Smart Design of Nanostructures for Boosting Tumor Immunogenicity in Cancer Immunotherapy
Abstract
1. Introduction
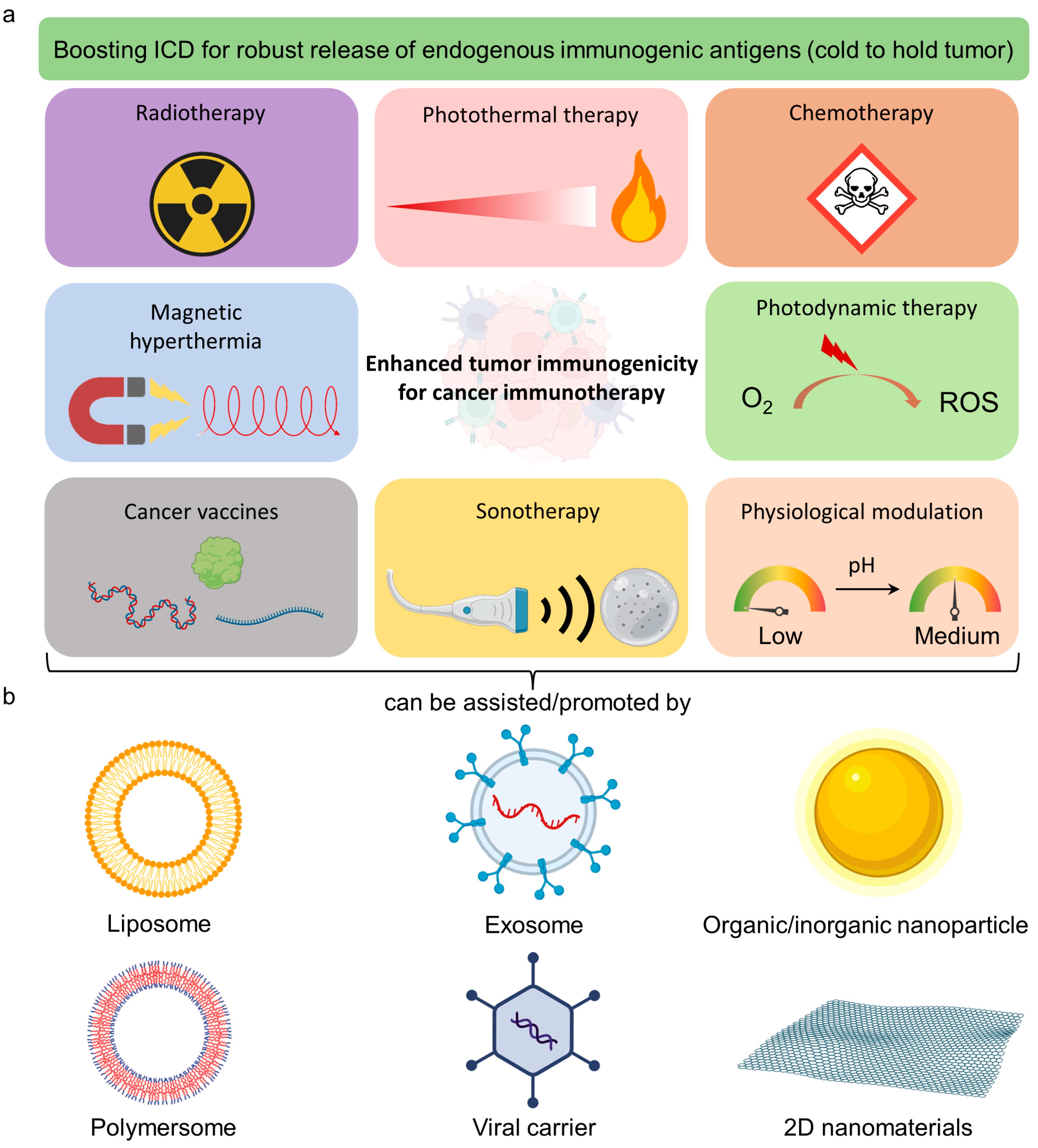
1.1. Emerging Immunogenic Boosters for Cancer and Their Limitations
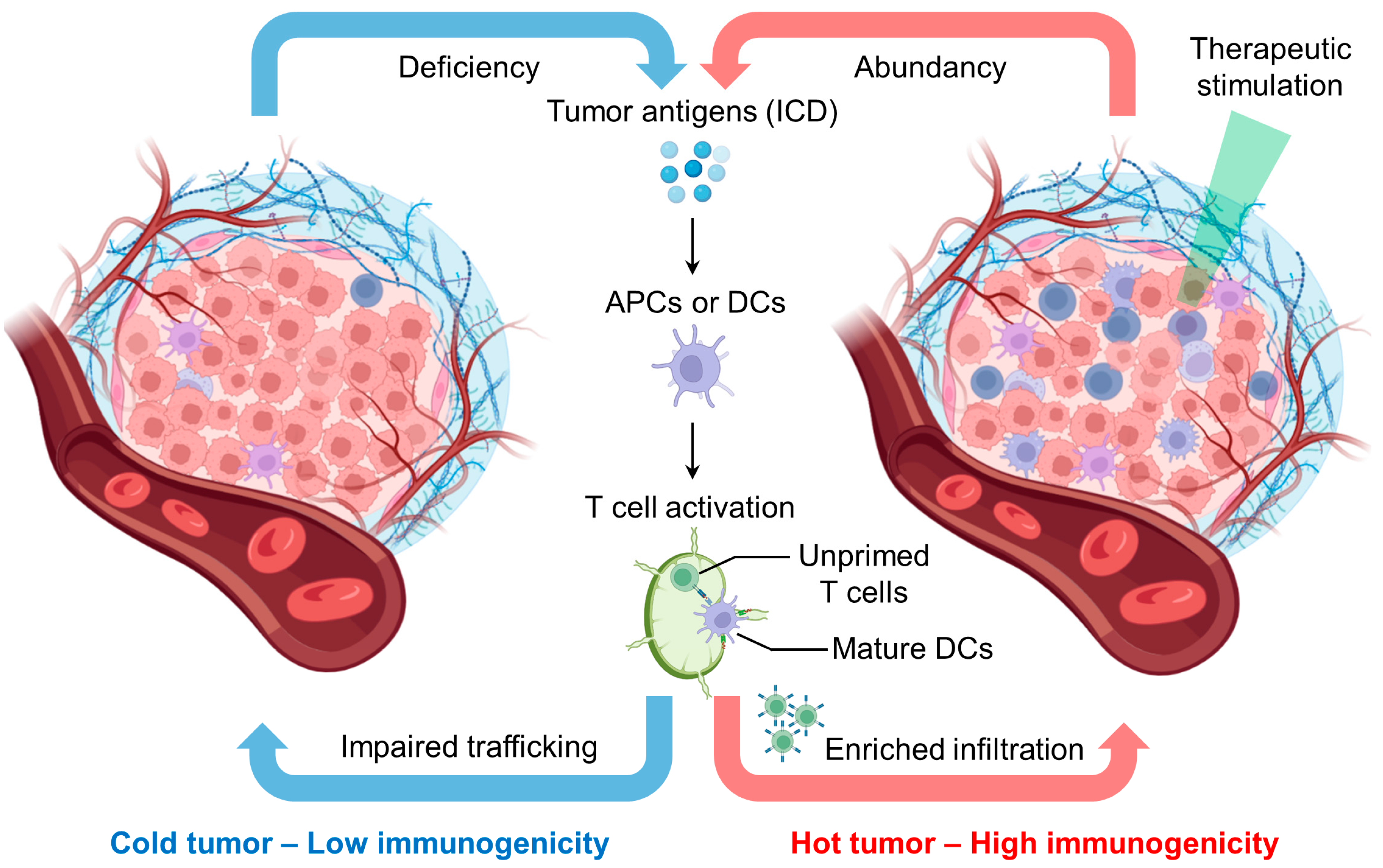
1.2. Reprogramming Tumor Immunogenicity for Enhanced Immunotherapy
1.3. Mechanism of Combined Therapy with ICB
2. Smart-Designed Nanobooster for Immunotherapy
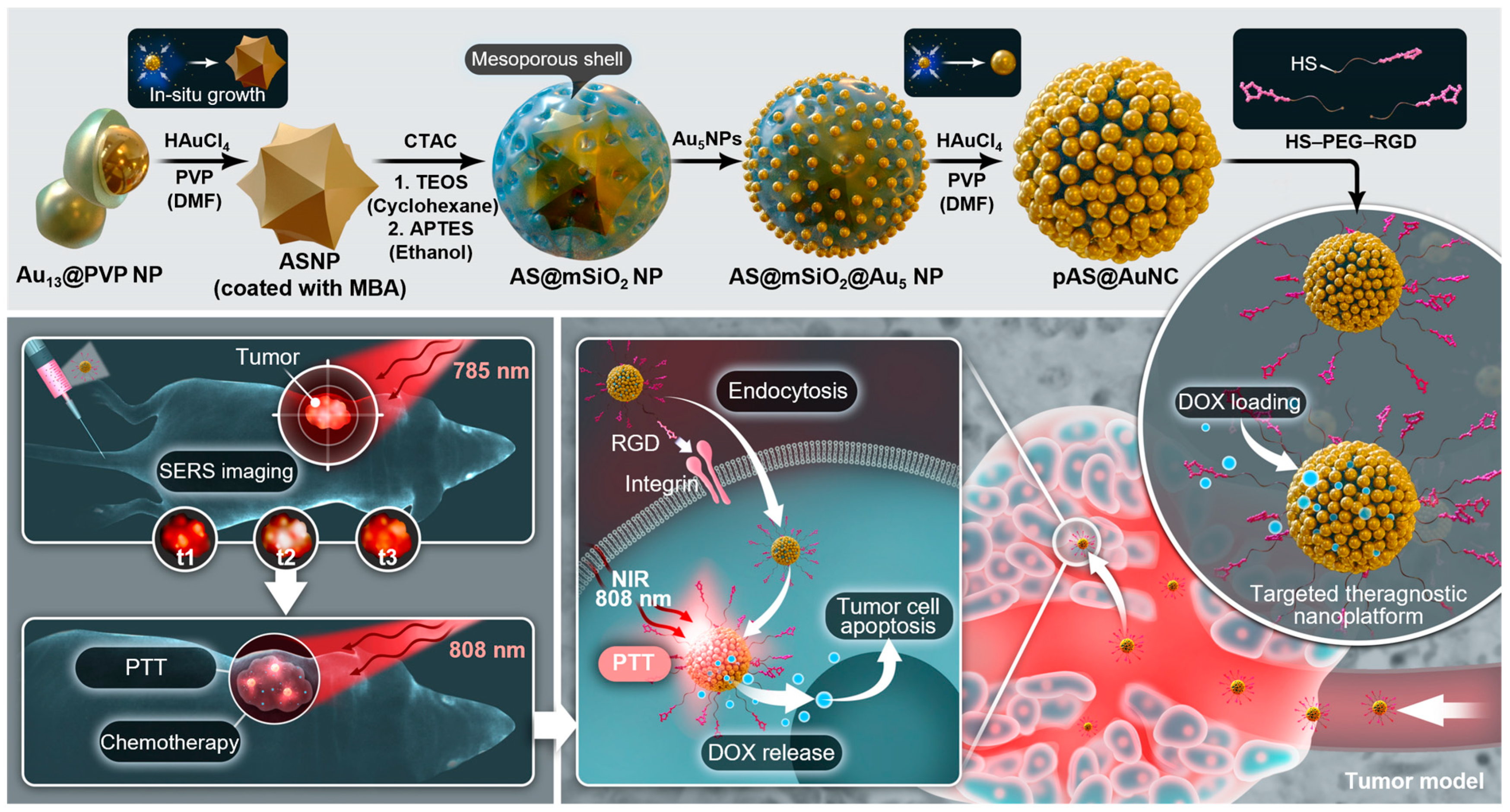
2.1. Unique Properties of Nanomaterials for Cancer Therapy
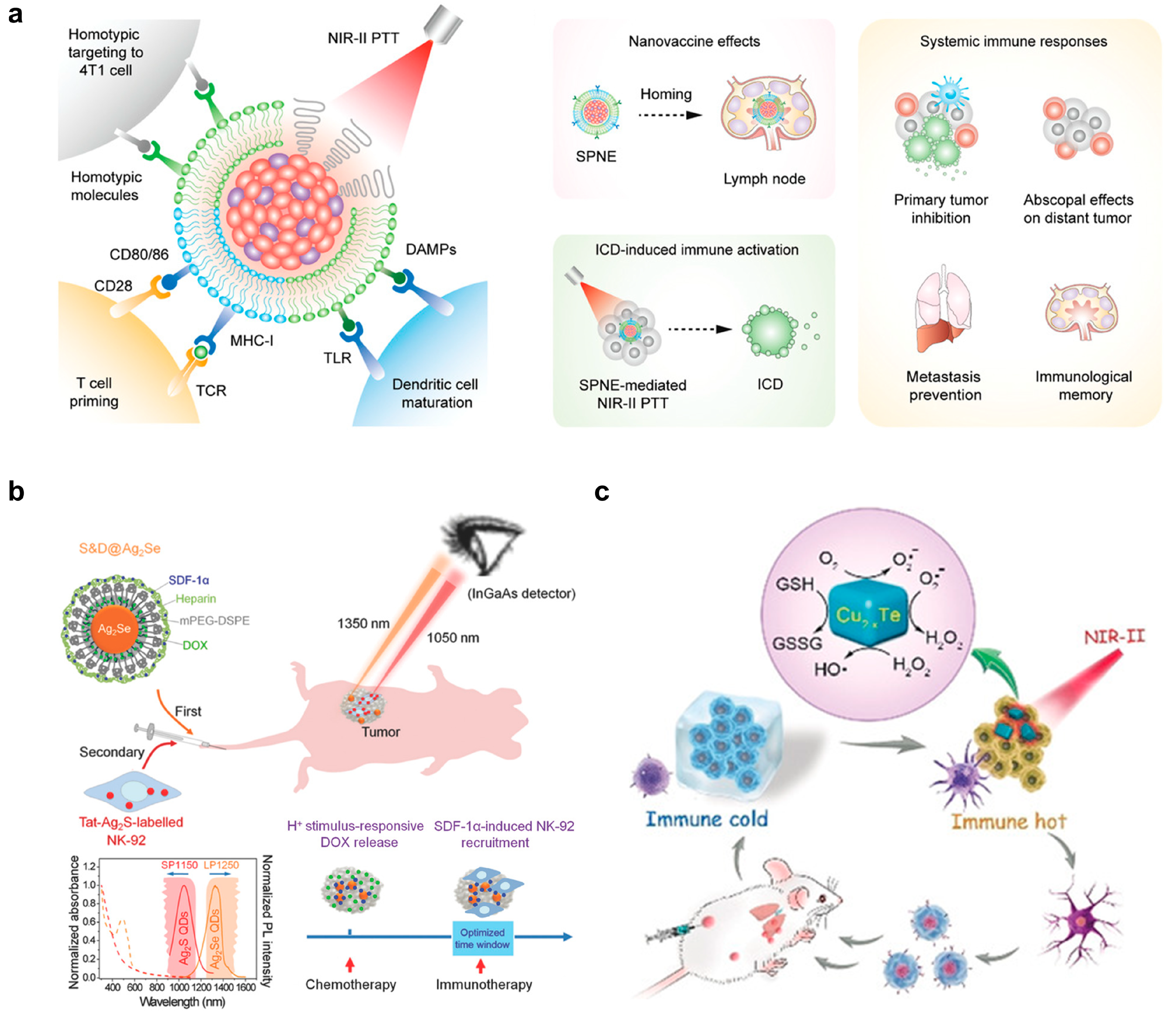
2.2. Light-Responsive Nanomaterials for Antitumor Therapy
2.3. pH-Responsive Nanomaterial for Antitumor Therapy
2.4. Nanocatalytic Activities for Tumor Therapy
2.5. Magnetic-Responsive Nanomaterials for Remote Control Antitumor Therapy

3. Conclusions
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIT | Cancer immunotherapy |
| ICB | Immune checkpoint blockade |
| CAR | Chimeric antigen receptor |
| CLTA4 | Cytotoxic T lymphocyte antigen 4 |
| PD1 | Programmed cell death 1 |
| PDL1 | Programmed cell death ligand 1 |
| FDA | Food and Drug Administration |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| ATP | Adenosine triphosphate |
| HMGB-1 | High-motility group box 1 |
| CRT | Calreticulin |
| APC | Antigen-presenting cell |
| DC | Dendritic cell |
| NK | natural killer |
| ICD | Immunogenic cell death |
| PEG | polyethylene) glycol |
| ROS | Reactive oxygen species |
| GSH) | Glutathione |
| MMP | Matrix metalloproteinase |
| ZnPcs | Zinc phthalocyanine |
| PLGA | polymers polylactic-co-glycolic acid) |
| TPP | Lipophilic triphenylphosphonium |
| DAMPs | Damage-associated molecules |
| AuNS | Gold nanostars |
| CRC | Colorectal Cancer |
| TSCC | Tongue Squamous Cell Carcinoma |
| NPt-Ca | Platinum Acetylacetonate with titania |
| NIR | Near-infrared |
| TLR | Toll-like receptor |
| pMHC | Peptide major histocompatibility complex |
| IL | Interleukin |
| PEGMA | Polyethylene glycol) methacrylate |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| DOX | Doxorubicin |
| LA | Lactic acid |
| LDHA | Lactate dehydrogenase A |
| DCA | Dichloroacetate |
| GOx | Glucose oxidase |
| AQ4N | Banoxantrone dihydrochloride |
| TCA | Tricarboxylic acid |
| CHC | α-cyano-4-hydroxycinnamate |
| γ-Fe2O3 | Maghemite |
| Fe3O4 | Magnetite |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| MRI | Magnetic resonance imaging |
| MH | Magnetic hyperthermia |
| AMF | Alternating magnetic field |
| MINP | Magnetic-responsive immunostimulatory nanoagent |
| CpG ODNs | Cytosine-phosphate-guanine oligodeoxynucleotides |
| PA | Photoacoustic |
| ZIF-8 | Zeolitic imidazolate framework-8 |
| ICG | Indocyanine green |
| OVA | Ovalbumin |
| cpMF | Circularly polarized magnetic field |
| HCSV | Hybrid core–shell vesicle |
| AA | Ascorbic acids |
| IONCs | Iron oxide nanocubes |
| UVB | Ultra-violet B |
| mPEG | Methoxy PEG |
| DSPE | 2-distearoyl-sn-glycero-3-phosphoethanolamine |
References
- Carlson, R.D.; Flickinger, J.C., Jr.; Snook, A.E. Talkin’ Toxins: From Coley’s to Modern Cancer Immunotherapy. Toxins 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G.; et al. A Bi-adjuvant Nanovaccine that Potentiates Immunogenicity of Neoantigen for Combination Immunotherapy of Colorectal Cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef] [PubMed]
- Finck, A.; Gill, S.I.; June, C.H. Cancer Immunotherapy Comes of Age and Looks for Maturity. Nat. Commun. 2020, 11, 3325. [Google Scholar] [CrossRef] [PubMed]
- Adamik, J.; Butterfield, L.H. What’s Next for Cancer Vaccines. Sci. Transl. Med. 2022, 14, eabo4632. [Google Scholar] [CrossRef]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In Vivo Characterization of the Physicochemical Properties of Polymer-Linked TLR Agonists that Enhance Vaccine Immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart Cancer Nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- The Two Directions of Cancer Nanomedicine. Nat. Nanotechnol. 2019, 14, 1083. [CrossRef]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The Foundations of Immune Checkpoint Blockade and the Ipilimumab Approval Decennial. Nat. Rev. Drug Discov. 2022, 21, 509–528. [Google Scholar] [CrossRef]
- Wong, W.K.; Yin, B.; Lam, C.Y.K.; Huang, Y.; Yan, J.; Tan, Z.; Wong, S.H.D. The Interplay between Epigenetic Regulation and CD8+ T Cell Differentiation/Exhaustion for T Cell Immunotherapy. Front. Cell Dev. Biol. 2022, 9, 3839. [Google Scholar] [CrossRef]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is Autoimmunity the Achilles’ Heel of Cancer Immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti–PD-1/PD-L1 Therapy of Human Cancer: Past, Present, and Future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Maus, M.V.; Plesa, G.; Johnson, L.A.; Zhao, Y.; Levine, B.L.; Grupp, S.A.; Porter, D.L. Engineered T Cells for Cancer Therapy. Cancer Immunol. Immunother. 2014, 63, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T Cells: The Promise and Challenges of Cancer Immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- Yin, B.; Ho, W.K.H.; Xia, X.; Chan, C.K.W.; Zhang, Q.; Ng, Y.M.; Lam, C.Y.K.; Cheung, J.C.W.; Wang, J.; Yang, M.; et al. A Multilayered Mesoporous Gold Nanoarchitecture for Ultraeffective Near-Infrared Light-Controlled Chemo/Photothermal Therapy for Cancer Guided by SERS Imaging. Small 2023, 19, e2206762. [Google Scholar] [CrossRef]
- Huang, J.; Yang, B.; Peng, Y.; Huang, J.; Wong, S.H.D.; Bian, L.; Zhu, K.; Shuai, X.; Han, S. Nanomedicine-Boosting Tumor Immunogenicity for Enhanced Immunotherapy. Adv. Funct. Mater. 2021, 31, 2011171. [Google Scholar] [CrossRef]
- Lam, C.Y.K.; Zhang, Q.; Yin, B.H.; Huang, Y.Y.; Wang, H.; Yang, M.; Wong, S.H.D. Recent Advances in Two-Dimensional Transition Metal Dichalcogenide Nanocomposites Biosensors for Virus Detection before and during COVID-19 Outbreak. J. Comp. Sci. 2021, 5, 190. [Google Scholar] [CrossRef]
- Wong, W.K.; Yin, B.; Rakhmatullina, A.; Zhou, J.; Wong, S.H.D. Engineering Advanced Dynamic Biomaterials to Optimize Adoptive T-Cell Immunotherapy. Eng. Regen. 2021, 2, 70–81. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning Cold Tumors Hot: From Molecular Mechanisms to Clinical Applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Z.J. Turning Cold Tumors into Hot Tumors by Improving T-Cell Infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.E.; Cano-Mejia, J.; Fernandes, R. Photothermal Therapy Generates a Thermal Window of Immunogenic Cell Death in Neuroblastoma. Small 2018, 14, e1800678. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Jin, L.; Shen, S.; Huang, Y.; Li, D.; Yang, X. Corn-Like Au/Ag Nanorod-Mediated NIR-II Photothermal/Photodynamic Therapy Potentiates Immune Checkpoint Antibody Efficacy by Reprogramming the Cold Tumor Microenvironment. Biomaterials 2021, 268, 120582. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Schwartz, M.; Zhang, Y.; Rosenblatt, J.D. B Cell Regulation of the Anti-Tumor Response and Role in Carcinogenesis. J. Immunother. Cancer 2016, 4, 40. [Google Scholar]
- Bruno, A.; Ferlazzo, G.; Albini, A.; Noonan, D.M. A think Tank of TINK/TANKs: Tumor-Infiltrating/Tumor-Associated Natural Killer Cells in Tumor Progression and Angiogenesis. J. Natl. Cancer Inst. 2014, 106, dju200. [Google Scholar] [CrossRef]
- Yin, B.; Chan, C.K.W.; Liu, S.; Hong, H.; Wong, S.H.D.; Lee, L.K.C.; Ho, L.W.C.; Zhang, L.; Leung, K.C.; Choi, P.C.; et al. Intrapulmonary Cellular-Level Distribution of Inhaled Nanoparticles with Defined Functional Groups and Its Correlations with Protein Corona and Inflammatory Response. ACS Nano 2019, 13, 14048–14069. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y.; Liu, X.; Fan, Y.; Zhang, Y.; Yi, C.; Cheng, C.; Yang, M. Near-Infrared Photothermally Enhanced Photo-Oxygenation for Inhibition of Amyloid-β Aggregation Based on RVG-Conjugated Porphyrinic Metal–Organic Framework and Indocyanine Green Nanoplatform. Int. J. Mol. Sci. 2022, 23, 10885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Oudeng, G.; Feng, H.; Liu, S.; Li, H.W.; Ho, Y.P.; Chen, Y.; Tan, Y.; Yang, M. 2D MOF Nanosensor-Integrated Digital Droplet Microfluidic Flow Cytometry for In Situ Detection of Multiple miRNAs in Single CTC Cells. Small 2022, 18, 2201779. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ho, L.W.C.; Liu, S.; Hong, H.; Tian, X.Y.; Li, H.; Choi, C.H.J. Sub-10 nm Substrate Roughness Promotes the Cellular Uptake of Nanoparticles by Upregulating Endocytosis-Related Genes. Nano Lett. 2021, 21, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting Kidney Mesangium by Nanoparticles of Defined Size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef]
- Yin, B.; Yang, H.; Yang, M. Integrating Soft Hydrogel with Nanostructures Reinforces Stem Cell Adhesion and Differentiation. J. Compos. Sci. 2022, 6, 19. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart Drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, G.; Li, W.; Wang, J.; Ren, H.; Zhao, Y. Stimuli-Responsive Gene Delivery Nanocarriers for Cancer Therapy. Nanomicro Lett. 2023, 15, 44. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef]
- Shen, H.; Sun, T.; Hoang, H.H.; Burchfield, J.S.; Hamilton, G.F.; Mittendorf, E.A.; Ferrari, M. In Enhancing Cancer Immunotherapy Through Nanotechnology-Mediated Tumor Infiltration and Activation of Immune Cells. Semin. Immunol. 2017, 34, 114–122. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, S.; Chatterjee, S.; Das, J.; Sil, P.C. Nanoparticles as Smart Carriers for Enhanced Cancer Immunotherapy. Front. Chem. 2020, 8, 597806. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, e1902333. [Google Scholar] [CrossRef]
- Wu, M.; Niu, X.; Zhang, R.; Ping Xu, Z. Two-Dimensional Nanomaterials for Tumor Microenvironment Modulation and Anticancer Therapy. Adv. Drug Deliv. Rev. 2022, 187, 114360. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing Factors and Strategies of Enhancing Nanoparticles into Tumors In Vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Wong, S.H.D.; Bian, L. Long-Term Detection of Oncogenic MicroRNA in Living Human Cancer Cells by Gold@ Polydopamine-Shell Nanoprobe. ACS Biomater. Sci. Eng. 2020, 6, 3778–3783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Cao, K.L.; Chen, M.; Wu, L.M. Synthesis of UV-Responsive Self-Healing Microcapsules and Their Potential Application in Aerospace Coatings. ACS Appl. Mater. Inter. 2019, 11, 33314–33322. [Google Scholar] [CrossRef]
- Zhang, J.; Wong, S.H.D.; Wu, X.; Lei, H.; Qin, M.; Shi, P.; Wang, W.; Bian, L.; Cao, Y. Engineering Photoresponsive Ligand Tethers for Mechanical Regulation of Stem Cells. Adv. Mater. 2021, 33, e2105765. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, Y.; Du, X.; Tan, Y.; Chen, W.; Yang, M. Wavelength-Regulated Switchable Photoelectrochemical System for Concurrent Detection of Dual Antibiotics. Biosens. Bioelectron. 2022, 202, 113999. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, B.; Hao, J.; Ma, L.; Huang, Y.; Shao, X.; Li, C.; Chu, Z.; Yi, C.; Wong, S.H.D. An AIEgen/Graphene Oxide Nanocomposite (AIEgen@ GO)-Based Two-Stage “Turn-On” Nucleic Acid Biosensor for Rapid Detection of SARS-CoV-2 Viral Sequence. Aggregate 2022, 4, e195. [Google Scholar] [CrossRef]
- Yin, C.; Wen, G.; Liu, C.; Yang, B.; Lin, S.; Huang, J.; Zhao, P.; Wong, S.H.D.; Zhang, K.; Chen, X.; et al. Organic Semiconducting Polymer Nanoparticles for Photoacoustic Labeling and Tracking of Stem Cells in the Second Near-Infrared Window. ACS Nano 2018, 12, 12201–12211. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman Spectroscopy to Characterize Biological Materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhao, X.H.; Huang, J.G.; Li, J.C.; Upputuri, P.K.; Sun, H.; Han, X.; Pramanik, M.; Miao, Y.S.; Duan, H.W.; et al. Transformable Hybrid Semiconducting Polymer Nanozyme for Second Near-Infrared Photothermal Ferrotherapy. Nat. Commun. 2020, 11, 1857. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Y.; Han, Y.; Pu, K.; Zhang, R. A Polymer Multicellular Nanoengager for Synergistic NIR-II Photothermal Immunotherapy. Adv. Mater. 2021, 33, e2008061. [Google Scholar] [CrossRef]
- Hao, X.; Li, C.; Zhang, Y.; Wang, H.; Chen, G.; Wang, M.; Wang, Q. Programmable Chemotherapy and Immunotherapy against Breast Cancer Guided by Multiplexed Fluorescence Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, e1804437. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, L.; Wang, Y.; Chen, Y.; Shi, J. Nanocatalytic Tumor Therapy by Single-Atom Catalysts. ACS Nano 2019, 13, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Ouyang, J.; Wei, C.; Li, H.; Chen, W.; Liu, Y.N. Artificial Enzyme Catalyzed Cascade Reactions: Antitumor Immunotherapy Reinforced by NIR-II Light. Angew. Chem. Int. Ed. Engl. 2019, 58, 17425–17432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.; Chen, L. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef]
- Sau, T.K.; Rogach, A.L.; Jackel, F.; Klar, T.A.; Feldmann, J. Properties and Applications of Colloidal Nonspherical Noble Metal Nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef]
- Yin, B.; Ho, W.K.H.; Zhang, Q.; Li, C.; Huang, Y.; Yan, J.; Yang, H.; Hao, J.; Wong, S.H.D.; Yang, M. Magnetic-Responsive Surface-Enhanced Raman Scattering Platform with Tunable Hot Spot for Ultrasensitive Virus Nucleic Acid Detection. ACS Appl. Mater. Interfaces 2022, 14, 4714–4724. [Google Scholar] [CrossRef]
- Chen, S.; Lei, Q.; Qiu, W.-X.; Liu, L.-H.; Zheng, D.-W.; Fan, J.-X.; Rong, L.; Sun, Y.-X.; Zhang, X.-Z. Mitochondria-Targeting “Nanoheater” for Enhanced Photothermal/Chemo-Therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic Extracellular Microenvironment and Cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic Tumor Microenvironment and pH-Sensing G Protein-Coupled Receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Van Sluis, R.; Bhujwalla, Z.M.; Raghunand, N.; Ballesteros, P.; Alvarez, J.; Cerdan, S.; Galons, J.P.; Gillies, R.J. In Vivo Imaging of Extracellular pH Using 1H MRSI. Magn. Reson. Med. 1999, 41, 743–750. [Google Scholar] [CrossRef]
- Li, H.J.; Du, J.Z.; Liu, J.; Du, X.J.; Shen, S.; Zhu, Y.H.; Wang, X.; Ye, X.; Nie, S.; Wang, J. Smart Superstructures with Ultrahigh pH-Sensitivity for Targeting Acidic Tumor Microenvironment: Instantaneous Size Switching and Improved Tumor Penetration. ACS Nano 2016, 10, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chao, Y.; Zhao, H.; Zhou, X.; Zhang, F.; Zhang, Z.; Li, Z.; Pan, J.; Wang, J.; Chen, Q.; et al. Smart Nanomedicine to Enable Crossing Blood-Brain Barrier Delivery of Checkpoint Blockade Antibody for Immunotherapy of Glioma. ACS Nano 2022, 16, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-kappaB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, Z.; Wang, Y.; Huang, J.; An, Y.; Wang, X.; Shuai, X. Codelivery of Anti-PD-1 Antibody and Paclitaxel with Matrix Metalloproteinase and pH Dual-Sensitive Micelles for Enhanced Tumor Chemoimmunotherapy. Small 2020, 16, e1906832. [Google Scholar] [CrossRef]
- Stephan, S.B.; Taber, A.M.; Jileaeva, I.; Pegues, E.P.; Sentman, C.L.; Stephan, M.T. Biopolymer Implants Enhance the Efficacy of Adoptive T-cell Therapy. Nat. Biotechnol. 2015, 33, 97–101. [Google Scholar] [CrossRef]
- Oh, J.; Xia, X.; Wong, W.K.R.; Wong, S.H.D.; Yuan, W.; Wang, H.; Lai, C.H.N.; Tian, Y.; Ho, Y.P.; Zhang, H.; et al. The Effect of the Nanoparticle Shape on T Cell Activation. Small 2022, 18, e2107373. [Google Scholar] [CrossRef]
- Deeg, J.; Axmann, M.; Matic, J.; Liapis, A.; Depoil, D.; Afrose, J.; Curado, S.; Dustin, M.L.; Spatz, J.P. T Cell Activation is Determined by the Number of Presented Antigens. Nano Lett. 2013, 13, 5619–5626. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Muller, J.; Depoil, D.; Mayya, V.; Sheetz, M.P.; Dustin, M.L.; Wind, S.J. Full Control of Ligand Positioning Reveals Spatial Thresholds for T Cell Receptor Triggering. Nat. Nanotechnol. 2018, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, Y.; Wang, D.; Liu, J.; An, J.; Li, Y.; Ma, C.; Pei, Y.; Zhang, Z.; Liu, J.; et al. In Vivo Activation of T-Cell Proliferation by Regulating Cell Surface Receptor Clustering Using a pH-Driven Interlocked DNA Nano-Spring. Nano Lett. 2022, 22, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key Chemokines Direct Migration of Immune Cells in Solid Tumors. Cancer Gene Ther. 2022, 29, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Abraham, W.D.; Zheng, Y.; Bustamante Lopez, S.C.; Luo, S.S.; Irvine, D.J. Active Targeting of Chemotherapy to Disseminated Tumors Using Nanoparticle-Carrying T Cells. Sci. Transl. Med. 2015, 7, 291ra94. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Shin, T.H.; Lee, J.H.; Cho, M.H.; Kim, I.S.; Kim, J.W.; Jung, K.; Lee, I.S.; Cheon, J.; Park, K.I. Design of Magnetically Labeled Cells (Mag-Cells) for in Vivo Control of Stem Cell Migration and Differentiation. Nano Lett. 2018, 18, 838–845. [Google Scholar] [CrossRef]
- Wong, S.H.D.; Wong, W.K.R.; Lai, C.H.N.; Oh, J.; Li, Z.; Chen, X.; Yuan, W.; Bian, L. Soft Polymeric Matrix as a Macroscopic Cage for Magnetically Modulating Reversible Nanoscale Ligand Presentation. Nano Lett. 2020, 20, 3207–3216. [Google Scholar] [CrossRef]
- Nie, W.; Wei, W.; Zuo, L.; Lv, C.; Zhang, F.; Lu, G.H.; Li, F.; Wu, G.; Huang, L.L.; Xi, X.; et al. Magnetic Nanoclusters Armed with Responsive PD-1 Antibody Synergistically Improved Adoptive T-Cell Therapy for Solid Tumors. ACS Nano 2019, 13, 1469–1478. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Dong, X.; Wang, Q.S.; Zhu, D.; Mei, L.; Yan, H.; Lv, F. A Platelet Intelligent Vehicle with Navigation for Cancer Photothermal-Chemotherapy. ACS Nano 2022, 16, 6359–6371. [Google Scholar] [CrossRef]
- Wojtkowiak, J.W.; Verduzco, D.; Schramm, K.J.; Gillies, R.J. Drug Resistance and Cellular Adaptation to Tumor Acidic pH Microenvironment. Mol. Pharm. 2011, 8, 2032–2038. [Google Scholar] [CrossRef]
- Choi, S.Y.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer-Generated Lactic Acid: A Regulatory, Immunosuppressive Metabolite? J. Pathol. 2013, 230, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer Acidity: An Ultimate Frontier of Tumor Immune Escape and a Novel Target of Immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, P.; Pearce, E.L. Lactic Acid and Lactate: Revisiting the Physiological Roles in the Tumor Microenvironment. Trends. Immunol. 2022, 43, 969–977. [Google Scholar] [CrossRef]
- Angelin, A.; Gil-de-Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J.; Kopinski, P.K.; Wang, L.; et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017, 25, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Quinn, W.J.; Jiao, J.; TeSlaa, T.; Stadanlick, J.; Wang, Z.; Wang, L.; Akimova, T.; Angelin, A.; Schafer, P.M.; Cully, M.D.; et al. Lactate Limits T Cell Proliferation via the NAD(H) Redox State. Cell Rep. 2020, 33, 108500. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Koyama, S.; Itahashi, K.; Tanegashima, T.; Lin, Y.T.; Togashi, Y.; Kamada, T.; Irie, T.; Okumura, G.; Kono, H.; et al. Lactic Acid Promotes PD-1 Expression in Regulatory T Cells in Highly Glycolytic Tumor Microenvironments. Cancer Cell 2022, 40, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zhao, Y.Y.; Shen, J.; Sun, X.; Liu, Y.; Liu, H.; Wang, Y.; Wang, J. Nanoenabled Modulation of Acidic Tumor Microenvironment Reverses Anergy of Infiltrating T Cells and Potentiates Anti-PD-1 Therapy. Nano Lett. 2019, 19, 2774–2783. [Google Scholar] [CrossRef]
- Kolb, D.; Kolishetti, N.; Surnar, B.; Sarkar, S.; Guin, S.; Shah, A.S.; Dhar, S. Metabolic Modulation of the Tumor Microenvironment Leads to Multiple Checkpoint Inhibition and Immune Cell Infiltration. ACS Nano 2020, 14, 11055–11066. [Google Scholar] [CrossRef]
- Tian, L.R.; Lin, M.Z.; Zhong, H.H.; Cai, Y.J.; Li, B.; Xiao, Z.C.; Shuai, X.T. Nanodrug Regulates Lactic Acid Metabolism to Reprogram the Immunosuppressive Tumor Microenvironment for Enhanced Cancer Immunotherapy. Biomater. Sci. 2022, 10, 3892–3900. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Dong, C.; Shi, S. Glucose Oxidase-Related Cancer Therapies. Adv. Ther. 2020, 3, 2000110. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS Stress in Cancer Cells and Therapeutic Implications. Drug Resist. Updat. 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, J.; Gao, W. Glucose Oxidase-Polymer Nanogels for Synergistic Cancer-Starving and Oxidation Therapy. ACS Appl. Mater. Interfaces 2017, 9, 23528–23535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Feng, L.; Dong, Z.; Wang, L.; Liang, C.; Chen, J.; Ma, Q.; Zhang, R.; Chen, Q.; Wang, Y.; et al. Glucose & Oxygen Exhausting Liposomes for Combined Cancer Starvation and Hypoxia-Activated Therapy. Biomaterials 2018, 162, 123–131. [Google Scholar]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Yanxiang Guo, J.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced Cancer Starvation Therapy by Simultaneous Deprivation of Lactate and Glucose Using a MOF Nanoplatform. Adv. Sci. 2021, 8, e2101467. [Google Scholar] [CrossRef] [PubMed]
- Alphandery, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of Magnetosomes Extracted from AMB-1 Magnetotactic Bacteria for Application in Alternative Magnetic Field Cancer Therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef] [PubMed]
- Day, N.B.; Wixson, W.C.; Shields, C.W. Magnetic Systems for Cancer Immunotherapy. Acta Pharm. Sin. B 2021, 11, 2172–2196. [Google Scholar] [CrossRef]
- Guo, Y.; Ran, Y.; Wang, Z.; Cheng, J.; Cao, Y.; Yang, C.; Liu, F.; Ran, H. Magnetic-Responsive and Targeted Cancer Nanotheranostics by PA/MR Bimodal Imaging-Guided Photothermally Triggered Immunotherapy. Biomaterials 2019, 219, 119370. [Google Scholar] [CrossRef]
- Ridolfi, R.; Riccobon, A.; Galassi, R.; Giorgetti, G.; Petrini, M.; Fiammenghi, L.; Stefanelli, M.; Ridolfi, L.; Moretti, A.; Migliori, G.; et al. Evaluation of In Vivo Labelled Dendritic Cell Migration in Cancer Patients. J. Transl. Med. 2004, 2, 27. [Google Scholar] [CrossRef]
- Jin, H.L.; Qian, Y.; Dai, Y.F.; Qiao, S.; Huang, C.; Lu, L.S.; Luo, Q.M.; Chen, J.; Zhang, Z.H. Magnetic Enrichment of Dendritic Cell Vaccine in Lymph Node with Fluorescent-Magnetic Nanoparticles Enhanced Cancer Immunotherapy. Theranostics 2016, 6, 2000–2014. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.B.; Ni, D.L.; Zhang, S.J.; Li, Q.; Yao, Z.W.; Zhang, J.W.; Yao, H.L.; Wang, Z.; Shi, J.L. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. Int. Ed. 2016, 55, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Liu, T.; Li, Y.; Lau, J.; Yang, Z.; Fan, W.P.; Zhou, Z.J.; Shi, C.R.; Ke, C.M.; Bregadze, V.I.; et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 2018, 12, 11355–11365. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Choi, B.S.; Li, W.G.; Kim, D.H. Magnetic Field Boosted Ferroptosis-Like Cell Death and Responsive MRI Using Hybrid Vesicles for Cancer Immunotherapy. Nat. Commun. 2020, 11, 3637. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized Cancer Vaccine Effectively Mobilizes Antitumor T Cell Immunity in Ovarian Cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Claims | Cancer Types | Patent No. and Published Year |
|---|---|---|---|
| Nanoparticles core consisting of metal/semiconductor atoms such as Au, Ag, Cu, Pd, Pt, Gd, and/or Fe and bear specific peptide sequence, FKLQTMVKLFNRIKNNVA and other antigens | At least one adjuvant can stimulate the immune system (T helper cells) response for the prevention and treatment of cancer. | Colon, pancreas, gut, lung, liver, ovary, or bladder cancer | JP2008514686A, 2008 |
| A nanoparticle consists of a polypeptide, FAEKFKEAVKDYFAKFWDGSGLTVSFWYLTVSPWY, with a cholesterol lipid-modified fluorescent dye molecule (DiR-BOA or Fluo-B0A) | The nanoparticle shows synergistic targeting, diagnosis, and treatment of nasopharyngeal cancer by the specific peptide sequence, LTVSPWYLTVSPWY, and fluorescent imaging with enhanced NK cell activity in the tumor environment. | Nasopharyngeal carcinoma | WO2013181934A1, 2013 |
| Zinc phthalocyanine (ZnPcs) and polymers poly(lactic-co-glycolic acid) (PLGA), polyethyleneglycol (PEG), and a lipophilic triphenylphosphonium (TPP) cation nanoparticles loaded with mitochondrial-targeted moiety | This nanoparticle can target TPP moiety in cancer and induce PDT for the production of reactive oxygen species (ROS) under laser stimulation to activate dendritic cells. | Breast cancer | US20150374714A1, 2015 |
| Nanoparticles based on human serum albumin that encapsulates chlorin and catalase and bond with pegylated anti-HER2 nano antibody | The nanoparticle can improve tumor hypoxia and promote immunogenic PDT to treat and inhibit ovarian tumor cells through enhanced immunogenic signals such as damage-associated molecules (DAMPs). | Ovarian cancer | CN113855788A, 2021 |
| Gold nanostars (AuNS) with the anti-PDL1 antibodies | Primary tumors and metastatic cancer sites can be targeted by plasmonics-active (plasmon peak at 600 to 1000 nm) AuNS with a mean tip-to-tip diameter from 10–200 nm and treated with laser-mediated PTT. The co-administration of anti-PDL1 targets the costimulatory molecules (e.g., PD-1 and PD-L1). | Metastatic breast cancer and/or bladder cancer | WO2016209936A1, 2022 |
| Compounds | Type of Disease | Number, Age and Sex of Participants | Purpose | Primary/Secondary Outcome Measures | Clinicaltrials.gov Identifier and Last Update Year |
|---|---|---|---|---|---|
| Ethylcellulose polymer encapsulating Cetuximab and decorated with somatostatin analog | Colorectal Cancer (CRC) | 30 adults from 20 years to 60 years with all sexes | To present a novel formulation for targeting and treating CRC safely in patients in a high dose with reduction of side effects to noncancer cells | Establishing pharmacokinetics parameters of Cetuximab in the target cells; determining the bioavailability of Cetuximab after oral and i.v. administration; determining the optimized formulation of Cetuximab | NCT03774680, 2019 (Recruiting) |
| Nano-scintillator fiber-optic dosimeter | Cancer of the Gastrointestinal, Genitourinary, or Gynecologic Systems | 13 adults and older adults of all sexes | To examine the real-time dosimetric monitoring of external beam radiotherapy | Dosimetric accuracy of the device with reference to a commercially available dosimeter; feasibility of clinical application of the nanomaterials for dosimetric monitoring of external beam radiotherapy | NCT02407977, 2018 (Completed) |
| Quercetin-encapsulated PLGA-PEG nanoparticles | Tongue Squamous Cell Carcinoma (TSCC) | 1,000,000 children, adults, and older adults of all sexes | To investigate the anticancer effects of Quercetin, either free or encapsulated by nanoparticles in TSCC cell line | Cytotoxicity, apoptosis, and the gene expression of BCL-2, Bax, and PI3K | NCT05456022, 2022 (Not yet recruiting) |
| Platinum Acetylacetonate with titania (NPt-Ca) | High-grade, recurrent brain tumor (brainstem glioma) in the central nervous system | 8 children (5–14 years old years) of all sexes | To study the enhanced therapeutic effect of NPt-Ca on carriers of the diagnosis of glioma brain stem that shows no response to conventional therapy, including surgery, radiation and chemotherapy | Change in the quality of life; Change in tumor size measured by brain magnetic resonance | NCT03250520, 2023 (Completed) |
| Components | Functions | Therapeutic Outcomes | Dosage | Ref. |
|---|---|---|---|---|
| Light-controlled materials | ||||
| poly(benzobisthiadiazole-alt-thiophene), silicon 2,3-naphthalocyanine bis(trihexylsilyloxide), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol), and 4T1 cell- and DC-derived membranes | NIR-II fluorescent imaging; Fused membrane to target 4T1 tumors and activate DCs and T cells; NIR-II photoirradiation to trigger PTT | SPNE directly accumulated in lymph nodes and tumors to exert dual vaccination effects; populations of mature DCs and activated T cells were higher; no recurrence in both primary and distant tumors 30 days post-treatment in 4T1-bearing Balb/c mice | 200 µg mL−1; 200 µL per mouse | [53] |
| Ag2Se and Ag2S QDs, heparin, DOX, mPEG-DSPE and SDF-1α | NIR-II fluorescent imaging; chemo- and immunotherapy; long-term tracking of NK-92 cells; attraction of NK-92 to tumors by chemotaxis | Significantly slowed down the regrowth of MDA-MB-231 tumors in nude mice | 1 mg mL−1; 200 µL per mouse | [54] |
| Cu2–xTe and DSPE-PEG | NIR-II induced PTT; enzyme-like activities to emulate glutathione oxidase for GSH depletion and peroxidase for ROS generation to kill tumor cells and boost immunomodulation of tumor-associated immune cells | 18.6% maturation ratio of DCs; the populations of tumor-infiltrating T helper cells and cytotoxic T lymphocytes were 10- and 11-fold higher than the control group; the growth of distant the tumor was delayed by 64%; the survival rate of mice was over 80% of 4T1-bearing Balb/c mice | 2.5 mg kg−1 | [56] |
| pH-responsive materials | ||||
| poly(ethylene glycol)-b-poly(2-azepane ethyl methacrylate)-modified polyamidoamine dendrimer with platinum prodrug conjugation | pH-dependent dissociation for enhanced NP penetration and drug delivery in an acidic environment | Enhanced platinum drug accumulation in BxPC-3 bearing Balb/c nude mice after intravenous injection | 40 μg of platinum per mouse bearing a BxPC-3 xenograft tumor | [65] |
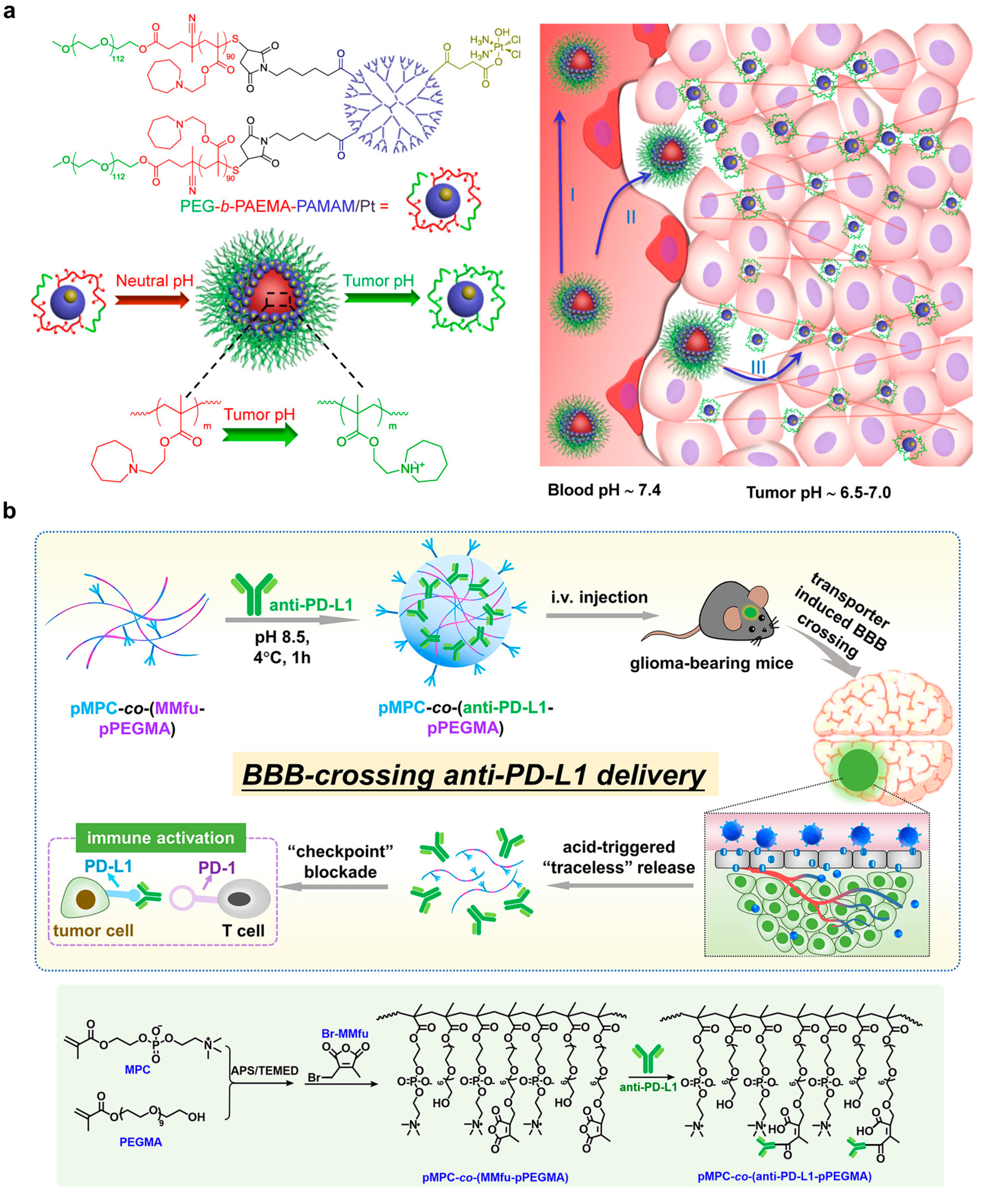
| Choline analogue 2-methacryloyloxyethyl phosphorylcholine presenting poly(ethylene glycol) with conjugation of anti-PD-1 via 3-(bromomethyl)-4-methyl-2,5-furandione | Crossing blood–brain barrier; pH-dependent release of anti-PD-1 for ICB immunotherapy | Promoted antibody accumulation in tumor and survival; enhanced tumor infiltrated CD8+ and CD4+ T cell proliferation (Ki67+); increased sera cytokine level (TNF-α and IFN-γ) in LCPN glioma bearing C57BL/6 mice after intravenous injection | 0.8 mg anti-PD-L1 per kg of mice | [66] |
| Interlocked DNA nanospring, conjugated with anti-CD3 and anti-CD28 | Activating T cells in an acidic environment for immunotherapy | Inhibited tumor growth; increased tumor-infiltrating CD8+ T cell population in B16F10-bearing C57BL/6 mice after intratumoral injection | 0.2 nmol DNA nanospring per mice; 50 µg BMS-1 (a PD-1/PD-L1 ICB drug) per mice | [73] |
| Metabolically modulating materials | ||||
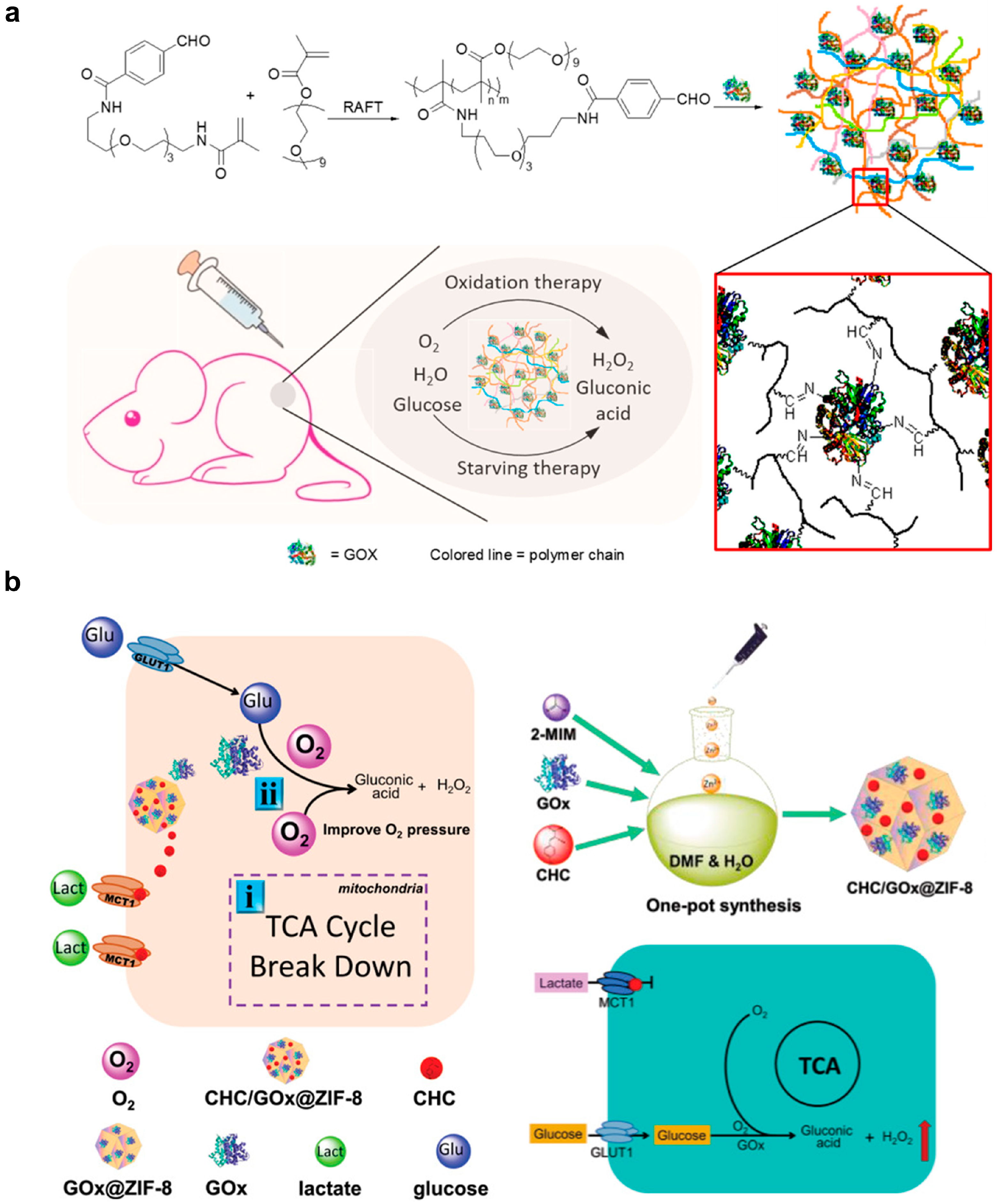
| Liposome for delivery of lonidamine and syrosingopine | Inhibition of lactate production in tumors for tumor control and immunomodulation | Inhibited tumor growth; prolonged survival; increased infiltrated M1-type macrophage and NK cell; reduced infiltrated M2-type macrophage and Treg cell in 4T1 tumor-bearing Balb/c mice after intravenous injection | 2.5 mg lonidamine and 1 mg syrosingopore per kg body weight | [89] |
| Separate liposomes for delivery of glucose oxidase and banoxantrone dihydrochloride | Glucose starvation, H2O2 generation and hypoxia-activated prodrug-mediated therapy | Inhibited tumor growth in 4T1 tumor bear balb/c nude mice after intravenous injection | 2 mg glucose oxidase and 5 mg banoxantrone dihydrochloride per kg body weight | [93] |
| Magnetic-responsive materials | ||||
| cytosine-phosphate-guanine oligodeoxynucleotides (CpG ODN), superparamagnetic iron oxide nanoparticles, and monomethoxypoly (ethylene glycol)-poly(lactic-co-glycolic acid)-poly-l-lysine (mPEG-PLGA-PLL) triblock copolymers | NIR-I (660 nm) mediated photoacoustic imaging and PTT to guide tumor therapy; contrast agents for MRI; load adjuvant to activate DCs via Toll-like receptor 9 | The size of primary and distant tumors decreased with a survival period over 60 days post-treatment; DC maturation level and | 5 mg per mouse | [98] |
| 1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine, 1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine, ICG, OVA peptide, α-APgp100 peptide and superparamagnetic oxide NPs | efficient delivery of indocyanine green/iron oxide/ovalbumin antigen to DCs and enhance the activation and migration efficiency of DCs to lymph nodes under magnetic control | 13.2% of the injected DC successfully migrated to lymph nodes by magnetic field compared to 2.6% of the control group; death rates of tumor cells reached 69%; inhibition efficiency of tumor growth was 96% | i.v. injection of 1.2 × 106 DCs treated with the nanoplatforms (16 µg/mL) in 50 µL PBS | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, B.; Wong, W.-K.; Ng, Y.-M.; Yang, M.; Leung, F.K.-C.; Wong, D.S.-H. Smart Design of Nanostructures for Boosting Tumor Immunogenicity in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1427. https://doi.org/10.3390/pharmaceutics15051427
Yin B, Wong W-K, Ng Y-M, Yang M, Leung FK-C, Wong DS-H. Smart Design of Nanostructures for Boosting Tumor Immunogenicity in Cancer Immunotherapy. Pharmaceutics. 2023; 15(5):1427. https://doi.org/10.3390/pharmaceutics15051427
Chicago/Turabian StyleYin, Bohan, Wai-Ki Wong, Yip-Ming Ng, Mo Yang, Franco King-Chi Leung, and Dexter Siu-Hong Wong. 2023. "Smart Design of Nanostructures for Boosting Tumor Immunogenicity in Cancer Immunotherapy" Pharmaceutics 15, no. 5: 1427. https://doi.org/10.3390/pharmaceutics15051427
APA StyleYin, B., Wong, W.-K., Ng, Y.-M., Yang, M., Leung, F. K.-C., & Wong, D. S.-H. (2023). Smart Design of Nanostructures for Boosting Tumor Immunogenicity in Cancer Immunotherapy. Pharmaceutics, 15(5), 1427. https://doi.org/10.3390/pharmaceutics15051427








