Dose-Ranging Plasma and Genital Tissue Pharmacokinetics and Biodegradation of Ultra-Long-Acting Cabotegravir In Situ Forming Implant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Performance Liquid Chromatography (HPLC)
2.3. Preparation of ISFI Formulation
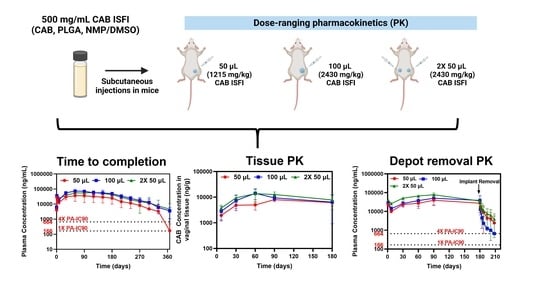
2.4. Pharmacokinetic (PK) Studies in Female BALB/c Mice
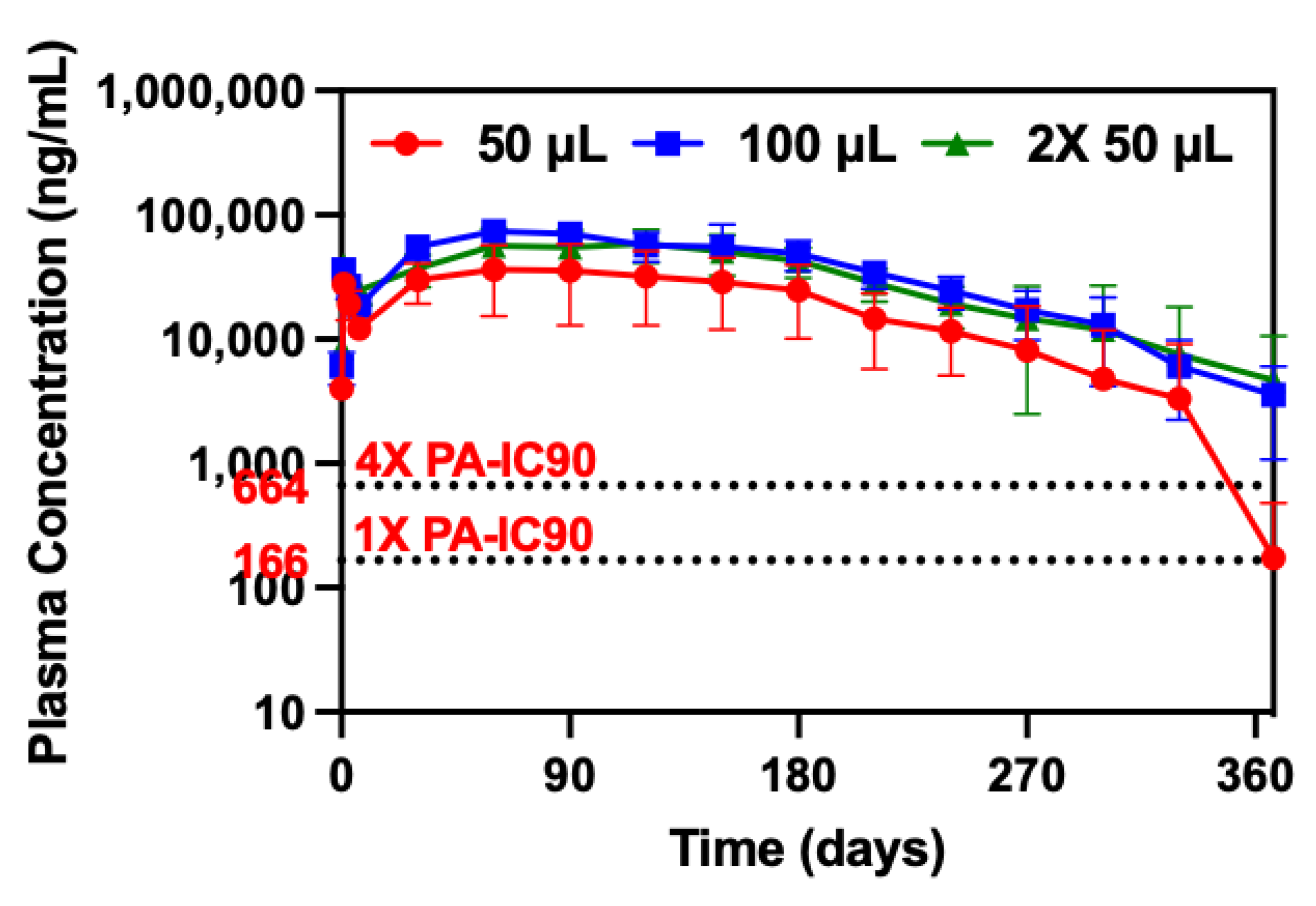
2.4.1. Long-Term Plasma PK and Non-Compartmental Analysis
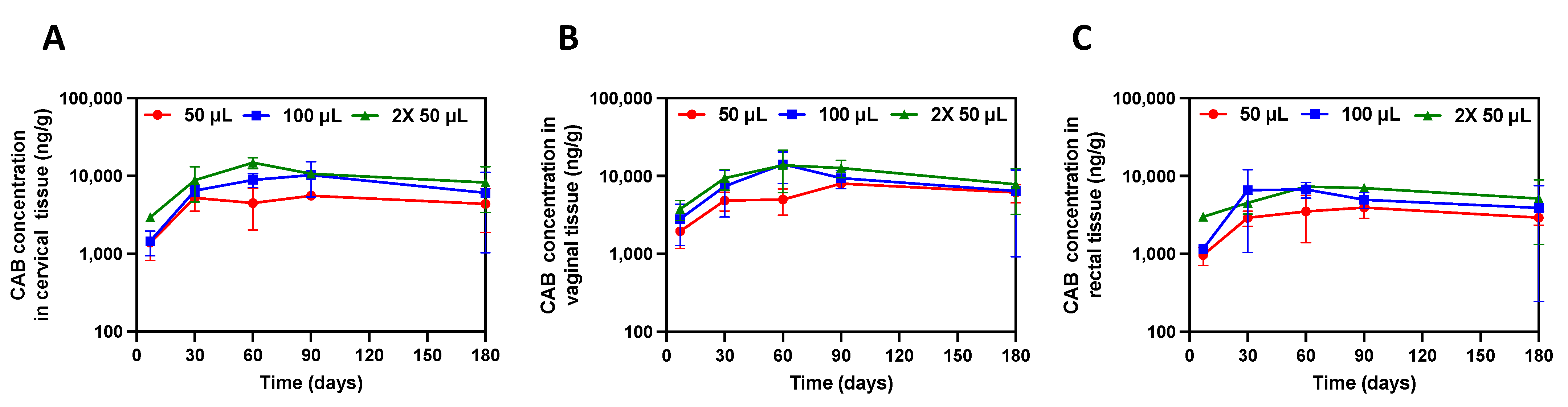
2.4.2. Tissue PK
2.4.3. Depot Removal PK and Assessment of PK Tail
2.5. Residual Drug Quantification
2.6. Gel Permeation Chromatography (GPC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Dose-Ranging Ultra-Long-Acting PK
3.2. Ultra-Long-Acting Genital Tissue PK
3.3. CAB ISFI Removal and Assessment of PK Tail
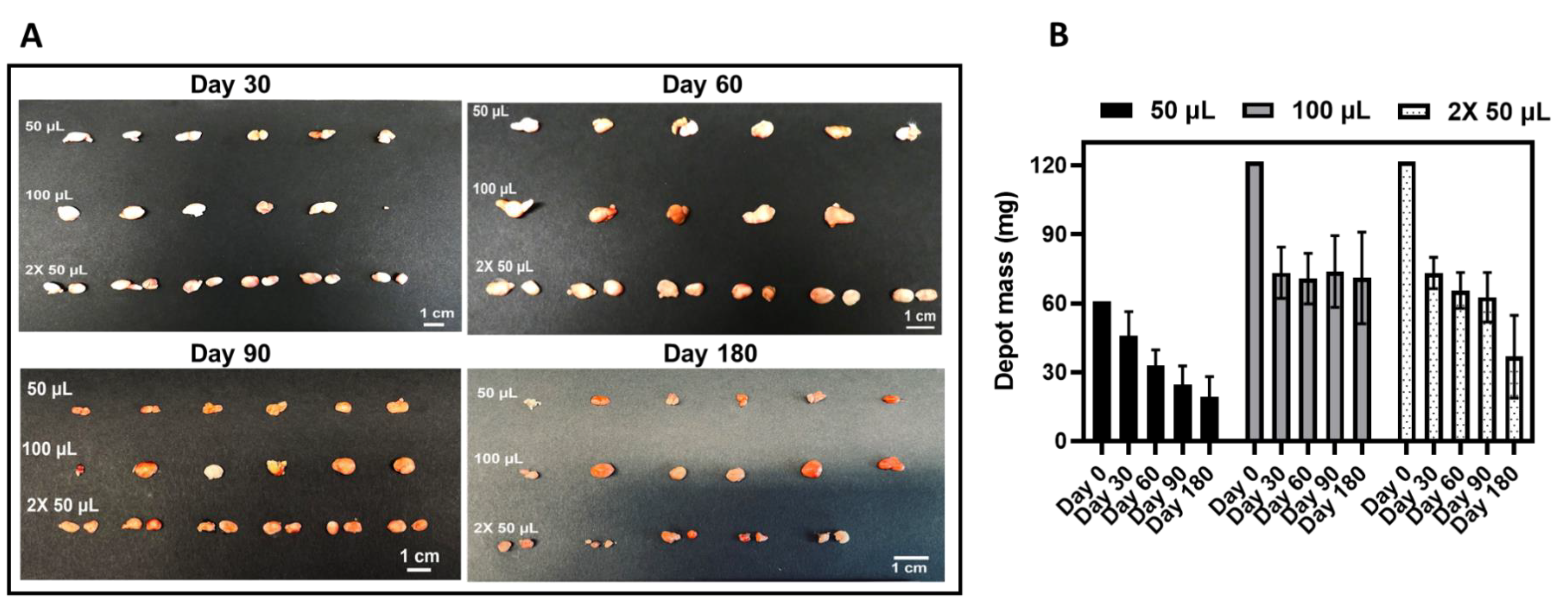
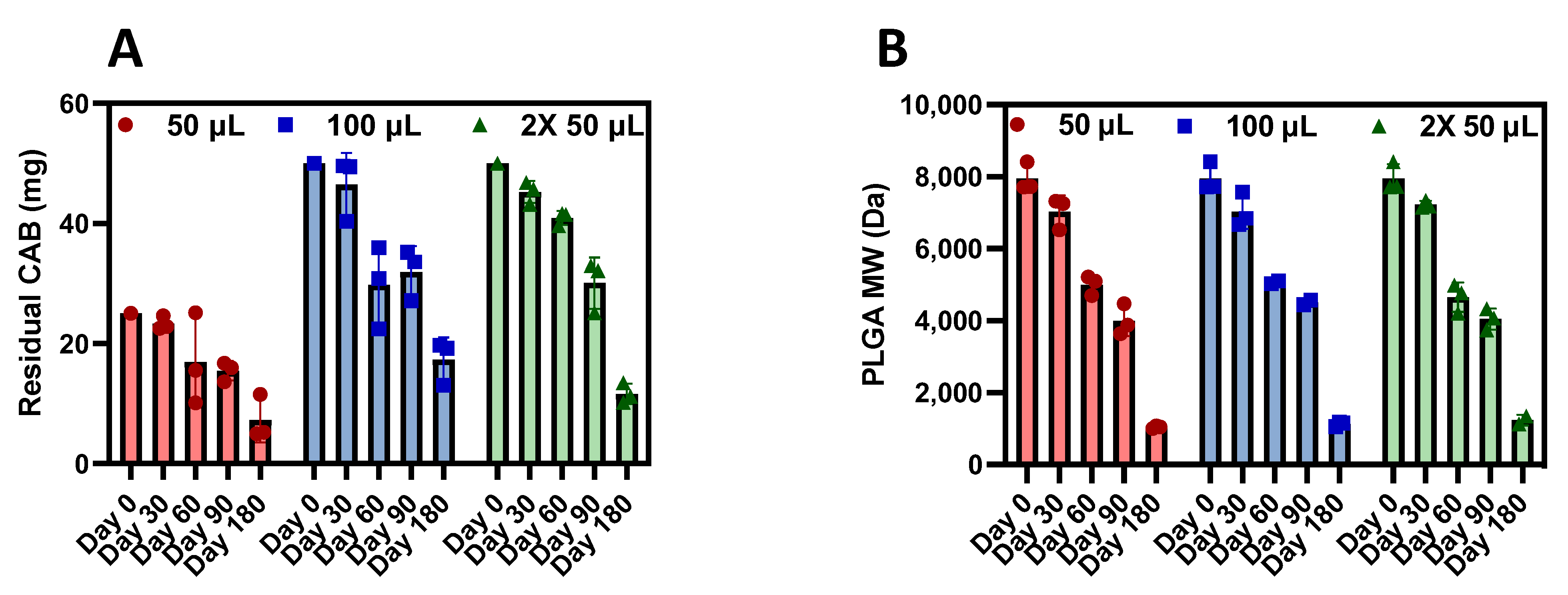
3.4. CAB ISFI Retrievability, Residual CAB, and Polymer Biodegradation
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV and AIDS Statistics. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 16 January 2023).
- Cobb, D.A.; Smith, N.A.; Edagwa, B.J.; McMillan, J.M. Long-acting approaches for delivery of antiretroviral drugs for prevention and treatment of HIV: A review of recent research. Expert Opin. Drug. Deliv. 2020, 17, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.D.; Harrigan, R.; Bangsberg, D.R.; Hogg, R.S.; Gross, R.; Yip, B.; Montaner, J.S. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J. Acquir. Immune. Defic. Syndr. 2009, 50, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Tolley, E.E.; Li, S.; Zangeneh, S.Z.; Atujuna, M.; Musara, P.; Justman, J.; Pathak, S.; Bekker, L.G.; Swaminathan, S.; Stanton, J.; et al. Acceptability of a long-acting injectable HIV prevention product among US and African women: Findings from a phase 2 clinical Trial (HPTN 076). J. Int. AIDS Soc. 2019, 22, e25408. [Google Scholar] [CrossRef] [PubMed]
- Minnis, A.M.; Montgomery, E.T.; Napierala, S.; Browne, E.N.; van der Straten, A. Insights for Implementation Science From 2 Multiphased Studies With End-Users of Potential Multipurpose Prevention Technology and HIV Prevention Products. J. Acquir. Immune. Defic. Syndr. 2019, 82 (Suppl. 3), S222–S229. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Donnell, D.; Clement, M.E.; Hanscom, B.; Cottle, L.; Coelho, L.; Cabello, R.; Chariyalertsak, S.; Dunne, E.F.; Frank, I.; et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N. Engl. J. Med. 2021, 385, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Li, S.; Grinsztejn, B.; Dawood, H.; Liu, A.Y.; Magnus, M.; Hosseinipour, M.C.; Panchia, R.; Cottle, L.; Chau, G.; et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018, 15, e1002690. [Google Scholar] [CrossRef] [PubMed]
- Trezza, C.; Ford, S.L.; Spreen, W.; Pan, R.; Piscitelli, S. Formulation and pharmacology of long-acting cabotegravir. Curr. Opin. HIV AIDS 2015, 10, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Li, S.; Eron, J.J., Jr.; Grinsztejn, B.; Dawood, H.; Liu, A.Y.; Magnus, M.; Hosseinipour, M.C.; Panchia, R.; Cottle, L.; et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: A secondary analysis of the HPTN 077 trial. Lancet HIV 2020, 7, e472–e481. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ibanescu, R.I.; Anstett, K.; Mesplede, T.; Routy, J.P.; Robbins, M.A.; Brenner, B.G.; Montreal Primary, H.I.V.C.S.G. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 2018, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Benhabbour, S.R.; Kovarova, M.; Jones, C.; Copeland, D.J.; Shrivastava, R.; Swanson, M.D.; Sykes, C.; Ho, P.T.; Cottrell, M.L.; Sridharan, A.; et al. Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat. Commun. 2019, 10, 4324. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, M.; Benhabbour, S.R.; Massud, I.; Spagnuolo, R.A.; Skinner, B.; Baker, C.E.; Sykes, C.; Mollan, K.R.; Kashuba, A.D.M.; Garcia-Lerma, J.G.; et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 2018, 9, 4156. [Google Scholar] [CrossRef] [PubMed]
- Young, I.C.; Massud, I.; Cottrell, M.L.; Shrivastava, R.; Maturavongsadit, P.; Prasher, A.; Wong-Sam, A.; Dinh, C.; Edwards, T.; Mrotz, V.; et al. Ultra-long-acting in-situ forming implants with cabotegravir protect female macaques against rectal SHIV infection. Nat. Commun. 2023, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, R.E.; Kost, J. Characterization of a polymeric PLGA-injectable implant delivery system for the controlled release of proteins. J. Biomed. Mater. Res. 2000, 50, 388–396. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.R.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.B.; Prasher, A.; Young, I.C.; Kim, J.; Shrivastava, R.; Maturavongsadit, P.; Benhabbour, S.R. Effects of Drug Physicochemical Properties on In-Situ Forming Implant Polymer Degradation and Drug Release Kinetics. Pharmaceutics 2022, 14, 1188. [Google Scholar] [CrossRef] [PubMed]
- Kempe, S.; Mader, K. In situ forming implants—An attractive formulation principle for parenteral depot formulations. J. Control. Release 2012, 161, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C.; Spainhour, C.B.; Shoemake, C.; Pallman, D.R.; Stricker-Krongrad, A.; Downing, P.A.; Seals, R.E.; Eagle, L.A.; Polhamus, K.; Daly, J. Tolerable Levels of Nonclinical Vehicles and Formulations Used in Studies by Multiple Routes in Multiple Species with Notes on Methods to Improve Utility. Int. J. Toxicol. 2016, 35, 95–178. [Google Scholar] [CrossRef] [PubMed]
- WHO. N-methyl-2-pyrrolidone. 2001. Available online: http://apps.who.int/iris/bitstream/handle/10665/42404/9241530359.pdf;jsessionid=4F6D612136E539740D11CC34EB09A7EC?sequence=1 (accessed on 5 November 2022).
- Becci, P.J.; Knickerbocker, M.J.; Reagan, E.L.; Parent, R.A.; Burnette, L.W. Teratogenicity study of N-methylpyrrolidone after dermal application to Sprague-Dawley rats. Fundam. Appl. Toxicol. 1982, 2, 73–76. [Google Scholar] [CrossRef] [PubMed]
- EPA. Tolerance for N-methylpyrrolidone (NMP; CAS Reg. No. 872-50-4). 2006. Available online: https://www.epa.gov/sites/default/files/2015-04/documents/methyl.pdf (accessed on 5 November 2022).
- Gaylord Chemical Company LLC. DMSO Health & Safety. 2021. Available online: https://www.gaylordchemical.com/environmental-health-safety/dmso-health-safety/ (accessed on 7 December 2021).


| Group | Dose (mg) | Median AUC0→367 (day × ng/mL) | 25% Percentile | 75% Percentile |
|---|---|---|---|---|
| 50 µL | 25 | 7,222,125 | 4,375,526 | 9,306,997 |
| 100 µL | 50 | 14,731,084 | 13,075,480 | 15,226,388 |
| 2× 50 µL | 50 | 12,634,860 | 11,474,641 | 12,906,369 |
| Group | Tissue | Day 7 | Day 30 | Day 60 | Day 90 | Day 180 |
|---|---|---|---|---|---|---|
| 50 µL | Cervical | 0.114 ± 0.05 | 0.175 ± 0.06 | 0.123 ± 0.07 | 0.157 ± 0.02 | 0.177 ± 0.10 |
| Vaginal | 0.160 ± 0.06 | 0.162 ± 0.04 | 0.137 ± 0.05 | 0.226 ± 0.03 | 0.247 ± 0.07 | |
| Rectal | 0.079 ± 0.02 | 0.097 ± 0.02 | 0.097 ± 0.06 | 0.111 ± 0.03 | 0.118 ± 0.02 | |
| 100 µL | Cervical | 0.078 ± 0.03 | 0.117 ± 0.02 | 0.120 ± 0.02 | 0.147 ± 0.07 | 0.124 ± 10 |
| Vaginal | 0.152 ± 0.08 | 0.133 ± 0.08 | 0.192 ± 0.08 | 0.134 ± 0.03 | 0.132 ± 0.11 | |
| Rectal | 0.063 ± 0.01 | 0.119 ± 0.10 | 0.091 ± 0.02 | 0.071 ± 0.02 | 0.080 ± 0.07 | |
| 2× 50 µL | Cervical | 0.119 ± 0.01 | 0.240 ± 0.11 | 0.263 ± 0.04 | 0.195 ± 0.01 | 0.193 ± 0.11 |
| Vaginal | 0.153 ± 0.04 | 0.255 ± 0.08 | 0.244 ± 0.14 | 0.232 ± 0.06 | 0.183 ± 0.11 | |
| Rectal | 0.121 ± 0.01 | 0.123 ± 0.03 | 0.129 ± 0.02 | 0.128 ± 0.01 | 0.120 ± 0.09 |
| Group | 30-Day Implant Removal | 60-Day Implant Removal | 90-Day Implant Removal | 180-Day Implant Removal |
|---|---|---|---|---|
| 50 µL | 10.11 ± 2.09 | 10.70 ± 3.55 | 28.68 ± 4.61 | 11.08 ± 4.81 |
| 100 µL | 5.23 ± 2.16 | 9.61 ± 4.19 | 22.06 ± 10.44 | 58.37 ± 19.54 |
| 2× 50 µL | 10.29 ± 1.87 | 13.92 ± 2.50 | 303.79 ± 36.41 | 10.81 ± 2.14 |
| Injection Volume | % CAB Remaining | |||
|---|---|---|---|---|
| 30 Days | 60 Days | 90 Days | 180 Days | |
| 50 µL | 93.2 ± 4.5% | 67.6 ± 30.4% | 61.7 ± 6.5% | 23.2 ± 3.4% |
| 100 µL | 92.3 ± 10.5% | 59.5 ± 13.6% | 63.8 ± 8.5% | 34.6 ± 7.4% |
| 2× 50 µL | 90.4 ± 3.6% | 81.7 ± 2.3% | 60.1 ± 8.5% | 29.0 ± 14.8% |
| Injection Volume | % PLGA MW Decrease | |||
|---|---|---|---|---|
| 30 Days | 60 Days | 90 Days | 180 Days | |
| 50 µL | 11.6 ± 5.6% | 37.1 ± 3.4% | 49.7 ± 5.4% | 86.9 ± 0.43% |
| 100 µL | 11.6 ± 5.9% | 36.3 ± 0.7% | 43.3 ± 1.1% | 85.8 ± 0.83% |
| 2× 50 µL | 9.1 ± 1.3% | 41.5 ± 5.1% | 49.1 ± 3.7% | 84.5 ± 1.89% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, I.C.; Thorson, A.L.; Shrivastava, R.; Sykes, C.; Schauer, A.P.; Cottrell, M.L.; Kashuba, A.D.M.; Benhabbour, S.R. Dose-Ranging Plasma and Genital Tissue Pharmacokinetics and Biodegradation of Ultra-Long-Acting Cabotegravir In Situ Forming Implant. Pharmaceutics 2023, 15, 1487. https://doi.org/10.3390/pharmaceutics15051487
Young IC, Thorson AL, Shrivastava R, Sykes C, Schauer AP, Cottrell ML, Kashuba ADM, Benhabbour SR. Dose-Ranging Plasma and Genital Tissue Pharmacokinetics and Biodegradation of Ultra-Long-Acting Cabotegravir In Situ Forming Implant. Pharmaceutics. 2023; 15(5):1487. https://doi.org/10.3390/pharmaceutics15051487
Chicago/Turabian StyleYoung, Isabella C., Allison L. Thorson, Roopali Shrivastava, Craig Sykes, Amanda P. Schauer, Mackenzie L. Cottrell, Angela D. M. Kashuba, and Soumya Rahima Benhabbour. 2023. "Dose-Ranging Plasma and Genital Tissue Pharmacokinetics and Biodegradation of Ultra-Long-Acting Cabotegravir In Situ Forming Implant" Pharmaceutics 15, no. 5: 1487. https://doi.org/10.3390/pharmaceutics15051487
APA StyleYoung, I. C., Thorson, A. L., Shrivastava, R., Sykes, C., Schauer, A. P., Cottrell, M. L., Kashuba, A. D. M., & Benhabbour, S. R. (2023). Dose-Ranging Plasma and Genital Tissue Pharmacokinetics and Biodegradation of Ultra-Long-Acting Cabotegravir In Situ Forming Implant. Pharmaceutics, 15(5), 1487. https://doi.org/10.3390/pharmaceutics15051487