Cannabinoids and Multiple Sclerosis: A Critical Analysis of Therapeutic Potentials and Safety Concerns
Abstract
1. Introduction
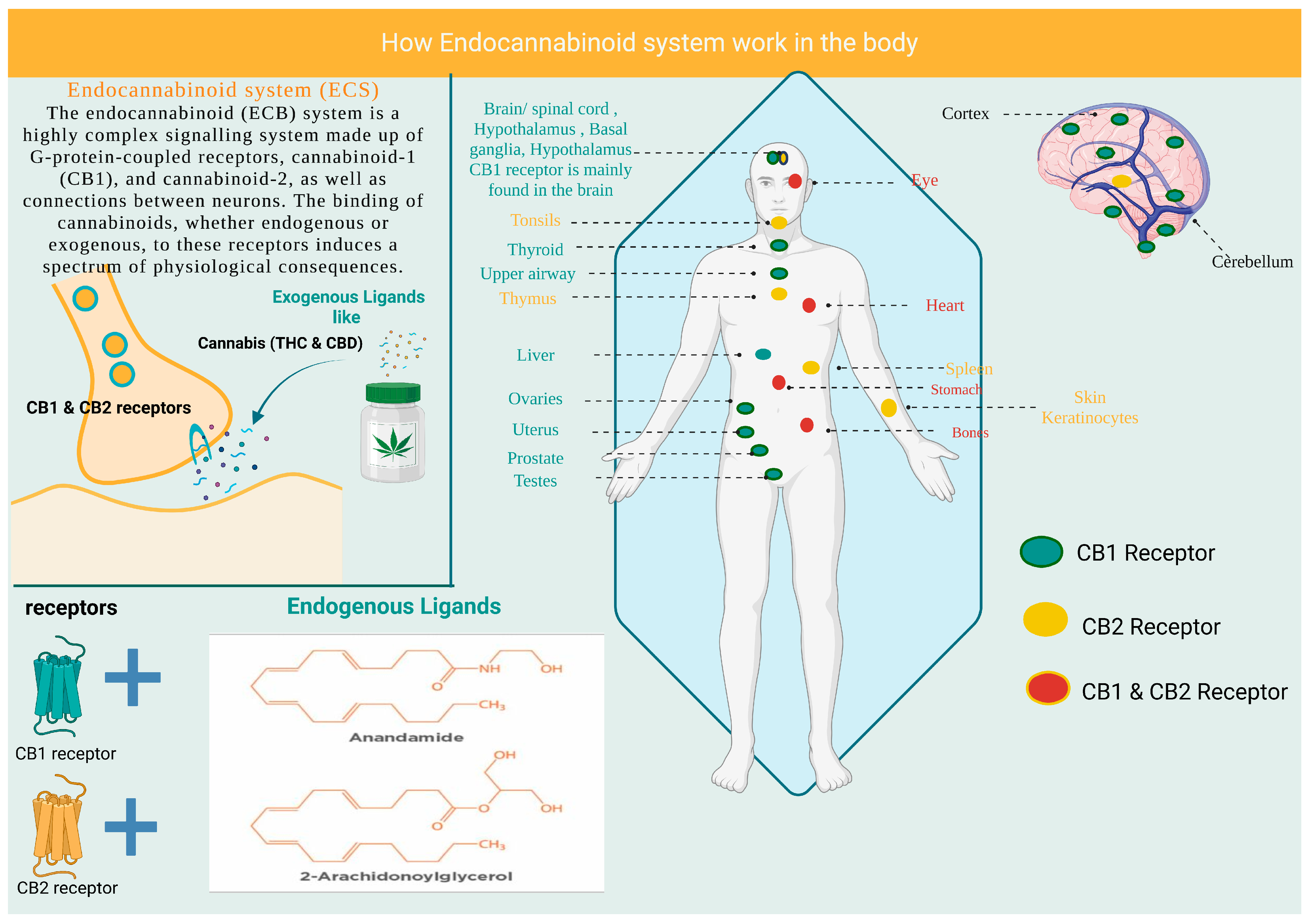
2. Cannabinoids and the Endocannabinoid System
3. Molecular Effect of Cannabinoids on the Central Nervous System
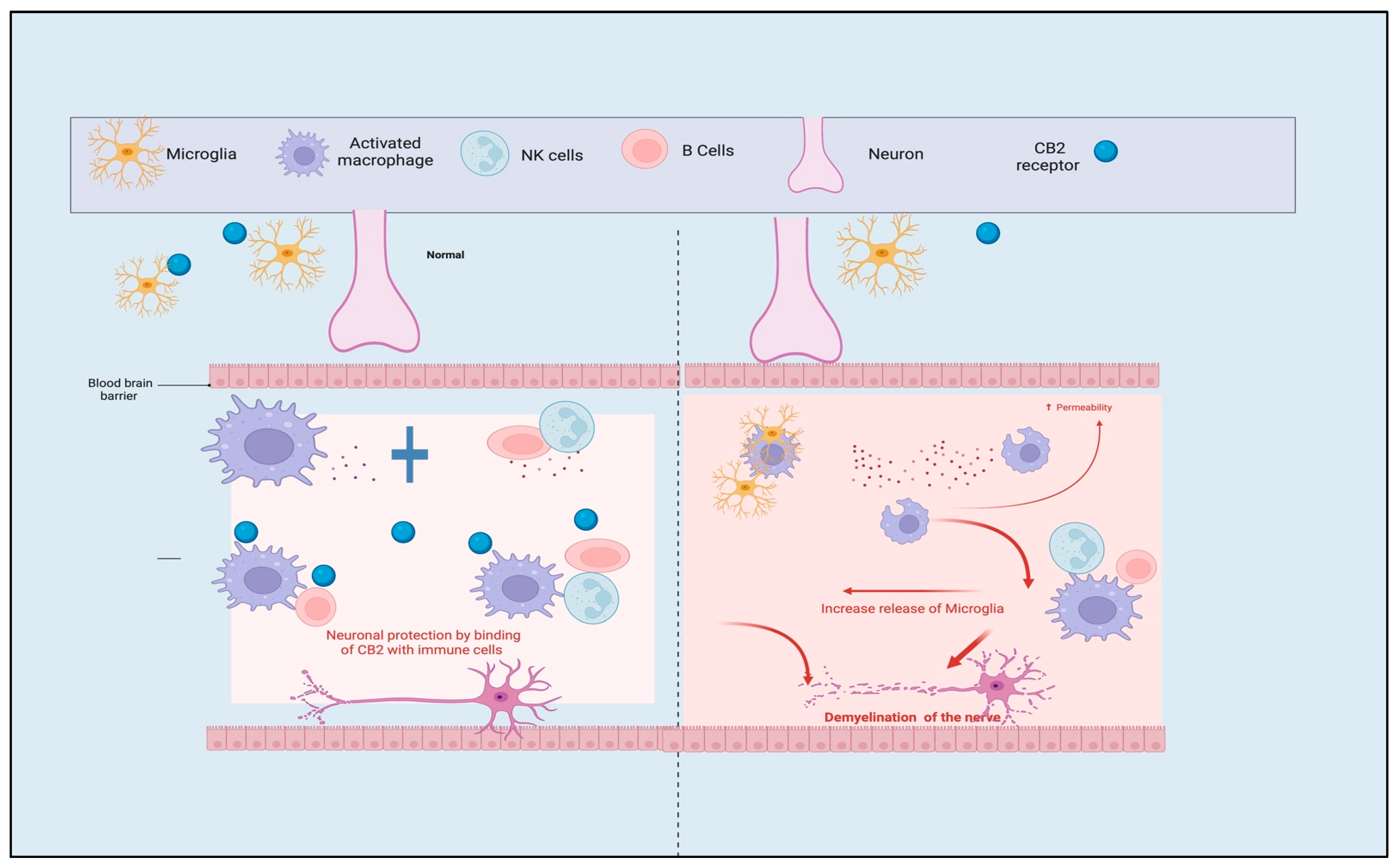
3.1. Role of CB1 and CB2 Receptors
3.2. Molecular Effect of Cannabinoids and ECB System in the Hippocampus
3.3. Effect of Cannabinoids on Mitochondria and Metabolic Pathway
4. Pharmacokinetics and Pharmacodynamics Characteristics of Cannabinoids
| PKM | Absorption | Bioavailability | Peak Plasma Concentration | Duration of Action | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|
| Dosage Form | |||||||
| Inhalation | High absorption = 10–60% | THC = 2–65%, CBD = 6–31% | Peak plasma concentrations of THC and CBD are reached quickly, within 3–10 min. | 1–4 h | Suitable bioavailability and rapid onset of action. | Variability between patients based on lung function. | |
| Oral | Low absorption = 2–14% | THC = 5–10%, CBD = 6–20% Due to the effect of 1st pass metabolism. | Achieve peak concentration (60–120 min). | 6 h | It provides a sufficient duration of action. | Delayed onset of action. | |
| Topical | Irregular absorption | Skin barriers hinder bioavailability due to the lipophilic nature of the drug. | Steady-state condition is achieved within = 17 h. | THC = 14 h, CBD = 72 h | Reduction in the side effects associated with systemic administration of the drugs. | Poor bioavailability due to skin barrier. | |
| Systemic intravenous | High absorption rate | High bioavailability like inhaled dosage form. | Within 10 min. | 4 h | Rapid action and high bioavailability. | Require an aqueous vehicle due to poor water solubility. | |
| Ref. | [15,43,44] | [15,47,48] | [15,49] | [49,50] | [50,51] | [15,52] | |
5. Pharmacogenetics and Cannabinoids
5.1. Variation of Genes That Code for Cannabinoid Receptors
5.1.1. Polymorphism of Cannabinoid Receptors CB1 and CB2
5.1.2. Transient Receptor Potential Cation Channel Subfamily V Member 1 (TRPV1)
5.2. Variation in Genes That Code for Metabolizing Enzymes
6. Adverse Effects of Cannabinoids
7. Cannabinoids and the Management of Multiple Sclerosis
| Drug (Active Constituent) | Dosage Form | Experimental Design | Outcomes | Ref. |
|---|---|---|---|---|
| THC (5–10 mg) | Oral | Double-blind study | Decrease muscle spasms and enhance the walking ability of the patient. | [106] |
| Nabilone (1 mg), a synthetic THC mimic | Oral | Open-Label study | Dimension in the pain with MS patients. | [107] |
| THC (10 mg oral or 15 mg rectal) | Oral/rectal | Open-Label study | Enhancement of walking ability, passive mobility, and dimension in the pain with young MS patients. | [108] |
| THC (7.5 mg) | Oral | Placebo-controlled, double-blind study | Improved muscle spasm perception. | [109] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Controlled double-blind, randomized study | It has an analgesic effect in addition to enhancing of quality of life of the patient (e.g., sleep improvement). | [110] |
| Tobacco and cannabis resin-containing smoking | Inhalation | Placebo-controlled trial | Decrease in the Nystagmus amplitude and enhancement of visual ability. | [111] |
| Dronabinol (>25 mg), a synthetic THC mimic | Oral | Randomized, double-blind controlled study | Significant analgesic effect. | [8] |
| THC (5–15 mg) | Oral | The single-blind study was a placebo-controlled trial | Enhancement of the patient’s ability in handwriting and a significant decrease in spasticity and tremors. | [112] |
| THC (5–10 mg) | Oral | Double-blind study | Improvement in tremors and ataxia with MS patient. | [113] |
| Nabilone (1 mg), a synthetic THC mimic | Oral | Double-blind controlled trials | Reduction in muscle spasms and tremors. | [114] |
| THC (10–15 mg oral or 2.5–5 mg rectal) | Oral/rectal | Open-Label study | Analgesic effect with MS patient. | [108] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Double-blind controlled trials | Improvement in muscle spasms in MS patients. | [115] |
| THC (1 mg) | Oral | Open-Label study | Have an analgesic effect on patients. | [107] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Double-blind controlled randomized trial | Improving the resistant MS spasticity more effectively and clinically. | [116] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Observational, prospective controlled trial | Improvement in the symptoms of MS in resistant patients. | [117] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Double-blind controlled trial | Improvement in the clinical states of MS patients. | [118] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Randomized controlled study | Decrees the neuropathic pain associated with MS patients. | [119] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Open-Label study | This study has proven the immunomodulatory effect of cannabinoids by detecting the gene expression of immune-related pathways. | [120] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Randomized controlled trial | A significant reduction in the pain associated with MS. | [121] |
| Sativex, a THC-CBD combination (2.7 mg: 2.5 mg) | Oromucosal | Controlled retrospective study | It demonstrates an efficient and safe reduction in muscle spasms. | [122] |
8. Functions of Cannabinoids in MS
9. The Growing Field of Cannabinoid Therapeutics
10. Future Research on Cannabinoids
11. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J. New Insights into the Burden and Costs of Multiple Sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Masuzzo, F.; Nair, G.; Sethi, V.; Kolb, H.; Ohayon, J.; Wu, T.; Cortese, I.C.M.; Reich, D.S. Association of Chronic Active Multiple Sclerosis Lesions with Disability In Vivo. JAMA Neurol. 2019, 76, 1474–1483. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, L.; Dorstyn, D.S.; Murphy, G.; Roberts, R.M. Neurological, Physical and Sociodemographic Correlates of Employment in Multiple Sclerosis: A Meta-Analysis. J. Health Psychol. 2020, 25, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, E.; Wood, T.; Motl, R.W.; Santinelli, F.B.; Barbieri, F.A.; Zanesco, A. The Importance of Promoting Physical Activity and Exercise Training as Adjuvant Therapy for People with Multiple Sclerosis. Motriz Rev. Educ. Fis. 2022, 28, e10220016021. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Broadley, S.A.; Barnett, M.H.; Boggild, M.; Brew, B.J.; Butzkueven, H.; Heard, R.; Hodgkinson, S.; Kermode, A.G.; Lechner-Scott, J.; Macdonell, R.A.L.; et al. Therapeutic Approaches to Disease Modifying Therapy for Multiple Sclerosis in Adults: An Australian and New Zealand Perspective Part 3 Treatment Practicalities and Recommendations. J. Clin. Neurosci. 2014, 21, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for Treatment of Spasticity and Other Symptoms Related to Multiple Sclerosis (CAMS Study): Multicentre Randomised Placebo-Controlled Trial. Lancet 2003, 362, 1517–1526. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Skrajda, M. Cannabinoids from Cannabis sp.: Mechanism of their activity and potential health benefits in human body. J. Educ. Health Sport 2017, 7, 936–945. [Google Scholar] [CrossRef]
- Hollister, L.E. Health Aspects of Cannabis. Pharmacol. Rev. 1986, 38, 1–20. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Smith, S.D. Cannabinoids Interaction with Transient Receptor Potential Family and Implications in the Treatment of Rosacea. Dermatol. Ther. 2021, 34, e15162. [Google Scholar] [CrossRef]
- Pennypacker, S.D.; Romero-Sandoval, E.A. CBD and THC: Do They Complement Each Other Like Yin and Yang? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 1152–1165. [Google Scholar] [CrossRef]
- Grotenhermen, F. Cannabinoids. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 507–530. [Google Scholar] [CrossRef]
- Hanuš, L.O. Pharmacological and Therapeutic Secrets of Plant and Brain (Endo)Cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Sales, P.; Murphy, S.; Averill, S.; Lau, N.; Sato, S.-O. Baby Boomers and Cannabis Delivery Systems. J. Drug Issues 2015, 45, 293–313. [Google Scholar] [CrossRef]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Int. J. Neuropsychopharmacol. 2018, 43, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W.; Mackie, K. Endocannabinoid System and Its Implications for Obesity and Cardiometabolic Risk. Eur. Heart J. Suppl. 2008, 10, B34–B41. [Google Scholar] [CrossRef]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the Cannabinoid CB2 Receptor in the Rat Cerebellum: An Immunohistochemical Study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef]
- Mitrirattanakul, S.; Ramakul, N.; Guerrero, A.V.; Matsuka, Y.; Ono, T.; Iwase, H.; Mackie, K.; Faull, K.F.; Spigelman, I. Site-Specific Increases in Peripheral Cannabinoid Receptors and Their Endogenous Ligands in a Model of Neuropathic Pain. Pain 2006, 126, 102–114. [Google Scholar] [CrossRef]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct Suppression of CNS Autoimmune Inflammation via the Cannabinoid Receptor CB1 on Neurons and CB2 on Autoreactive T Cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Rangananth, C.; Redish, A.D. Viewpoints: How the Hippocampus Contributes to Memory, Navigation and Cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, R.P. Hippocampus as a Wormhole: Gateway to Consciousness. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8, e1446. [Google Scholar] [CrossRef] [PubMed]
- Scarante, F.F.; Vila-Verde, C.; Detoni, V.L.; Ferreira-Junior, N.C.; Guimarães, F.S.; Campos, A.C. Cannabinoid Modulation of the Stressed Hippocampus. Front. Mol. Neurosci. 2017, 10, 411. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Desprez, T.; Metna-Laurent, M.; Bellocchio, L.; Marsicano, G.; Soria-Gomez, E. Dissecting the Cannabinergic Control of Behavior: The Where Matters. Bioessays 2015, 37, 1215–1225. [Google Scholar] [CrossRef]
- Hampson, R.E.; Deadwyler, S.A. Cannabinoids, Hippocampal Function and Memory. Life Sci. 1999, 65, 715–723. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.; Bonilla-Del Río, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Robin, L.M.; et al. Localization of the Cannabinoid Type-1 Receptor in Subcellular Astrocyte Compartments of Mutant Mouse Hippocampus. Glia 2018, 66, 1417–1431. [Google Scholar] [CrossRef]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose Metabolism Links Astroglial Mitochondria to Cannabinoid Effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Ortega-Gutiérrez, S.; van der Stelt, M.; et al. CB1 Cannabinoid Receptors and On-Demand Defense against Excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Soria-Gomez, E.; Metna, M.; Bellocchio, L.; Busquets-Garcia, A.; Marsicano, G. The Endocannabinoid System in the Control of Behavior. In Handbook of Neurobehavioral Genetics and Phenotyping; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 323–355. [Google Scholar] [CrossRef]
- Degroot, A.; Köfalvi, A.; Wade, M.R.; Davis, R.J.; Rodrigues, R.J.; Rebola, N.; Cunha, R.A.; Nomikos, G.G. CB1 Receptor Antagonism Increases Hippocampal Acetylcholine Release: Site and Mechanism of Action. Mol. Pharmacol. 2006, 70, 1236–1245. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Wade, M.; Nomikos, G.G. Biphasic Effects of Cannabinoids on Acetylcholine Release in the Hippocampus: Site and Mechanism of Action. J. Neurosci. 2003, 23, 9374–9384. [Google Scholar] [CrossRef] [PubMed]
- Wyrofsky, R.R.; Reyes, B.A.S.; Zhang, X.Y.; Bhatnagar, S.; Kirby, L.G.; van Bockstaele, E.J. Endocannabinoids, Stress Signaling, and the Locus Coeruleus-Norepinephrine System. Neurobiol. Stress 2019, 11, 100176. [Google Scholar] [CrossRef] [PubMed]
- MacAskill, A.F.; Kittler, J.T. Control of Mitochondrial Transport and Localization in Neurons. Trends Cell Biol. 2010, 20, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Laughlin, S.B. An Energy Budget for Signaling in the Grey Matter of the Brain. J. Cereb. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef]
- Laughlin, S.B.; de Ruyter Van Steveninck, R.R.; Anderson, J.C. The Metabolic Cost of Neural Information. Nat. Neurosci. 1998, 1, 36–41. [Google Scholar] [CrossRef]
- Loureiro, M.; Renard, J.; Zunder, J.; Laviolette, S.R. Hippocampal Cannabinoid Transmission Modulates Dopamine Neuron Activity: Impact on Rewarding Memory Formation and Social Interaction. Int. J. Neuropsychopharmacol. 2014, 40, 1436–1447. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A Cannabinoid Link between Mitochondria and Memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Whyte, D.A.; Al-Hammadi, S.; Balhaj, G.; Brown, O.M.; Penefsky, H.S.; Souid, A.K. Cannabinoids Inhibit Cellular Respiration of Human Oral Cancer Cells. Pharmacology 2010, 85, 328–335. [Google Scholar] [CrossRef]
- Rubens, M. Political and Medical Views on Medical Marijuana and Its Future. Soc. Work Public Health 2014, 29, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and Potential Therapeutic Role in Epilepsy and Other Neuropsychiatric Disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Kalant, H. Medicinal Use of Cannabis: History and Current Status. Pain Res. Manag. 2001, 6, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannibinol, Cannabidiol and Cannabinol. Handb. Exp. Pharmacol. 2005, 168, 657–690. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Cannabinoids for therapeutic use. Am. J. Drug Deliv. 2004, 2, 229–240. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Potvin, S. Cannabidiol in Humans—The Quest for Therapeutic Targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180. [Google Scholar]
- Badowski, M.E. A Review of Oral Cannabinoids and Medical Marijuana for the Treatment of Chemotherapy-Induced Nausea and Vomiting: A Focus on Pharmacokinetic Variability and Pharmacodynamics. Cancer Chemother. Pharmacol. 2017, 80, 441–449. [Google Scholar] [CrossRef]
- De Luis, D.A.; Sagrado, M.G.; Aller, R.; Izaola, O.; Conde, R.; Romero, E. C358A Missense Polymorphism of the Endocannabinoid Degrading Enzyme Fatty Acid Amide Hydrolase (FAAH) and Insulin Resistance in Patients with Diabetes Mellitus Type 2. Diabetes Res. Clin. Pract. 2010, 88, 76–80. [Google Scholar] [CrossRef]
- Pellesi, L.; Licata, M.; Verri, P.; Vandelli, D.; Palazzoli, F.; Marchesi, F.; Cainazzo, M.M.; Pini, L.A.; Guerzoni, S. Pharmacokinetics and Tolerability of Oral Cannabis Preparations in Patients with Medication Overuse Headache (MOH)—A Pilot Study. Eur. J. Clin. Pharmacol. 2018, 74, 1427–1436. [Google Scholar] [CrossRef]
- Arnold, J.C.; Hone, P.; Holland, M.L.; Allen, J.D. CB2 and TRPV1 Receptors Mediate Cannabinoid Actions on MDR1 Expression in Multidrug Resistant Cells. Pharmacol. Rep. 2012, 64, 751–757. [Google Scholar] [CrossRef]
- Agrawal, A.; Wetherill, L.; Dick, D.M.; Xuei, X.; Hinrichs, A.; Hesselbrock, V.; Kramer, J.; Nurnberger, J.I., Jr.; Schuckit, M.; Bierut, L.J.; et al. Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am. J. Med. Genet. 2009, 150B, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Harismendy, O.; Bansal, V.; Bhatia, G.; Nakano, M.; Scott, M.; Wang, X.; Dib, C.; Turlotte, E.; Sipe, J.C.; Murray, S.S.; et al. Population Sequencing of Two Endocannabinoid Metabolic Genes Identifies Rare and Common Regulatory Variants Associated with Extreme Obesity and Metabolite Level. Genome Biol. 2010, 11, R118. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Beatriz Fagundo, A.; Cuenca, A.; Rodriguez, J.; Cuyás, E.; Langohr, K.; de Sola Llopis, S.; Civit, E.; Farré, M.; Peña-Casanova, J.; et al. COMT Val158met and 5-HTTLPR Genetic Polymorphisms Moderate Executive Control in Cannabis Users. Int. J. Neuropsychopharmacol. 2013, 38, 1598–1606. [Google Scholar] [CrossRef]
- Agrawal, A.; Edenberg, H.J.; Foroud, T.; Bierut, L.J.; Dunne, G.; Hinrichs, A.L.; Nurnberger, J.I.; Crowe, R.; Kuperman, S.; Schuckit, M.A.; et al. Association of GABRA2 with Drug Dependence in the Collaborative Study of the Genetics of Alcoholism Sample. Behav. Genet. 2006, 36, 640–650. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.; Cubells, J. Genetics of Two μ Opioid Receptor Gene (OPRM1) Exon I Polymorphisms: Population Studies, and Allele Frequencies in Alcohol- and Drug-Dependent Subjects. Mol. Psychiatry 1999, 4, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, B.Z.; Kranzler, H.R.; Oslin, D.; Anton, R.; Farrer, L.A.; Gelernter, J. Linkage Analysis Followed by Association Show NRG1 Associated with Cannabis Dependence in African Americans. Biol. Psychiatry 2012, 72, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.W.; Ishiguro, H.; Ohtsuki, T.; Hess, J.; Carillo, F.; Walther, D.; Onaivi, E.S.; Arinami, T.; Uhl, G.R. Human Cannabinoid Receptor 1: 5′ Exons, Candidate Regulatory Regions, Polymorphisms, Haplotypes and Association with Polysubstance Abuse. Mol. Psychiatry 2004, 9, 916–931. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid Control of Food Intake and Energy Balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, X.; Zhu, Z.H.; Zhao, J.; Hu, X.; Ball, D.M.; Sham, P.C.; Collier, D.A. No Association between (AAT)n Repeats in the Cannabinoid Receptor Gene (CNR1) and Heroin Abuse in a Chinese Population. Mol. Psychiatry 2000, 5, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Covault, J.; Gelernter, J.; Kranzler, H. Association Study of Cannabinoid Receptor Gene (CNR1) Alleles and Drug Dependence. Mol. Psychiatry 2001, 6, 501–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bühler, K.M.; Huertas, E.; Echeverry-Alzate, V.; Giné, E.; Moltó, E.; Montoliu, L.; López-Moreno, J.A. Risky Alcohol Consumption in Young People Is Associated with the Fatty Acid Amide Hydrolase Gene Polymorphism C385A and Affective Rating of Drug Pictures. Mol. Genet. Genom. 2014, 289, 279–289. [Google Scholar] [CrossRef]
- Hartman, C.A.; Hopfer, C.J.; Haberstick, B.; Rhee, S.H.; Crowley, T.J.; Corley, R.P.; Hewitt, J.K.; Ehringer, M.A. The Association between Cannabinoid Receptor 1 Gene (CNR1) and Cannabis Dependence Symptoms in Adolescents and Young Adults. Drug Alcohol Depend. 2009, 104, 11–16. [Google Scholar] [CrossRef]
- Carrasquer, A.; Nebane, N.M.; Williams, W.M.; Song, Z.H. Functional Consequences of Nonsynonymous Single Nucleotide Polymorphisms in the CB2 Cannabinoid Receptor. Pharmacogenet. Genom. 2010, 20, 157–166. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Marincsák, R.; Dobrosi, N.; Géczy, T.; Paus, R. TRP Channels as Novel Players in the Pathogenesis and Therapy of Itch. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Scheidweiler, K.B.; Wohlfarth, A.; Zhu, M.; Pang, S.; Huestis, M.A. Strategies to Distinguish New Synthetic Cannabinoid FUBIMINA (BIM-2201) Intake from Its Isomer THJ-2201: Metabolism of FUBIMINA in Human Hepatocytes. Forensic Toxicol. 2016, 34, 256–267. [Google Scholar] [CrossRef]
- Stout, S.M.; Cimino, N.M. Exogenous Cannabinoids as Substrates, Inhibitors, and Inducers of Human Drug Metabolizing Enzymes: A Systematic Review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef]
- Evans, W.E.; Relling, M.V. Pharmacogenomics: Translating Functional Genomics into Rational Therapeutics. Science 1999, 286, 487–491. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, X.; Wang, Y.; Yan, W.; Yang, J. Comprehensive Analysis of UGT1A1 Genetic Polymorphisms in Chinese Tibetan and Han Populations. Biochem. Genet. 2012, 50, 967–977. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic Susceptibility to Adverse Effects of Drugs and Environmental Toxicants: The Role of the CYP Family of Enzymes. Mutat. Res. Fund. Mol. M 2001, 482, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Saghafi, F.; Salehifar, E.; Janbabai, G.; Zaboli, E.; Hedayatizadeh-Omran, A.; Amjadi, O.; Moradi, S. CYP2D6*3 (A2549del), *4 (G1846A), *10 (C100T) and *17 (C1023T) Genetic Polymorphisms in Iranian Breast Cancer Patients Treated with Adjuvant Tamoxifen. Biomed. Rep. 2018, 9, 446. [Google Scholar] [CrossRef]
- Murayama, N.; Nakamura, T.; Saeki, M.; Soyama, A.; Saito, Y.; Sai, K.; Ishida, S.; Itoda, M.; Ozawa, S.; Sawada, J.I.; et al. CYP3A4 Gene Polymorphisms Influence Testosterone 6β-Hydroxylation. Drug Metab. Pharmacokinet. 2002, 17, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, J.; Medwid, S.; Facey, A.; Sung, I.; Russell, L.E.; Tirona, R.G.; Kim, R.B.; Schwarz, U.I. In-Vitro Characterization of Coding Variants with Predicted Functional Implications in the Efflux Transporter Multidrug Resistance Protein 4 (MRP4, ABCC4). Pharm. Genet. Genom. 2022, 32, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.P.; Wang, Y.H.; Wang, S.H.; Geng, P.W.; Hu, L.M.; Hu, G.X.; Cai, J.P. In Vitro Functional Characterization of 37 CYP2C9 Allelic Isoforms Found in Chinese Han Population. Acta Pharmacol. 2013, 34, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, L.; Girard, H.; Fortier, L.C.; Gagné, J.F.; Guillemette, C. Novel Functional Polymorphisms in the UGT1A7 and UGT1A9 Glucuronidating Enzymes in Caucasian and African-American Subjects and Their Impact on the Metabolism of 7-Ethyl-10-Hydroxycamptothecin and Flavopiridol Anticancer Drugs. J. Pharmacol. Exp. Ther. 2003, 307, 117–128. [Google Scholar] [CrossRef]
- Saeki, M.; Saito, Y.; Jinno, H.; Sai, K.; Ozawa, S.; Sawada, J.I.; Komamura, K.; Kamakura, S.; Kitakaze, M.; Ueno, K.; et al. Three Novel Single Nucleotide Polymorphisms in UGT1A9. Drug Metab. Pharmacokinet. 2003, 18, 146–149. [Google Scholar] [CrossRef]
- Jinno, H.; Saeki, M.; Saito, Y.; Tanaka-Kagawa, T.; Hanioka, N.; Sai, K.; Kaniwa, N.; Ando, M.; Shirao, K.; Minami, H.; et al. Functional Characterization of Human UDP-Glucuronosyltransferase 1A9 Variant, D256N, Found in Japanese Cancer Patients. J. Pharmacol. Exp. Ther. 2003, 306, 688–693. [Google Scholar] [CrossRef]
- Bosma, P.J. Inherited Disorders of Bilirubin Metabolism. J. Hepatol. 2003, 38, 107–117. [Google Scholar] [CrossRef]
- Moghrabi, N.; Sutherland, L.; Wooster, R.; Povey, S.; Boxer, M.; Burchell, B. Chromosomal Assignment of Human Phenol and Bilirubin UDP-Glucuronosyltransferase Genes (UGT1A-Subfamily). Ann. Hum. Genet. 1992, 56, 81–91. [Google Scholar] [CrossRef]
- Miles, K.; Stern, S.; Smith, P.; Kessler, F.; Ali, S.; Ritter, J. An investigation of human and rat liver microsomal mycophenolic acid glucuronidation: Evidence for a principal role of UGT1A enzymes and species differences in UGT1A specificity. Drug Metab. Dispos. 2005, 33, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R.B. Adverse Health Effects of Marijuana Use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Carliner, H.; Brown, Q.L.; Sarvet, A.L.; Hasin, D.S. Cannabis Use, Attitudes, and Legal Status in the US: A Review. Prev. Med. 2017, 104, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yuecel, M.; Solowij, N. Acute and Chronic Effects of Cannabinoids on Human Cognition—A Systematic Review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef]
- Duperrouzel, J.C.; Granja, K.; Pacheco-Colón, I.; Gonzalez, R. Adverse Effects of Cannabis Use on Neurocognitive Functioning: A Systematic Review of Meta-Analytic Studies. J. Dual Diagn. 2020, 16, 43–57. [Google Scholar] [CrossRef]
- Lorenzetti, V.; Chye, Y.; Silva, P.; Solowij, N.; Roberts, C.A. Does Regular Cannabis Use Affect Neuroanatomy? An Updated Systematic Review and Meta-Analysis of Structural Neuroimaging Studies. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, A.T.; Whitney, P.; Cuttler, C.; Spradlin, A.; Hinson, J.M.; McLaughlin, R.J. Altered Attentional Control Strategies but Spared Executive Functioning in Chronic Cannabis Users. Drug Alcohol Depend. 2017, 181, 116–123. [Google Scholar] [CrossRef]
- Becker, M.P.; Collins, P.F.; Luciana, M. Neurocognition in College-Aged Daily Marijuana Users. J. Clin. Exp. Neuropsychol. 2014, 36, 379–398. [Google Scholar] [CrossRef]
- Gonzalez, R.; Schuster, R.M.; Mermelstein, R.J.; Vassileva, J.; Martin, E.M.; Diviak, K.R. Performance of Young Adult Cannabis Users on Neurocognitive Measures of Impulsive Behavior and Their Relationship to Symptoms of Cannabis Use Disorders. J. Clin. Exp. Neuropsychol. 2012, 34, 962–976. [Google Scholar] [CrossRef]
- Grant, J.E.; Chamberlain, S.R.; Schreiber, L.; Odlaug, B.L. Neuropsychological Deficits Associated with Cannabis Use in Young Adults. Drug Alcohol Depend. 2012, 121, 159–162. [Google Scholar] [CrossRef]
- Griffith-Lendering, M.F.H.; Huijbregts, S.C.J.; Vollebergh, W.A.M.; Swaab, H. Motivational and Cognitive Inhibitory Control in Recreational Cannabis Users. J. Clin. Exp. Neuropsychol. 2012, 34, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, J.; Holroyd, S.; Heffernan, T.M. Does Cannabis Use Affect Prospective Memory in Young Adults? J. Psychopharmacol. 2010, 24, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Huestegge, L.; Kunert, H.-J.; Radach, R. Long-Term Effects of Cannabis on Eye Movement Control in Reading. Psychopharmacology 2010, 209, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.A.; Parsons, L.H. Keep off the Grass? Cannabis, Cognition and Addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Ganzer, F.; Bröning, S.; Kraft, S.; Sack, P.-M.; Thomasius, R. Weighing the Evidence: A Systematic Review on Long-Term Neurocognitive Effects of Cannabis Use in Abstinent Adolescents and Adults. Neuropsychol. Rev. 2016, 26, 186–222. [Google Scholar] [CrossRef]
- Lisdahl, K.M.; Wright, N.E.; Medina-Kirchner, C.; Maple, K.E.; Shollenbarger, S. Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Curr. Addict. Rep. 2014, 1, 144–156. [Google Scholar] [CrossRef]
- Lubman, D.I.; Cheetham, A.; Yücel, M. Cannabis and Adolescent Brain Development. Pharmacol. Ther. 2015, 148, 1–16. [Google Scholar] [CrossRef]
- Landrigan, J.; Bessenyei, K.; Leitner, D.; Yakovenko, I.; Fisk, J.D.; Prentice, J.L. A Systematic Review of the Effects of Cannabis on Cognition in People with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103338. [Google Scholar] [CrossRef]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef]
- Baxter, A.G. The Origin and Application of Experimental Autoimmune Encephalomyelitis. Nat. Rev. Immunol. 2007, 7, 904–912. [Google Scholar] [CrossRef]
- Lyman, W.; Sonett, J.; Brosnan, C.; Bornstein, M. Δ9-Tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1989, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wirguin, I.; Mechoulam, R.; Breuer, A.; Schezen, E.; Weidenfeld, J.; Brenner, T. Suppression of Experimetal Autoimmune Encephalomyelitis by Cannabinoids. Immunopharmacology 1994, 28, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Miron, S.; Lavie, V.; Margalit, R.; Biegon, A. Dexanabinol (HU-211) Effect on Experimental Autoimmune Encephalomyelitis: Implications for the Treatment of Acute Relapses of Multiple Sclerosis. J. Neuroimmunol. 2000, 102, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Musty, R.; Rein, J.; Tillery, W.; Pertwee, R. The Perceived Effects of Smoked Cannabis on Patients with Multiple Sclerosis. Eur. Neurol. 1997, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Petro, D.J.; Ellenberger, C. Treatment of Human Spasticity with Δ9-Tetrahydrocannabinol. J. Clin. Pharmacol. 1981, 21, 413S–416S. [Google Scholar] [CrossRef] [PubMed]
- Hamann, W.; di Vadi, P.P. Analgesic Effect of the Cannabinoid Analogue Nabilone Is Not Mediated by Opioid Receptors. Lancet 1999, 353, 560. [Google Scholar] [CrossRef]
- Brenneisen, R.; Egli, A.; Elsohly, M.A.; Henn, V.; Spiess, Y. The Effect of Orally and Rectally Administered Delta 9-Tetrahydrocannabinol on Spasticity: A Pilot Study with 2 Patients. Int. J. Clin. Pharmacol. Ther. 1996, 34, 446–452. [Google Scholar]
- Ungerleider, J.T.; Andyrsiak, T.; Fairbanks, L.; Ellison, G.W.; Myers, L.W. Delta-9-THC in the Treatment of Spasticity Associated with Multiple Sclerosis. Adv. Alcohol Subst. Abuse 1988, 7, 39–50. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, Controlled Trial of Cannabis-Based Medicine in Central Pain in Multiple Sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef]
- Schon, F.; Hart, P.E.; Hodgson, T.L.; Pambakian, A.L.M.; Ruprah, M.; Williamson, E.M.; Kennard, C. Suppression of Pendular Nystagmus by Smoking Cannabis in a Patient with Multiple Sclerosis. Neurology 1999, 53, 2209. [Google Scholar] [CrossRef]
- Clifford, D.B. Tetrahydrocannabinol for Tremor in Multiple Sclerosis. Ann. Neurol. 1983, 13, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Meinck, H.M.; Schönle, P.W.; Conrad, B. Effect of Cannabinoids on Spasticity and Ataxia in Multiple Sclerosis. J. Neurol. 1989, 236, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Martyn, C.N.; Illis, L.S.; Thom, J. Nabilone in the Treatment of Multiple Sclerosis. Lancet 1995, 345, 579. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A Preliminary Controlled Study to Determine Whether Whole-Plant Cannabis Extracts Can Improve Intractable Neurogenic Symptoms. Clin. Rehabil. 2003, 17, 21–29. [Google Scholar] [CrossRef]
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sativex® as Add-on Therapy vs. Further Optimized First-Line ANTispastics (SAVANT) in Resistant Multiple Sclerosis Spasticity: A Double-Blind, Placebo-Controlled Randomised Clinical Trial. Int. J. Neurosci. 2019, 129, 119–128. [Google Scholar] [CrossRef]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Nabiximols (THC/CBD Oromucosal Spray, Sativex®) in Clinical Practice—Results of a Multicenter, Non-Interventional Study (MOVE 2) in Patients with Multiple Sclerosis Spasticity. Eur. Neurol. 2014, 71, 271–279. [Google Scholar] [CrossRef]
- Squintani, G.; Donato, F.; Turri, M.; Deotto, L.; Teatini, F.; Moretto, G.; Erro, R. Cortical and Spinal Excitability in Patients with Multiple Sclerosis and Spasticity after Oromucosal Cannabinoid Spray. J. Neurol. Sci. 2016, 370, 263–268. [Google Scholar] [CrossRef]
- Russo, M.; Naro, A.; Leo, A.; Sessa, E.; D’Aleo, G.; Bramanti, P.; Calabrò, R.S. Evaluating Sativex® in Neuropathic Pain Management: A Clinical and Neurophysiological Assessment in Multiple Sclerosis. Pain Med. 2016, 17, 1145–1154. [Google Scholar] [CrossRef]
- Sorosina, M.; Clarelli, F.; Ferr, L.; Osiceanu, A.M.; Unal, N.T.; Mascia, E.; Martinelli, V.; Comi, G.; Benigni, F.; Esposito, F.; et al. Clinical Response to Nabiximols Correlates with the Downregulation of Immune Pathways in Multiple Sclerosis. Eur. J. Neurol. 2018, 25, 70. [Google Scholar] [CrossRef]
- Turri, M.; Teatini, F.; Donato, F.; Zanette, G.; Tugnoli, V.; Deotto, L.; Bonetti, B.; Squintani, G. Pain Modulation after Oromucosal Cannabinoid Spray (SATIVEX®) in Patients with Multiple Sclerosis: A Study with Quantitative Sensory Testing and Laser-Evoked Potentials. Medicines 2018, 5, 59. [Google Scholar] [CrossRef]
- Patti, F.; Messina, S.; Solaro, C.; Amato, M.P.; Bergamaschi, R.; Bonavita, S.; Bossio, R.B.; Morra, V.B.; Costantino, G.F.; Cavalla, P.; et al. Efficacy and Safety of Cannabinoid Oromucosal Spray for Multiple Sclerosis Spasticity. J. Neurol. Neurosurg. Psychiatry 2016, 87, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L.F. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. 2021, 16, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of Cannabinoids, Δ9-Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation through Regulation of MiRNA-Mediated Signaling Pathways. Front. Immunol. 2019, 10, 1921. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.; Friedman, H. Cannabinoid Receptors and Immunity. Immunol. Today 1998, 19, 373–381. [Google Scholar] [CrossRef]
- Nahas, G.G.; Morishima, A.; Desoize, B. Effects of Cannabinoids on Macromolecular Synthesis and Replication of Cultured Lymphocytes. Fed. Proc. 1977, 36, 1748–1752. [Google Scholar]
- Klein, T.W.; Kawakami, Y.; Newton, C.; Friedman, H. Marijuana Components Suppress Induction and Cytolytic Function of Murine Cytotoxic T Cells In Vitro and In Vivo. J. Toxicol. Environ. Health 1991, 32, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bam, M.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol Regulates Gene Expression in Encephalitogenic T Cells Using Histone Methylation and Noncoding RNA during Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2019, 9, 15780. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids Decrease the Th17 Inflammatory Autoimmune Phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- Manera, C.; Bertini, S. Cannabinoid-Based Medicines and Multiple Sclerosis. Adv. Exp. Med. Biol. 2021, 1264, 111–129. [Google Scholar] [CrossRef]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an Agonist Replacement Therapy during Cannabis Withdrawal: A Randomized Clinical Trial. JAMA Psychiatry 2014, 71, 281–291. [Google Scholar] [CrossRef]
- Nahas, G.G.; Frick, H.C.; Lattimer, J.K.; Latour, C.; Harvey, D. Pharmacokinetics of THC in Brain and Testis, Male Gametotoxicity and Premature Apoptosis of Spermatozoa. Hum. Psychopharmacol. Clin. Exp. 2002, 17, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sieradzan, K.A.; Fox, S.H.; Hill, M.; Dick, J.P.R.; Crossman, A.R.; Brotchie, J.M. Cannabinoids Reduce Levodopa-Induced Dyskinesia in Parkinson’s Disease: A Pilot Study. Neurology 2001, 57, 2108–2111. [Google Scholar] [CrossRef]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J. Effects of Dronabinol on Anorexia and Disturbed Behavior in Patients with Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Walther, S.; Mahlberg, R.; Eichmann, U.; Kunz, D. Delta-9-Tetrahydrocannabinol for Nighttime Agitation in Severe Dementia. Psychopharmacology 2006, 185, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P. Oral Nabilone Capsules in the Treatment of Chemotherapy-Induced Nausea and Vomiting and Pain. Expert Opin. Investig. Drugs 2008, 17, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New Opportunities and Challenges of Natural Products Research: When Target Identification Meets Single-Cell Multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
| Protein | Gene | Function | Variation | Effect | Ref. |
|---|---|---|---|---|---|
| CB1 | CNR1 | Receptor | 63-9597T > C | Cannabinoids addiction | [54] |
| CB2 | CNR2 | Receptor | 946C > T | The effect and the main function of the CB2 receptor are altered | [54] |
| FAAH protein | Fatty Acid Amide Hydrolase FAAH | Biotransformation | 385C > A | It is associated with drug abuse | [55] |
| Catechol-O-methyltransferase enzyme | COMT | The regulation and inactivation of catecholamine neurotransmitters in the brain | 472A > G | Modest controllable effect of cannabinoids consumption on executive functions | [56] |
| GABA | GABRA2 | Receptor | 231A > G | No significant effect on the drug dependence | [57] |
| Mu opioid receptor | OPRM1 | Receptor | 118A > G | No significant effect | [58] |
| ErbB3, ErbB4 | NRG1 | Promotes the growth, differentiation, and survival of a wide range of cell types | 122-16329C > T | Associated with cannabinoids dependence | [59] |
| Gene | Allele | Nucleotide Change | Effect | Ref. |
|---|---|---|---|---|
| CYP2D6 | CYP2D6*3 | A2549del | It will produce a protein with little or no function, which means increased activity of cannabinoids in the body. | [73] |
| CYP2D6 | CYP2D6*4 | G1846A | The activity of the enzyme is reduced, causing drug accumulation in the body. | [73] |
| CYP2C9 | CYP2C9*2 | c.430C > T | The activity of the enzyme is reduced, causing drug accumulation in the body. | [74] |
| CYP2C9 | CYP2C9*3 | c.1075A | The activity of the enzyme is reduced, causing drug accumulation in the body. | [74] |
| CYP3A4 | CYP3A4*2 | 664 T-C | The activity of the enzyme is reduced, causing an increase in the drug’s half-life time. | [74] |
| CYP3A4 | CYP3A4*11 | 1088 C-T | The activity of the enzyme is reduced, causing an increase in the drug’s half-life time. | [75] |
| CYP3A4 | CYP3A4*12 | 1117 C-T | The activity of the enzyme is reduced, causing an increase in the drug’s half-life time. | [76] |
| CYP3A4 | CYP3A4*17 | 566 T-C | Decrease in the enzyme activity, which increases the half-life of the drug. | [69] |
| UGT1A | UGT1A9*3a | 98T-C | Reduction or inactivation of the enzyme. | [77] |
| UGT1A | UGT1A9*4 | 726 T-G | Reduction or inactivation of the enzyme. | [78] |
| UGT1A | UGT1A9* 5 | 766 G-A | Reduction or inactivation of the enzyme. | [79] |
| UGT1A | UGT1A1*3 | 1124 C-T | It will lead to the inactivation of the enzyme. | [80] |
| UGT1A | UGT1A1*10 | 1021C-T | Dimension in the enzyme activity. | [81] |
| UGT1A | UGT1A1*13 | 508-510del | Inactivation of the enzyme. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouh, R.A.; Kamal, A.; Abdelnaser, A. Cannabinoids and Multiple Sclerosis: A Critical Analysis of Therapeutic Potentials and Safety Concerns. Pharmaceutics 2023, 15, 1151. https://doi.org/10.3390/pharmaceutics15041151
Nouh RA, Kamal A, Abdelnaser A. Cannabinoids and Multiple Sclerosis: A Critical Analysis of Therapeutic Potentials and Safety Concerns. Pharmaceutics. 2023; 15(4):1151. https://doi.org/10.3390/pharmaceutics15041151
Chicago/Turabian StyleNouh, Roua A., Ahmed Kamal, and Anwar Abdelnaser. 2023. "Cannabinoids and Multiple Sclerosis: A Critical Analysis of Therapeutic Potentials and Safety Concerns" Pharmaceutics 15, no. 4: 1151. https://doi.org/10.3390/pharmaceutics15041151
APA StyleNouh, R. A., Kamal, A., & Abdelnaser, A. (2023). Cannabinoids and Multiple Sclerosis: A Critical Analysis of Therapeutic Potentials and Safety Concerns. Pharmaceutics, 15(4), 1151. https://doi.org/10.3390/pharmaceutics15041151





