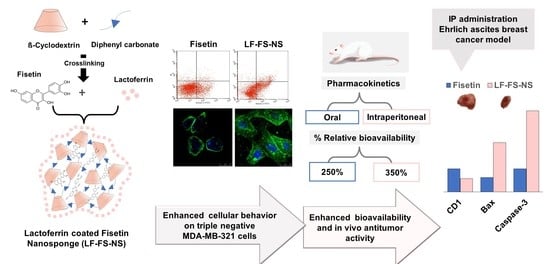
Targeted Fisetin-Encapsulated β-Cyclodextrin Nanosponges for Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blank Nanosponges
2.3. Ferric Chloride Test
2.4. Preparation of Fisetin-Loaded Nanosponges
2.5. Preparation of Lactoferrin (LF)-Coated FS-NS
2.6. Physicochemical Characterization
2.6.1. Surface Area and Porosity Analysis
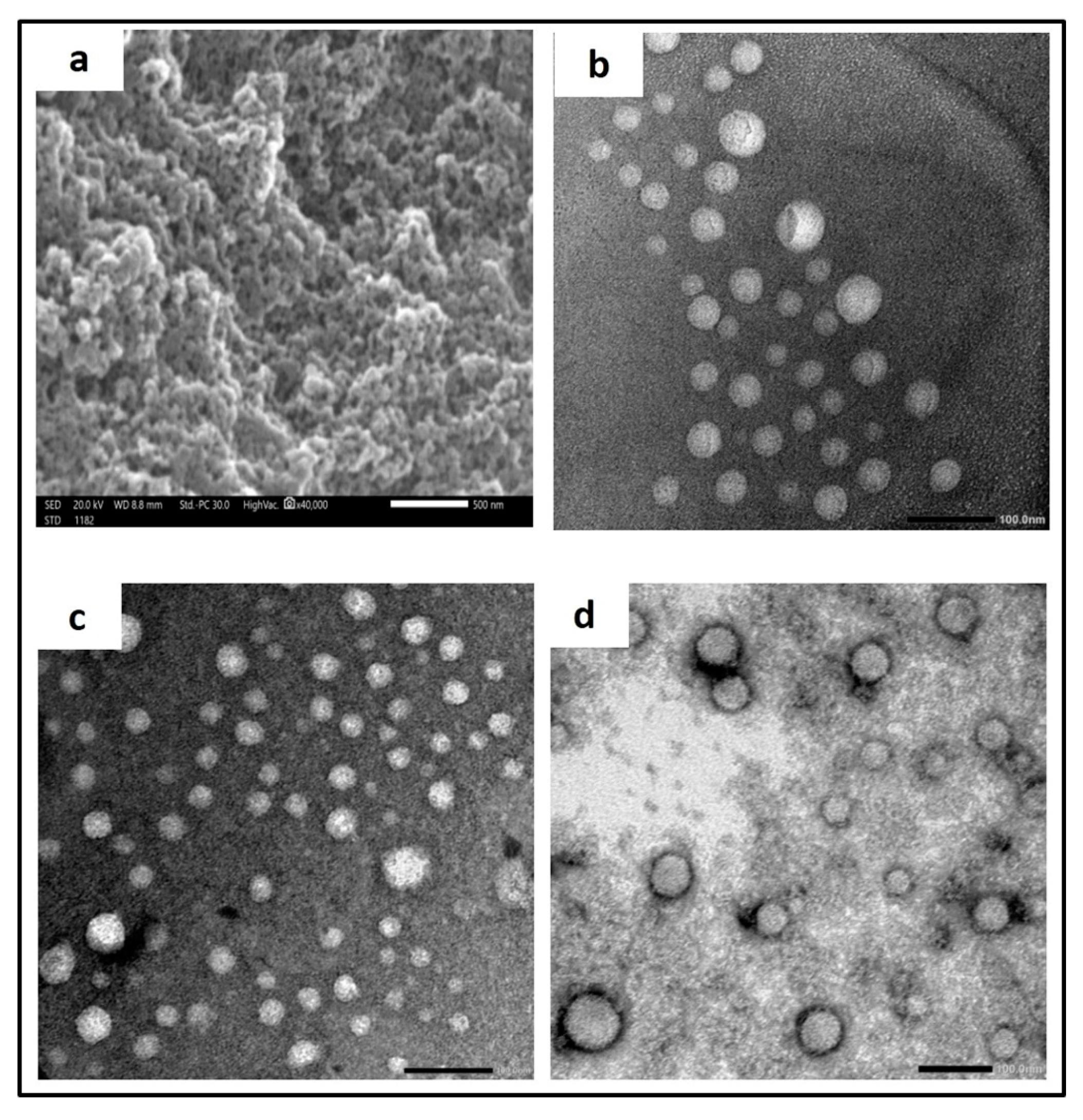
2.6.2. Microscopical Examination
Scanning Electron Microscopy (SEM)
Transmission Electron Microscopy (TEM)
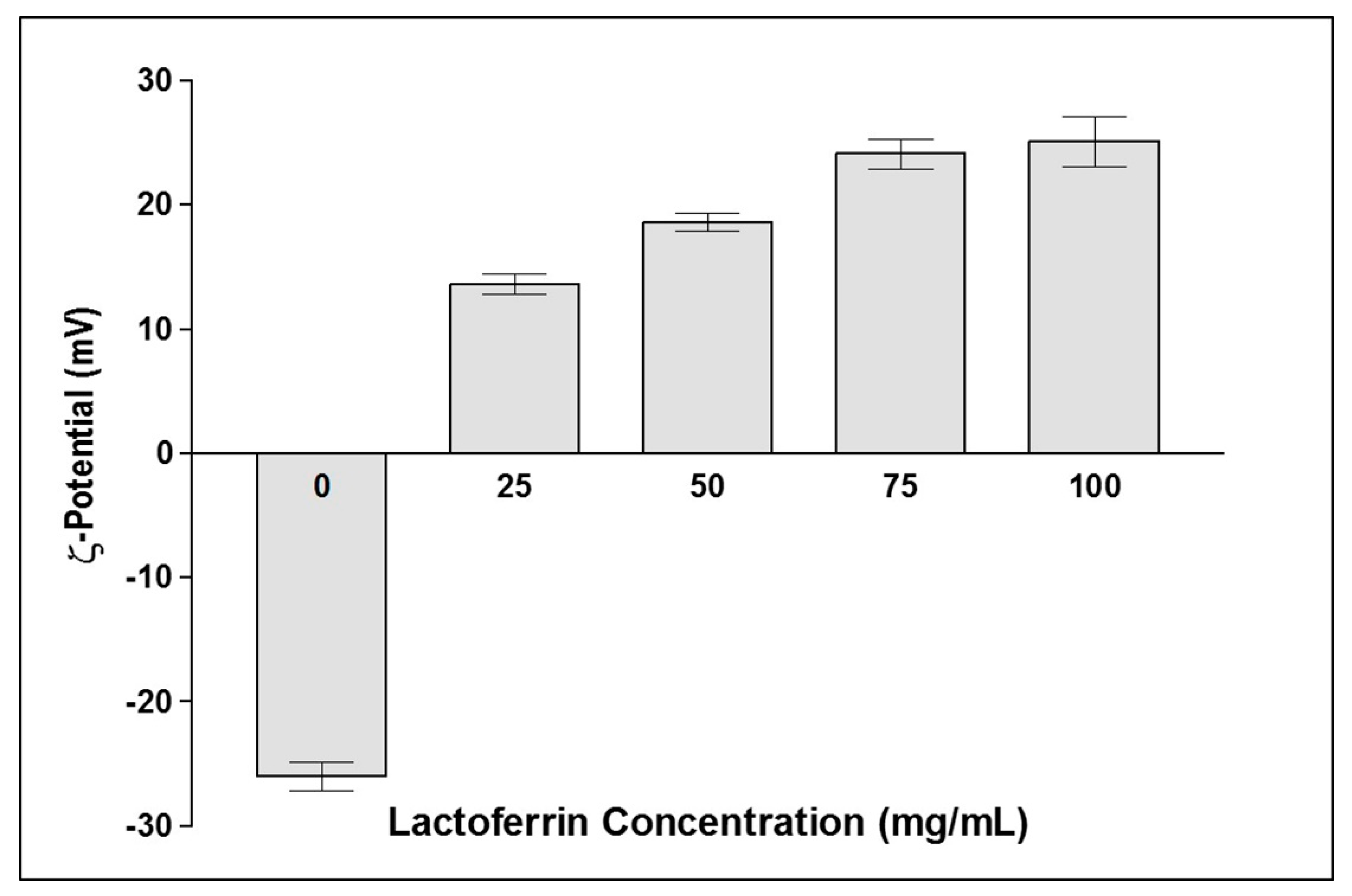
2.6.3. ξ-Potential Measurement
2.6.4. Entrapment Efficiency and Drug Loading Determination
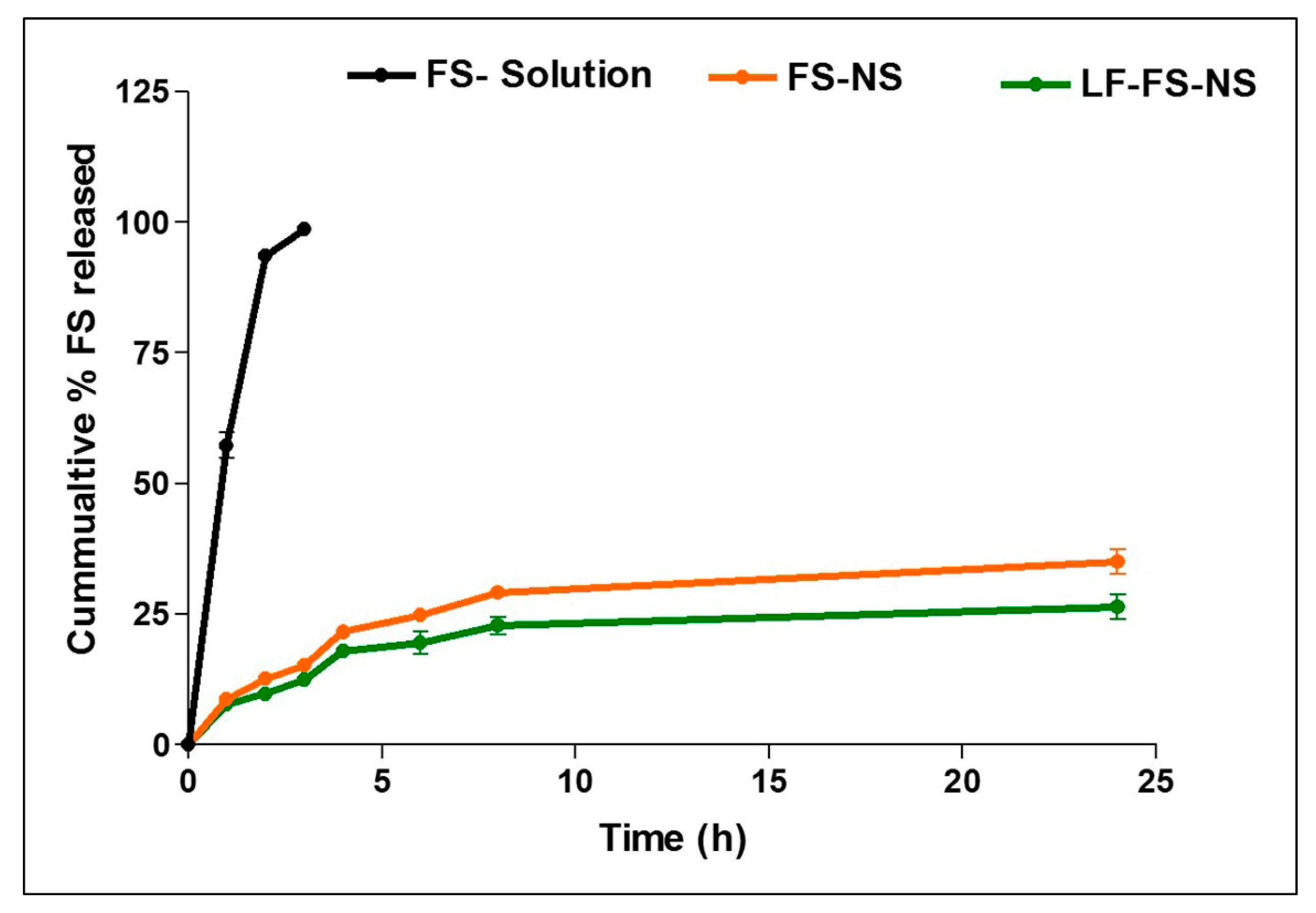
2.6.5. In Vitro Drug Release
2.6.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.7. X-ray Powder Diffractometry (XRD)
2.7. In Vitro Cell Culture Studies
2.7.1. MTT Cytotoxicity Assay
2.7.2. Apoptosis Assay (Annexin V-FTIC/Propidium Iodide Assay)
2.7.3. Cell Migration by Wound Healing Assay
2.7.4. Cellular Uptake
2.8. In Vivo Studies
2.8.1. Pharmacokinetic Study
Study Design
Quantification of FS in Plasma Samples
Pharmacokinetic Data Analysis
2.8.2. Antitumor Efficacy Evaluation
Experimental Design
Assessment of Tumor Growth
Tumor Biomarkers
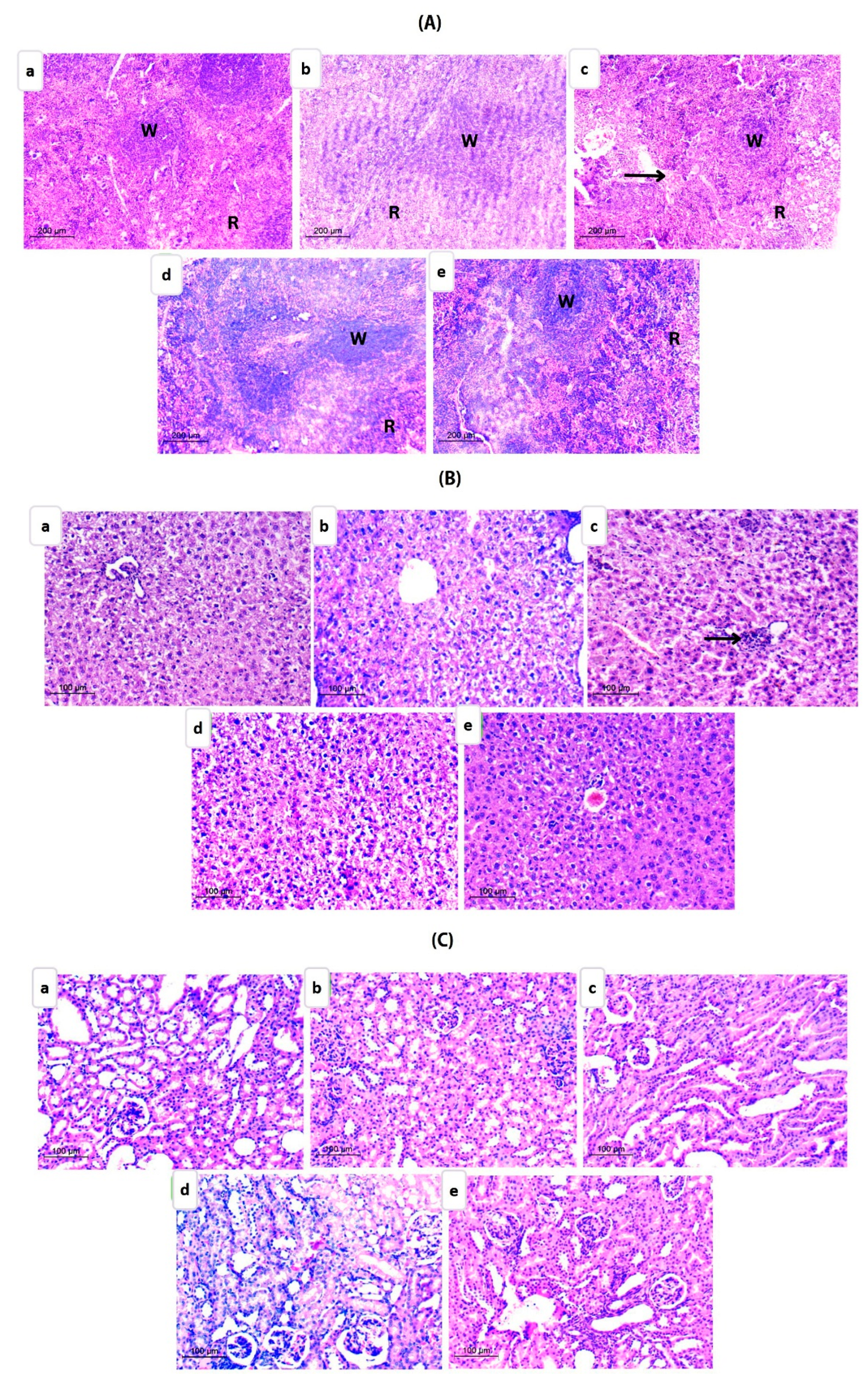
Histopathological Examination
2.8.3. In Vivo Toxicity Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Optimization of β-CD-NS
3.2. Preparation of LF-Coated FS-NS
3.3. Physicochemical Characterization
3.3.1. Colloidal Properties and Entrapment Efficiency
3.3.2. Analysis of Surface Area and Porosity of NS
3.3.3. Microscopical Examination
3.3.4. In Vitro Drug Release
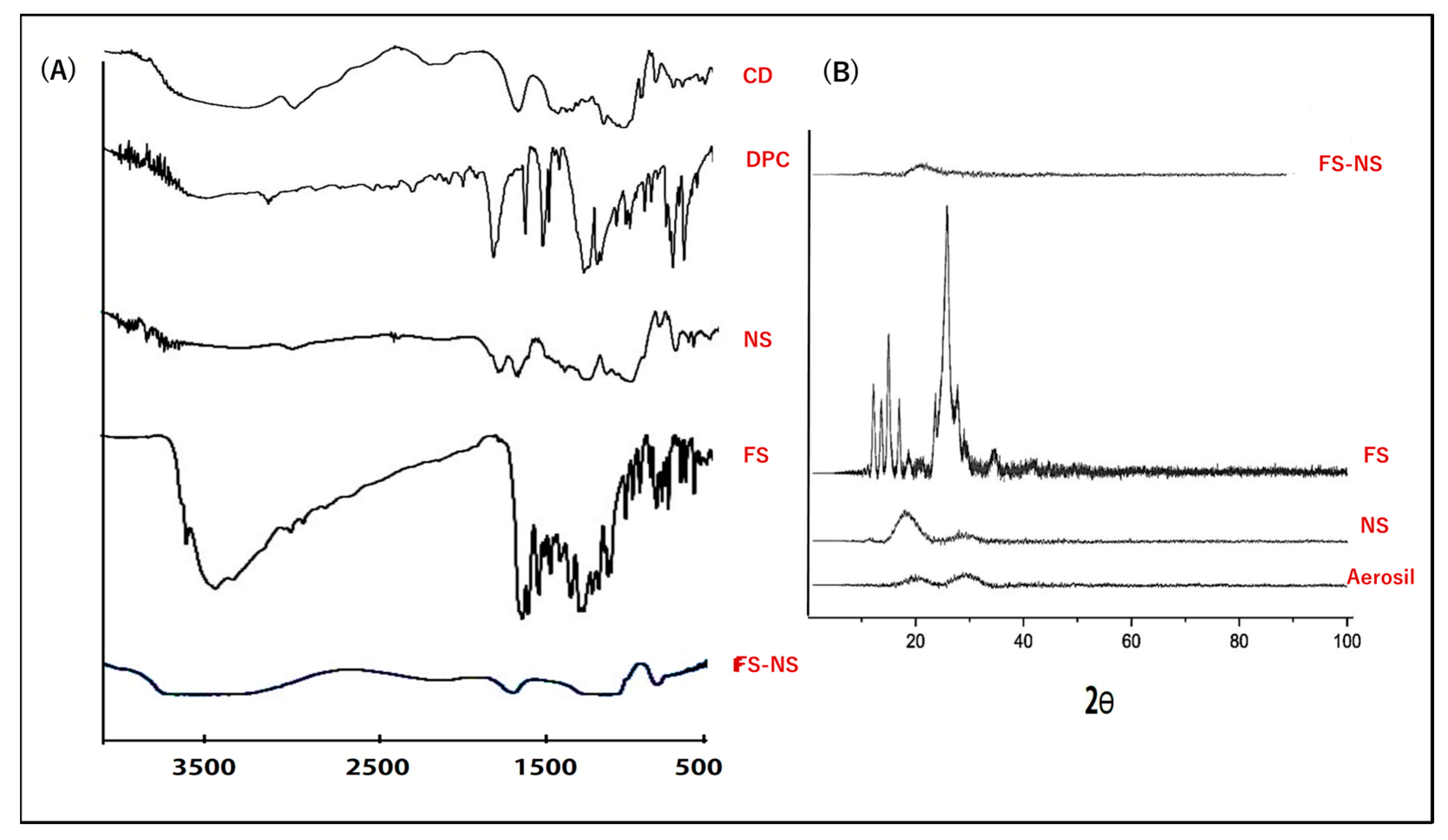
3.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.6. X-ray Diffractometry (XRD)
3.4. Cell Line Studies
3.4.1. Cytotoxicity Evaluation
3.4.2. In Vitro Apoptosis Assay
3.4.3. Cell Migration
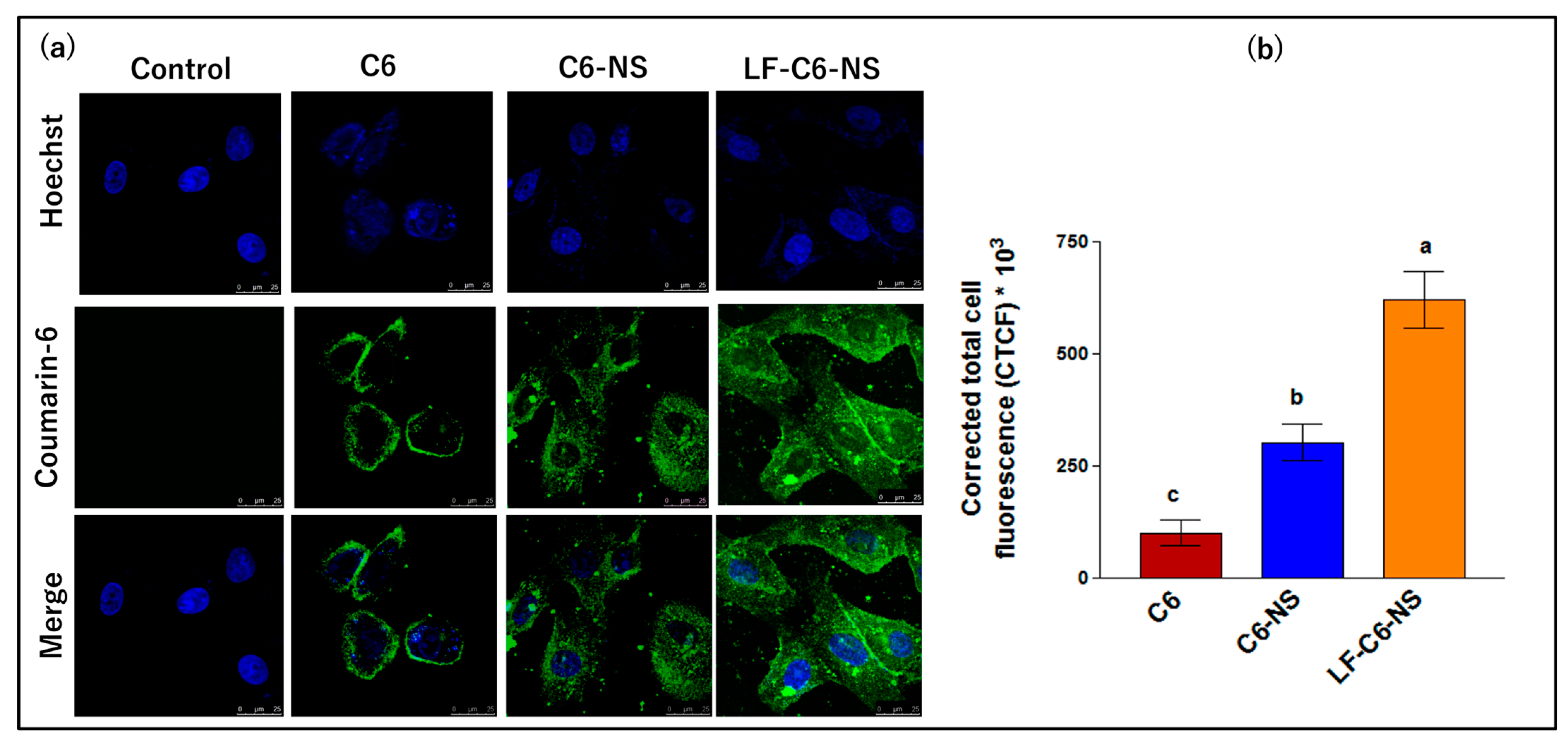
3.4.4. Cellular Uptake
3.5. In Vivo Studies
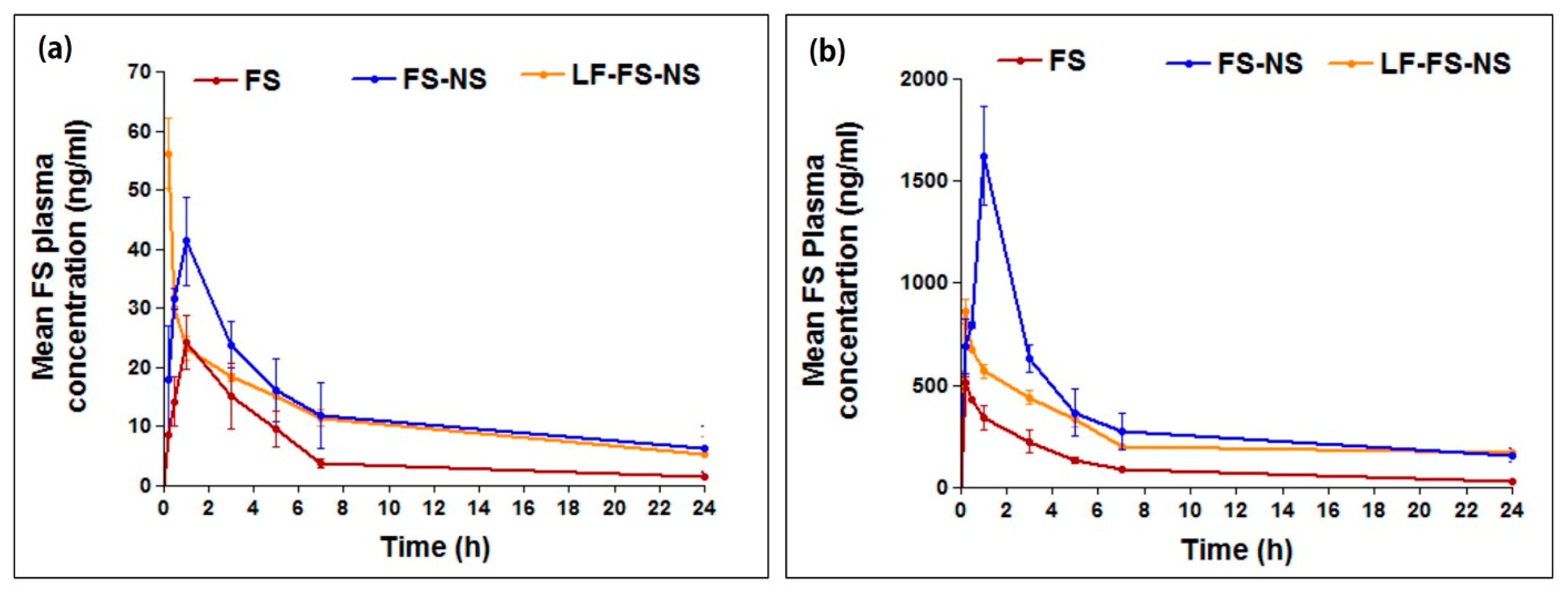
3.5.1. Pharmacokinetic Study
3.5.2. In Vivo Evaluation of Anticancer Potential
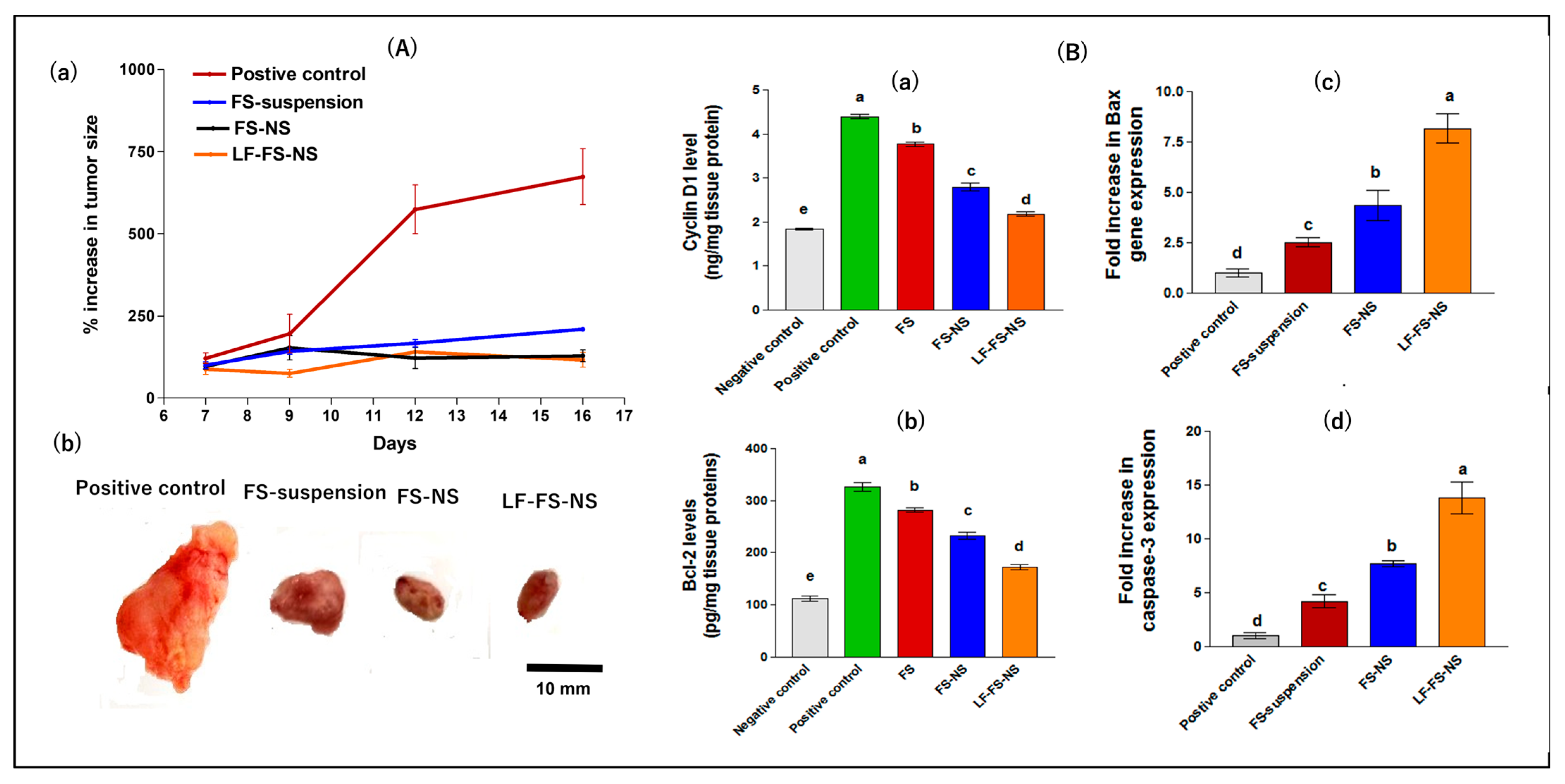
Tumor Growth Inhibition
Assessment of Tumor Biomarkers
Histopathological Evaluation
3.5.3. In Vivo Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davankov, V.A.; Ilyin, M.M.; Tsyurupa, M.P.; Timofeeva, G.I.; Dubrovina, L.V. From a Dissolved Polystyrene Coil to an Intramolecularly-Hyper-Cross-Linked “Nanosponge”. Macromolecules 1996, 29, 8398–8403. [Google Scholar] [CrossRef]
- Anwer, M.K.; Fatima, F.; Ahmed, M.M.; Aldawsari, M.F.; Alali, A.S.; Kalam, M.A.; Alshamsan, A.; Alkholief, M.; Malik, A.; Az, A.; et al. Abemaciclib-loaded ethylcellulose based nanosponges for sustained cytotoxicity against MCF-7 and MDA-MB-231 human breast cancer cells lines. Saudi Pharm. J. 2022, 30, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Carboxylate cross-linked cyclodextrin: A nanoporous scaffold for enhancement of rosuvastatin oral bioavailability. Eur. J. Pharm. Sci. 2018, 111, 1–12. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Asela, I.; Donoso-Gonzalez, O.; Yutronic, N.; Sierpe, R. beta-Cyclodextrin-Based Nanosponges Functionalized with Drugs and Gold Nanoparticles. Pharmaceutics 2021, 13, 513. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for Drug Delivery and Cancer Therapy: Recent Advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef]
- Abou Taleb, S.; Moatasim, Y.; GabAllah, M.; Asfour, M.H. Quercitrin loaded cyclodextrin based nanosponge as a promising approach for management of lung cancer and COVID-19. J. Drug Deliv. Sci. Technol. 2022, 77, 103921. [Google Scholar] [CrossRef]
- Kapil, G.; Sankha, B.; Bhupendra, P. Recent Pharmaceutical Developments in the Treatment of Cancer Using Nanosponges. In Advanced Drug Delivery Systems; Chapter 4; Bhupendra, P., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Anwer, M.K.; Ahmed, M.M.; Aldawsari, M.F.; Iqbal, M.; Kumar, V. Preparation and Evaluation of Diosmin-Loaded Diphenylcarbonate-Cross-Linked Cyclodextrin Nanosponges for Breast Cancer Therapy. Pharmaceuticals 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Khazaei Monfared, Y.; Mahmoudian, M.; Cecone, C.; Caldera, F.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers 2022, 14, 594. [Google Scholar] [CrossRef]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the Anti-Tumor Efficacy of Doxorubicin Nanosponges in In Vitro and in Mice Bearing Breast Tumor Models. Cancers 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Benoni, G.; Cuzzolin, L. Safety and Efficacy of Phytomedicines in Cancer Prevention and Treatment. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 207–220. [Google Scholar]
- Vachetta, V.S.; Marder, M.; Troncoso, M.F.; Elola, M.T. Opportunities, obstacles and current challenges of flavonoids for luminal and triple-negative breast cancer therapy. Eur. J. Med. Chem. Rep. 2022, 6, 100077. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Kubina, R.; Krzykawski, K.; Kabala-Dzik, A.; Wojtyczka, R.D.; Chodurek, E.; Dziedzic, A. Fisetin, a Potent Anticancer Flavonol Exhibiting Cytotoxic Activity against Neoplastic Malignant Cells and Cancerous Conditions: A Scoping, Comprehensive Review. Nutrients 2022, 14, 2604. [Google Scholar] [CrossRef]
- Li, J.; Gong, X.; Jiang, R.; Lin, D.; Zhou, T.; Zhang, A.; Li, H.; Zhang, X.; Wan, J.; Kuang, G.; et al. Fisetin Inhibited Growth and Metastasis of Triple-Negative Breast Cancer by Reversing Epithelial-to-Mesenchymal Transition via PTEN/Akt/GSK3β Signal Pathway. Front. Pharmacol. 2018, 9, 772. [Google Scholar] [CrossRef]
- Khozooei, S.; Lettau, K.; Barletta, F.; Jost, T.; Rebholz, S.; Veerappan, S.; Franz-Wachtel, M.; Macek, B.; Iliakis, G.; Distel, L.V.; et al. Fisetin induces DNA double-strand break and interferes with the repair of radiation-induced damage to radiosensitize triple negative breast cancer cells. J. Exp. Clin. Cancer Res. 2022, 41, 256. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Gilani, S.A.; Shariati, M.A.; Imran, A.; Afzaal, M.; Atif, M.; Tufail, T.; Anjum, F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021, 9, 3–16. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef]
- Krishnakumar, I.M.; Jaja-Chimedza, A.; Joseph, A.; Balakrishnan, A.; Maliakel, B.; Swick, A. Enhanced bioavailability and pharmacokinetics of a novel hybrid-hydrogel formulation of fisetin orally administered in healthy individuals: A randomised double-blinded comparative crossover study. J. Nutr. Sci. 2022, 11, e74. [Google Scholar] [CrossRef] [PubMed]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Talaat, S.M.; Elnaggar, Y.S.R.; El-Ganainy, S.O.; Gowayed, M.A.; Abdel-Bary, A.; Abdallah, O.Y. Novel bio-inspired lipid nanoparticles for improving the anti-tumoral efficacy of fisetin against breast cancer. Int. J. Pharm. 2022, 628, 122184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Kumar, H.; Bhatt, T.; Jain, R.; Panchal, K.; Chaurasiya, A.; Jain, V. Fisetin in Cancer: Attributes, Developmental Aspects, and Nanotherapeutics. Pharmaceuticals 2023, 16, 196. [Google Scholar] [CrossRef]
- Halder, A.; Jethwa, M.; Mukherjee, P.; Ghosh, S.; Das, S.; Helal Uddin, A.B.M.; Mukherjee, A.; Chatterji, U.; Roy, P. Lactoferrin-tethered betulinic acid nanoparticles promote rapid delivery and cell death in triple negative breast and laryngeal cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1362–1371. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Garrido, B.; González, S.; Hermosilla, J.; Millao, S.; Quilaqueo, M.; Guineo, J.; Acevedo, F.; Pesenti, H.; Rolleri, A.; Shene, C.; et al. Carbonate-β-Cyclodextrin-Based Nanosponge as a Nanoencapsulation System for Piperine: Physicochemical Characterization. J. Soil Sci. Plant Nutr. 2019, 19, 620–630. [Google Scholar] [CrossRef]
- El-Habashy, S.; Eltaher, H.; Gaballah, A.; Mehanna, R.; El-Kamel, A.H. Biomaterial-Based Nanocomposite for Osteogenic Repurposing of Doxycycline. Int. J. Nanomed. 2021, 16, 1103–1126. [Google Scholar] [CrossRef]
- Shende, P.K.; Trotta, F.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Biasizzo, M. Influence of different techniques on formulation and comparative characterization of inclusion complexes of ASA with β-cyclodextrin and inclusion complexes of ASA with PMDA cross-linked β-cyclodextrin nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 447–454. [Google Scholar] [CrossRef]
- Kharouba, M.; El-Kamel, A.; Mehanna, R.; Thabet, E.; Heikal, L. Pitavastatin-loaded bilosomes for oral treatment of hepatocellular carcinoma: A repurposing approach. Drug Deliv. 2022, 29, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.M.M.; Gowayed, M.A.; El-Ganainy, S.O.; Sheta, E.; Elnaggar, Y.S.R.; Abdallah, O.Y. Pectin coated nanostructured lipid carriers for targeted piperine delivery to hepatocellular carcinoma. Int. J. Pharm. 2022, 619, 121712. [Google Scholar] [CrossRef]
- Lu, Q.; Lai, Y.; Zhang, H.; Ren, K.; Liu, W.; An, Y.; Yao, J.; Fan, H. Hesperetin Inhibits TGF-β1-Induced Migration and Invasion of Triple Negative Breast Cancer MDA-MB-231 Cells via Suppressing Fyn/Paxillin/RhoA Pathway. Integr. Cancer Ther. 2022, 21, 15347354221086900. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.E.-S.; El-Moslemany, R.M.; Mehanna, R.A.; Thabet, E.H.; Abdelfattah, E.-Z.A.; El-Kamel, A. Chitosan-coated bovine serum albumin nanoparticles for topical tetrandrine delivery in glaucoma: In vitro and in vivo assessment. Drug Deliv. 2022, 29, 1150–1163. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Kapoor, B.; Vyas, M.; Khursheed, R.; et al. Enhanced oral bioavailability and neuroprotective effect of fisetin through its SNEDDS against rotenone-induced Parkinson’s disease rat model. Food Chem. Toxicol. 2020, 144, 111590. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Zidan, M.F.; Ibrahim, H.M.; Afouna, M.I.; Ibrahim, E.A. In vitro and in vivo evaluation of cyclodextrin-based nanosponges for enhancing oral bioavailability of atorvastatin calcium. Drug Dev. Ind. Pharm. 2018, 44, 1243–1253. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Sharma, K.; Kadian, V.; Kumar, A.; Mahant, S.; Rao, R. Evaluation of solubility, photostability and antioxidant activity of ellagic acid cyclodextrin nanosponges fabricated by melt method and microwave-assisted synthesis. J. Food Sci. Technol. 2022, 59, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Gholibegloo, E.; Mortezazadeh, T.; Salehian, F.; Ramazani, A.; Amanlou, M.; Khoobi, M. Improved curcumin loading, release, solubility and toxicity by tuning the molar ratio of cross-linker to β-cyclodextrin. Carbohydr. Polym. 2019, 213, 70–78. [Google Scholar] [CrossRef]
- Shilpi, S.; Vimal, V.D.; Soni, V. Assessment of lactoferrin-conjugated solid lipid nanoparticles for efficient targeting to the lung. Prog. Biomater. 2015, 4, 55–63. [Google Scholar] [CrossRef]
- Zhang, J.-q.; Jiang, K.-m.; An, K.; Ren, S.-H.; Xie, X.-g.; Jin, Y.; Lin, J. Novel water-soluble fisetin/cyclodextrins inclusion complexes: Preparation, characterization, molecular docking and bioavailability. Carbohydr. Res. 2015, 418, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Q.; Ahmad, M.; Minhas, M.U.; Batool, F.; Malik, N.S.; Rehman, M. Novel β-cyclodextrin nanosponges by chain growth condensation for solubility enhancement of dexibuprofen: Characterization and acute oral toxicity studies. J. Drug Deliv. Sci. Technol. 2021, 61, 102089. [Google Scholar] [CrossRef]
- Smith, M.L.; Murphy, K.; Doucette, C.D.; Greenshields, A.L.; Hoskin, D.W. The Dietary Flavonoid Fisetin Causes Cell Cycle Arrest, Caspase-Dependent Apoptosis, and Enhanced Cytotoxicity of Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells. J. Cell Biochem. 2016, 117, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G.; et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Zhu, J.; Gao, Z.; Lai, X.; Zhu, X.; Mao, G. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomed. 2018, 13, 3039–3051. [Google Scholar] [CrossRef]
- Pereira, C.S.; Guedes, J.P.; Gonçalves, M.; Loureiro, L.; Castro, L.; Gerós, H.; Rodrigues, L.R.; Côrte-Real, M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget 2016, 7, 62144–62158. [Google Scholar] [CrossRef]
- Shahi Thakuri, P.; Gupta, M.; Singh, S.; Joshi, R.; Glasgow, E.; Lekan, A.; Agarwal, S.; Luker, G.D.; Tavana, H. Phytochemicals inhibit migration of triple negative breast cancer cells by targeting kinase signaling. BMC Cancer 2020, 20, 4. [Google Scholar] [CrossRef]
- Abdelmoneem, M.A.; Abd Elwakil, M.M.; Khattab, S.N.; Helmy, M.W.; Bekhit, A.A.; Abdulkader, M.A.; Zaky, A.; Teleb, M.; Elkhodairy, K.A.; Albericio, F.; et al. Lactoferrin-dual drug nanoconjugate: Synergistic anti-tumor efficacy of docetaxel and the NF-κB inhibitor celastrol. Mater. Sci. Eng. C 2021, 118, 111422. [Google Scholar] [CrossRef]
- Du, X.J.; Wang, J.L.; Iqbal, S.; Li, H.J.; Cao, Z.T.; Wang, Y.C.; Du, J.Z.; Wang, J. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater. Sci. 2018, 6, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Chellampillai, B.; Pawar, A.P. Improved bioavailability of orally administered andrographolide from pH-sensitive nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 2011, 35, 123–129. [Google Scholar] [CrossRef] [PubMed]
- El-Lakany, S.A.; Elgindy, N.A.; Helmy, M.W.; Abu-Serie, M.M.; Elzoghby, A.O. Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expert Opin. Drug Deliv. 2018, 15, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.P.; Trotta, F.; Kushwah, V.; Devasari, N.; Singh, C.; Suresh, S.; Jain, S. Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr. Polym. 2016, 137, 339–349. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Claassen, V. Neglected Factors in Pharmacology and Neuroscience Research: Biopharmaceutics, Animal Characteristics, Maintenance, Testing Conditions, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 12. [Google Scholar]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef] [PubMed]
- El-Far, S.W.; Helmy, M.W.; Khattab, S.N.; Bekhit, A.A.; Hussein, A.A.; Elzoghby, A.O. Phytosomal bilayer-enveloped casein micelles for codelivery of monascus yellow pigments and resveratrol to breast cancer. Nanomedicine 2018, 13, 481–499. [Google Scholar] [CrossRef]
- Allahyari, S.; Zahednezhad, F.; Khatami, M.; Hashemzadeh, N.; Zakeri-Milani, P.; Trotta, F. Cyclodextrin nanosponges as potential anticancer drug delivery systems to be introduced into the market, compared with liposomes. J. Drug Deliv. Sci. Technol. 2022, 67, 102931. [Google Scholar] [CrossRef]
- Jasim, I.K.; Abd Alhammid, S.N.; Abdulrasool, A.A. Synthesis and evaluation of B-cyclodextrin Based Nanosponges of 5- Fluorouracil by Using Ultrasound Assisted Method. Iraqi J. Pharm. Sci. 2020, 29, 88–98. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wu, B. The Association between Splenocyte Apoptosis and Alterations of Bax, Bcl-2 and Caspase-3 mRNA Expression, and Oxidative Stress Induced by Dietary Nickel Chloride in Broilers. Int. J. Environ. Res. Public Health 2013, 10, 7310–7326. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shen, S.C.; Lee, W.R.; Lin, H.Y.; Ko, C.H.; Shih, C.M.; Yang, L.L. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch. Toxicol. 2002, 76, 351–359. [Google Scholar] [CrossRef]
- Monfared, Y.K.; Pedrazzo, A.R.; Mahmoudian, M.; Caldera, F.; Zakeri-Milani, P.; Valizadeh, H.; Cavalli, R.; Matencio, A.; Trotta, F. Oral supplementation of solvent-free kynurenic acid/cyclodextrin nanosponges complexes increased its bioavailability. Colloids Surf. B Biointerfaces 2023, 222, 113101. [Google Scholar] [CrossRef]
- Khazaei Monfared, Y.; Mahmoudian, M.; Caldera, F.; Pedrazzo, A.R.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Nisin delivery by nanosponges increases its anticancer activity against in-vivo melanoma model. J. Drug Deliv. Sci. Technol. 2023, 79, 104065. [Google Scholar] [CrossRef]
- Alsawaf, S.; Alnuaimi, F.; Afzal, S.; Thomas, R.M.; Chelakkot, A.L.; Ramadan, W.S.; Hodeify, R.; Matar, R.; Merheb, M.; Siddiqui, S.S.; et al. Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology 2022, 11, 1717. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward | Reverse |
|---|---|---|
| Bax | GCTGACATGTTTGCTGATGG | GATCAGCTCGGGCACTTTAG |
| Caspase-3 | AGGGGTCATTTATGGGACA | TACACGGGATCTGTTTCTTTG |
| GAPDH | TCACCACCATGGAGAAGGC | GCTAAGCAGTTGGTGGTGCA |
| Formulation Code | DMF Volume (mL) | Reaction Temperature (°C) | Reaction Time (h) | Ferric Chloride Test | Gelification |
|---|---|---|---|---|---|
| F1 | 3 | 90 | 2 | Negative | No |
| F2 | 3 | 90 | 5 | Negative | No |
| F3 | 3 | 120 | 2 | Negative | No |
| F4 | 3 | 120 | 5 | Negative | No |
| F5 | 6 | 90 | 2 | Negative | No |
| F6 | 6 | 90 | 5 | Positive | Yes |
| F7 | 6 | 120 | 2 | Positive | Yes |
| F8 | 6 | 120 | 5 | Positive | Yes |
| F9 | 6 | 150 | 2 | Positive | Yes |
| F10 | 6 | 150 | 5 | Positive | Yes |
| Formulation | Size (nm) | PDI | ζ-Potential (mV) | EE% | DL% |
|---|---|---|---|---|---|
| NS | 46.1 ± 6.2 | 0.13 | −22 ± 1.8 | - | - |
| FS-NS | 38.2 ± 3.8 | 0.19 | −26 ± 6.5 | 96.1 ± 0.3 | 23.8 ± 0.2 |
| LF-FS-NS | 52.7 ± 7.2 | 0.09 | 24 ± 1.1 | 95.9 ± 0.2 | - |
| Oral | Intraperitoneal | |||||
|---|---|---|---|---|---|---|
| Parameter | FS Suspension | FS-NS | LF-FS-NS | FS Suspension | FS-NS | LF-FS-NS |
| t1/2 (h) | 7.2 c ± 1.5 | 15.4 a ± 3.5 | 11.2 b± 2.1 | 8.5 c ± 2.1 | 17.7 a ± 2.5 | 12.2 b ± 1.2 |
| Tmax (h) | 1 | 1 | 0.25 | 0.25 | 1 | 0.25 |
| Cmax (ng/mL) | 24.3 c ± 4.6 | 41.3 b ± 7.4 | 56.2 a ± 5.9 | 509.8 c ± 31.6 | 1622.4 a ± 242.9 | 862.9 b ± 60.1 |
| AUC 0–24 (ng/mL × h) | 135.1 b ± 4.2 | 312.1a ± 116.8 | 274.1 a ± 18.5 | 2538.8 b ± 121.6 | 8474.5 a ± 1834.9 | 6160.1 a ± 121.9 |
| AUC 0–∞ (ng/mL x h) | 149.5 b ± 2.7 | 457.6 a ± 251.5 | 368.7 a ± 28.5 | 2874.6 c ± 254.1 | 12,371.5 a ± 2143.5 | 9126.1 b ± 565.1 |
| * Relative bioavailability | 3.1 | 2.5 | 4.3 | 3.2 | ||
| Group | ALT (U/mL) | AST (U/mL) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|
| Negative control | 85.5 ± 2.9 | 60.1 ± 5.5 | 43 ± 2.4 | 0.94 ± 0.1 |
| Positive control | 92.1 ± 5.1 | 60.5 ± 2.8 | 48 ± 3.8 | 1 ± 0.3 |
| FS suspension | 91.5 ± 4.3 | 61.6 ± 5.2 | 44 ± 3.5 | 1.07 ± 0.2 |
| FS-NS | 90.3 ± 6.2 | 62.2 ± 3.6 | 49.2 ± 4.6 | 1.05 ± 0.2 |
| LF-FS-NS | 91.8 ± 3.4 | 61.2 ± 6.1 | 47 ± 2.2 | 1.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboushanab, A.R.; El-Moslemany, R.M.; El-Kamel, A.H.; Mehanna, R.A.; Bakr, B.A.; Ashour, A.A. Targeted Fisetin-Encapsulated β-Cyclodextrin Nanosponges for Breast Cancer. Pharmaceutics 2023, 15, 1480. https://doi.org/10.3390/pharmaceutics15051480
Aboushanab AR, El-Moslemany RM, El-Kamel AH, Mehanna RA, Bakr BA, Ashour AA. Targeted Fisetin-Encapsulated β-Cyclodextrin Nanosponges for Breast Cancer. Pharmaceutics. 2023; 15(5):1480. https://doi.org/10.3390/pharmaceutics15051480
Chicago/Turabian StyleAboushanab, Alaa R., Riham M. El-Moslemany, Amal H. El-Kamel, Radwa A. Mehanna, Basant A. Bakr, and Asmaa A. Ashour. 2023. "Targeted Fisetin-Encapsulated β-Cyclodextrin Nanosponges for Breast Cancer" Pharmaceutics 15, no. 5: 1480. https://doi.org/10.3390/pharmaceutics15051480
APA StyleAboushanab, A. R., El-Moslemany, R. M., El-Kamel, A. H., Mehanna, R. A., Bakr, B. A., & Ashour, A. A. (2023). Targeted Fisetin-Encapsulated β-Cyclodextrin Nanosponges for Breast Cancer. Pharmaceutics, 15(5), 1480. https://doi.org/10.3390/pharmaceutics15051480