Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
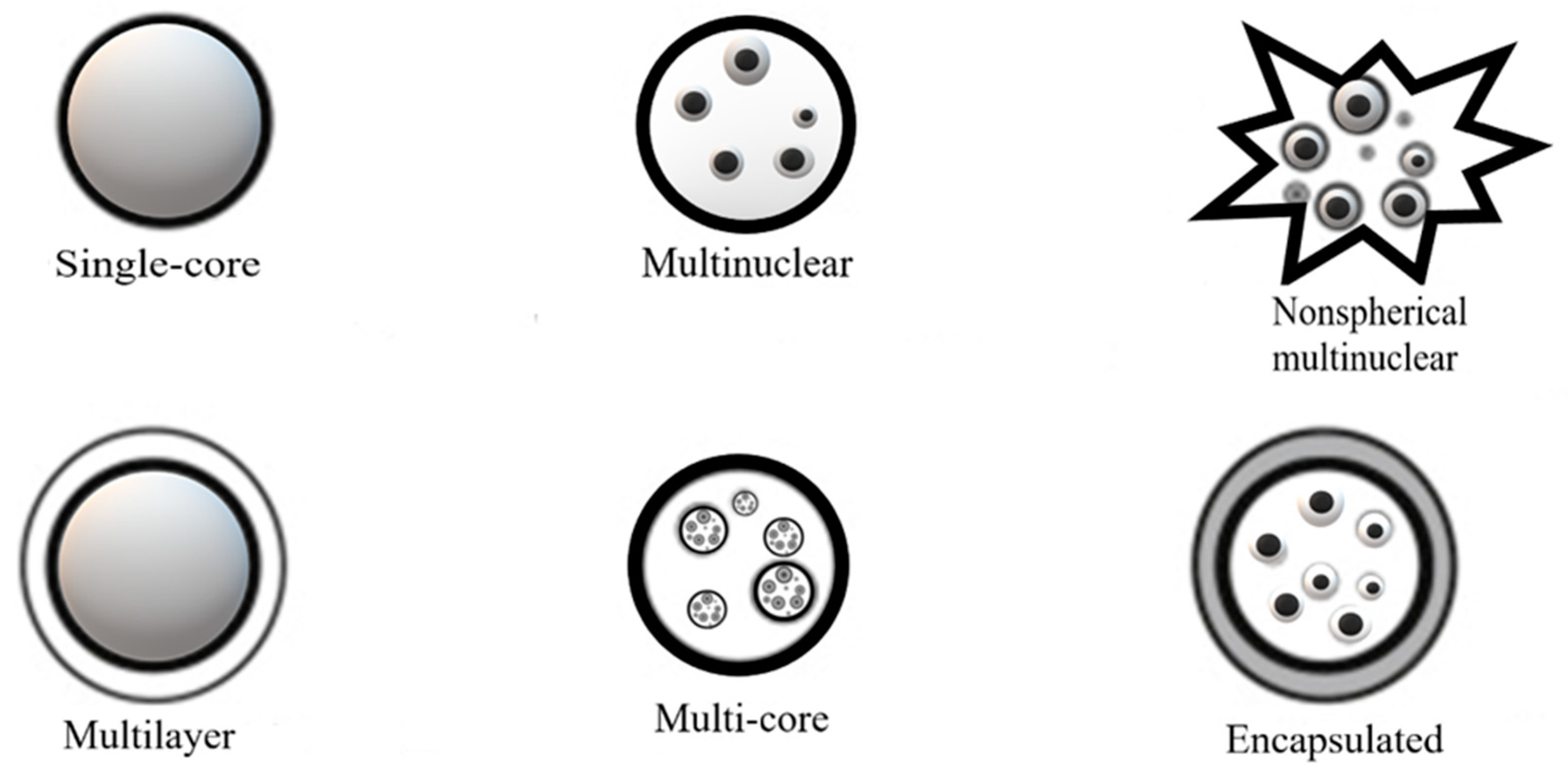
2.2. Theortical Framework of the Microencapsulation
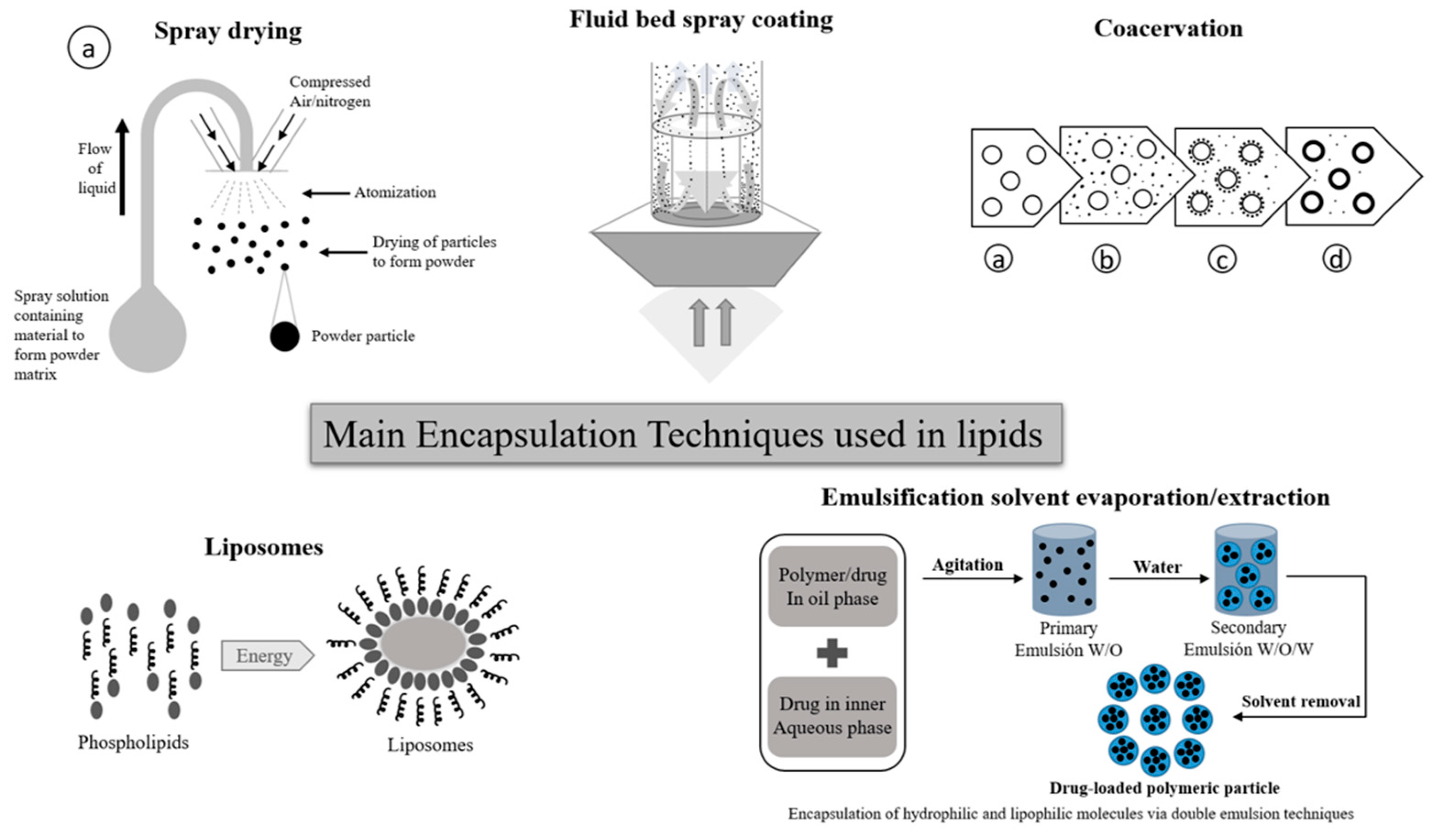
2.2.1. Representative Encapsulation Techniques
- -
- Spray drying is a widely used manufacturing technique used in food, agri-food, and the pharmaceutical industry, among others. This technology allows to obtain a powder product, starting from a concentrated liquid solution or suspension. Its operating principle is based on the atomization of the solution, thus generating small micro droplets upon contact with a sprayed stream of hot air (between 150 °C to 300 °C) [45].
- -
- In the case of coacervation, a homogeneous solution of charged macromolecules separates into two liquid phases in equilibrium, a colloidal suspension in which the more concentrated phase is known as the “coacervated phase”, and the other is known as the “equilibrium phase”. During the simple coacervation process of a polyelectrolyte, the addition of salt or alcohol normally promotes phase segregation, through the self-neutralization of the loads [45,46]. Additionally, the coacervation complex involves the interaction of at least two biopolymers fillers. Parameters such as concentration, biopolymers ratio, temperature, pH, ionic strength, and charge density, must be balanced for an efficient phase separation [47,48].
- -
- Liposomes are vesicular structures made up of lipids organized in bilayers or multilayers. These structures are similar to the structure of the membrane lipid. In the medical field, model studies have been developed of the physical behavior and chemistry of the cell membrane, cell compartments, and the cells themselves vesicular transport structures in and out of the cell. Liposomes are considered vesicles with a unilamellar spherical structure or multilamellar; that is, its vesicular lipid conformation may have a bilayer lipid or several concentric lipid bilayers. By their conformation, unilamellar liposomes are classified into small (SUV), medium (MUV), large (LUV), or giant (GUV) [49,50].
- -
- Spray coating in a fluidized bed system allows for obtaining a suitable surface coating by uniformly applying the coating material. In this method, the coating material, which is in liquid state, is sprayed onto all the particles as they move in the fluidized bed [51,52], at the time that the aqueous or organic solution is evaporated, forming the coating layer. With this technique, it is possible to obtain particle size of 100 μm up to 3 mm [52,53]. On the other hand, emulsification solvent evaporation/extraction consists in forming an emulsion by combining a polymer and a volatile organic solvent. By heating the emulsion, the solvent is evaporated [54,55]. The solution is formed by the dispersion of the active component and the encapsulating agent, this dispersion is emulsified in an external aqueous phase, in which the polymer is insoluble. In this technique, the use of stabilizing agents that favor the formation of the particles and the retention of the bioactive compound inside each capsule is common [55]. This technique is normally used to microencapsulate hydrophobic bioactive compounds; it can additionally perform both a single emulsion and a double emulsion, also known as water-in-oil-in-water (w/o/w) [56].
2.2.2. Spray Drying Encapsulation
3. Results
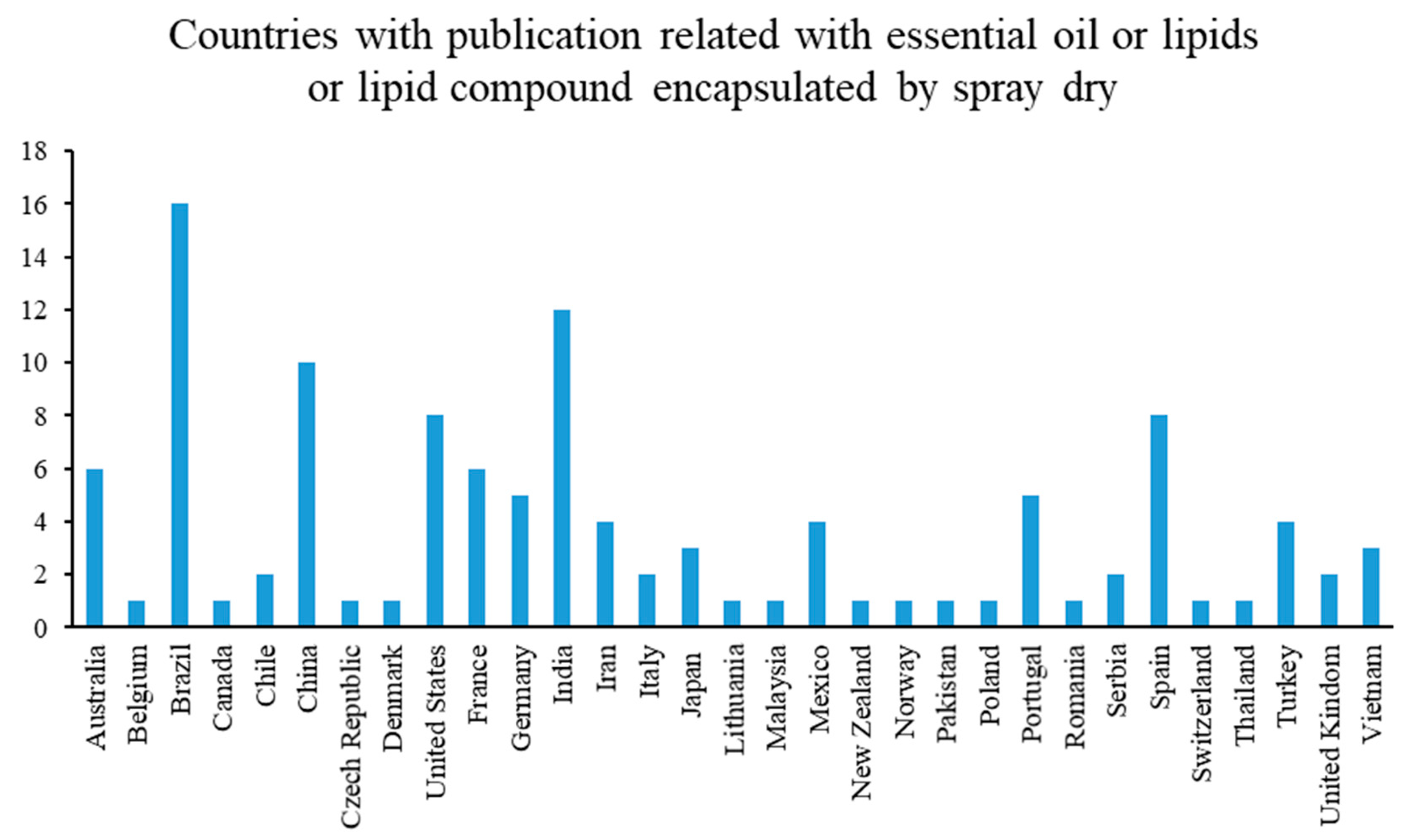
3.1. Relevance of Technology per Geographical Regions and Market Push
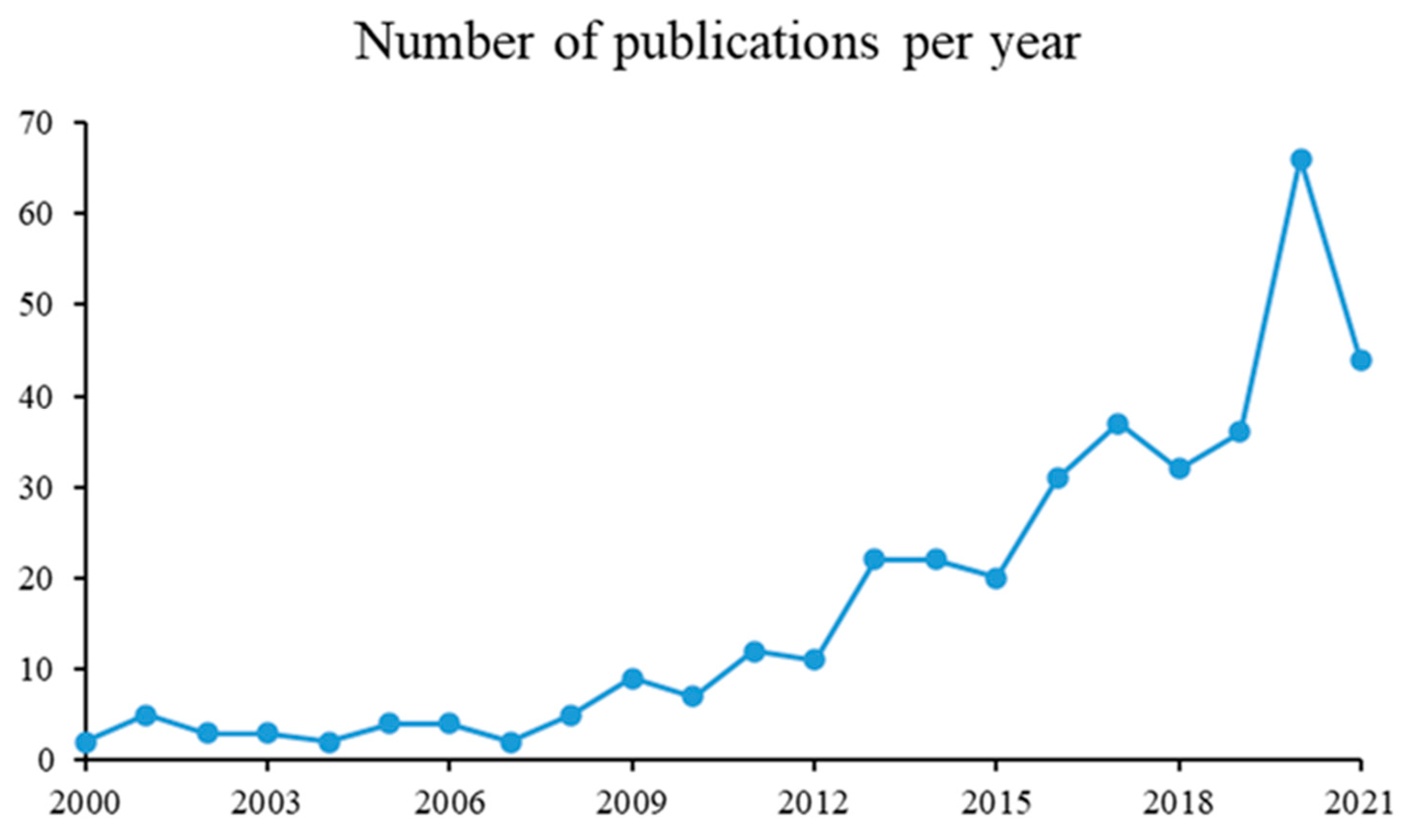
3.2. Years and Publications
3.3. Journals
3.3.1. Q1 Articles
3.3.2. Q2, Q3, and Q4 Articles
4. Discussion
4.1. Substrates Used in Microencapsulation
4.1.1. Natural Polymers
4.1.2. Synthetic Polymers
4.2. Active Ingredients
4.2.1. Liposoluble Vitamins
Vitamin A
Vitamin D
Vitamin E
4.2.2. Essential Oils
4.2.3. Polyunsaturated Fatty Acids (PUFA)
4.2.4. Structured Lipids
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, L. Functional Foods: Designer Foods, Pharmafoods, Nutraceuticals; Springer Science & Business Media: Cham, Switzerland, 2012. [Google Scholar]
- GlobeNewswire. Functional Food Market Size Worth Around USD 309 Bn by 2027 2020. Available online: https://www.globenewswire.com/news-release/2020/11/20/2130656/0/en/Functional-Food-Market-Size-Worth-Around-USD-309-Bn-by-2027.html?faodatalab=2020-11-20-1 (accessed on 25 March 2021).
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional Foods: Trends and Development. Ital. J. Food Sci. 2016, 28, 338–352. [Google Scholar]
- Murúa-Pagola, B.; Beristain-Guevara, C.I.; Martínez-Bustos, F. Preparation of starch derivatives using reactive extrusion and evaluation of modified starches as shell materials for encapsulation of flavoring agents by spray drying. J. Food Eng. 2009, 91, 380. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Okuro, P.K.; Eustáquio de Matos, F.; Favaro-Trindade, C.S. Technological challenges for spray chilling encapsulation of functional food ingredients. Food Technol. Biotechnol. 2013, 51, 171–182. [Google Scholar]
- Azimova, S.S.; Glushenkova, A.I.; Vinogradova, V.I. Lipids, Lipophilic Components and Essential Oils from Plant Sources; Springer: London, UK, 2011. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Valenzuela, B.A.; Sanhueza, C.J. Estructuracion de lipidos y sustitutos de grasas, ¿lipidos del futuro? Rev. Chil. Nutr. 2008, 35, 394–405. [Google Scholar] [CrossRef]
- Mishra, M. Handbook of Encapsulation and Controlled Release. Available online: https://books.google.com/books?id=pY7wCgAAQBAJ&pgis=1 (accessed on 23 March 2021).
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter. R. Soc. Chem. 2020, 16, 570–590. [Google Scholar]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Khan, H.; Naseem, S. Encapsulation and characterization of controlled release flurbiprofen loaded microspheres using beeswax as an encapsulating agent. J. Mater. Sci. Mater. Med. 2010, 21, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Risch, S. Encapsulation: Overview of uses and techniques. ACS Symp. Ser. 1995, 2–7. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Arici, M.; Topbas, O.; Karavana, S.Y.; Ertan, G.; Sariisik, M.; Ozturk, C. Preparation of naproxen–ethyl cellulose microparticles by spray-drying technique and their application to textile materials. J. Microencapsul. 2014, 31, 654. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Gimeno, M. An overview of the latest development of microencapsulation for agricultural products. J. Environ. Sci. Heal. Part B Pestic Food Contam. Agric. Wastes 1996, 31, 407–420. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic encapsulation technology: From microencapsulation to release into the gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef]
- Dubey, R.; Shami, T.C.; Bhasker Rao, K.U. Microencapsulation Technology and Applications. Def. Sci. J. 2009, 59, 82–95. [Google Scholar]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Marinković, S.Š.; Bezbradica, D.; Škundrić, P. Microencapsulation in the textile industry. Chem. Ind. Chem. Eng. Q. 2006, 12, 58–62. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Le, T.V.A.; Dang, N.N.; Nguyen, D.C.; Nguyen, P.T.N.; Tran, T.T.; Nguyen, Q.V.; Bach, L.G.; Thuy Nguyen Pham, T.D. Microencapsulation of Essential Oils by Spray-Drying and Influencing Factors. J. Food Qual. 2021, 2021, 5525879. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A Review of Applications in the Food and Pharmaceutical Industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Miyazawa, K.; Yajima, I.; Kaneda, I.; Yanaki, T. Preparation of a new soft capsule for cosmetics. J. Cosmet. Sci. 2000, 51, 239–252. [Google Scholar]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Tekin, R.; Bac, N.; Erdogmus, H. Microencapsulation of fragrance and natural volatile oils for application in cosmetics, and household cleaning products. Macromol. Symp. 2013, 333, 35–40. [Google Scholar] [CrossRef]
- Nguyen, P.; Vo, T.; Tran, T.; Le, T.; Mai, H.; Long, G. Encapsulation efficiency and thermal stability of lemongrass (Cymbopogon citratus) essential oil microencapsulated by the spray-drying process. Food Res. 2021, 5, 195–202. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Boi, S.; Read, J.; Gould, D.; Szewczyk, P.K.; Stachewicz, U.; Kiryukhin, M.V.; Pastorino, L.; Sukhorukov, G.B. Micro-sized “pelmeni”—A universal microencapsulation approach overview. Mater. Des. 2021, 202, 109527. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An overview of microencapsulation in the food industry: Opportunities, challenges, and innovations. Eur. Food Res. Technol. 2020, 246, 1371. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Stafussa, A.P.; de Cassia Bergamasco, R.; Madrona, G.S. Chia seed mucilage as a potential encapsulating agent of a natural food dye. J. Food Eng. 2020, 285, 110101. [Google Scholar] [CrossRef]
- Chiou, D.; Langrish, T.A.G. Development and characterisation of novel nutraceuticals with spray drying technology. J. Food Eng. 2007, 82, 84–91. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Fanger, G.O. Microencapsulation: A brief history and introduction. Microencapsulation 1974, 1–20. [Google Scholar] [CrossRef]
- Korma, S.A.; Wei, W.; Ali, A.; Abed, S.M.; Zheng, L.; Jin, Q.; Wang, X. Spray-dried novel structured lipids enriched with medium-and long-chain triacylglycerols encapsulated with different wall materials: Characterization and stability. Food Res. Int. 2019, 116, 538. [Google Scholar] [CrossRef]
- Kanakdande, D.; Bhosale, R.; Singhal, R.S. Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohydr. Polym. 2007, 67, 536–541. [Google Scholar] [CrossRef]
- Linke, A.; Weiss, J.; Kohlus, R. Oxidation rate of the non-encapsulated- and encapsulated oil and their contribution to the overall oxidation of microencapsulated fish oil particles. Food Res. Int. 2020, 127, 108705. [Google Scholar] [CrossRef]
- Santos, M.B.; Garcia-Rojas, E.E. Recent advances in the encapsulation of bioactive ingredients using galactomannans-based as delivery systems. Food Hydrocoll. 2021, 18, 106815. [Google Scholar] [CrossRef]
- Gupta, A.; Bohidar, H.B. Kinetics of phase separation in systems exhibiting simple coacervation. Phys. Rev. E Stat. Nonlinear Soft Matter. Phys. 2005, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras Castillo, C.J. Microencapsulation of propolis extract by complex coacervation. LWT Food. Sci. Technol. 2011, 44, 429. [Google Scholar] [CrossRef]
- Glomm, W.R.; Molesworth, P.P.; Sandru, E.M.; Truong, L.T.; Brunsvik, A.; Johnsen, H. Microencapsulation of peppermint oil by complex coacervation and subsequent spray drying using bovine serum albumin/gum acacia and an oxidized starch crosslinker. Appl. Sci. 2021, 11, 3956. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Jafari, S.M.; Vakili, S.; Dehnad, D. Production of a Functional Yogurt Powder Fortified with Nanoliposomal Vitamin D Through Spray Drying. Food Bioprocess Technol. Food Bioprocess Technol. 2019, 12, 1220–1231. [Google Scholar] [CrossRef]
- Link, K.C.; Schlünder, E.U. Fluidized bed spray granulation Investigation of the coating process on a single sphere. Chem. Eng. Process Process Intensif. 1997, 36, 443–457. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Dewettinck, K.; Huyghebaert, A. Fluidized bed coating in food technology. Trends Food Sci. Technol. 1999, 10, 163–168. [Google Scholar] [CrossRef]
- Boury, F.; Marchais, H.; Benoit, J.P.; Proust, J.E. Surface characterization of poly(α-hydroxy acid) microspheres prepared by a solvent evaporation/extraction process. Biomaterials 1997, 18, 125–136. [Google Scholar] [CrossRef]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control Release 2005, 102, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2004; pp. 162–164. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Lucas, J.; Ralaivao, M.; Estevinho, B.N.; Rocha, F. A new approach for the microencapsulation of curcumin by a spray drying method, in order to value food products. Powder Technol. 2020, 362, 428. [Google Scholar] [CrossRef]
- Methaapanon, R.; Kornbongkotmas, S.; Ataboonwongse, C.; Soottitantawat, A. Microencapsulation of n-octadecane and methyl palmitate phase change materials in silica by spray drying process. Powder Technol. 2020, 361, 910. [Google Scholar] [CrossRef]
- Bhandari, B.; Bansal, N.; Zhang, M.; Schuck, P. Handbook of Food Powders, Processes and Properties; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ozdemir, N.; Bayrak, A.; Tat, T.; Altay, F.; Kiralan, M.; Kurt, A. Microencapsulation of basil essential oil: Utilization of gum arabic/whey protein isolate/maltodextrin combinations for encapsulation efficiency and in vitro release. J. Food Meas. Charact. 2021, 15, 1865–1876. [Google Scholar] [CrossRef]
- Desobry Sa Netto, F.M.; Labuza, T.P. Comparison of Spray-drying, Drum-drying and Freeze-drying for b-Carotene Encapsulation and Preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Nedović, V.; Kalušević, A.; Manojlović, V.; Petrović, T.; Bugarski, B. Encapsulation Systems in the Food Industry. In Advances in Food Process Engineering Research and Applications; Springer: Boston, MA, USA, 2013; Volume 229, Available online: https://books.google.com/books?id=rxO4BAAAQBAJ&pgis=1 (accessed on 12 June 2021).
- Zhang, C.; Ada Khoo, S.L.; Chen, X.D.; Quek, S.Y. Microencapsulation of fermented noni juice via micro-fluidic-jet spray drying: Evaluation of powder properties and functionalities. Powder Technol. 2020, 361, 995. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Market Data Forecast. Latin America Food Encapsulation Market | 2021–2026 | Mexico, Brazil, Argentina, Chile. Available online: https://www.marketdataforecast.com/market-reports/latin-america-food-encapsulation-market/request-sample (accessed on 21 August 2021).
- Forero-Peñuela, L.Y.; Forero-Peñuela, Z.L.; Deschamps, C.; Alves-Porsse, A. Análisis exploratorio de las exportaciones de aceites esenciales en Brasil: Evidencia desde 2000 hasta. Idesia 2017. [CrossRef]
- Naciones Únicas—CEPAL. Perspectivas del Comercio Internacional de América Latina y el Caribe. 2020. Available online: https://www.cepal.org/es/publicaciones/46613-perspectivas-comercio-internacional-america-latina-caribe-2020-la-integracion (accessed on 24 July 2021).
- Bussineswire. Global Food Encapsulation Market (2020 to 2025)—Players Profiled Include Friesl and Campina, DSM & Ingredion Incorporated Among Others—ResearchAndMarkets.com. 2021. Available online: https://www.businesswire.com/news/home/20200407005388/en (accessed on 24 July 2021).
- Naciones Unidas. DESAFÍOS GLOBALES Población. Nac. Unidas. 2021. Available online: https://www.un.org/es/global-issues/population (accessed on 24 August 2021).
- Abhishek Parameswaran. Olive Oil Times. 2018. Available online: https://www.oliveoiltimes.com/es/business/is-india-poised-for-an-edible-oils-revolution/62877 (accessed on 24 August 2021).
- Poshadri, A.; Aparna, K. Microencapsulation technology: A review. J. Res. Angrau. 2010, 38, 86–102. [Google Scholar]
- De Jesus Freitas, T.; Assunção, L.S.; de Lima Silva, V.; Oliveira, T.S.; Conceição, I.S.; Machado, B.A.S.; Nunes, I.L.; Otero, D.M.; Ribeiro, C.D.F. Prospective study on microencapsulation of oils and its application in foodstuffs. Recent Pat. Nanotechnol. 2021, 16, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.; Sood, M.; Singh, J.; Bandral, J.D.; Gupta, N.; Bhat, A. Microencapsulation and its applications in food industry. J. Pharmacogn. Phytochem. 2019, 8, 32. [Google Scholar]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef] [PubMed]
- Scimago. Trends in Food Science and Technology Scimago. 2021. Available online: https://www.scimagojr.com/journalsearch.php?q=22475&tip=sid&clean=0 (accessed on 29 August 2021).
- Scimago. Food Research International. Scimago. 2021. Available online: https://www.scimagojr.com/journalsearch.php?q=23180&tip=sid&clean=0 (accessed on 29 August 2021).
- Scimago. International Journal of Cosmetic Science. Scimago. 2021. Available online: https://www.scimagojr.com/journalsearch.php?q=26568&tip=sid&clean=0 (accessed on 29 August 2021).
- Vijeth, S.; Heggannavar, G.B.; Kariduraganavar, M.Y. Encapsulating Wall Materials for Micro-/Nanocapsules; IntechOpen: London, UK, 2019. [Google Scholar]
- Xiao, Z.; Liu, W.; Zhu, G.; Zhou, R.; Niu, Y. A review of the preparation and application of flavour and essential oils microcapsules based on complex coacervation technology. J. Sci. Food Agric. 2014, 94, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Mali Snehal, D.; Khochage Swapna, R.; Nitalikar Manoj, M.; Magdum Chandrakant, S. Microencapsulation: A review. Res. J. Pharm. Technol. 2013, 6, 954–961. [Google Scholar]
- Huang, H.J.; Chen, X.D.; Yuan, W.K. Microencapsulation based on emulsification for producing pharmaceutical products: A literature review. Dev. Chem. Eng. Miner. Process. 2006, 14, 515–544. [Google Scholar] [CrossRef]
- Wang, R.; Tian, Z.; Chen, L. A novel process for microencapsulation of fish oil with barley protein. Food Res. Int. 2011, 44, 2735–2741. [Google Scholar] [CrossRef]
- Scremin, F.R.; Veiga, R.S.; Silva-Buzanello, R.A.; Becker-Algeri, T.A.; Corso, M.P.; Torquato, A.S.; Bittencourt, P.R.S.; Flores, E.L.M.; Canan, C. Synthesis and characterization of protein microcapsules for eugenol storage. J. Therm. Anal. 2017, 131, 653–660. [Google Scholar] [CrossRef]
- Baik, M.-Y.; Suhendro, E.L.; Nawar, W.W.; McClements, D.; Decker, E.; Chinachoti, P. Effects of antioxidants and humidity on the oxidative stability of microencapsulated fish oil. J. Am. Oil Chem. Soc. 2004, 81, 355–360. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Tamm, F.; Guadix, A.; Drusch, S. Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem. 2015, 194, 1208–1216. [Google Scholar] [CrossRef]
- Shaikh, J.; Bhosale, R.; Singhal, R. Microencapsulation of black pepper oleoresin. Food Chem. 2006, 94, 105–110. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Bigeard, F.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Influence of emulsion and powder size on the stability of encapsulated d-limonene by spray drying. Innov. Food Sci. Emerg. Technol. 2005, 6, 107–114. [Google Scholar] [CrossRef]
- Faria, A.F.; Mignone, R.A.; Montenegro, M.A.; Mercadante, A.Z.; Borsarelli, C.D. Characterization and Singlet Oxygen Quenching Capacity of Spray-Dried Microcapsules of Edible Biopolymers Containing Antioxidant Molecules. J. Agric. Food Chem. 2010, 58, 8004–8011. [Google Scholar] [CrossRef] [PubMed]
- de Barros Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Asensio, C.M.; Paredes, A.J.; Martin, M.P.; Allemandi, D.A.; Nepote, V.; Grosso, N.R. Antioxidant Stability Study of Oregano Essential Oil Microcapsules Prepared by Spray-Drying. J. Food Sci. 2017, 82, 2864–2872. [Google Scholar] [CrossRef]
- Luna-Guevara, J.; Ochoa-Velasco, C.; Hernández-Carranza, P.; Guerrero-Beltrán, J. Microencapsulation of walnut, peanut and pecan oils by spray drying. Food Struct. 2017, 12, 26–32. [Google Scholar] [CrossRef]
- Mehrad, B.; Shabanpour, B.; Jafari, S.M.; Pourashouri, P. Characterization of dried fish oil from menhaden encapsulated by spray drying. AACL Bioflux. 2015, 8, 57–69. [Google Scholar]
- Jafari, S.M.; Assadpoor, E.; Bhandari, B.; He, Y. Nano-particle encapsulation of fish oil by spray drying. Food Res. Int. 2008, 41, 172–183. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Roccia, P.; Salgado, D.; Leon, A.; Pedroza-Islas, R. Utilization of Different Wall Materials to Microencapsulate Fish Oil Evaluation of its Behavior in Bread Products. Am. J. Food Technol. 2008, 3, 384–393. [Google Scholar] [CrossRef]
- Kolanowski, W.; Ziolkowski, M.; Weißbrodt, J.; Kunz, B.; Laufenberg, G. Microencapsulation of fish oil by spray drying--impact on oxidative stability. Part 1. Eur. Food Res. Technol. 2005, 222, 336–342. [Google Scholar] [CrossRef]
- Rascón, M.P.; Bonilla, E.; García, H.S.; Salgado, M.A.; González-Arnao, M.T.; Beristain, C.I. Tg and aw as criteria for the oxidative stability of spray-dried encapsulated paprika oleoresin. Eur. Food Res. Technol. 2015, 241, 217–225. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schmidt-Hansberg, B.; Kind, M.; Schwarz, K. Process engineering parameters and type of n-octenylsuccinate-derivatised starch affect oxidative stability of microencapsulated long chain polyunsaturated fatty acids. J. Food Eng. 2009, 95, 386–392. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Dewettinck, K.; Verhé, R. Properties of oregano (Origanum vulgare L.), citronella (Cymbopogon nardus G.) and marjoram (Majorana hortensis L.) flavors encapsulated into milk protein-based matrices. Food Res. Int. 2006, 39, 413–425. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. The correlation of wall material composition with flow characteristics and encapsulation behavior of fish oil emulsion. Food Res. Int. 2012, 49, 379–388. [Google Scholar] [CrossRef]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
- Drusch, S.; Serfert, Y.; Scampicchio, M.; Schmidt-Hansberg, B.; Schwarz, K. Impact of Physicochemical Characteristics on the Oxidative Stability of Fish Oil Microencapsulated by Spray-Drying. J. Agric. Food Chem. 2007, 55, 11044–11051. [Google Scholar] [CrossRef]
- Polavarapu, S.; Oliver, C.M.; Ajlouni, S.; Augustin, M.A. Physicochemical characterisation and oxidative stability of fish oil and fish oil–extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 2011, 127, 1694–1705. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Chen, X.D.; Selomulya, C. Micro-encapsulation and stabilization of DHA containing fish oil in protein-based emulsion through mono-disperse droplet spray dryer. J. Food Eng. 2016, 175, 74–84. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol. J. Food Eng. 2015, 144, 93–102. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food. Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- de Melo Ramos, F.; Júnior, V.S.; Prata, A.S. Impact of vacuum spray drying on encapsulation of fish oil: Oxidative stability and encapsulation efficiency. Food Res. Int. 2021, 143, 110283. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Teleky, B.E.; Ranga, F.; Simon, E.; Pop, O.L.; Babalau-Fuss, V.; Kapsalis, N.; Vodnar, D.C. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. LWT 2021, 152, 112285. [Google Scholar] [CrossRef]
- Aguiar, M.C.S.; das Graças Fernandes da Silva, M.F.; Fernandes, J.B.; Forim, M.R. Evaluation of the microencapsulation of orange essential oil in biopolymers by using a spray-drying process. Sci. Rep. 2020, 10, 11799. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by spray-drying, using binary and ternary blends of gum arabic, starch and maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribes-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of Vitamins A and E as spray-dried additives for the feed industry. Molecules 2020, 25, 1357. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-F.; Liu, X.; Zhou, M.-L.; Zhang, Q.-L. Preparation of vitamin A acetate microcapsules and process optimization. J. Chem. Eng. Chin. Univ. 2019, 33, 400–409. [Google Scholar]
- Li, X.; Feng, Y.; Ting, S.; Jiang, J.; Liu, Y. Effect of processing conditions on the physiochemical properties and nutrients retention of spray-dried microcapsules using mixed protein system. CYTA J. Food 2019, 17, 25–35. [Google Scholar] [CrossRef]
- Correâ-Filho, L.C.; Lourenço, M.M.; Moldaõ-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef]
- O’Connor, G.; Krishnan, N.; Fagan-Murphy, A.; Cassidy, J.; O’Leary, S.; Robertson, B.D.; Keane, J.; O’Sullivan, M.P.; Cryan, S.A. Inhalable poly (lactic-co-glycolic acid)(PLGA) microparticles encapsulating all-trans-Retinoic acid (ATRA) as a host-directed, adjunctive treatment for Mycobacterium tuberculosis infection. Eur. J. Pharm. Biopharm. 2019, 134, 153–165. [Google Scholar] [CrossRef]
- Garcia, C.M.; Fernandez, M.; Lopez, O.D.; Castiñeira, M.; Martinez, B.; Nogueira, A.; Turiño, L. Microencapsulation of shark liver oil pool by spray drying. Lat. Am. Appl. Res. 2018, 48, 89–93. [Google Scholar] [CrossRef]
- Kosasih, E.A.; Warjito Imansyah, I.H.; Ruhyat, N. Use of a Double Condenser in a Dehumidifier with a Spray Dryer for Vitamin A Extraction in Tomato as a Heat-Sensitive Material. In AIP Conference Proceedings; AIP Publishing LLC: Anchorage, AK, USA, 2017. [Google Scholar]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Design and characterization of controlled-release vitamin A microparticles prepared by a spray-drying process. Powder Technol. 2017, 305, 411–417. [Google Scholar] [CrossRef]
- Xie, Y.L.; Zhou, H.M.; Liang, X.H.; He, B.S.; Han, X.X. Study on the morphology, particle size and thermal properties of Vitamin A microencapsulated by starch octenylsucciniate. Agric. Sci. China 2010, 9, 1058–1064. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Marathe, S.J.; Singhal, R.S. Co-encapsulation of vitamins B12 and D3 using spray drying: Wall material optimization, product characterization, and release kinetics. Food Chem. 2021, 335, 127642. [Google Scholar] [CrossRef]
- Shi, X.; Tan, T. Preparation of chitosan/ethylcellulose complex microcapsule and its application in controlled release of Vitamin D2. Biomaterials 2002, 23, 4469–4473. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Chr, P. Spray- or freeze-drying of casein micelles loaded with vitamin D2: Studies on storage stability and in vitro digestibility. LWT Food Sci. Technol. 2018, 97, 87–93. [Google Scholar] [CrossRef]
- Mahdi Jafari, S.; Masoudi, S.; Bahrami, A. A Taguchi approach production of spray-dried whey powder enriched with nanoencapsulated vitamin D3. Dry Technol. 2019, 37, 2059. [Google Scholar] [CrossRef]
- Dadkhodazade, E.; Mohammadi, A.; Shojaee-Aliabadi, S.; Mortazavian, A.M.; Mirmoghtadaie, L.; Hosseini, S.M. Yeast Cell Microcapsules as a Novel Carrier for Cholecalciferol Encapsulation: Development, Characterization and Release Properties. Food Biophys. 2018, 13, 404–411. [Google Scholar] [CrossRef]
- Wei-hong, T.; Min-chang, G.; Zhen, X.; Jie, S. Pharmacological and pharmacokinetic studies with vitamin D-loaded nanoemulsions in asthma mode. Inflammation 2014, 37, 723–728. [Google Scholar] [CrossRef]
- Górska, A.; Szulc, K.; Ostrowska-Ligęza, E.; Wirkowska, M.; Bryś, J. The influence of trehalose–maltodextrin and lactose–maltodextrin matrices on thermal and sorption properties of spray-dried β-lactoglobulin–vitamin D 3 complexes. J. Therm. Anal. Calorim. 2013, 112, 429–436. [Google Scholar] [CrossRef]
- Budinčić, J.M.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; You, Y.J.; Quek, S.Y.; Wu, W.D.; Chen, X.D. Interplaying Effects of Wall and Core Materials on the Property and Functionality of Microparticles for Co-Encapsulation of Vitamin E with Coenzyme Q Food Bioprocess Technol. Food Bioprocess. Technol. 2020, 13, 705–721. [Google Scholar] [CrossRef]
- Surini, S.; Khotima, N.H. Stability study of ethylcellulose coated-tocotrienol microcapsules prepared by solvent evaporation and spray drying techniques. Int. J. Appl. Pharm. 2020, 12, 197–201. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation of a starch-based carrier for oral delivery of Vitamin E to the small intestine. Food Hydrocoll. 2019, 91, 26–33. [Google Scholar] [CrossRef]
- Huang, E.; Quek, S.Y.; Fu, N.; Wu, W.D.; Chen, X.D. Co-encapsulation of coenzyme Q10 and vitamin E: A study of microcapsule formation and its relation to structure and functionalities using single droplet drying and micro-fluidic-jet spray drying. J. Food Eng. 2019, 247, 45–55. [Google Scholar] [CrossRef]
- Selamat, S.N.; Mohamad, S.N.H.; Muhamad, I.I.; Khairuddin, N.; Md Lazim, N.A. Characterization of Spray-Dried Palm Oil Vitamin E Concentrate. Arab. J. Sci. Eng. 2018, 43, 6165–6169. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Kaban, J.; Zulmi, R. Microencapsulation of Vitamin E from palm fatty acid distillate with galactomannan and gum acacia using spray drying method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012095. [Google Scholar] [CrossRef]
- Gangurde, A.B.; Ali, M.T.; Pawar, J.N.; Amin, P.D. Encapsulation of vitamin E acetate to convert oil to powder microcapsule using different starch derivatives. J. Pharm. Investig. 2017, 47, 559–574. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Anandharamakrishnan, C. Enhancement of oral bioavailability of vitamin E by spray-freeze drying of whey protein microcapsules. Food Bioprod. Process. 2016, 100, 469–476. [Google Scholar]
- Hategekimana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Encapsulation of vitamin E: Effect of physicochemical properties of wall material on retention and stability. Carbohydr. Polym. 2015, 124, 172–179. [Google Scholar] [CrossRef]
- Ikematsu, Y.; Uchida, S.; Namiki, N. Preparation and evaluation of orally disintegrating tablets containing vitamin e as a model fat-soluble drug. Chem. Pharm. Bull. 2015, 63, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control Release 2010, 146, 276. [Google Scholar] [CrossRef] [PubMed]
- National Coordinating Committee on Food and Nutrition. Vitamin A. Recommended Nutrition Intakes Malaysia. 2005; pp. 111–120. Available online: https://www.moh.gov.my/moh/images/gallery/rni/insert.pdf (accessed on 6 August 2021).
- Vilanova, N.; Solans, C. Vitamin A, Palmitate-B-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Shah, N.P. Microencapsulation of vitamins. Int. Food Res J. 2007, 14, 1–14. [Google Scholar]
- Yoshida, K.; Sekine, T.; Matsuzaki, F.; Yanaki, T.; Yamaguchi, M. Stability of vitamin A in oil-in-water-in-oil-type multiple emulsions. J. Am. Oil Chem. Soc. 1999, 76, 1–6. [Google Scholar] [CrossRef]
- Bartilucci, A.; Foss, N.E. Cyanocobalamin (Vitamin B12). J. Am. Pharm. Assoc. 1954, 43, 159. [Google Scholar] [CrossRef]
- Romo-Hualde, A.; Yetano-Cunchillos, A.I.; González-Ferrero, C.; Sáiz-Abajo, M.J.; González-Navarro, C.J. Supercritical fluid extraction and microencapsulation of bioactive compounds from red pepper (Capsicum annum L.) by-products. Food Chem. 2012, 133, 1045. [Google Scholar] [CrossRef]
- Zanuy, M.V.; Carranza, F.H. Metabolismo, fuentes endógenas y exógenas de vitamina D. Rev. Española Enfermedades Metabólicas Óseas 2007, 16, 63–70. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Conti, M.; Patrono, D.; Mancini, R.; Pession, A.; Puggioli, C.; Bersani, G. Potential intake of vitamins “A” and “D” through branded intravenous lipid emulsions: Liquid Chromatography-Tandem Mass Spectrometry Analysis. Clin. Nutr. 2017, 36, 530. [Google Scholar] [CrossRef]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2019, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Blumberg, J.B. Vitamin E and the Immune Response. In Nutrient Modulation of the Immune Response; CRC Press: Boca Raton, FL, USA, 2020; pp. 223–238. [Google Scholar]
- Constantinou, C.; Charalambous, C.; Kanakis, D. Vitamin E and cancer: An update on the emerging role of γ and δ tocotrienols. Eur. J. Nutr. 2020, 59, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.F.; Rossi, G.C.M.; Campagna, G.; Capobianco, D.; Costagliola, C.; Qualicos Study Group. Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma—A Preliminary Study. Molecules 2020, 25, 5614. [Google Scholar] [CrossRef] [PubMed]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s disease: The mediating role of cellular aging. Aging Clin. Exp. Res. 2020, 32, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the >control of postharvest diseases of fresh horticultural produce. Postharvest. Biol. Technol. 2016, 122, 41. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.; et al. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef]
- Veiga RDSDa Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Chraibi, S.; Rosière, R.; Larbanoix, L.; Gérard, P.; Hennia, I.; Laurent, S.; Vermeersch, M.; Amighi, K.; Wauthoz, N. The combination of an innovative dry powder for inhalation and a standard cisplatin-based chemotherapy in view of therapeutic intensification against lung tumours. Eur. J. Pharm. Biopharm. 2021, 164, 93–104. [Google Scholar] [CrossRef]
- Nafee, N.; Gaber, D.M.; Elzoghby, A.O.; Helmy, M.W.; Abdallah, O.Y. Promoted Antitumor Activity of Myricetin against Lung Carcinoma Via Nanoencapsulated Phospholipid Complex in Respirable Microparticles. Pharm. Res. 2020, 37, 1–24. [Google Scholar] [CrossRef]
- Khan, I.; Lau, K.; Bnyan, R.; Houacine, C.; Roberts, M. A Facile and Novel Approach to Manufacture Paclitaxel-Loaded Proliposome Tablet Formulations of Micro or Nano Vesicles for Nebulization. Pharm. Res. 2020, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Meola, T.R.; Paxton, K.; Joyce, P.; Schultz, H.B.; Prestidge, C.A. The effect of drug ionization on lipid-based formulations for the oral delivery of anti-psychotics. ADMET DMPK 2020, 8, 437–451. [Google Scholar] [PubMed]
- Dening, T.J.; Joyce, P.; Kovalainen, M.; Gustafsson, H.; Prestidge, C.A. Spray Dried Smectite Clay Particles as a Novel Treatment against obesity. Pharm. Res. 2018, 36, 21. [Google Scholar] [CrossRef] [PubMed]
- Meola, T.R.; Abuhelwa, A.Y.; Joyce, P.; Clifton, P.; Prestidge, C.A. A safety, tolerability, and pharmacokinetic study of a novel simvastatin silica-lipid hybrid formulation in healthy male participants. Drug Deliv. Transl. Res. 2021, 11, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Momordica, D. Antidiabetic, hypolipidemic, antioxidant and anti-inflammatory effects of Momordica charantia L. foliage extract. J. Pharm. Pharmacogn. Res. 2021, 9, 537–548. [Google Scholar]
- Rodrigues, S.; Cunha, L.; Kollan, J.; Neumann, P.R.; da Costa, A.M.R.; Dailey, L.A.; Grenha, A. Cytocompatibility and cellular interactions of chondroitin sulfate microparticles designed for inhaled tuberculosis treatment. Eur. J. Pharm. Biopharm. 2021, 163, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dormenval, C.; Lokras, A.; Cano-Garcia, G.; Wadhwa, A.; Thanki, K.; Rose, F.; Thakur, A.; Franzyk, H.; Foged, C. Identification of factors of importance for spray drying of small interfering RNA-loaded lipidoid-polymer hybrid nanoparticles for inhalation. Pharm. Res. 2019, 36, 1–15. [Google Scholar] [CrossRef]
- Wijayadi, L.J.; Rusli, T.R. Encapsulated Lime Peel Essential Oil (Citrus hystrix) into Chitosan Nanoparticle: New entity to enhanced effectivity against Propionil bacterium acne in vitro. IOP Conf. Ser. Mater. Sci. Eng. 2020, 852, 012016. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer pâté. LWT 2020, 125, 109223. [Google Scholar] [CrossRef]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Shahidi Noghabi, M.; Molaveisi, M. Microencapsulation optimization of cinnamon essential oil in the matrices of gum Arabic, maltodextrin, and inulin by spray-drying using mixture design. J. Food Process Eng. 2020, 43, 1–13. [Google Scholar] [CrossRef]
- Adamiec, J.; Kalemba, D. Analysis of microencapsulation ability of essential oils during spray drying. Dry Technol. 2006, 24, 1127–1132. [Google Scholar] [CrossRef]
- Thuong Nhan, N.P.; Tan Thanh, V.; Huynh Cang, M.; Lam, T.D.; Cam Huong, N.; Hong Nhan, L.T.; Thanh Truc, T.; Tran, Q.T.; Bach, L.G. Microencapsulation of lemongrass (Cymbopogon citratus) essential oil via spray drying: Effects of feed emulsion parameters. Processes 2020, 8, 40. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; de Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1. [Google Scholar] [CrossRef]
- Hashim, N.A.; Abdul Mudalip, S.K.; Sulaiman, S.Z.; Md Shaarani, S. Nutritional values and microencapsulation techniques of fish oil from different sources: A mini review. Mater. Today Proc. 2021, 42, 222–228. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868. [Google Scholar] [CrossRef]
- Damerau, A.; Ogrodowska, D.; Banaszczyk, P.; Dajnowiec, F.; Tańska, M.; Linderborg, K.M. Baltic herring (Clupea harengus membras) oil encapsulation by spray drying using a rice and whey protein blend as a coating material. J. Food Eng. 2022, 314, 110769. [Google Scholar] [CrossRef]
- Copado, C.N.; Julio, L.M.; Diehl, B.W.K.; Ixtaina, V.Y.; Tomás, M.C. Multilayer microencapsulation of chia seed oil by spray-drying using electrostatic deposition technology. LWT 2021, 152, 112206. [Google Scholar] [CrossRef]
- Jia, C.; Huang, S.; Liu, R.; You, J.; Xiong, S.; Zhang, B.; Rong, J. Storage stability and in-vitro release behavior of microcapsules incorporating fish oil by spray drying. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127234. [Google Scholar] [CrossRef]
- Yang, W.; Shan, Z. Application of wool keratin: An anti-ultraviolet wall material in spray drying. J. Food Sci. Technol. 2021, 58, 4235–4244. [Google Scholar] [CrossRef]
- Castejón, N.; Luna, P.; Señoráns, F.J. Microencapsulation by spray drying of omega-3 lipids extracted from oilseeds and microalgae: Effect on polyunsaturated fatty acid composition. LWT 2021, 148, 111789. [Google Scholar] [CrossRef]
- Cui, T.; Chen, C.; Jia, A.; Li, D.; Shi, Y.; Zhang, M.; Bai, X.; Liu, X.; Liu, C. Characterization and human microfold cell assay of fish oil microcapsules: Effect of spray drying and freeze-drying using konjac glucomannan (KGM)-soybean protein isolate (SPI) as wall materials. J. Funct. Foods 2021, 83, 104542. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, Y.; Wen, J.; Quek, S.Y. A Comparison of Microfluidic-Jet Spray Drying, Two-Fluid Nozzle Spray Drying, and Freeze-Drying for Co-Encapsulating β-Carotene, Lutein, Zeaxanthin, and Fish Oil. Foods 2021, 10, 1522. [Google Scholar] [CrossRef]
- Yu, F.; Xue, C.; Zhang, Z. Mechanical characterization of fish oil microcapsules by a micromanipulation technique. LWT 2021, 144, 111194. [Google Scholar] [CrossRef]
- Paulo, B.B.; Alvim, I.D.; Reineccius, G.; Prata, A.S. Barrier properties of spray-dried emulsions containing flavorings or unsaturated triglycerides. LWT 2021, 142, 111040. [Google Scholar] [CrossRef]
- Encina, C.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Vergara, C.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Hydroxypropyl-inulin as a novel encapsulating agent of fish oil by conventional and water-free spray drying. Food Hydrocoll. 2021, 113, 106518. [Google Scholar] [CrossRef]
- Linke, A.; Weiss, J.; Kohlus, R. Impact of the oil load on the oxidation of microencapsulated oil powders. Food Chem. 2021, 341, 128153. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, L.; McClements, D.J.; Wang, X.; Ye, J.; Liu, C. Spray drying and rehydration of macadamia oil-in-water emulsions: Impact of macadamia protein isolate to chitosan hydrochloride ratio. Food Chem. 2021, 342, 128380. [Google Scholar] [CrossRef] [PubMed]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Ahmed, I.A.M.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, S.; Berdejo, D.; Pagán, E.; García-Gonzalo, D.; Pagán, R. Modified cyclodextrin type and dehydration methods exert a significant effect on the antimicrobial activity of encapsulated carvacrol and thymol. J. Sci. Food Agric. 2021, 101, 3827–3835. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; da Rocha, M.G.; Lima, L.R.; Cunha, A.P.; de Oliveira, J.S.; de Andrade, A.R.C.; Ricardo, N.M.P.S.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Rocha, M.F.G.; et al. Essential oils encapsulated in chitosan microparticles against Candida albicans biofilms. Int. J. Biol. Macromol. 2021, 166, 621–632. [Google Scholar] [CrossRef]
- Umaña, M.; Wawrzyniak, P.; Rosselló, C.; Llavata, B.; Simal, S. Evaluation of the addition of artichoke by-products to O/W emulsions for oil microencapsulation by spray drying. LWT 2021, 151, 112146. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Greque, L.; Santos, M.D.F.C.; Novais, L.M.R.; D’Oca, C.D.R.M.; Prentice, C.; Salas-Mellado, M.D.L.M. Effect of the spray drying conditions on the physicochemical and structural characteristics and the stability of chia oil microparticles. J. Appl. Polym. Sci. 2021, 138, 1–14. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Pérez-Pérez, N.C.; Villalobos-Castillejos, F.; Alamilla-Beltrán, L.; Tovar-Benítez, T. Influence of Sechium edule starch on the physical and chemical properties of multicomponent microcapsules obtained by spray-drying. Food Biosci. 2021, 43, 101275. [Google Scholar] [CrossRef]
- Partheniadis, I.; Vergkizi, S.; Lazari, D.; Reppas, C.; Nikolakakis, I. Formulation, characterization and antimicrobial activity of tablets of essential oil prepared by compression of spray-dried powder. J. Drug Deliv. Sci. Technol. 2019, 50, 226. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Z.; Xie, Y.; Ma, A.; Zhang, H.; Rao, P.; Wang, Q. Synthesis, physicochemical properties, and health aspects of structured lipids: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 759–800. [Google Scholar] [CrossRef]
- Longhi, R. Trans Fatty Acid in the Liver and Central Nervous System. In Dietary Interventions in Liver Disease; Ronald, R.W., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 275–286. [Google Scholar]
- Lee, Y.-Y.; Tang, T.-K.; Tan, C.-P.; Alitheen, N.B.M.; Phuah, E.-T.; Ab Karim, N.A.; Lai, O.-M. Entrapment of Palm-Based Medium- and Long-Chain Triacylglycerol via Maillard Reaction Products. Food Bioprocess Technol. 2015, 8, 1571–1582. [Google Scholar] [CrossRef]
- Baldim, I.; Souza, C.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Oliveira, W. Spray-dried structured lipid carriers for the loading of rosmarinus officinalis: New nutraceutical and food preservative. Foods 2020, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhang, G.; Li, C.; Liu, L.; Sun, L.; Liu, N.; Li, X. Characteristic of microencapsulated 1,3-dioleoyl-2-palmitoylglycerol and its application in infant formula powder. Int. J. Food Prop. 2018, 21, 2355–2365. [Google Scholar] [CrossRef]
- Wei, W.; Li, C.; Zhang, W.; Liu, N. Strengthening oxidative stability of the infant liquid milk by structured lipid microcapsules. J. Chin. Inst. Food Sci. Technol. 2014, 14, 180–189. [Google Scholar]
- Nagachinta, S.; Akoh, C.C. Spray-dried structured lipid containing long-chain polyunsaturated fatty acids for use in infant formulas. J. Food Sci. 2013, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nagachinta, S.; Akoh, C.C. Synthesis of structured lipid enriched with omega fatty acids and sn -2 palmitic acid by enzymatic esterification and its incorporation in powdered infant formula. J. Agric. Food Chem. 2013, 61, 4455–4463. [Google Scholar] [CrossRef]



| Encapsulating Agent | Reference |
|---|---|
| Barley protein | [83] |
| Carrageenan, rice bran | [84] |
| Corn syrup: Sodium caseinate: Lecithin | [85] |
| Glucose syrup | [86] |
| Gum arabic and modified starch | [87] |
| Gum arabic and modified starch (CAPSUL and HI-CAP 100) | [88] |
| Gum Arabic or maltodextrin 20 dextrose equivalent | [89] |
| Gum arabic/starch/maltodextrin/inulin | [90] |
| Hydroxypropyl methyl cellulose, maltodextrin, and, colloidal silicon dioxide | [91] |
| Maltodextrin | [92] |
| Maltodextrin: Fish gelatin: k carragenan | [93] |
| Maltodextrin: n-OSA starch:Whey protein concentrate | [94] |
| Methylcellulose: Calcium-gelatin casein: Whey protein concentrate Maltodextrin Soy lecithin | [95] |
| Methylcellulose: Maltodextrin: Lecithin | [96] |
| Modified starch (Capsul®) | [97] |
| n-OSA starch: Glucose syrup | [98] |
| Powder milk (SMP) and whey protein concentrate (WPC) | [99] |
| Skim milk powder: Whey protein concentrate: Whey protein isolate: Milk protein concentrate: Sodium caseinate | [100] |
| Soy fiber: Maltodextrin: Hydroxypropyl bcyclodextrin: n-OSA starch | [101] |
| Sugar beet pectin: Glucose syrup | [102] |
| Sugar beet pectin: Glucose syrup | [103] |
| Whey protein and maltodextrin | [104] |
| Whey protein isolate | [105] |
| Zein/casein complex | [106] |
| Encapsulated Material | Encapsulation Efficiency | Essential Oil, Lipids or Lipidic Compound | Encapsulation Conditions | Principal Results | Reference |
|---|---|---|---|---|---|
| Gum arabic, maltodextrin and starch | 88–98% | Vitamin A | In the encapsulation process, 150 °C was established as the inlet temperature. Air pressure and aspiration rate were set to 5–6 bar and 100% (36 m3/h), respectively. | It is possible to prove the viability and integrity of the vitamin A particles produced in the current work, which revealed great encapsulation efficiency values and, at the same time, a total release of the active compound when placed in the proper medium. | [111] |
| Capsul-CAP®, sodium caseinate-SC in combination with Tween 80 (TW) as an emulsifier and maltodextrin (MD) | Vitamin A: 23–100%; Vitamin E: 29–48% | Vitamin E and Vitamin A | Nozzle air flow-rate of 1.052 m3/h and aspiration of 80% (32 m3/h). The inlet and outlet temperatures were 120 ± 1 °C and 74 ± 1 °C, respectively. | The proposed encapsulation methodology is, therefore, a feasible alternative for the stabilization of vitamin A and E and protection against oxidation processes in the feed manufacturing industry. | [112] |
| Gelatin | - | Vitamin A acetate (VA) | Inlet temperature = 120–140 °C, feeding rate = 20 mL/min and solid content = 25–30%. | The optimized emulsion conditions are: mass ratio of gelatin: VA = 4:1; emulsion temperature = 60 °C, emulsion pH = 4.5, emulsion time = 40 min and shear rate = 10,000 r/min. | [113] |
| Sodium caseinate and pea protein | 95–98% | Vitamin A | The inlet air temperature of the dryer was set for five different levels from 187 °C to 127 °C, and the outlet temperature was at 80 °C. The targeted moisture content of final spray-dried powder was set at 2.5%. | The results also indicated a potential inherent correlation between properties of liquid emulsion and powdered microcapsules. On the other hand, a lower spray drying inlet temperature at 127 °C increased the moisture content and water activity and decrease the glass transition temperature of spray-dried powders, which consequently resulted in powder caking and nutrients degradation. | [114] |
| Arabic gum | 5.1 and 33.9% | β-carotene (Precursor or vitamin A) | The drying air flow rate was set at 47 m3/h. The feed solution was kept under magnetic stirring. The pressure of the compressed air set at 1.7 bar and had a maximum flow rate of 73 m3/h. The inlet temperature ranged between 110 and 200 °C. | The drying inlet temperature of 173 °C and the Arabic gum concentration of 11.9% were those that allow obtaining higher β-carotene content, higher encapsulation efficiency, and higher drying yield. | [115] |
| Poly(D,L-Lactide–co-glycolide) (PLGA) | 70.5 ± 2.3% | Metabolite of vitamin A | The spray dryer was operated using Nitrogen at 670 L/h (55 mm), an aspirator rate of 100%, an inlet temperature of 50 °C and a solution feed rate of 4 mL/min (15%). | The results also show the benefit of all-trans-retinoic acid (ATRA) as a practical treatment post-infection, and high light the importance of appropriate nutrition in host-protective immune responses to tuberculosis disease. | [116] |
| Gum arabic and maltodextrin | 44.1% | Vitamin A (shark liver oil) | Inlet temperature, 150 °C; outlet temperature, 90 °C; air flow rate, 600 L/h; and drying air flow rate, 60 m3/h. | Best encapsulation efficiency and moisture content for its conservation, the combination of gum Arabic and maltodextrin, as encapsulation agents, should be maintained at 47% and 23%, respectively. | [117] |
| Maltodextrin | - | Vitamin A | Inlet temperature: 90–120 °C | Spray drying, combined with a dehumidifier and a double condenser to test vitamin A concentrations in a mixture of tomato juice and maltodextrin, can be operated up to a temperature of 90 °C. | [118] |
| Arabic gum | - | Vitamin A (Retinol) | The air and solution flow rates, air pressure, inlet and outlet temperature were set at 35 m3/h (90%), 3–6 mL/min (between 10 and 20%), 5–6 bar, around 150 °C and around 88 °C | Vitamin A release assays showed that the usage of 2, 5 and 10% (w/v) of Arabic gum do not ensure an efficient protection and stabilization of vitamin A. It was necessary to increase the encapsulating agent concentration until 15 and 20% in order to obtain the release of initial amount of vitamin A used in the assays. | [119] |
| HI-CAP 100 (starch octenylsucciniate, OSA-starch) | 96.38% | Vitamin A | The emulsions were spray-dried at a feed rate of 1000 mL/min. The optimum air inlet and outlet temperature were 182 and 82 °C, respectively | Vitamin A microcapsules produced with HI-CAP 100 exhibited spherical shapes with characteristic dents, which was attributed to drying and cooling solidification involved during spray-drying. The vibrating frequency of the centrifugal granulation had effect on the particle size distribution of microcapsules (p < 0.05). | [120] |
| Encapsulanting Agent | Encapsulation Efficiency | Essential Oil, Lipids or Lipidic Compound | Encapsulation Conditions | Principal Results | Reference |
|---|---|---|---|---|---|
| Gum acacia: Hi-Cap® 100: maltodextrin = 38:60:2 | 77–93% | Vitamin D3 | The aspirator and feed rate set at 1400 rpm and 20 rpm, respectively, and compressed air flow pressure was adjusted to 2 bar. | A wall material combination of 38:60:2 ratio of gum acacia, Hi-Cap® 100, and maltodextrin showed best physico functional properties for co-encapsulation of vitamins B12 and D3 as seen from the physico functional parameters such as entrapment efficiency, total encapsulation efficiency, and process efficiency of the microcapsules as well as the storage and thermal stability of the vitamins entrapped therein. | [121] |
| Chitosan/ethylcellulose | 95% | Vitamin D2 | Solutions were spray-dried at a feed rate of 5 mL/min. The air inlet temperature was 168 °C and pressure 0.38 MPa. | The drug loading of this system was more than 86%. | [122] |
| Casein micelles (CM) | 88% | Vitamin D2 | The inlet air temperature was 180 °C and the outlet air temperature was 80 °C. The suspension was pumped with a gear pump which operated at 285–410 rpm. between 191 and 203 g/min, approximately 6.7 kg per minute. | The recovery rates for Vit. D2 were 76% (spray-dried powders), The Vit. D2 content stayed constant in all powders during four months of storage, 90% of the Vit. D2 added as encapsulated product in dried CM remained active after in vitro proteolysis. | [123] |
| Maltodextrin (MD), gum Arabic (GA), modified starch (MS), and whey protein concentrate (WPC) | 96.4% | Vitamin D3 | Air gauge pressure was kept at 0.06 MPa and air flow rate at 73 m3/h. The inlet temperature was set at four different levels (160, 170, 180, and 190 °C) and outlet temperature at 80 ± 5 °C. | Vitamin D3 was encapsulated into nanoliposomes and then formulated by biopolymers including MD, GA, MS, and WPC. Finally, the produced feed solutions were turned into powders through spray drying. The results showed that the inlet air temperature and carrier agents had a significant effect on whey powder characteristics loaded with nanoliposomal vitamin D3. | [124] |
| Milk protein concentrate, modified starch content, gum Arabic and maltodextrin | - | Vitamin D | Feed mixture was atomized from the nozzle into a vertical co-current drying chamber with 2 m height, while the hot air flowrate, atomizing air pressure and outlet air temperature were fixed at 550 L/h, 0.3 bar and 90 °C. For all experiments, the type and concentration of drying air as well as inlet air temperature (160, 170, 180 or 190 °C) were independent variables. | Properties of yogurt powders fortified with encapsulated vitamin D are significantly dependent on drying conditions and feed mix ture, and achieving favorable properties of fortified yogurt powders is made possible through optimization of independent variables. | [50] |
| Saccharomyces cerevisiae yeast cells | - | Vitamin D | Inlet temperature 130 °C, outlet temperature 75–77 °C, feed flow rate 6.08 mL/min, nozzle diameter 0.7 mm, dry air flow rate 568 L/h, aspirator 90% and pump rate 25%. | Yeast based microencapsulation technique was used success-fully for encapsulation of cholecalciferol. The Saccharomyces cerevisiae yeast cell microcapsules could serve as a novel carrier for encapsulation of cholecalciferolin order to increase its bioavailability for using in food and pharmaceutical industries. | [125] |
| Ovalbumin | - | Vitamin D | Inlet temperature 60 °C, outlet temperature 35 °C, aspiration 85%, feeding rate of the suspension 5 mL/min. | The result revealed that VD-loaded nanoemulsions (VDNM) led to an improvement in oral bioavailability (BA) of Vitamin D in amurine ovalbumin-induced asthma model. These data provided an important proof that VDNM might be a new potential therapy for the management of asthma in humans. | [126] |
| Trehalose–maltodextrin and lactose–maltodextrin | - | Vitamin D3 | The operational conditions of the spray drying were air inlet temperature: 120 °C and flowrate: 51.4 mL/min. | β-lactoglobulin (β-LG) has been reported to be capable of binding a variety of fat-soluble ligands, including vitamin D3. The importance of the binding property is that it is possible to deliver vitamin D3 using β-LG as a carrier without the presence of the fat in which it normally associates. | [127] |
| Encapsulating Agent | Encapsulation Efficiency | Essential Oil, Lipids or Lipidic Compound | Encapsulation Conditions | Principal Results | Reference |
|---|---|---|---|---|---|
| Chitosan and sodium lauryl ether sulfate (SLES) | 73% | Vitamin E | Aspiration (0.6 m3/min) and feeding (2.2 mL/min). Inlet temperature 160 °C and the outlet temperature 100 °C. | The use of aldehydes as cross-linking agents and found that chitosan/SLES complex can be used as wall material for the microencapsulation of hydrophobic active molecules in cosmetic industry. | [128] |
| Whey protein isolate (WPI), WPI/soluble corn fiber (SCF), and WPI/maltodextrin | 87.4 and 91.0% | Vitamin E with coenzyme Q10 | Nozzle 100-μm and spray drying temperature was 190 and 90 °C for the inlet and outlet, respectively. | The composition and property of wall material governed most powder properties and influenced some important functionalities such as proneness to digestion-induced disintegration. Core material impacted on particle morphology and color and played a key role on stabilizing powder functionalities during storage. | [129] |
| Ethylcellulose (EC) | 21.60 and 99.75%. | Tocotrienol (vitamin E compound) | Inlet temperature 80–90 °C, outlet temperature 70–80 °C; feed flow 5 mL/min; pressure 3 bar. | The microencapsulation of tocotrienol with EC using SE (Solvent Evaporation) and spray drying techniques produced a solid form of tocotrienol that was considerably more stable than the natural form of tocotrienol. | [130] |
| Capsul-CAP®, sodium caseinate-(SC) in combination with tween 80 (TW) as an emulsifier and maltodextrin (MD) | Vitamin A: 23–100%; Vitamin E: 29–48% | Vitamin E and vitamin A | Nozzle air flow-rate of 1.052 m3/h and aspiration of 80% (32 m3/h). The inlet and outlet temperatures were 120 ± 1 °C and 74 ± 1 °C, respectively. | The proposed encapsulation methodology is therefore, a feasible alternative for the stabilization of vitamin A and E and protection against oxidation processes in the feed manufacturing industry. | [112] |
| Carboxymethyl starch (H-CMS) and xanthan gum (XG) | 57–67% | Vitamin E | The inlet and outlet temperatures of spray-drying were 190 ± 5 °C and 80 ± 5 °C, respectively. | H-CMS may be used to construct a pH-sensitive functional 399 factor delivery system, which further expands its practical application and has a certain guiding 400 significance for the use of starch in the production of value-added products. | [131] |
| OSA (octenyl succinic anhydride) modified starch (HICAP100) | 98–99% | Coenzyme Q10 (CoQ10) and vitamin E (VE) | Inlet/outlet temperatures of 160/70 and 190/90 °C, respectively. Airflow rate was set at 250 L/min, with a feed rate of 1.5 mL/min. | The CoQ10 and VE retention, antioxidant capacities and color of the microcapsules were relatively stable when spray-dried at 190 °C than at 160 °C. | [132] |
| Maltodextrin and sodium caseinate | 60–71% | Vitamin E | Inlet temperature 110 °C, air pressure 55 kgf/cm2, and atomizer speed 20,000–25,000 rpm, nozzle 1.5 mm. | The best core/wall ratio obtained in this experiment is 1.0 for its efficiency and physical characteristic although it showed the tendency of agglomeration. | [133] |
| Gum acacia (GA) and mixed of galactomannan from Arenga pinnata (GAP) with GA | 60–70% | Vitamin E | Initial temperature 70 °C for 15 min. Inlet temperature (180–200 °C). | The increment of GAP decreasing moisture content and the particle size from 16 μm to 11 μm, the yield of microcapsule, encapsulation efficiency, the amount of vitamin E absorbed and oxidation stability of vitamin E were increased. | [134] |
| Cremophore RH 40, tween 80, maltodextrin, OSA-modified starches (Capsul and Hicap100) | 53–63% | Vitamin E acetate | Inlet and outlet temperatures were 110–130 °C and 55–60 °C, feed rate 1–5 mL min−1, atomization air pressure 2–3 kg cm−2 and aspiration rate 40–45%. | The microcapsules packed in amber colored glass bottles exhibited no significant change in moisture content and drug content indicated microcapsules were stable for 3 months at accelerated conditions. | [135] |
| Whey protein | 89% | Vitamin E | Inlet and outlet temperatures 100 °C and 80 °C, respectively. The feed liquid flow rate 4 mL/min. | It was demonstrated that pharmacokinetic parameters were improved using the spray freeze drying technique over that of spray drying and freeze-drying techniques. The spray freeze-dried vitamin E microcapsules were able to increase the oral bioavailability by 1.13 and 1.19-fold compared to spray dried, and freeze-dried microcapsules respectively. Thus, this study indicated that spray freeze drying technique could be potentially employed for encapsulating poorly water-soluble bioactive compounds. | [136] |
| Octenyl succinic anhydride (OSA) modified starches | - | Vitamin E | Inlet and outlet temperatures were 150 °C and 85 °C respectively, feed rate 10 mL/min. | This study might be useful to service providers interested in delivering Vitamin E in form of nanocapsules in identifying appropriate modified starch to act as emulsifier and wall material. | [137] |
| Hydrolyzed gelatin | - | Vitamin E (VE) (d-α-tocopheryl acetate and d-α-tocopheryl acid succinate) | Inlet temperature 200 °C, outlet temperature 100 °C, and the feeding rate 1.5 mL/min. | Tablet porosity of 30 to 35% and tensile strength of 7 kg/cm2 or greater are required for VE orally desintegrating tablets (ODTs) to rapidly disintegrate and have sufficient strength. It has also been demonstrated that, for the addition of VE, VE spray drying granules of small particle size and powder VE are the most suitable. | [138] |
| Encapsulated Material | Encapsulation Efficiency | Essential Oil, Lipids or Lipidic Compound | Encapsulation Conditions | Principal Results | Reference |
|---|---|---|---|---|---|
| Rice and whey protein | 40–50% | Baltic herring (BH) oil | Inlet air temperature in the range of 123–129 °C, and outlet temperature in the range of 72–78 °C. | Production of emulsions with BH oil and whey protein concentrate and rice protein concentrate (RPC) mixture as wall material components, resulted in stable emulsions with relatively small droplet size and large dispersion. However, while RPC was shown to either agglomerate or stay non-dissolved at pH 3, but surprisingly, at pH 3, the most stable emulsion was obtained. | [179] |
| Hydrolyzed sunflower lecithins, chitosan and chia mucilage | 84.11–99.37% | Chia seed oil | Feed rate of 0.6 L/h, and air inlet/outlet temperatures of 170 and 75 °C, respectively | Chia oil microcapsules with appropriate physicochemical stability were obtained through the spray drying of multilayer emulsions pre-pared using adequate electrostatic deposition by the layer by layer technique. A high microencapsulation efficiency was obtained, suggesting that the type and concentration of wall materials were suitable in trapping and containing the lipid nucleus. | [180] |
| Octenyl succinic anhydride–linked starch (OSA-S) and maltodextrin (MD) | Not reported | Fish oil | Inlet air temperature 180–190 °C, outlet air temperature 80–90 °C, feeding speed 20 mL/min, and atomizer speed 200–300 r/min | The microcapsules were not resistant to acid treatment and had a lower oxidation rate in neutral condition. Moreover, the results of in vitro digestion investigations showed that the fish oil microcapsules were easily dissolved and released in simulated gastric fluid, which was also confirmed with confocal laser scanning microscopy (CLSM). | [181] |
| Low-molecular-weight keratin (LMWK) | Not reported | Fish oil | The diameter of the feed nozzle was 0.75 mm and the air pressure was 0.6 bar. The inlet and outlet temperatures were 175 and 80 °C, respectively. | In the present work, LMWK was successfully applied as part of the wall material during the spray drying process for fish oil encapsulation. Under the same drying conditions microcapsules containing LWMK illustrated lower moisture content and higher encapsulation efficiency and anti-ultraviolet capability. The beneficial effects of LMWK were enhanced with increasing proportionality. | [182] |
| Sodium caseinate and lactose | 58.8–76.9% | Omega-3 (lipids from oil seeds and microalgae) | Air inlet temperature 170 °C, compressed air pressure 5 bar, air flow 700 L/min and aspiration 70% | Microencapsulation efficiency depended on the type of lipid extract to encapsulate and varied from 57.0 to 76.9%. The highest microencapsulation efficiency was found for chia fatty acid ethyl esters microcapsules (76.9%), while echium microcapsules showed the highest payload (142 mg/g). | [183] |
| Konjac glucomannan (KGM) and soybean protein isolate (SPI) | 90.10% | Fish oil | The pump rotation speed at 20 mL/min, the temperature of the air at the inlet and outlet of the dryer were 200 and 80 °C, respectively | Release kinetics test further indicated retention rate of core materials for microcapsules prepared with spray drying were better than with freeze-drying. In addition, a human epithelial microfold cell (M-cell) transcytotic assay demonstrated that the M-cells had greater transport activity for the exogenous microcapsules. | [184] |
| Whey protein isolate (WPI) and octenylsuccinic anhydride (OSA) modified starch | 94.0–95.1% | β-carotene, lutein, zeaxanthin, and fish oil | The flow pressure was 0.4 psi, inlet temperature was 180 °C, and outlet temperature was controlled in a range of 85–90 °C | This study has provided an alternative way of delivering visual-beneficial compounds via a novel drying method, which is fundamentally essential in both areas of microencapsulation application and functional food development. | [185] |
| Gelatin, gum Arabic and maltodextrin | 83–95% | Fish oil | The inlet air temperatures were 190 ± 2 °C, and the feed flow rate of the emulsion was 3 mL/min, leading to the recorded outlet air temperature of 60 ± 2 °C | The microcapsules prepared by coacervation of gelatin and gum Arabic followed by spray coating with a mixture of gelation and maltodextrin were the strongest and stiffest based on the calculated nominal rupture stress and Young’s modulus, respectively. | [186] |
| Maltodextrin and modified starch | 69–87% | Fish oil | VSD process is carried out under low evaporation temperature (around 30 °C) and airflow (only atomization air of 20 L/min | The oxidative stability of the oil was greater in the vacuum spray drying (VSD) particles confirmed by Rancimat and Oxipres methodologies. Regarding the consolidation of VSD as a commercially competitive dryer, modifications must be made to your project with the aim of improving the transfer of heat and mass and achieving at least feed rate ranges similar to those employed in the spray drying. | [108] |
| Whey protein isolate, gelatin and Capsul® | 42.5–94.6% | Unsaturated triglyceride (fish oil) and (orange essential oil) | Iinlet and outlet air drying temperature of 180 °C and 90 ± 3 °C, respectively | The interfacial membrane surrounding the oil droplets is suggested to be determinant in the oxidative stability. The protein matrices showed antioxidant capacity that also can contribute to high protection. | [187] |
| Hydroxypropyl-inulin (HPI) | Fish oil (FO) | The inlet gas temperature was from 150 to 200 °C (conventional spray drying) and from 75 to 135 °C (water- free spray drying | FO-conventional spray drying and FO-water-free spray drying microparticle systems showed encapsulation efficiency values of FO above 80%, in spite of the different FO encapsulation mechanism (emulsion retention and triglyceride-HPI interactions, respectively). However, the type of solvent slightly affected the microparticle properties (Tg, moisture, hygroscopicity, FO release and FO oxidative stability) | [188] | |
| Maltodextrin and soy protein isolate | 90–94% | Fish oil | The airflow rate was set to 250 kg/h and emulsions dried at an inlet and outlet temperature of 180 and 87 °C, respectively | Due to the standardization of the particle size and the determination of oxidation products in the total- and encapsulated oil, the influence of size and non-encapsulated oil could be eliminated. The oxidation of encapsulated lipids is limited by the oxygen availability and supply rather than by the oil load. This is explained by two effects, the oxygen diffusion and a scavenging activity of the oil located in the outer particle region consuming the penetrating oxygen and thereby protecting oil droplets in the particle center. | [189] |
| Gum Arabic and maltodextrin | Not reported | Carotenoids | 6 bars air pressure and 740 L/h pressured gas flow feed. In respect to the airflow and the inlet and outlet temperatures of drying air were at a first trial 160 ± 2 °C and 70 ± 2 °C, respectively | The results related to Individual carotenoids content of the microcapsules, however, presented a considerably diminished lycopene content after atomization. Furthermore, undetectable quantities of β-carotene were observed in the gastric phase of the simulated digestion of the microcapsules indicating a strong degradation process in the acidic environment. | [109] |
| Deoiled or hydrolyzed sunflower lecithins, chitosan and chia mucilage | 84–99% | Chia oil | Feed rate of 0.6 L/h, and air inlet/outlet temperatures of 170 and 75 °C | All the microcapsules studied were efficient to protect chia oil against lipid oxidation (<10 meq hydroperoxides/kg oil), mainly the three-layer ones. The omega-3 PUFAs content after storage presented the highest levels in the three-layer microcapsules and decreased only in the monolayer system. | [180] |
| Maltodextrin and modified starch | 69–87% | Fish oil | The fresh emulsion was fed into the drying chamber at a 0.012 L/min | Particles had a lower mean diameter (6.9 μm) when compared to spray drying particles (14.6 μm), which favors the reduction of occluded oxygen. Both samples showed a continuous wall with no apparent cracks, which is an important factor to provide better protection of active. The oxidative stability of the oil was greater in the vacuum spray drying particles confirmed by Rancimat and Oxipres methodologies. | [108] |
| Macadamia protein isolate (MPI) and chitosan hydrochloride (CHC) | 94.2% | Macadamia oil | Flow rate of 5 mL/min. The aspirator was set at 100%, the actual air flow rate was 538 L/h, the inlet air temperature was set at 160 °C and the outlet air temperature at 85 ± 2 °C. Macadamia oil powders were collected and stored at 4 °C before being analyzed. | Optimum MPI/CHC level of 5:1 for producing the macadamia oil microcapsules because this gave a high encapsulation efficiency, strong protection against lipid oxidation, and good storage stability after rehydration. | [190] |
| Gum Arabic (GA), whey protein isolate (WPI), maltodextrin (MD) | 82.34–87.19% | Basil essential oil (BEO) | Finally, the GA:WPI:MD formulation demonstrated a high product yield and encapsulation efficiency with better physicochemical properties for encapsulation of BEO. | [61] | |
| Maltodextrin, gum Arabic and whey protein | 92.80–97.38% | Seed oil | The temperature of inlet air was maintained at 180 ± 1 °C, the outlet temperature was 80 ± 1 °C, and the direction of hot air was co-current. The atomizing air inlet speed was 3 m3/h, while the feeding speed was 20 mL/min | Carbohydrate-based microencapsulation showed the highest relative crystallinity, the temperature of the glass transition (Tg), which indicated good stability. Carbohydrate-based microencapsulation greatly improved the oxidative stability of gurum seeds oil suggesting better safeguarding of this sensitive oil. | [191] |
| Hydroxypropyl (HP) α-, HP β- and HP-γ-CD, cyclodextrins (CDs) | Carvacrol (95.7–98.1%), Thymol (70.2–79.8%) | Carvacrol and thymol | Inlet air temperature, 170 °C; outlet air temperature, 68 °C; nlet air flow 35 m3/h, pump flow 5 mL/min | Spray-drying method, mainly combined with HP-γ-CD, allows for obtaining solid complexes that maintain an antimicrobial activity of a level comparable to that displayed by compounds in a free state. | [192] |
| Maltodextrin, SAC (whey protein isolate, gelatin or Capsul®) | 48–95% | Fish oil, orange essential oil (OEO) | Inlet air temperature, 180 °C; outlet air temperature, 90 °C. | The protein matrices showed antioxidant capacity that also can contribute to high protection. This work provided insights about the understanding of how barrier properties of powders affect oxidation. | [187] |
| Gum Arabic (GA), maltodextrin (MD), and whey protein isolate (WPI) | 82.34 and 87.19% | Basil (ocimum basilicum L.) essential oil (BEO) | Inlet air temperature, 150 °C; feed rate of 3 mL/min, drying air flow rate of 40 Kg/h | The GA:WPI:MD formulation demonstrated a high product yield and encapsulation efficiency with better physicochemical properties for encapsulation of BEO. | [61] |
| Maltodextrin | 92% | Lemongrass (cymbopogon citratus) essential oi | Inlet temperatures: 60, 100, 140, and 180 °C | When the temperature and the time increased, the color of powder became dark and OR values were rapidly reduced. The selected optimal temperature and time was 100 °C and 20 min. | [33] |
| Bovine serum albumin, gum acacia and oxidized starch crosslinker | Not reported | Peppermint Oil | The inlet air temperature was varied from 135 to 145 °C and the aspirator rate was maintained at 100% | After complex coacervation, all the reactant ratios used here resulted in stable spherical mononuclear core-shell capsules, with no measurable loss of peppermint oil compared to the parent emulsion. Samples without crosslinker did not withstand spray drying, thus demonstrating the need for reinforcing the complex coacervate walls with a cross linker or with a common additive such as a sugar. | [48] |
| Chitosan tween-80 | 39% | Lemongrass essential oil | Temperature input 160–165 °C | Chitosan microparticles loaded with essential oil (CMEOs) had higher thermal stability and presented better colloidal stability than chitosan microparticles and pure oils. The results also demonstrated that the proposed system allows controlled release of the bioactive com-pound. | [193] |
| Octenyl succinic anhydride–linked starch (OSA- S) and maltodextrin (MD) | Not reported | Fish oil | Inlet air temperature 180–190 °C; outlet air temperature 80–90 °C; feeding speed 20 mL/min; and atomized speed 200–300 r/min. | Different temperatures and pH had significant effects on the oxidation stability of fish oil microcapsules as observed an upward trend for peroxide value, acid value, and thiobarbituric acid test during storage. The microcapsules were not resistant to acid treatment and had a lower oxidation rate in neutral condition. | [181] |
| Artichoke bracts flour, maltodextrin, tween 20 | 65–79% | Sunflower oil | Inlet air temperature 175 °C; outlet air temperature 100 °C aspirator 80% | Artichoke bracts are a healthy alternative to synthetic emulsifiers and could be successfully combined with common wall materials for lipids microencapsulation. | [194] |
| Maltodextrin and gum Arabic | 87% | Chia oil | Inlet air temperature 100–120 °C; drying airflow of 1.65 m3/min; air atomizing pressure of 4 bar | Treatment 4 (120 °C for inlet temperature and 0.1 L/h of feed rate) is the most indicated for application in food products. | [195] |
| S. edule fruit starch (SS) in combination with whey protein (WPC) and gum Arabic (GA) | 60–78% | Cinnamon oleoresin | The drying conditions were inlet/outlet air drying temperatures of 150/85 °C, using a parallel arrangement of the nozzle concerning the drying airflow, and a nozzle pressure of 2.5 kPa. Throughout the drying process, the emulsion was continuously agitated | Cinnamon oleoresin microcapsules synthesized with ternary formulations stabilize the phenolic compounds in cinnamon oleoresin and reach higher encapsulation efficiency values. Based on these results, we suggest that S. edule fruit can be used as a starch source. When combined with other wall materials. | [196] |
| Biopolymers, gelatin and lignin | 97.0% | Orange essential oil | Inlet air temperature 110–150 °C; Feed flow rate 0.15–0.45 L/h; Drying air flow 301–536 L/h | It was possible to prepare microparticles showing microspherical morphology, with the average particle size of less than 4 μm (gelatin) and 3 μm (lignin), with an oil content of 90% (w/w) for gelatin and powder recovery of 72% (w/w) for lignin as wall material. | [110] |
| Whey protein isolate (WPI) as the primary wall material by prebiotic carbohydrates, such as maltodextrin (MD) and inulin (IN) | 89.10% | Structured lipids (SLs) enriched with medium and long chain triacylglycerols (MLCTs) | The spray dryer was operated at inlet and outlet temperatures of 180 ± 5 °C and 80 ± 5 °C, respectively with the airflow rate set at 300 NL/min | The microcapsules produced by using WPI/IN (1:1) were selected as the best treatment based on the highest microencapsulation efficiency and the lowest level of peroxide value. The finding of this study could promote the possibility of a new combination of wall materials and places IN with its high nutritional and functional properties as a substituent secondary wall material. | [42] |
| Gum Arabic, maltodextrin, and inulin | 40–95% | Cinnamon essential oil | The inlet temperature was set at 170 °C, and the feed rate used was 0.8 L/h. An atomizing air flow rate of 35 L/min was selected and maintained. | The results demonstrated that the cinnamon essential oil microcapsules with the least amount of the surface cinnamaldehyde and with the greatest amount of the encapsulation efficiency of innam aldehyde, the release of cinnamaldehyde, and powder recovery could be efficiently produced using the selected wall materials. | [172] |
| Silica | 70% | N-octadecane and methyl palmitate | The spray dryer was operated under a nitrogen atmosphere to prevent high-temperature combustion of the ethanol by-product of the sol-gel process 35 m3/h of N2 gas entered the dryer at 160 °C, to dehumidify the liquid droplets, and exited at 100 °C | The highest phase change materials (PCM) encapsulation efficiency could be achieved with the initial PCM to silica weight ratio of 0.25. | [59] |
| Arabic gum, maltodextrin, and modified starch | 97.9–98.3% | Oregano essential oil | Feed rate 5 mL/min, inlet air temperature 180 °C, outlet 117 °C, aspiration 100% and airflow 600 L/h. | The release profile of essential oil (EO) from tablets prepared from spray drying powder with 10% or 20% w/w EO containing 5% w/w croscarmellose sodium was fast and similar to the profile obtained from spray-dried powder with 20% EO content. | [197] |
| Encapsulating Agent | Encapsulation Efficiency | Structured Lipid | Encapsulation Conditions | Principal Results | Reference |
|---|---|---|---|---|---|
| Whey protein and gum Arabic | Not shown | Rosmarinus officinal | Feed flow rate of 4 g/min, atomizer 0.5 mm, inlet temperature at 90 °C, flow rate 60 m3/h, pressure and flow of 3 kgf/cm2 and 17 Lpm, respectively. | Powdered redispersible lipid-based compositions entrapping antioxidants from Rosmarinus officinalis extract were successfully generated by spray-drying. | [201] |
| Whey protein isolate (WPI) as the primary wall material by prebiotic carbohydrates, such as maltodextrin (MD) and inulin (IN) | 89.10 ± 1.03% | Structured lipids (SLs) enriched with medium-and long-chain triacylglycerols (MLCTs) | Inlet and outlet temperatures of 180 °C ± 5 °C and 80 ± 5 °C, respectively with the air flow rate set at 300 NL/min. | The microcapsules produced by using WPI/IN (1:1) were selected as the best treatment based on the highest microencapsulation efficiency and the lowest level of peroxide value. The finding of this study could promote the possibility of a new combination of wall materials, and places IN with its high nutritional and functional properties as a substituent secondary wall material. | [42] |
| Whey protein isolate and maltodextrin | Not shown | 1,3-Dioleoyl-2-palmitoylglycerol (OPO) | Inlet air temperature at 184 °C and outlet air temperature at 89 °C. | In this study, the addition of microencapsulated OPO in infant formula can significantly improve the oxidative stability of infant formula and extend the shelf life of the product. Infant formula with microencapsulated OPO possess better sense state and provide a broader space for its industrial production. | [202] |
| Sodium caseinate (SC), soy protein (SP) and maltodextrin (M) | 42–82% | Structured lipid palm-based medium- and long-chain triacylglycerol (MLCT) | Flow rate of 15 mL/min and atomizer 0.7-mm standard diameter nozzle. The inlet and outlet temperature 140 and 110 °C, respectively. | Binary mixture of sodium caseinate and soy protein with maltodextrin when heated in solution at certain temperature and time period produces Maillard reaction products that can be used as natural emulsifier as well as encapsulating agent with improved physical properties. | [200] |
| Not shown | Not shown | 1,3-oleic acid-2-palmitic acid structured lipid | Not shown. | The oxidative stability of the infant liquid milk added the structured lipid microcapsules has been significantly improved. Provide a theoretical basis for industrial production. | [203] |
| Whey protein isolate and corn syrup solids (CSS) | 90% | Structured lipids (SLs) containing long-chain polyunsatured fatty acids (LCPUFAs) | Inlet temperature of 180 °C and an outlet temperature of 80 °C at a feeding rate of 5 mL/min. | Structured lipids (SLs) containing long chain polyunsatured fatty acids (LCPUFAs) were successfully microencapsulated in Maillard reaction products (MRPs) obtained from heated whey protein isolates and corn syrup solids solution with high microencapsulation efficiency. | [204] |
| Non-fat dry milk, whey protein isolate, lactose, maltodextrin | Not shown | Structured lipid (SL) enriched with arachidonic (ARA) and docosahexaenoic (DHA) | Two different combinations of spray-drying inlet−outlet temperature (120−70 °C vs. 180−80 °C) were used. | Formula prepared with microencapsulated SL and the dry-blending method had better oxidative stability and color quality. | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Osorno, D.M.; López-Jaramillo, M.C.; Caicedo Paz, A.V.; Villa, A.L.; Peresin, M.S.; Martínez-Galán, J.P. Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review. Pharmaceutics 2023, 15, 1490. https://doi.org/10.3390/pharmaceutics15051490
Sánchez-Osorno DM, López-Jaramillo MC, Caicedo Paz AV, Villa AL, Peresin MS, Martínez-Galán JP. Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review. Pharmaceutics. 2023; 15(5):1490. https://doi.org/10.3390/pharmaceutics15051490
Chicago/Turabian StyleSánchez-Osorno, Diego Mauricio, María Camila López-Jaramillo, Angie Vanesa Caicedo Paz, Aída Luz Villa, María S. Peresin, and Julián Paul Martínez-Galán. 2023. "Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review" Pharmaceutics 15, no. 5: 1490. https://doi.org/10.3390/pharmaceutics15051490
APA StyleSánchez-Osorno, D. M., López-Jaramillo, M. C., Caicedo Paz, A. V., Villa, A. L., Peresin, M. S., & Martínez-Galán, J. P. (2023). Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review. Pharmaceutics, 15(5), 1490. https://doi.org/10.3390/pharmaceutics15051490









