.A.; Fox, H.G.; Geller, D.E.; Haarman, E.G.; Hebestreit, H.U.; Lapey, A.; Schou, I.M.; et al. Long-term inhaled dry powder mannitol in cystic fibrosis: An international randomized study. Am. J. Respir. Crit. Care Med. 2012, 185, 645–652. [Google Scholar] [CrossRef]
- Greenwood, J.; Schwarz, C.; Sommerwerck, U.; Nash, E.F.; Tamm, M.; Cao, W.; Mastoridis, P.; Debonnett, L.; Hamed, K. Ease of use of tobramycin inhalation powder compared with nebulized tobramycin and colistimethate sodium: A crossover study in cystic fibrosis patients with pulmonary Pseudomonas aeruginosa infection. Ther. Adv. Respir. Dis. 2017, 11, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Kbah, N.; Kuzmov, A.; Pogrebnyak, N.; Pozharov, V.; Minko, T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J. Control. Release 2019, 296, 225–231. [Google Scholar] [CrossRef]
- Guan, J.; Yuan, H.; Yu, S.; Mao, S.; Tony Zhou, Q. Spray dried inhalable ivacaftor co-amorphous microparticle formulations with leucine achieved enhanced in vitro dissolution and superior aerosol performance. Int. J. Pharm. 2022, 622, 121859. [Google Scholar] [CrossRef]
- Fohner, A.E.; McDonagh, E.M.; Clancy, J.P.; Whirl Carrillo, M.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ivacaftor pathway, pharmacokinetics/pharmacodynamics. Pharm. Genom. 2017, 27, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Personalized or Precision Medicine? The Example of Cystic Fibrosis. Front. Pharmacol. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, C.; Tindall, J.M.; Hong, J.S.; Sorscher, E.J. Making precision medicine personal for cystic fibrosis. Science 2019, 365, 220–221. [Google Scholar] [CrossRef]
- Accurso, F.J.; Rowe, S.M.; Clancy, J.P.; Boyle, M.P.; Dunitz, J.M.; Durie, P.R.; Sagel, S.D.; Hornick, D.B.; Konstan, M.W.; Donaldson, S.H.; et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 2010, 363, 1991–2003. [Google Scholar] [CrossRef]

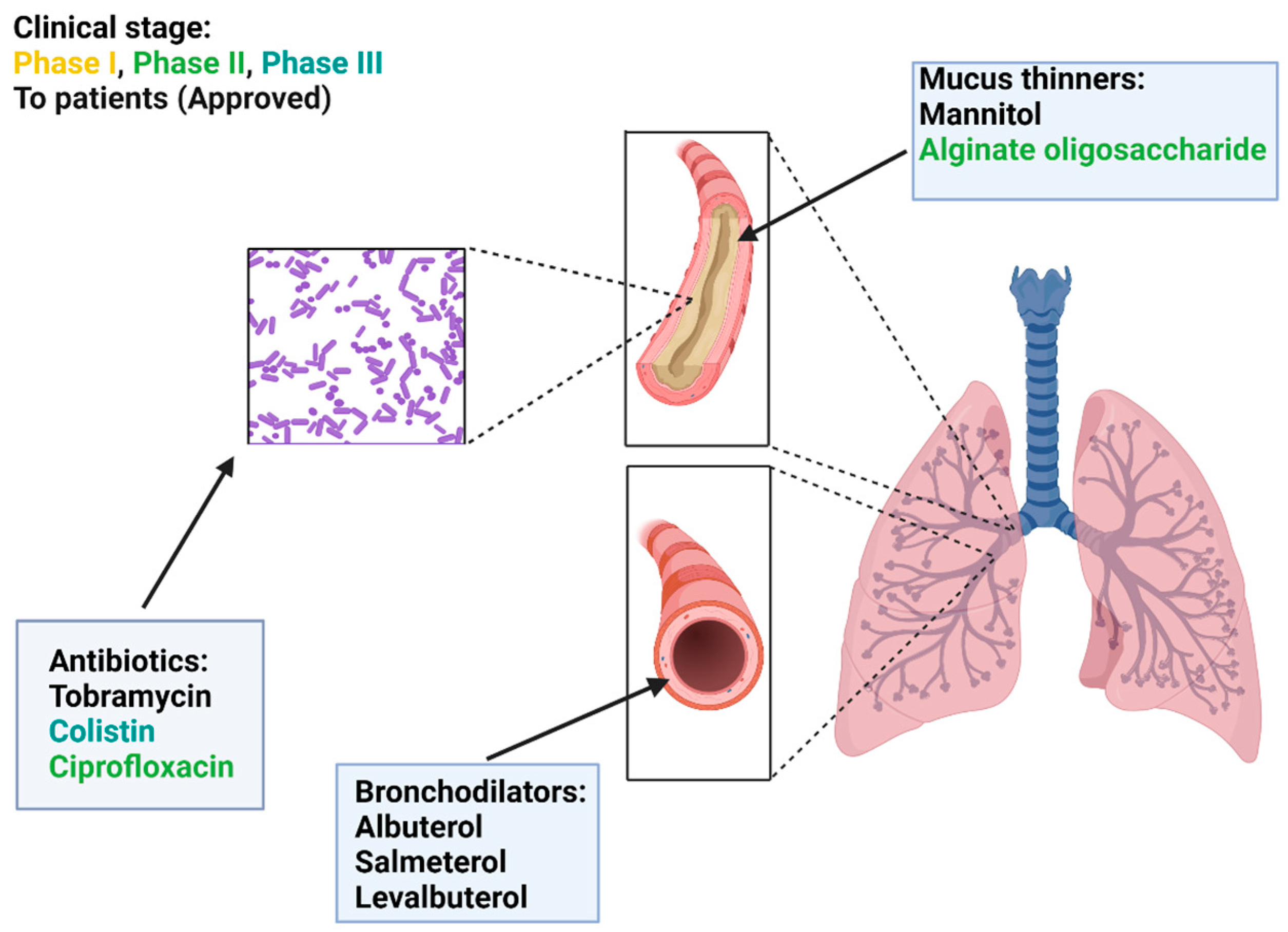
| Type | Treatment | Mechanism of Action | Status: |
|---|---|---|---|
| Mucus thinners | Mannitol | Mucus hydration | To patients FDA/EMA |
| Alginate oligosaccharide | Macromolecules degradation | Phase II | |
| Bronchodilators | Albuterol | Relaxation of smooth muscle | To patients FDA/EMA |
| Salmeterol | Relaxation of smooth muscle | To patients FDA/EMA | |
| Levalbuterol | Relaxation of smooth muscle | To patients FDA/EMA | |
| Antibiotics | Tobramycin | Inhibition of protein synthesis | To patients FDA/EMA |
| Colistin | Disruption of the outer cell membrane | To patients EMA/Phase III USA | |
| Ciprofloxacin | Inhibition of bacterial DNA replication | Phase II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Li, D.; Reyes-Ortega, F.; Schneider-Futschik, E.K. Dry Powder Inhalation for Lung Delivery in Cystic Fibrosis. Pharmaceutics 2023, 15, 1488. https://doi.org/10.3390/pharmaceutics15051488
Han X, Li D, Reyes-Ortega F, Schneider-Futschik EK. Dry Powder Inhalation for Lung Delivery in Cystic Fibrosis. Pharmaceutics. 2023; 15(5):1488. https://doi.org/10.3390/pharmaceutics15051488
Chicago/Turabian StyleHan, Xiaoxuan, Danni Li, Felisa Reyes-Ortega, and Elena K. Schneider-Futschik. 2023. "Dry Powder Inhalation for Lung Delivery in Cystic Fibrosis" Pharmaceutics 15, no. 5: 1488. https://doi.org/10.3390/pharmaceutics15051488
APA StyleHan, X., Li, D., Reyes-Ortega, F., & Schneider-Futschik, E. K. (2023). Dry Powder Inhalation for Lung Delivery in Cystic Fibrosis. Pharmaceutics, 15(5), 1488. https://doi.org/10.3390/pharmaceutics15051488







