Basic Properties of Adipose-Derived Mesenchymal Stem Cells of Rheumatoid Arthritis and Osteoarthritis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. ASC Isolation and Culture
2.3. Flow Cytometry Analysis of ASCs Phenotype
2.4. Adipogenic Differentiation of ASCs
2.5. Osteogenic Differentiation of ASCs
2.6. Chondrogenic Differentiation of ASCs
2.7. Gene Expression Analysis
2.8. Co-Cultures of ASCs with Purified Allogeneic CD4+ T-Cells or PBMCs and Proliferation Assay
2.9. Determination of the Concentration of Soluble Factors Involved in the Antiproliferative Properties of ASCs
2.10. Blocking Experiments
2.11. Statistical Analysis
3. Results
3.1. Phenotype
3.2. ASC Differentiation
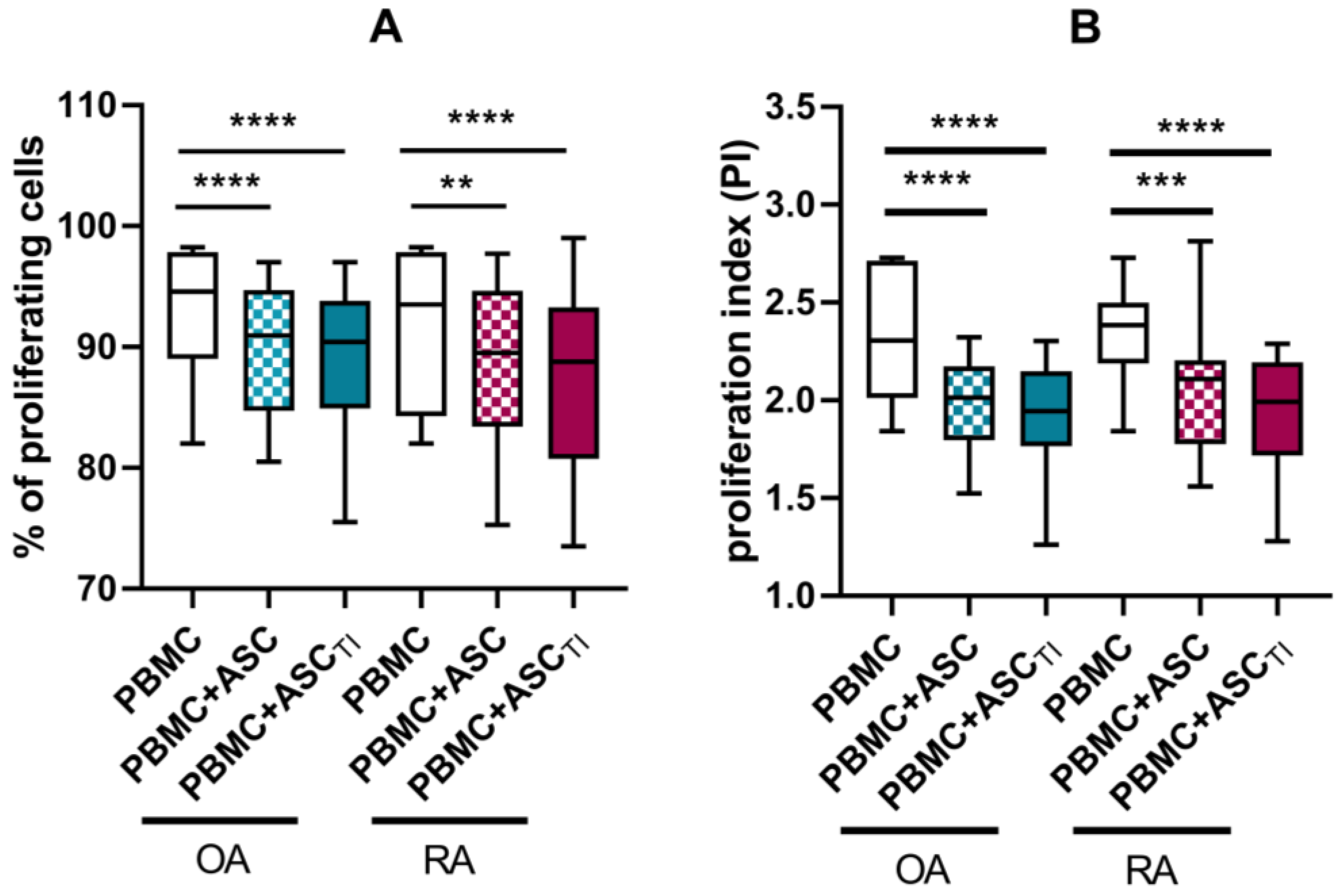
3.3. Effect of ASCs on Activated T Cell Proliferation
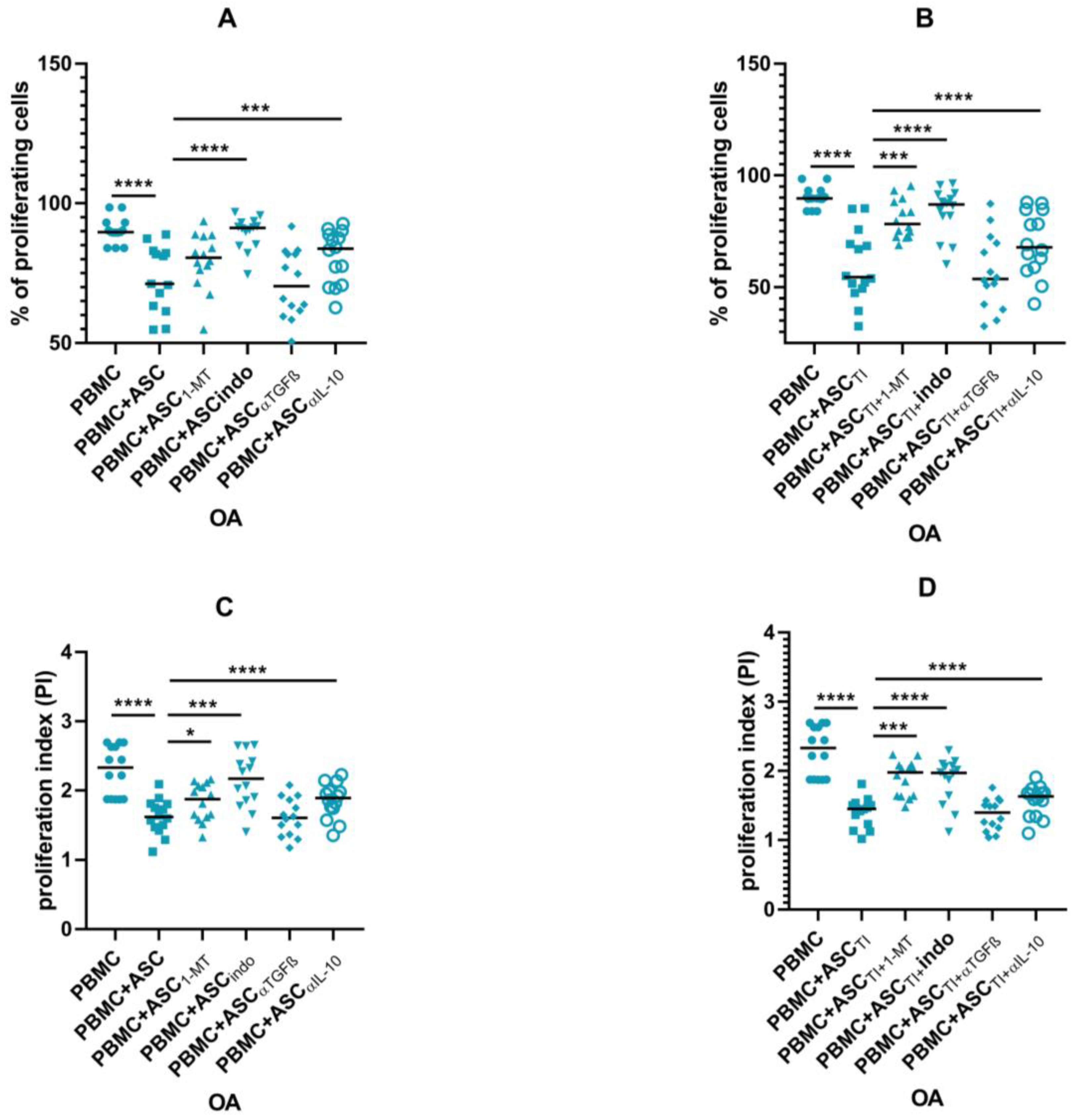
3.4. Soluble Factors Involved in Antiproliferative Capacities of ASCs
3.5. Blocking Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Sang-Cheol, B.; Ranjeny, T.; Deane, K.D.; Deshiré Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Firestein, G.S.; McInnes, I.B. The pathogenesis of rheumatoid arthritis. Immunity 2022, 55, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Korb, A.; Pavenstädt, H.; Pap, T. Cell death in rheumatoid arthritis. Apoptosis 2009, 14, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.; Schönfeld, C. Novel Insights into the Pathogenesis of Osteoarthritis. Curr. Rheumatol. 2018, 14, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Fraser, J.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Zuk, P.; Zhu, M.; Mizuno, H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Xiaowen, B.; Eckhard, A. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem. Biophys. Res. Comm. 2010, 401, 321–326. [Google Scholar]
- Kim, N.; Cho, S. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. 2013, 28, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P. The adipose-derived stem cell: Looking back and looking ahead. Mol. Biol. Cell 2010, 21, 1783–1787. [Google Scholar] [CrossRef]
- Kokai, L.; Marra, K.; Rubin, J. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Jorgensen, C.; Noel, D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013, 95, 2229–2234. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Fearon, U.; Canavan, M.; Biniecka, M.; Veale, D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, E.; Hobiger, S.; Despot-Slade, E.; Pichler, M.; Zenobi-Wong, M. Hypoxia regulates RhoA and Wnt/beta-catenin signaling in a context-dependent way to control redifferentiation of chondrocytes. Sci. Rep. 2017, 7, 9032. [Google Scholar] [CrossRef]
- Antebi, B.; Rodriguez, L.A.; Walker, K.P. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef]
- Govoni, M.; Muscari, C.; Bonafè, F.; Morselli, P.G.; Cortesi, M.; Dallari, D.; Giordano, E. A brief very-low oxygen tension regimen is sufficient for the early chondrogenic commitment of human adipose-derived mesenchymal stem cells. Adv. Med. Sci. 2021, 66, 98–104. [Google Scholar] [CrossRef]
- Bura, A.; Planat-Benard, V.; Bourin, P.; Silvestre, J.S.; Gross, F.; Grolleau, J.L.; Saint-Lebese, B.; Peyrafitte, J.A.; Fleury, S.; Gadelorge, M.; et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non revascularizable critical limb ischemia. Cytotherapy 2014, 16, 245–257. [Google Scholar] [CrossRef]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Cranio-Maxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Marra, K.; Rubin, P. The potential of adipose-derived stem cells in craniofacial repair and regeneration. Birth Defects Res. 2012, 96, 95–97. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011, 7, 269–291. [Google Scholar] [CrossRef]
- Jonathan, K.; Katherine, S. Upchurch, ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar]
- Peat, G.; Thomas, E.; Duncan, R.; Wood, L.; Hay, E.; Croft, P. Clinical classification criteria for knee osteoarthritis: Performance in the general population and primary care. Ann. Rheum. Dis. 2006, 65, 1363–1367. [Google Scholar] [CrossRef]
- Skalska, U.; Kontny, E.; Prochorec-Sobieszek, M.; Maśliński, W. Intra-articular adipose-derived mesenchymal stem cells from rheumatoid arthritis patients maintain the function of chondrogenic differentiation. Rheumatology 2012, 51, 1757–1764. [Google Scholar] [CrossRef]
- Kuca-Warnawin, E.; Plebańczyk, M.; Bonek, K.; Kontny, E. Inhibition of Allogeneic and Autologous T Cell Proliferation by Adipose-Derived Mesenchymal Stem Cells of Ankylosing Spondylitis Patients. Stem Cells Int. 2021, 2021, 6637328. [Google Scholar] [CrossRef]
- Kuca-Warnawin, E.; Skalska, U.; Janicka, I.; Musiałowicz, U.; Bonek, K.; Głuszko, P.; Szczęsny, P.; Olesińska, M.; Kontny, E. The phenotype and secretory activity of adipose-derived mesenchymal stem cells (ASCs) of patients with rheumatic diseases. Cells 2019, 8, 1659. [Google Scholar] [CrossRef]
- Kuca-Warnawin, E.; Olesińska, M.; Szczȩsny, P.; Kontny, E. Impact and Possible Mechanism(s) of Adipose Tissue-Derived Mesenchymal Stem Cells on T-Cell Proliferation in Patients With Rheumatic Disease. Front. Physiol. 2022, 12, 749481. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2002, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell. Prolif. 2020, 53, e127122020. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. Osteoarthritis: Inflammation and fibrosis in adipose tissue of osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 325–326. [Google Scholar] [CrossRef]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Meligy, F.Y.; Shigemura, K.; Behnsawy, H.M.; Fujisawa, M.; Kawabata, M.; Shirakawa, T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 203–215. [Google Scholar] [CrossRef]
- Varma, M.J.; Breuls, R.G.; Schouten, T.E.; Jurgens, W.J.; Bontkes, H.J.; Schuurhuis, G.J.; van Ham, S.M.; van Milligen, F.J. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, G.; Ural, E.; Ural, D.; Aksoy, A.; Kozdağ, G.; Gacar, G.; Karaöz, E. Comparative analysis of apoptotic resistance of mesenchymal stem cells isolated from human bone marrow and adipose tissue. Sci. World J. 2012, 2012, 105698. [Google Scholar] [CrossRef]
- Ribeiro, A.; Laranjeira, P.; Mendes, S.; Velada, I.; Leite, C.; Andrade, P.; Santos, F.; Henriques, A.; Grãos, M.; Cardoso, C.M.P.; et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. Ther. 2013, 4, 125. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Adesida, A.B.; Tew, S.R.; Andrew, J.G.; Hardingham, T.E. The epitope characterisation and the osteogenic differentiation potential of human fat pad-derived stem cells is maintained with ageing in later life. Injury 2009, 40, 150–157. [Google Scholar] [CrossRef]
- Garcia, J.; Mennan, C.; McCarthy, H.S.; Roberts, S.; Richardson, J.B.; Wright, K.T. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016, 2016, 6969726. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Piccione, M.; Belluzzi, E.; Petrelli, L.; Pozzuoli, A.; Ramonda, R.; Rossato, M.; Favero, M.; Ruggieri, P.; et al. Infrapatellar Fat Pad Stem Cells Responsiveness to Microenvironment in Osteoarthritis: From Morphology to Function. Front. Cell Dev. Biol. 2019, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytom. Part A 2009, 9999A, 22–30. [Google Scholar] [CrossRef]
- Skalska, U.; Prochorec-Sobieszek, M.; Kontny, E. Osteoblastic potential of infrapatellar fat pad-derived mesenchymal stem cells from rheumatoid arthritis and osteoarthritis patients. Int. J. Rheum. Dis. 2016, 19, 577–585. [Google Scholar] [CrossRef]
- Dudics, V.; Kunstár, A.; Kovács, J.; Lakatos, T.; Géher, P.; Gömör, B.; Monostori, Ė.; Uher, F. Chondrogenic Potential of Mesenchymal Stem Cells from Patients with Rheumatoid Arthritis and Osteoarthritis: Measurements in a Microculture System. Cells Tissues Organs 2009, 189, 307–316. [Google Scholar] [CrossRef]
- Morimoto, D.; Kuroda, S.; Kizawa, T.; Nomura, K.; Higuchi, C.; Yoshikawa, H.; Tomita, T. Equivalent osteoblastic differentiation function of human mesenchymal stem cells from rheumatoid arthritis in comparison with osteoarthritis. Rheumatology 2009, 48, 643–649. [Google Scholar] [CrossRef]
- Bravo, B.; Argüello, J.M.; Gortazar, A.R.; Forriol, F.; Vaquero, J. Modulation of gene expression in infrapatellar fat pad-derived mesenchymal stem cells in osteoarthritis. Cartilage 2016, 9, 55–62. [Google Scholar] [CrossRef]
- Yan, B.; Lv, S.; Tong, P.; Yan, L.; Chen, Z.; Zhou, L.; Yuan, Q.; Guo, L.; Shan, L. Intra-Articular Injection of Adipose-Derived Stem Cells Ameliorates Pain and Cartilage Anabolism/Catabolism in Osteoarthritis: Preclinical and Clinical Evidences. Front. Pharmacol. 2022, 13, 854025. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, Y.C.; Yu, S.N.; Lai, W.L.; Shen, Y.S.; Shen, P.C.; Lin, S.H.; Chang, C.H.; Lee, S.M. Infrapatellar fat pad-derived mesenchymal stromal cell product for treatment of knee osteoarthritis: A first-in-human study with evaluation of the potency marker. Cytotherapy 2021, 24, 72–85. [Google Scholar] [CrossRef]
- Djouad, F.; Bouffi, C.; Ghannam, S.; Noël, D.; Jorgensen, C. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 392–399. [Google Scholar] [CrossRef]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.J.; van Buul, G.M.; Lubberts, E.; Bos, P.K.; Verhaar, J.A.; Hoogduijn, M.J.; van Osch, G.J. Effect of Arthritic Synovial Fluids on the Expression of Immunomodulatory Factors by Mesenchymal Stem Cells: An Explorative in vitro Study. Front. Immunol. 2012, 3, 231. [Google Scholar] [CrossRef] [PubMed]
- Cuerquis, J.; Romieu-Mourez, R.; François, M.; Routy, J.P.; Young, Y.K.; Zhao, J.; Eliopoulos, N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: Effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy 2014, 16, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, Y.; Li, W.; Song, B.; Yang, R. Interleukin-17A- or tumor necrosis factor α-mediated increase in proliferation of T cells cocultured with synovium-derived mesenchymal stem cells in rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R1692013. [Google Scholar] [CrossRef]
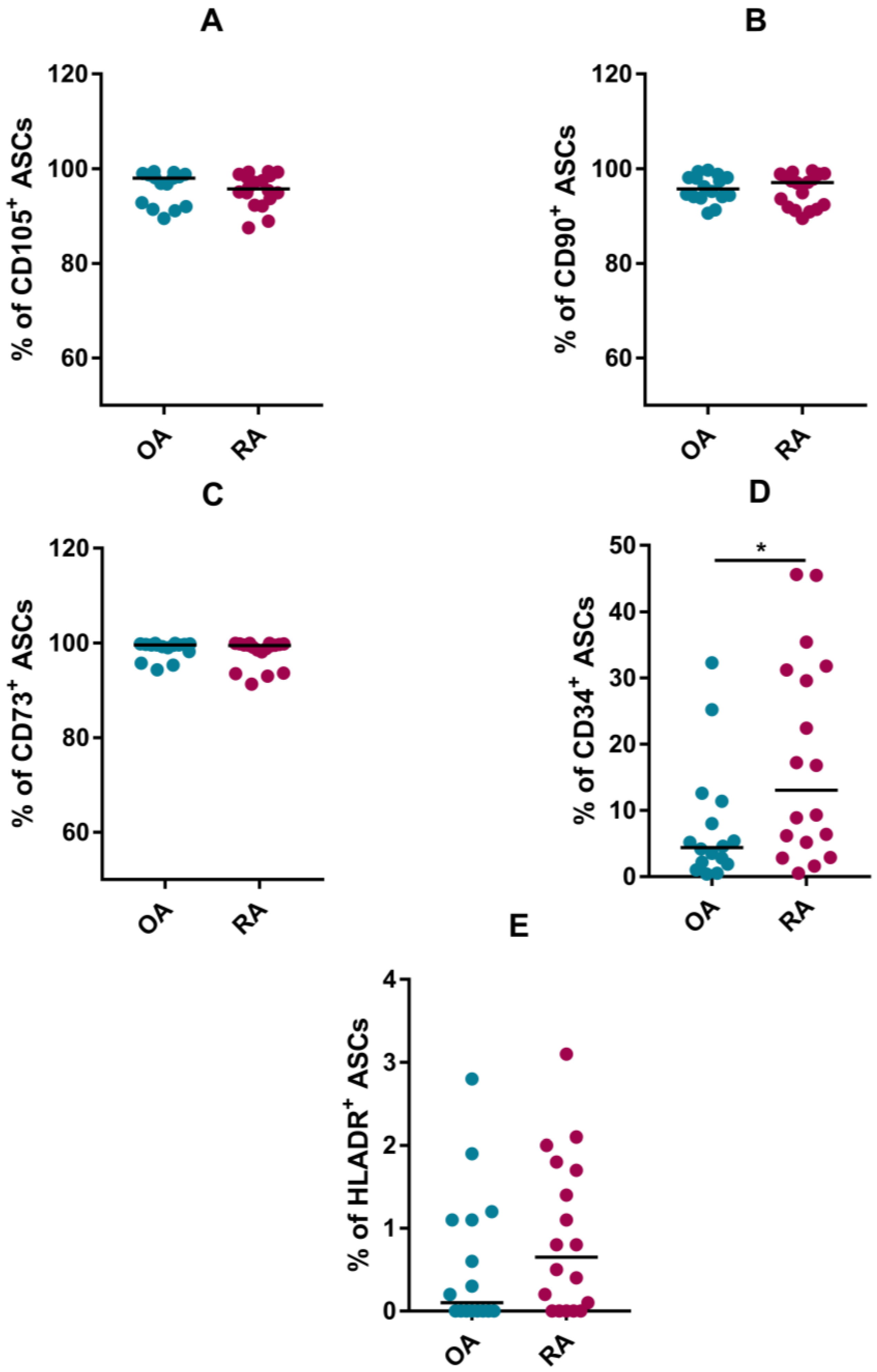
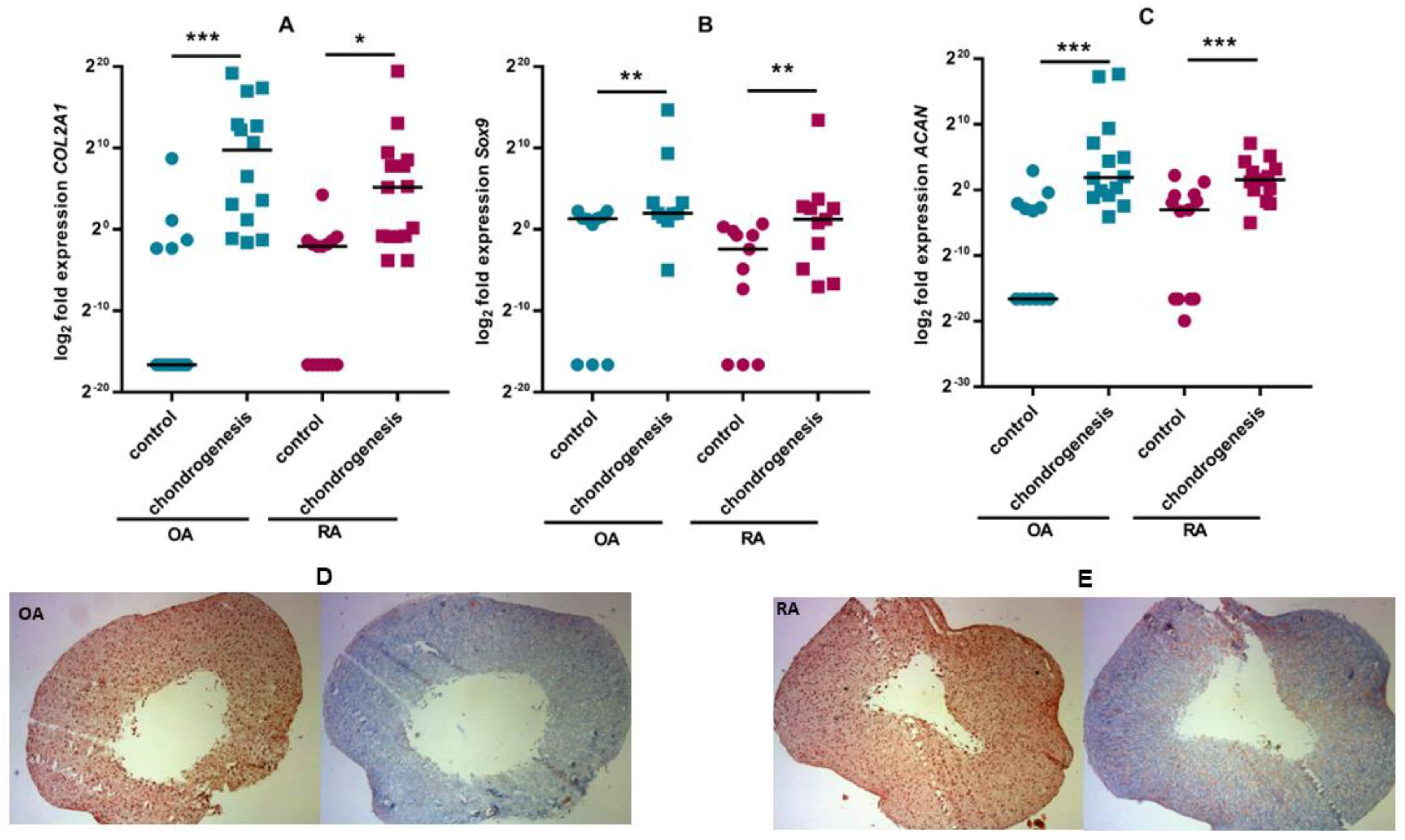

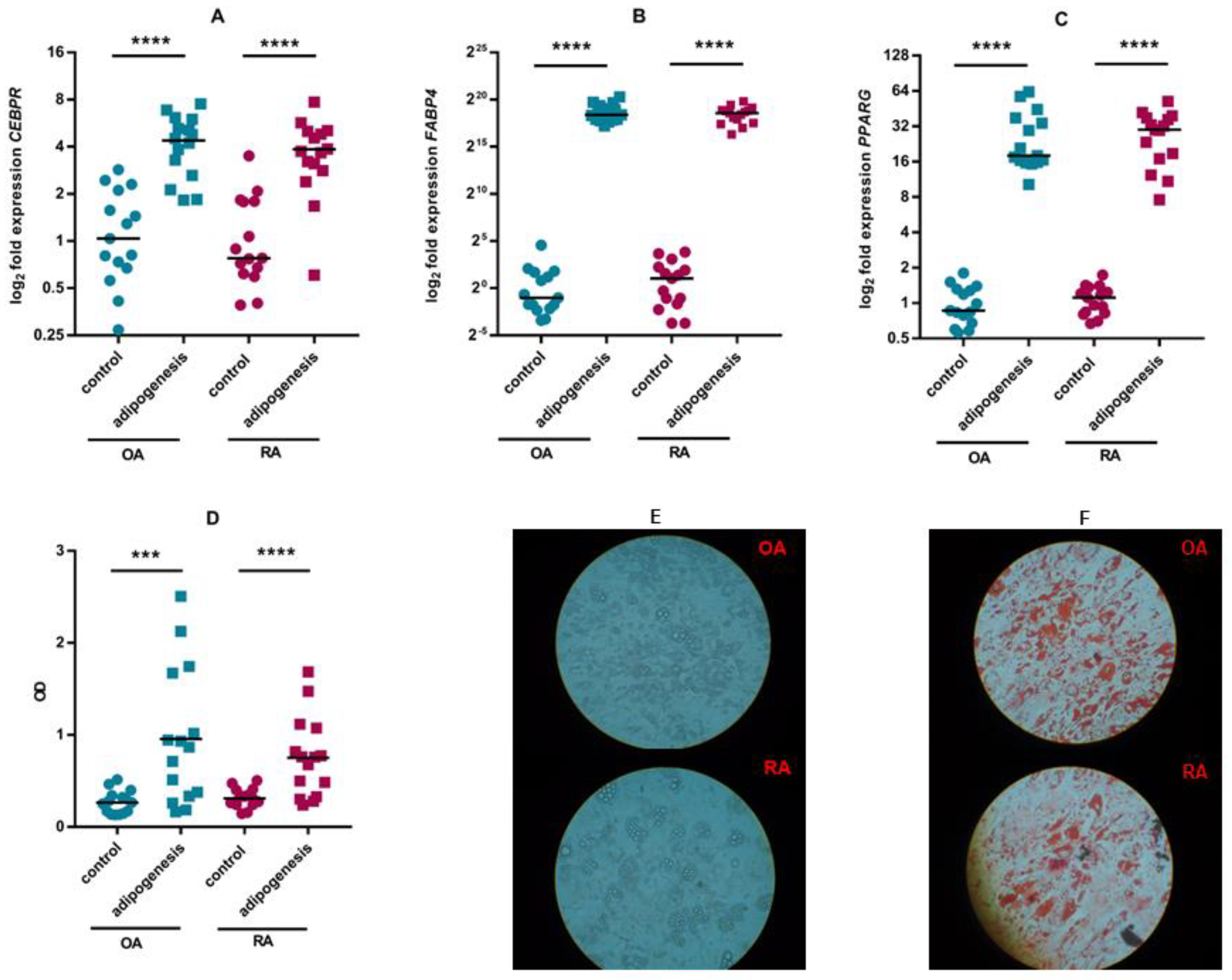
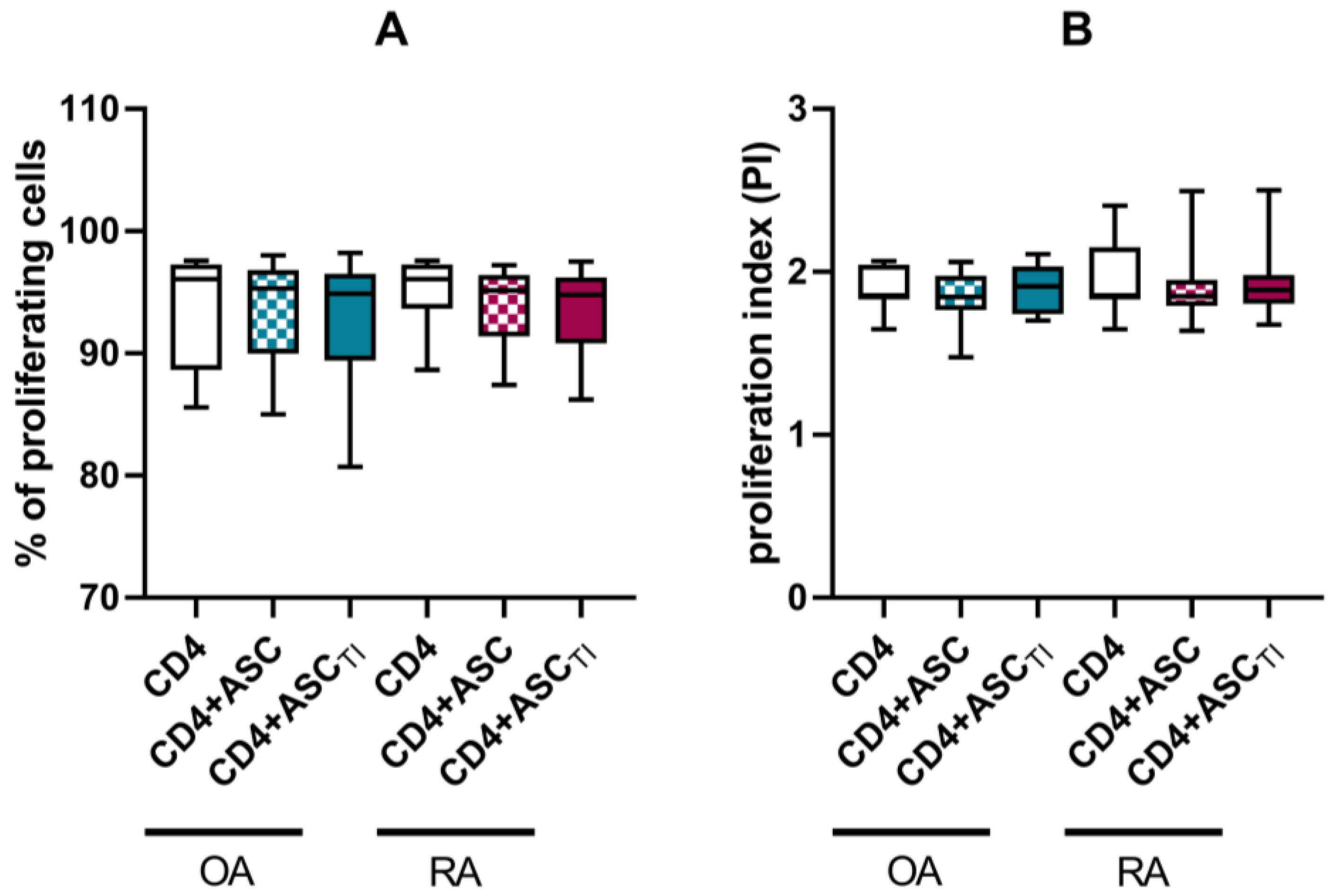



| Rheumatoid Arthritis (RA) (n = 18) | Osteoarthritis (OA) (n = 16) | |
|---|---|---|
| Demographics | ||
| Age, years | 61 (44–69) | 64 (39–69) |
| Sex, female (F)/male (M), n | 12 F/6 M | 10 F/6 M |
| BMI, kg/m2 | 28.4 (23.3–37.5) | 34.2 (27.7–45.5) # |
| Laboratory values | ||
| CRP, mg/L | 13.2 (2–36) | 4 (1–14) ### |
| ESR, mm/h | 19 (5–77) | 14 (2–32) # |
| Treatment, % | ||
| Methotrexate | 55% | 0% |
| Glucocorticoids | 66% | 0% |
| Sulfasalazine | 33% | 0% |
| NSAIDs | 66% | 81% |
| Gene Symbol | Assay ID | Gene Name |
|---|---|---|
| Markers of adipogenesis | ||
| CEBPB | Hs00942496_s1 | CCAAAT/enhancer-binding protein beta |
| FABP4 | Hs01086177_m1 | Fatty acid binding protein 4 |
| PPARG | Hs01115513_m1 | Peroxisome proliferator-activated receptor gamma |
| Markers of osteogenesis | ||
| RUNX2 | Hs01047973_m1 | Runt-related transcription factor 2 |
| OPN | Hs00959010_m1 | Osteopontin |
| COL1A1 | Hs00164004_m1 | Collagen type I α 1 |
| OSX | Hs01866874_s1 | osterix |
| ALPL | Hs01029144_m1 | Alkaline phosphatase |
| Markers of chondrogenesis | ||
| SOX9 | Hs00165814_m1 | SRY (sex determining region Y)-box 9 |
| ACAN | Hs00153936_m1 | Aggrecan |
| COL2A1 | Hs00264051_m1 | Collagen type II α 1 |
| Reference gene | ||
| GUSB | Hs00939627_m1 | Glucuronidase β |
| RPL13A | Hs04194366_g1 | Ribosomal protein L13a |
| TBP | Hs00427620_m1 | TATA-binding protein |
| eEF-1 | Hs02339452_g1 | eukaryotic elongation factor 1 gamma-like protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuca-Warnawin, E.; Kurowska, W.; Plebańczyk, M.; Wajda, A.; Kornatka, A.; Burakowski, T.; Janicka, I.; Syrówka, P.; Skalska, U. Basic Properties of Adipose-Derived Mesenchymal Stem Cells of Rheumatoid Arthritis and Osteoarthritis Patients. Pharmaceutics 2023, 15, 1003. https://doi.org/10.3390/pharmaceutics15031003
Kuca-Warnawin E, Kurowska W, Plebańczyk M, Wajda A, Kornatka A, Burakowski T, Janicka I, Syrówka P, Skalska U. Basic Properties of Adipose-Derived Mesenchymal Stem Cells of Rheumatoid Arthritis and Osteoarthritis Patients. Pharmaceutics. 2023; 15(3):1003. https://doi.org/10.3390/pharmaceutics15031003
Chicago/Turabian StyleKuca-Warnawin, Ewa, Weronika Kurowska, Magdalena Plebańczyk, Anna Wajda, Anna Kornatka, Tomasz Burakowski, Iwona Janicka, Piotr Syrówka, and Urszula Skalska. 2023. "Basic Properties of Adipose-Derived Mesenchymal Stem Cells of Rheumatoid Arthritis and Osteoarthritis Patients" Pharmaceutics 15, no. 3: 1003. https://doi.org/10.3390/pharmaceutics15031003
APA StyleKuca-Warnawin, E., Kurowska, W., Plebańczyk, M., Wajda, A., Kornatka, A., Burakowski, T., Janicka, I., Syrówka, P., & Skalska, U. (2023). Basic Properties of Adipose-Derived Mesenchymal Stem Cells of Rheumatoid Arthritis and Osteoarthritis Patients. Pharmaceutics, 15(3), 1003. https://doi.org/10.3390/pharmaceutics15031003






