Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Preparation of Conditioned Medium
2.3. ELISA for Angiogenic Cytokines
2.4. Immunofluorescence
2.5. Western Blot Analysis
2.6. In Vitro Tube Formation Assay
2.7. Rat Hindlimb Ischemia Model
2.8. Quantification of Capillary Vessels and Proliferating Cells
2.9. Statistical Analysis
3. Results
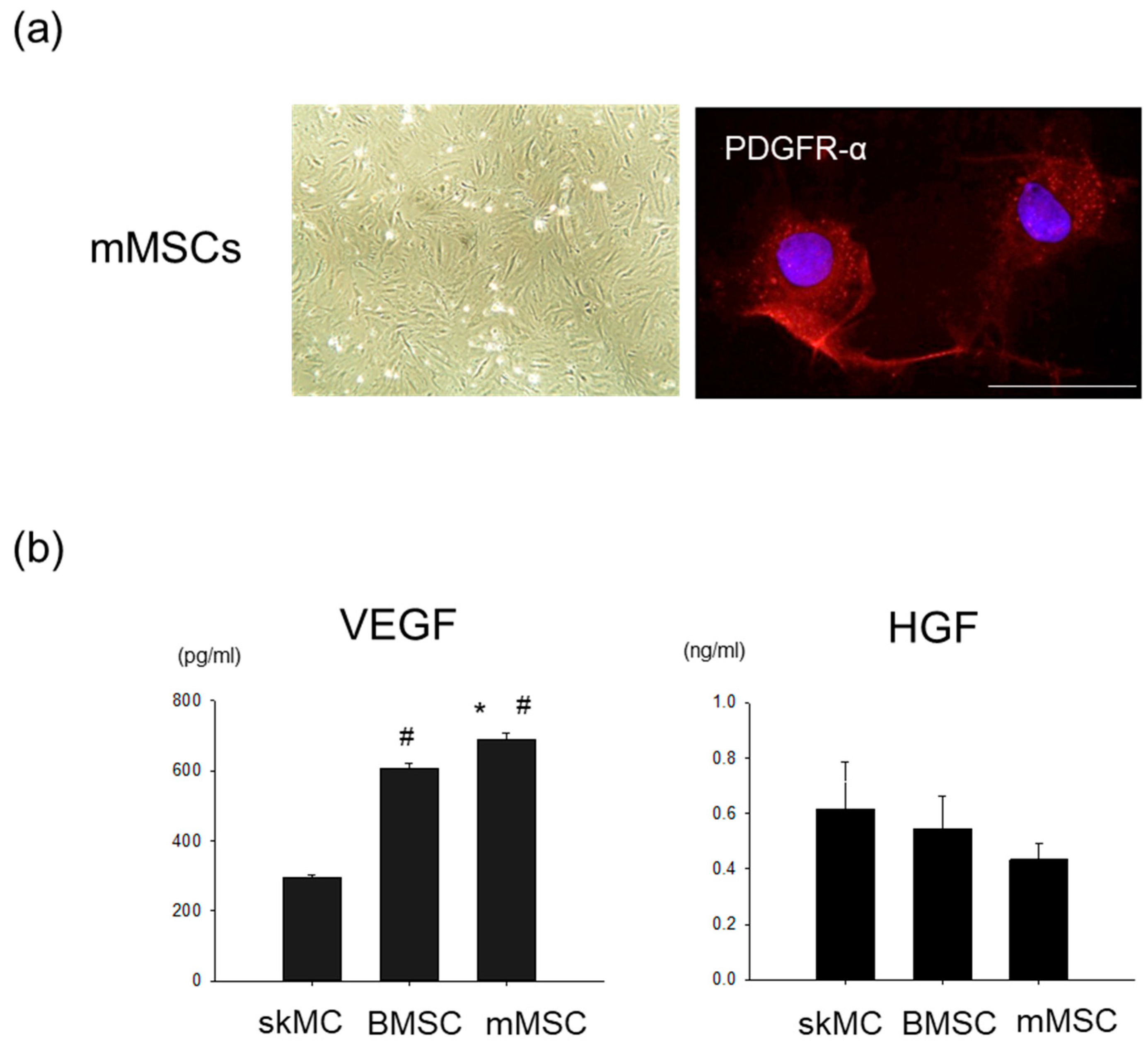
3.1. Pro-Angiogenic Potential in Cultured Rat MSCs Extracted from Skeletal Muscle
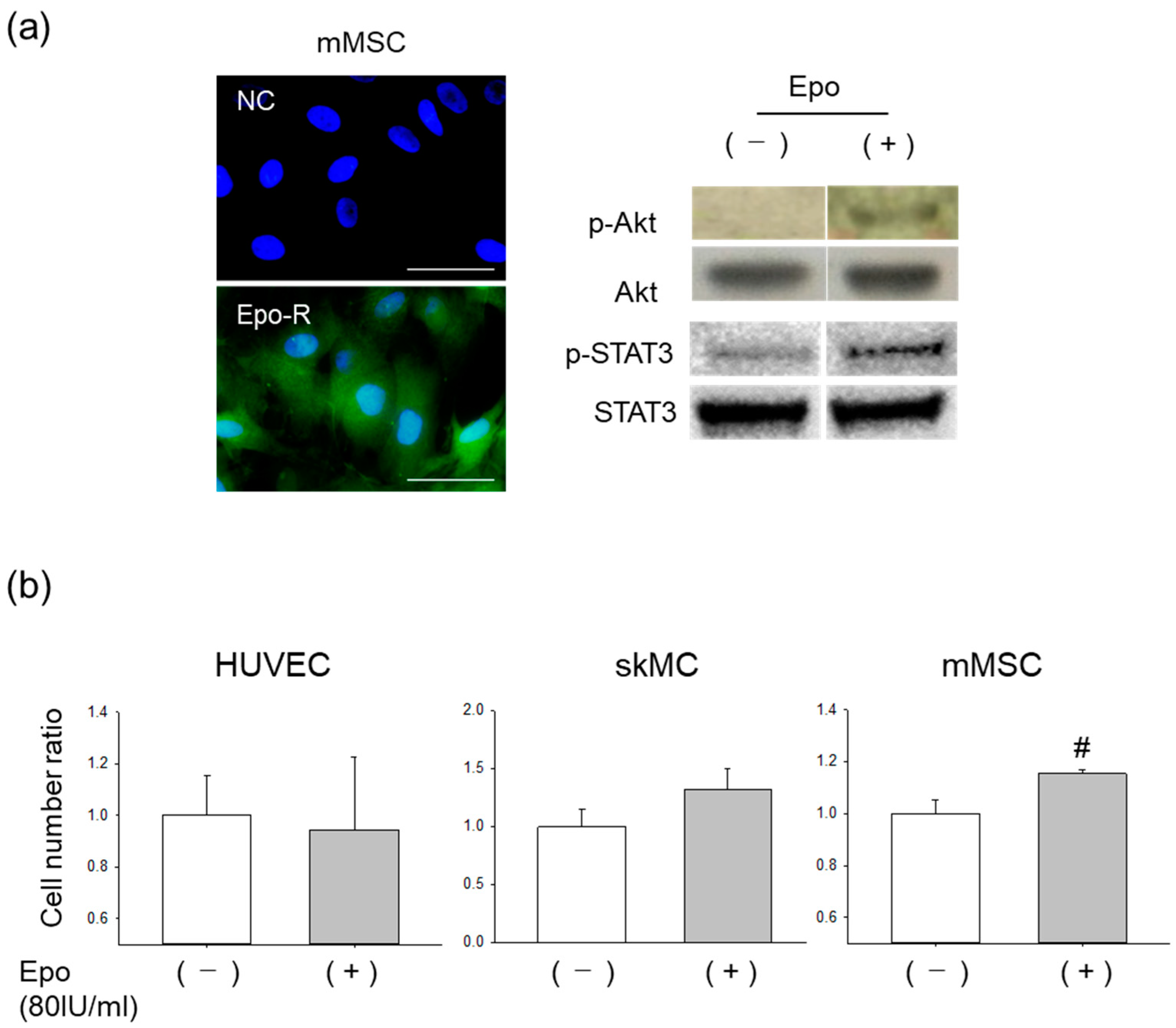
3.2. Effects of Epo on mMSCs In Vitro
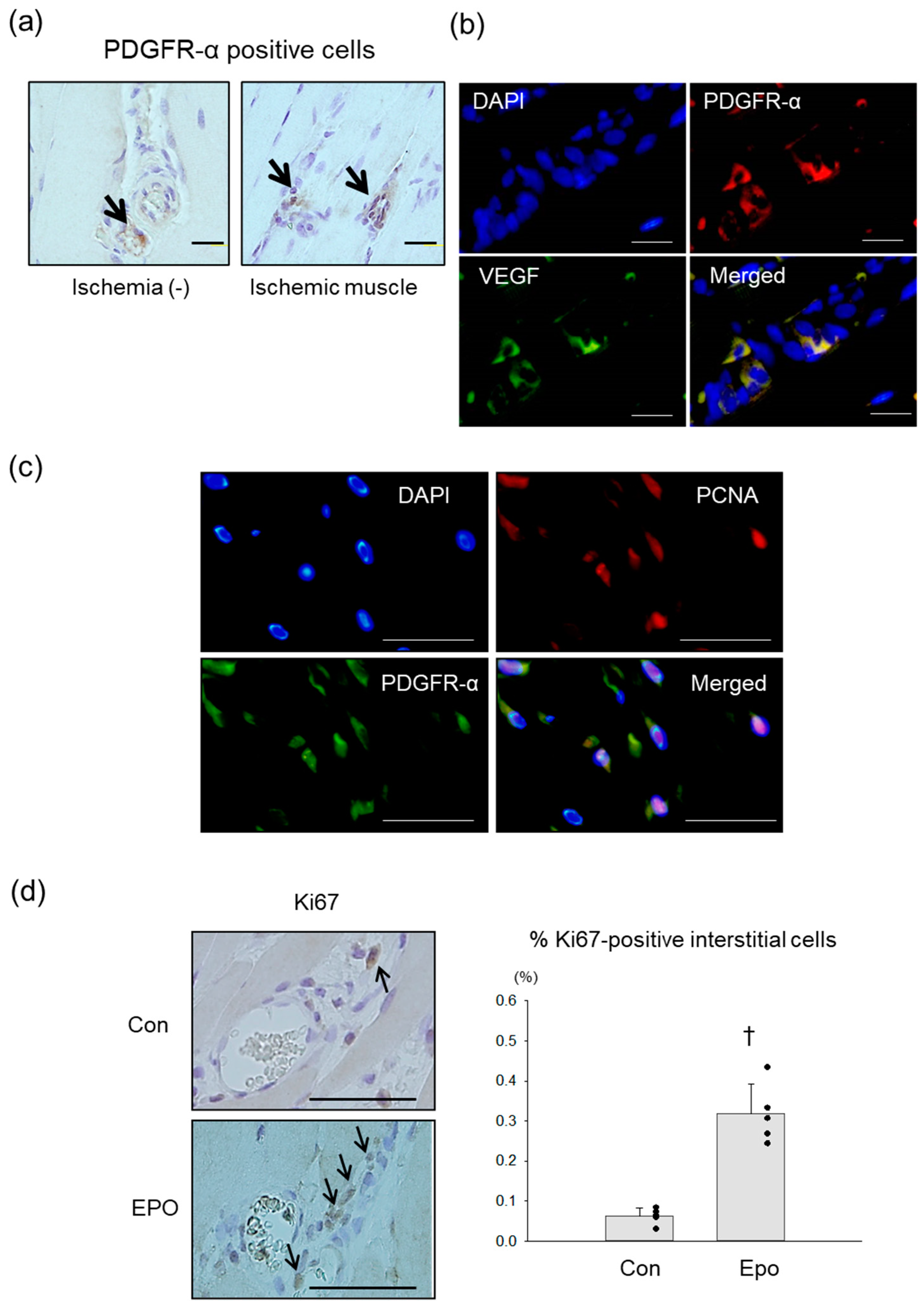
3.3. PDGFR-α Positive MSCs in Rat Ischemic Limb Treated with Epo
3.4. Angiogenic Effect of Epo in Rat Ischemic Limb
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.40. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kondo, K.; Hayashida, R.; Sasaki, K.I.; Ohtsuka, M.; Fukumoto, Y.; Takashima, S.; Inoue, O.; Usui, S.; Takamura, M.; et al. Therapeutic angiogenesis for patients with no-option critical limb ischemia by adipose-derived regenerative cells: TACT-ADRC multicenter trial. Angiogenesis 2022, 25, 535–546. [Google Scholar] [CrossRef]
- Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Higashide, S.; Ioji, T.; Fujita, Y.; et al. Autologous granulocyte colony-stimulating factor-mobilized peripheral blood CD34 positive cell transplantation for hemodialysis patients with critical limb ischemia: A prospective phase II clinical trial. Stem. Cells Transl. Med. 2018, 7, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Shirbaghaee, Z.; Hassani, M.; Heidari Keshel, S.; Soleimani, M. Emerging roles of mesenchymal stem cell therapy in patients with critical limb ischemia. Stem. Cell Res. Ther. 2022, 13, 462. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal stem cell-based therapy for cardiovascular disease: Progress and challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Iso, Y.; Spees, J.L.; Serrano, C.; Bakondi, B.; Pochampally, R.; Song, Y.H.; Sobel, B.E.; Delafontaine, P.; Prockop, D.J. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007, 354, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Iso, Y. Clinical application of vascular regenerative therapy for peripheral artery disease. Biomed. Res. Int. 2013, 2013, 179730. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Iso, Y.; Uyama, T.; Kawachi, K.; Wakabayashi, K.; Omori, Y.; Soda, T.; Shoji, M.; Koba, S.; Yokoyama, S.; et al. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab. Investig. 2011, 91, 553–564. [Google Scholar] [CrossRef]
- Mizukami, T.; Iso, Y.; Sato, C.; Sasai, M.; Spees, J.L.; Toyoda, M.; Umezawa, A.; Miyazaki, A.; Suzuki, H. Priming with erythropoietin enhances cell survival and angiogenic effect of mesenchymal stem cell implantation in rat limb ischemia. Regen. Ther. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair-current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Deonarine, A.; Jones, J.O.; Denton, A.E.; Feig, C.; Lyons, S.K.; Espeli, M.; Kraman, M.; McKenna, B.; Wells, R.J.; et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef]
- Sato, C.; Iso, Y.; Mizukami, T.; Otabe, K.; Sasai, M.; Kurata, M.; Sanbe, T.; Sekiya, I.; Miyazaki, A.; Suzuki, H. Fibroblast growth factor-23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 470, 657–662. [Google Scholar] [CrossRef]
- Iso, Y.; Usui, S.; Toyoda, M.; Spees, J.L.; Umezawa, A.; Suzuki, H. Bone marrow-derived mesenchymal stem cells inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia after arterial injury in rats. Biochem. Biophys. Rep. 2018, 16, 79–87. [Google Scholar] [CrossRef]
- Iso, Y.; Rao, K.S.; Poole, C.N.; Zaman, A.K.; Curril, I.; Sobel, B.E.; Kajstura, J.; Anversa, P.; Spees, J.L. Priming with ligands secreted by human stromal progenitor cells promotes grafts of cardiac stem/progenitor cells after myocardial infarction. Stem Cells 2014, 32, 674–683. [Google Scholar] [CrossRef]
- Usui, S.; Iso, Y.; Sasai, M.; Mizukami, T.; Mori, H.; Watanabe, T.; Shioda, S.; Suzuki, H. Kisspeptin-10 induces endothelial cellular senescence and impaired endothelial cell growth. Clin. Sci. 2014, 127, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Ikemoto-Uezumi, M.; Nakatani, M.; Morita, M.; Yamaguchi, A.; Yamada, H.; Nishino, I.; Hamada, Y.; et al. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014, 5, e1186. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ikemoto-Uezumi, M.; Tsuchida, K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front. Physiol. 2014, 5, 68. [Google Scholar] [CrossRef]
- Markel, T.A.; Wang, Y.; Herrmann, J.L.; Crisostomo, P.R.; Wang, M.; Novotny, N.M.; Herring, C.M.; Tan, J.; Lahm, T.; Meldrum, D.R. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2308-14. [Google Scholar] [CrossRef]
- Broxmeyer, H.E. Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013, 210, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kimáková, P.; Solár, P.; Solárová, Z.; Komel, R.; Debeljak, N. Erythropoietin and its angiogenic activity. Int. J. Mol. Sci. 2017, 18, 1519. [Google Scholar] [CrossRef]
- Ashley, R.A.; Dubuque, S.H.; Dvorak, B.; Woodward, S.S.; Williams, S.K.; Kling, P.J. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr. Res. 2002, 51, 472–478. [Google Scholar] [CrossRef]
- Ueda, K.; Takano, H.; Niitsuma, Y.; Hasegawa, H.; Uchiyama, R.; Oka, T.; Miyazaki, M.; Nakaya, H.; Komuro, I. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. J. Clin. Investig. 2010, 120, 2016–2029. [Google Scholar] [CrossRef]
- Alvarez Arroyo, M.V.; Castilla, M.A.; González Pacheco, F.R.; Tan, D.; Riesco, A.; Casado, S.; Caramelo, C. Role of vascular endothelial growth factor on erythropoietin-related endothelial cell proliferation. J. Am. Soc. Nephrol. 1998, 9, 1998–2004. [Google Scholar] [CrossRef]
- Baker, J.M.; Parise, G. Skeletal muscle erythropoietin expression is responsive to hypoxia and exercise. Med. Sci. Sports Exerc. 2016, 48, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.C.; Huntsman, H.D.; Liu, J.; Zou, K.; Boppart, M.D. Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS ONE 2012, 7, e29760. [Google Scholar] [CrossRef] [PubMed]
- Iso, Y.; Suzuki, H. Exercise therapy for intermittent claudication in peripheral artery disease. E-J. Cardiol. Pract. 2015, 13, 34. [Google Scholar]
- Duscha, B.D.; Robbins, J.L.; Jones, W.S.; Kraus, W.E.; Lye, R.J.; Sanders, J.M.; Allen, J.D.; Regensteiner, J.G.; Hiatt, W.R.; Annex, B.H. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Morrow, D.A.; Jarolim, P.; Sloan, S.; Hoffman, E.B.; O'Donnell, T.F.; Vora, A.N.; Omland, T.; Solomon, S.D.; Pfeffer, M.A.; et al. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J. Am. Coll. Cardiol. 2014, 63, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hu, X.; Ma, X.; Su, H.; Ying, L.; Peng, J.; Pan, X.; Bao, Y.; Zhou, J.; Jia, W. Elevated serum fibroblast growth factor 23 levels as an indicator of lower extremity atherosclerotic disease in Chinese patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2017, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef]
- Neri, S.; Borzì, R.M. Molecular Mechanisms Contributing to Mesenchymal Stromal Cell Aging. Biomolecules 2020, 10, 340. [Google Scholar] [CrossRef]
- Efimenko, A.; Dzhoyashvili, N.; Kalinina, N.; Kochegura, T.; Akchurin, R.; Tkachuk, V.; Parfyonova, Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 2014, 3, 32–41. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iso, Y.; Usui, S.; Suzuki, H. Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin. Pharmaceutics 2023, 15, 1049. https://doi.org/10.3390/pharmaceutics15041049
Iso Y, Usui S, Suzuki H. Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin. Pharmaceutics. 2023; 15(4):1049. https://doi.org/10.3390/pharmaceutics15041049
Chicago/Turabian StyleIso, Yoshitaka, Sayaka Usui, and Hiroshi Suzuki. 2023. "Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin" Pharmaceutics 15, no. 4: 1049. https://doi.org/10.3390/pharmaceutics15041049
APA StyleIso, Y., Usui, S., & Suzuki, H. (2023). Mesenchymal Stem/Stromal Cells in Skeletal Muscle Are Pro-Angiogenic, and the Effect Is Potentiated by Erythropoietin. Pharmaceutics, 15(4), 1049. https://doi.org/10.3390/pharmaceutics15041049




