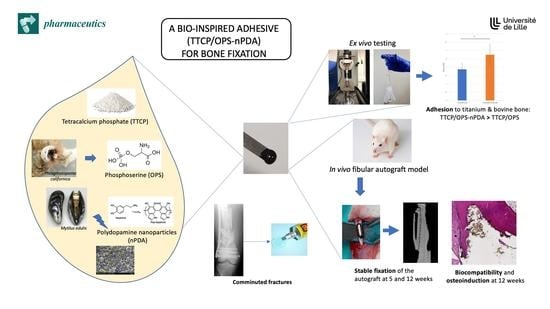
In Vitro and In Vivo Evaluation of a Bio-Inspired Adhesive for Bone Fixation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glue Fabrication
2.3. Impact of the Formulation on the Adhesion Force of the Glue
2.3.1. Tested Glue Formulations
2.3.2. Instrumental Tensile Adhesion Test on Glued Titanium Samples
2.4. Characterization of the Optimized Glue
2.4.1. Ex Vivo Instrumental Tensile Adhesion Test on Glued Bovine Bone
2.4.2. Ex Vivo Manual Tensile Adhesion Test on Glued Rat Tibia/Fibula
2.4.3. Compression Test
2.4.4. Test to Determine Setting Time
2.4.5. Biomineralization Test
2.4.6. Cytotoxicity Test
2.5. In Vivo Evaluation of the Efficacy of the Bone Glue for Autograft Fixation
2.5.1. Animal Model
2.5.2. Surgical Procedure
2.5.3. Euthanasia Procedure and Clinical Evaluation
2.5.4. Microcomputed Tomography (microCT) Evaluation
2.5.5. Histological Evaluation
2.6. Statistics
3. Results
3.1. Characterization of Synthesized nPDA
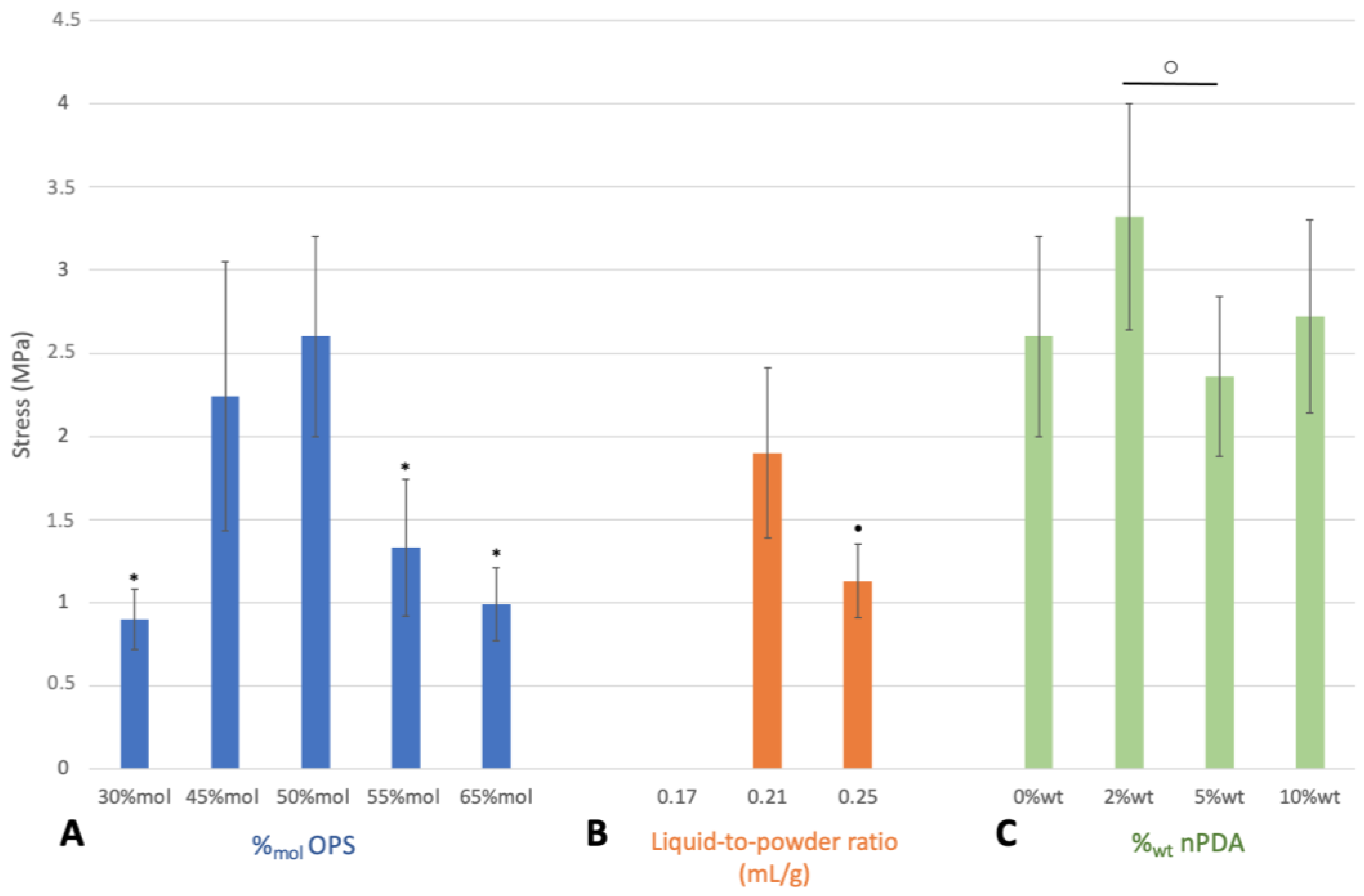
3.2. Impact of Glue Formulation on Adhesion
3.2.1. Instrumental Tensile Adhesion Test for Formulation Screening and Optimization
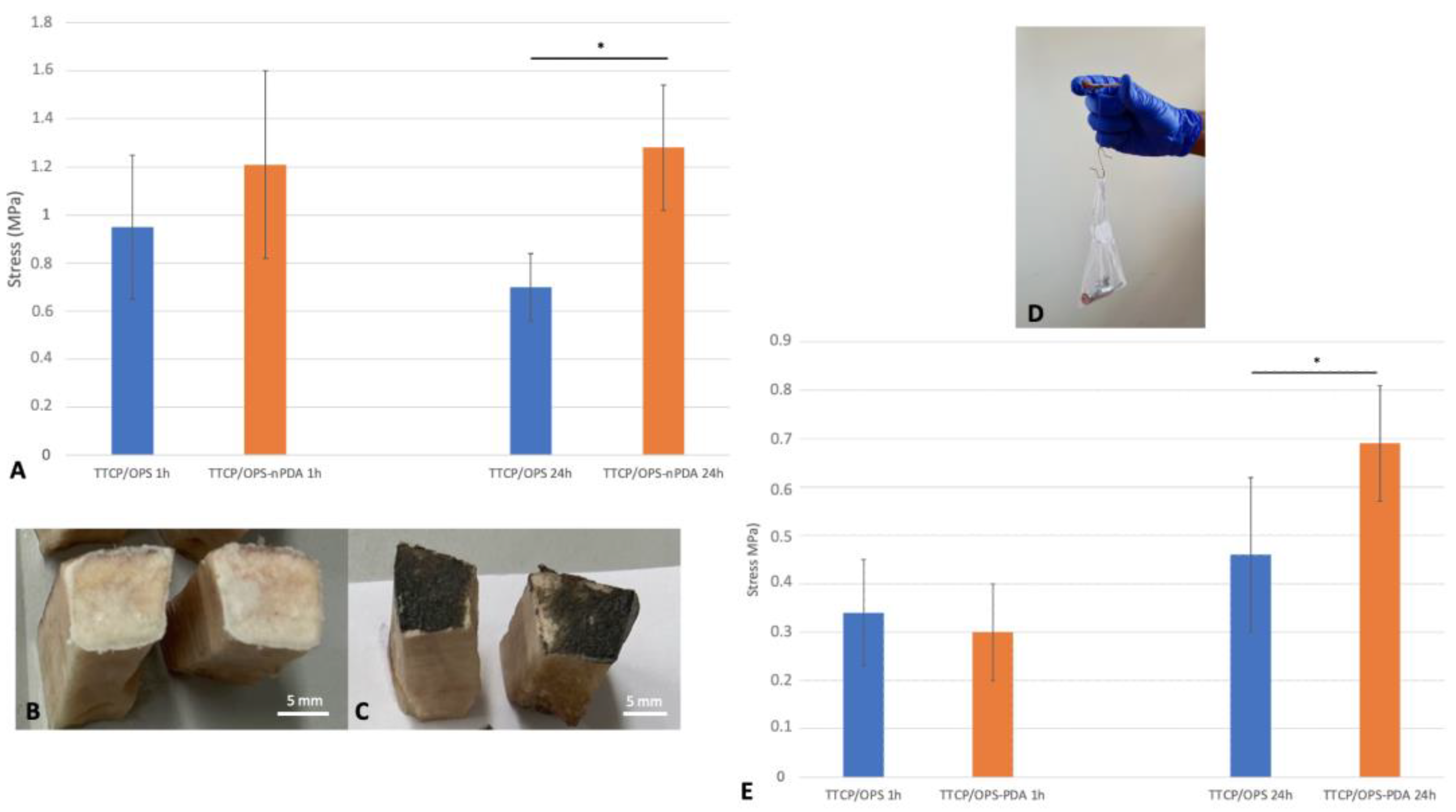
3.2.2. Impact of Immersion Time on Adhesion of Optimal Glue Formulation
3.3. Characterization of Glue Performance with Optimal Formulation
3.3.1. Adhesion to Bovine Bone: Ex Vivo Instrumental Tensile Adhesion Test
3.3.2. Adhesion of Glued Rat Tibia/Fibula: Ex Vivo Manual Tensile Adhesion Test
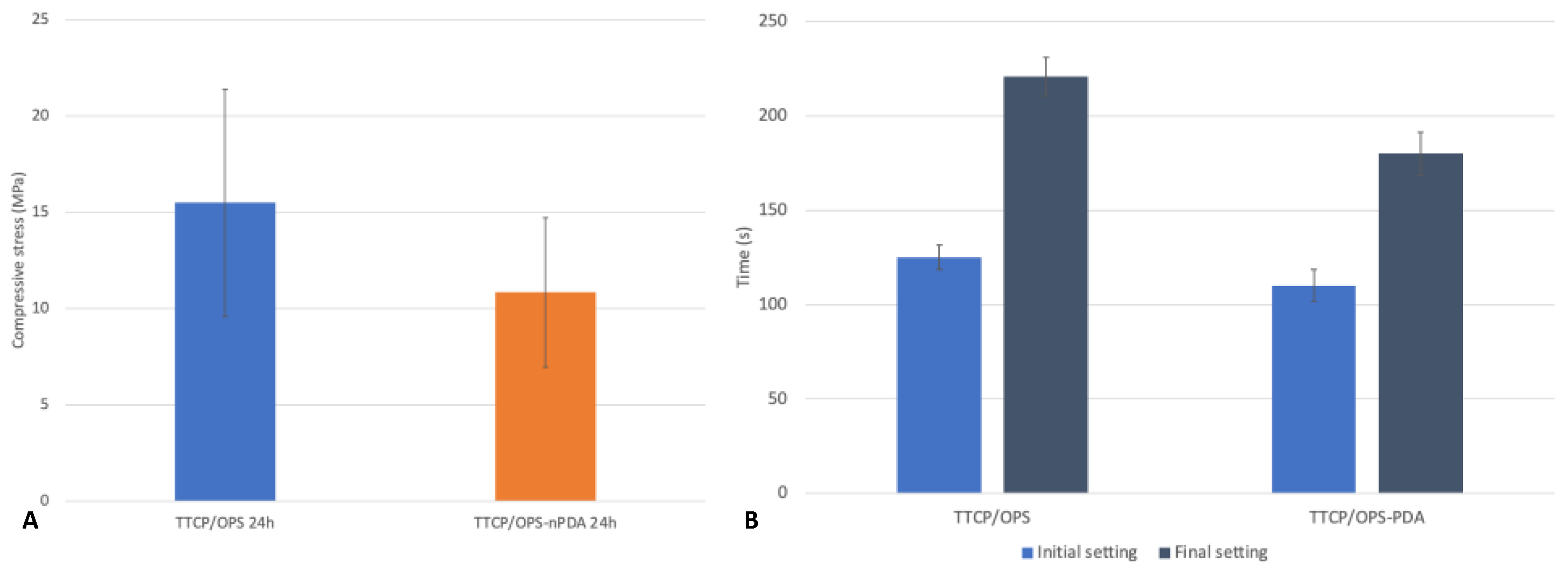
3.3.3. Cohesive Strength of the Glue: Instrumental Compression Test
3.3.4. Setting Time
3.3.5. Biomineralization Test
3.3.6. Cytotoxicity Test
3.4. In Vivo Evaluation
3.4.1. Clinical Observation
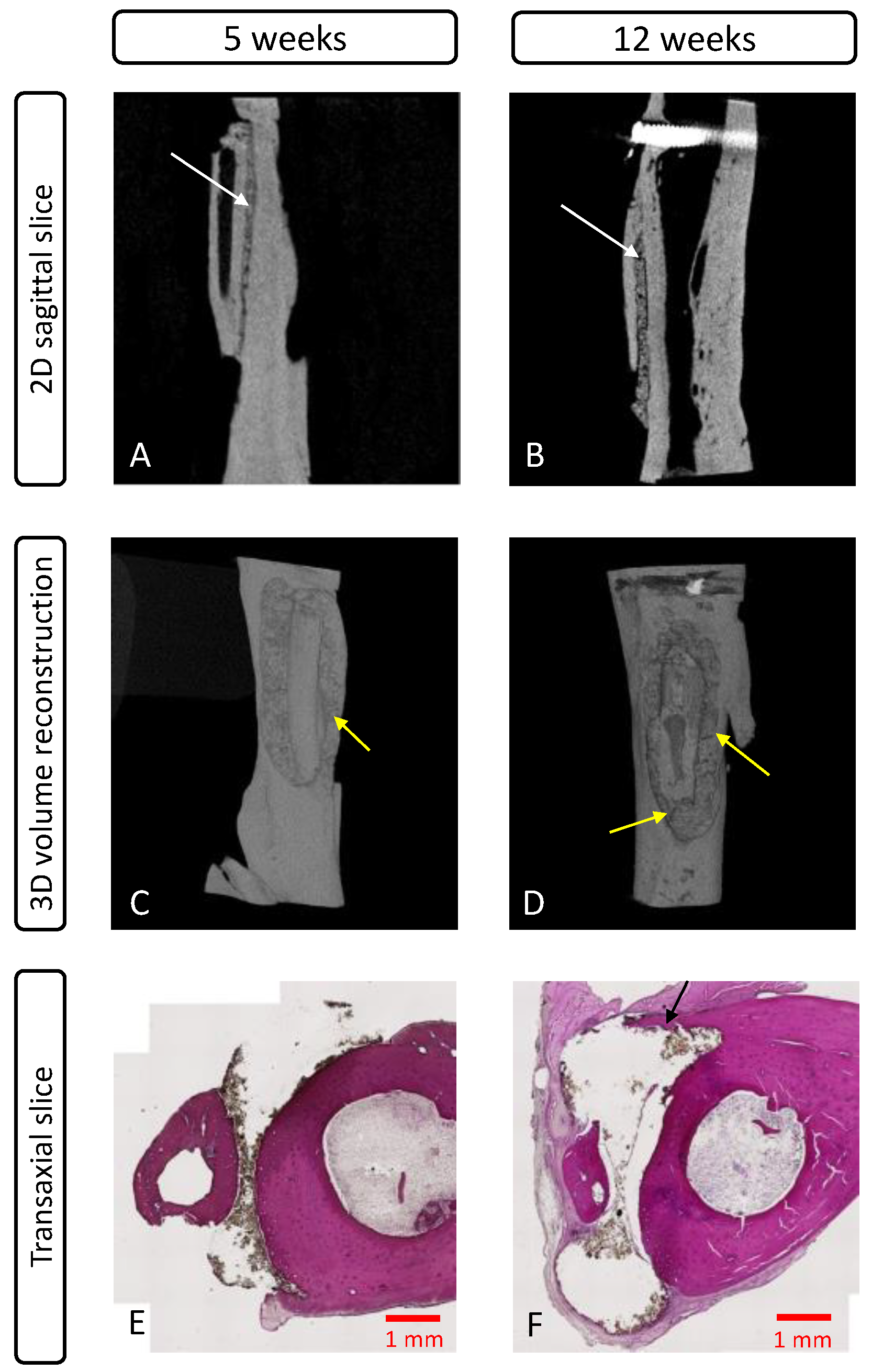
3.4.2. MicroCT Evaluation
3.4.3. Histological Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Bergin, P.F.; Weber, T.G.; Gerow, D.E.; Spitler, C.A.; Graves, M.L.; Russell, G.V. Intraosseous Plating for the Management of Cortical Defects. J. Orthop. Trauma 2018, 32 (Suppl. 1), S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Skroch, L.; Fischer, I.; Meisgeier, A.; Kozolka, F.; Apitzsch, J.; Neff, A. Condylar Remodeling after Osteosynthesis of Fractures of the Condylar Head or Close to the Temporomandibular Joint. J. Craniomaxillofac. Surg. 2020, 48, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Pontell, M.E.; Niklinska, E.B.; Braun, S.A.; Jaeger, N.; Kelly, K.J.; Golinko, M.S. Resorbable Versus Titanium Hardware for Rigid Fixation of Pediatric Upper and Midfacial Fractures: Which Carries a Lower Risk Profile? J. Oral Maxillofac. Surg. 2021, 79, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Acklin, Y.P.; Bircher, A.; Morgenstern, M.; Richards, R.G.; Sommer, C. Benefits of Hardware Removal after Plating. Injury 2018, 49 (Suppl. 1), S91–S95. [Google Scholar] [CrossRef] [PubMed]
- Kellam, P.J.; Harrast, J.; Weinberg, M.; Martin, D.F.; Davidson, N.P.; Saltzman, C.L. Complications of Hardware Removal. J. Bone Jt. Surg. Am. 2021, 103, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Lalli, T.A.J.; Matthews, L.J.; Hanselman, A.E.; Hubbard, D.F.; Bramer, M.A.; Santrock, R.D. Economic Impact of Syndesmosis Hardware Removal. Foot 2015, 25, 131–133. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone Adhesives for Trauma Surgery: A Review of Challenges and Developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F.; et al. Current State of Bone Adhesives-Necessities and Hurdles. Materials 2019, 12, 3975. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium Phosphate Cements for Bone Substitution: Chemistry, Handling and Mechanical Properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A Water-Borne Adhesive Modeled after the Sandcastle Glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Bioinspired Mineral-Organic Bioresorbable Bone Adhesive. Adv. Health Mater. 2018, 7, e1800467. [Google Scholar] [CrossRef] [PubMed]
- Pujari-Palmer, M.; Guo, H.; Wenner, D.; Autefage, H.; Spicer, C.D.; Stevens, M.M.; Omar, O.; Thomsen, P.; Edén, M.; Insley, G.; et al. A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials. Materials 2018, 11, 2492. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Kay, G.W.; Brown, M.C.; Cochran, D.L. Bone Glue—The Final Frontier for Fracture Repair and Implantable Device Stabilization. Int. J. Adhes. Adhes. 2020, 102, 102647. [Google Scholar] [CrossRef]
- Kirillova, A.; Nillissen, O.; Liu, S.; Kelly, C.; Gall, K. Reinforcement and Fatigue of a Bioinspired Mineral-Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2021, 10, e2001058. [Google Scholar] [CrossRef]
- Geddes, A.T.; Thatcher, G.P.; Hetzel, S.; McCabe, R.P.; Vandereby, R.; Snyder, C.J. Biomechanical Testing of a Calcium Phosphate-Phosphoserine–Based Mineral-Organic Adhesive for Non-Invasive Fracture Repair of Mandibular Fractures in Dogs. Front. Vet. Sci. 2020, 7, 59. [Google Scholar] [CrossRef]
- Foley, K.T.; Woodard, E.J.; Slotkin, J.R.; Mayotte, C.K.; Baldwin, A.C.; Brown, M.C.; Hess, B.J. Cranial Flap Fixation in Sheep Using a Resorbable Bone Adhesive. J. Neurosurg. 2020, 134, 621–629. [Google Scholar] [CrossRef]
- Pujari-Palmer, M.; Giró, R.; Procter, P.; Bojan, A.; Insley, G.; Engqvist, H. Factors That Determine the Adhesive Strength in a Bioinspired Bone Tissue Adhesive. ChemEngineering 2020, 4, 19. [Google Scholar] [CrossRef]
- Vrchovecká, K.; Pávková-Goldbergová, M.; Engqvist, H.; Pujari-Palmer, M. Cytocompatibility and Bioactive Ion Release Profiles of Phosphoserine Bone Adhesive: Bridge from In Vitro to In Vivo. Biomedicines 2022, 10, 736. [Google Scholar] [CrossRef]
- Hulsart-Billström, G.; Stelzl, C.; Procter, P.; Pujari-Palmer, M.; Insley, G.; Engqvist, H.; Larsson, S. In Vivo Safety Assessment of a Bio-Inspired Bone Adhesive. J. Mater. Sci. Mater. Med. 2020, 31, 24. [Google Scholar] [CrossRef] [PubMed]
- Procter, P.; Hulsart-Billström, G.; Alves, A.; Pujari-Palmer, M.; Wenner, D.; Insley, G.; Engqvist, H.; Larsson, S. Gluing Living Bone Using a Biomimetic Bioadhesive: From Initial Cut to Final Healing. Front. Bioeng. Biotechnol. 2021, 9, 728042. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Mussel Adhesion—Essential Footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Pardeshi, S.; Sharma, J.G.; Lee, S.H.; Choi, E.H. Biomedical and Clinical Importance of Mussel-Inspired Polymers and Materials. Mar. Drugs 2015, 13, 6792–6817. [Google Scholar] [CrossRef]
- Huang, S.; Liang, N.; Hu, Y.; Zhou, X.; Abidi, N. Polydopamine-Assisted Surface Modification for Bone Biosubstitutes. Biomed. Res. Int. 2016, 2016, 2389895. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2020, 11, 607099. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Han, P.; Liu, X.; Xu, M.; Tian, T.; Chang, J.; Xiao, Y. Mussel-Inspired Bioceramics with Self-Assembled Ca-P/Polydopamine Composite Nanolayer: Preparation, Formation Mechanism, Improved Cellular Bioactivity and Osteogenic Differentiation of Bone Marrow Stromal Cells. Acta Biomater. 2014, 10, 428–438. [Google Scholar] [CrossRef]
- Liu, Z.; Qu, S.; Zheng, X.; Xiong, X.; Fu, R.; Tang, K.; Zhong, Z.; Weng, J. Effect of Polydopamine on the Biomimetic Mineralization of Mussel-Inspired Calcium Phosphate Cement in Vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.; Zhang, G.; Zhao, J.; Fu, R.; Tang, K.; Zhi, W.; Duan, K.; Weng, J.; Li, W.; et al. Enhanced Repairing of Critical-Sized Calvarial Bone Defects by Mussel-Inspired Calcium Phosphate Cement. ACS Biomater. Sci. Eng. 2018, 4, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles Modified by Polydopamine: Working as “Drug” Carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Zhang, Y.; Jiang, Y.; Lu, X.; Fang, L.; Gan, D.; Lv, C.; Zhang, H.; Qu, S. Protein-Affinitive Polydopamine Nanoparticles as an Efficient Surface Modification Strategy for Versatile Porous Scaffolds Enhancing Tissue Regeneration. Part. Part. Syst. Charact. 2016, 33, 89–100. [Google Scholar] [CrossRef]
- Xie, X.; Tang, J.; Xing, Y.; Wang, Z.; Ding, T.; Zhang, J.; Cai, K. Intervention of Polydopamine Assembly and Adhesion on Nanoscale Interfaces: State-of-the-Art Designs and Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002138. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General Functionalization Route for Cell Adhesion on Non-Wetting Surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Bou-Francis, A.; Ghanem, A. Standardized Methodology for in Vitro Assessment of Bone-to-Bone Adhesion Strength. Int. J. Adhes. Adhes. 2017, 77, 96–101. [Google Scholar] [CrossRef]
- Procter, P.; Pujari-Palmer, M.; Hulsart-Billström, G.; Wenner, D.; Insley, G.; Larsson, S.; Engqvist, H. A Biomechanical Test Model for Evaluating Osseous and Osteochondral Tissue Adhesives. BMC Biomed. Eng. 2019, 1, 11. [Google Scholar] [CrossRef]
- Bojan, A.J.; Stadelmann, V.A.; Wu, D.; Pujari-Palmer, M.; Insley, G.; Sundh, D.; Persson, C.; Engqvist, H.; Procter, P. A New Bone Adhesive Candidate-Does It Work in Human Bone? An Ex-Vivo Preclinical Evaluation in Fresh Human Osteoporotic Femoral Head Bone. Injury 2022, 53, 1858–1866. [Google Scholar] [CrossRef]
- Wu, D.; Pujari-Palmer, M.; Bojan, A.; Palmquist, A.; Procter, P.; Öhman-Mägi, C.; Ferguson, S.J.; Isaksson, P.; Persson, C. The Effect of Two Types of Resorbable Augmentation Materials—A Cement and an Adhesive—On the Screw Pullout Pullout Resistance in Human Trabecular Bone. J. Mech. Behav. Biomed. Mater. 2020, 110, 103897. [Google Scholar] [CrossRef]
- Van Erk, M.; Van Luijk, J.; Yang, F.; Leeuwenburgh, S.C.G.; Sánchez-Fernández, M.J.; Hermans, E.; Félix Lanao, R.P.; Van Goor, H. A Systematic Review and Meta-Analyses on Animal Models Used in Bone Adhesive Research. J. Orthop. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kesseli, F.P.; Lauer, C.S.; Baker, I.; Mirica, K.A.; Van Citters, D.W. Identification of a Calcium Phosphoserine Coordination Network in an Adhesive Organo-Apatitic Bone Cement System. Acta Biomater. 2020, 105, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.-H.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef]
- Short, H.L.; Fevrier, H.B.; Meisel, J.A.; Santore, M.T.; Heiss, K.F.; Wulkan, M.L.; Raval, M.V. Defining the Association between Operative Time and Outcomes in Children’s Surgery. J. Pediatr. Surg. 2017, 52, 1561–1566. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Fernández, E.; Boltong, M.G.; Bermúdez, O.; Planell, J.A.; Driessens, F.C. Compliance of an Apatitic Calcium Phosphate Cement with the Short-Term Clinical Requirements in Bone Surgery, Orthopaedics and Dentistry. Clin. Mater. 1994, 17, 99–104. [Google Scholar] [CrossRef]
- Takadama, H.; Hashimoto, M.; Mizuno, M.; Kokubo, T. Round-robin test of SBF for in vitro measurement of apatite-forming ability of synthetic materials. Phosphorus Res. Bull. 2004, 17, 119–125. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.; Radwan, M.M.; Abo-Almaged, H.H. In Vitro Bioactivity Study of Calcium Aluminate/Calcium Phosphate. Interceram.—Int. Ceram. Rev. 2019, 68, 36–43. [Google Scholar] [CrossRef]
- Kwon, K.-D.; Chang, J.-S.; Lee, S.-H.; Lee, D.-H.; Lee, K.-S.; Hwang, J.-H.; Lee, H.-S.; Byun, S.-E. The Effect of Cefazolin on Mechanical Properties and Antibacterial Reactions of Calcium Phosphate Cement. J. Korean Orthop. Assoc. 2011, 46, 273–281. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Vega, M.A.; Enrique, J.; Marcelo, G.; Martín del Valle, E.M. Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors. Cancers 2019, 11, 11679. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Valle, E.M.M. del Polydopamine Nanoparticles Kill Cancer Cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient near-Infrared Photothermal Therapeutic Agent for in Vivo Cancer Therapy. Adv. Mater. Weinh. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Zeng, Y.; Wu, L.; Wang, Q.; Han, X.; Liu, X.; Liu, J. Chlorin E6 Conjugated Poly(Dopamine) Nanospheres as PDT/PTT Dual-Modal Therapeutic Agents for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 8176–8187. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zou, L.; Li, B.; Hu, M.; Ye, W.; Ji, J. Photothermal Killing of Methicillin-Resistant Staphylococcus Aureus by Bacteria-Targeted Polydopamine Nanoparticles with Nano-Localized Hyperpyrexia. ACS Biomater. Sci. Eng. 2019, 5, 5169–5179. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G.; Tiffany, S.M.; Bell, W.R.; Gutknecht, W.F. Autoxidation versus Covalent Binding of Quinones as the Mechanism of Toxicity of Dopamine, 6-Hydroxydopamine, and Related Compounds toward C1300 Neuroblastoma Cells in Vitro. Mol. Pharm. 1978, 14, 644–653. [Google Scholar]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Tessier, P.; Kawamoto, H.; Matthews, D.; Posnick, J.; Raulo, Y.; Tulasne, J.F.; Wolfe, S.A. Autogenous Bone Grafts and Bone Substitutes--Tools and Techniques: I. A 20,000-Case Experience in Maxillofacial and Craniofacial Surgery. Plast. Reconstr. Surg. 2005, 116, 6S–24S. [Google Scholar] [CrossRef] [PubMed]
- LaTrenta, G.S.; McCarthy, J.G.; Breitbart, A.S.; May, M.; Sissons, H.A. The Role of Rigid Skeletal Fixation in Bone-Graft Augmentation of the Craniofacial Skeleton. Plast. Reconstr. Surg. 1989, 84, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Moseke, C.; Gbureck, U. Tetracalcium Phosphate: Synthesis, Properties and Biomedical Applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef]
- Ying, X.; Chen, X.; Cheng, S.; Guo, X.; Chen, H.; Xu, H.Z. Phosphoserine Promotes Osteogenic Differentiation of Human Adipose Stromal Cells through Bone Morphogenetic Protein Signalling. Cell. Biol. Int. 2014, 38, 309–317. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.-K.; Fan, J.; Fartash, A.; Aghaloo, T.L.; Lee, M. Photocrosslinkable Chitosan Hydrogels Functionalized with the RGD Peptide and Phosphoserine to Enhance Osteogenesis. J. Mater. Chem. B 2016, 4, 5289–5298. [Google Scholar] [CrossRef] [PubMed]
- Nunamaker, D.M. Experimental Models of Fracture Repair. Clin. Orthop. Relat. Res. 1998, 355, S56–S65. [Google Scholar] [CrossRef]
- Mills, L.A.; Simpson, A.H.R.W. In Vivo Models of Bone Repair. J. Bone Jt. Surg. Br. 2012, 94, 865–874. [Google Scholar] [CrossRef]
- Moss, M.L. A Functional Analysis of Fusion of the Tibia and Fibula in the Rat and Mouse. Acta Anat. 1977, 97, 321–332. [Google Scholar] [CrossRef]
- Yeroslavsky, G.; Girshevitz, O.; Foster-Frey, J.; Donovan, D.M.; Rahimipour, S. Antibacterial and Antibiofilm Surfaces through Polydopamine-Assisted Immobilization of Lysostaphin as an Antibacterial Enzyme. Langmuir 2015, 31, 1064–1073. [Google Scholar] [CrossRef]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and Management of Fracture-Related Infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Mosser, P.; Kohn, D. Infections after High Tibial Osteotomy. Knee Surg. Sport. Traumatol. Arthrosc. 2013, 21, 161–169. [Google Scholar] [CrossRef]
- Ferri, J.; Druelle, C.; Schlund, M.; Bricout, N.; Nicot, R. Complications in Orthognathic Surgery: A Retrospective Study of 5025 Cases. Int. Orthod. 2019, 17, 789–798. [Google Scholar] [CrossRef]
- Schlund, M.; Meeus, J.; Politis, C.; Ferri, J. Management of Sinus Graft Infection-a Systematic Review. Int. J. Oral Maxillofac. Surg. 2022, 51, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic Penetration into Bone and Joints: An Updated Review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine Antibacterial Materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Yang, K.; Shin, J.; Cho, S.-W. Polydopamine-Assisted Osteoinductive Peptide Immobilization of Polymer Scaffolds for Enhanced Bone Regeneration by Human Adipose-Derived Stem Cells. Biomacromolecules 2013, 14, 3202–3213. [Google Scholar] [CrossRef]
- Ahmad, T.; Byun, H.; Shin, H.J.; Lee, J.; Madhurakkat Perikamana, S.K.; Kim, E.M.; Shin, Y.M.; Shin, H. Polydopamine-Assisted One-Step Modification of Nanofiber Surfaces with Adenosine to Tune the Osteogenic Differentiation of Mesenchymal Stem Cells and the Maturation of Osteoclasts. Biomater. Sci. 2020, 8, 2825–2839. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The Role of Peptides in Bone Healing and Regeneration: A Systematic Review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef]




| No Fixation | TTCP/OPS-nPDA | |
|---|---|---|
| 5 weeks | 0% (0/3 sites) | 86% (6/7 sites) |
| 12 weeks | 0% (0/3 sites) | 71% (5/7 sites) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlund, M.; Dartus, J.; Defrançois, S.; Ferri, J.; Delattre, J.; Blanchemain, N.; Woisel, P.; Lyskawa, J.; Chai, F. In Vitro and In Vivo Evaluation of a Bio-Inspired Adhesive for Bone Fixation. Pharmaceutics 2023, 15, 1233. https://doi.org/10.3390/pharmaceutics15041233
Schlund M, Dartus J, Defrançois S, Ferri J, Delattre J, Blanchemain N, Woisel P, Lyskawa J, Chai F. In Vitro and In Vivo Evaluation of a Bio-Inspired Adhesive for Bone Fixation. Pharmaceutics. 2023; 15(4):1233. https://doi.org/10.3390/pharmaceutics15041233
Chicago/Turabian StyleSchlund, Matthias, Julien Dartus, Sarah Defrançois, Joël Ferri, Jérôme Delattre, Nicolas Blanchemain, Patrice Woisel, Joël Lyskawa, and Feng Chai. 2023. "In Vitro and In Vivo Evaluation of a Bio-Inspired Adhesive for Bone Fixation" Pharmaceutics 15, no. 4: 1233. https://doi.org/10.3390/pharmaceutics15041233
APA StyleSchlund, M., Dartus, J., Defrançois, S., Ferri, J., Delattre, J., Blanchemain, N., Woisel, P., Lyskawa, J., & Chai, F. (2023). In Vitro and In Vivo Evaluation of a Bio-Inspired Adhesive for Bone Fixation. Pharmaceutics, 15(4), 1233. https://doi.org/10.3390/pharmaceutics15041233