Collagen Functionalization of Polymeric Electrospun Scaffolds to Improve Integration into Full-Thickness Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffold Preparation
2.2. Excisional Wound Model
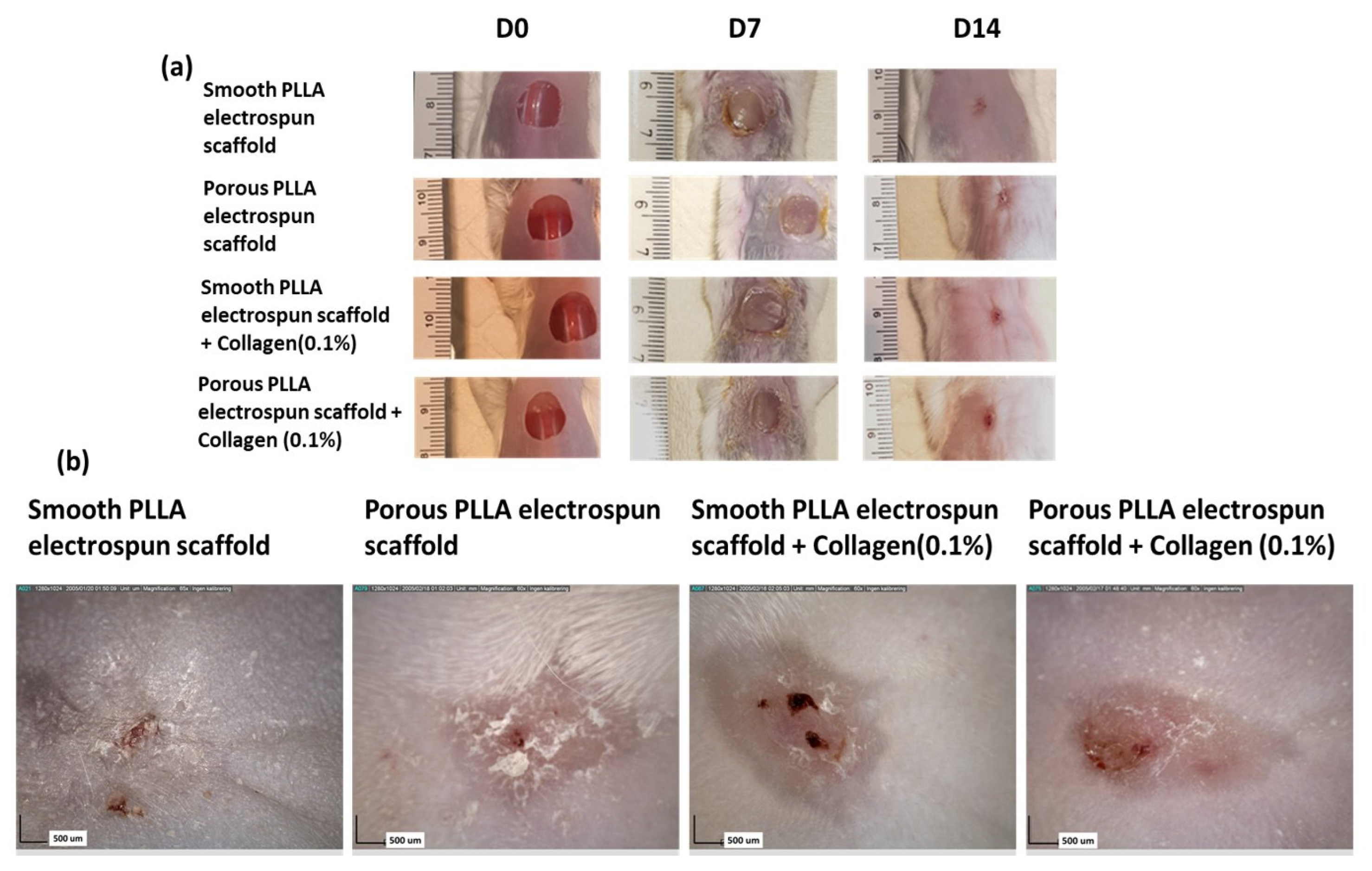
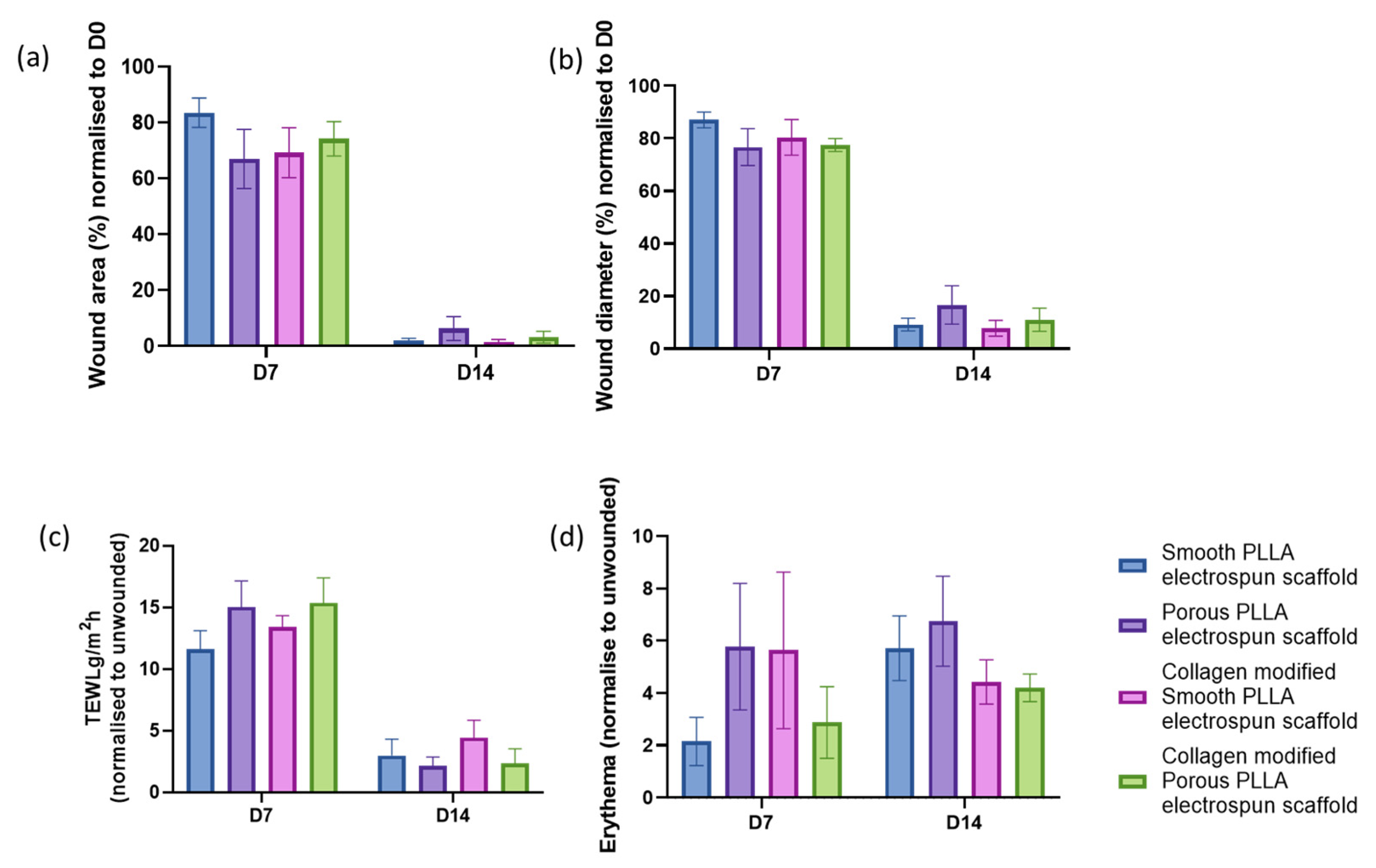
2.3. Macroscopic Wound Assessment
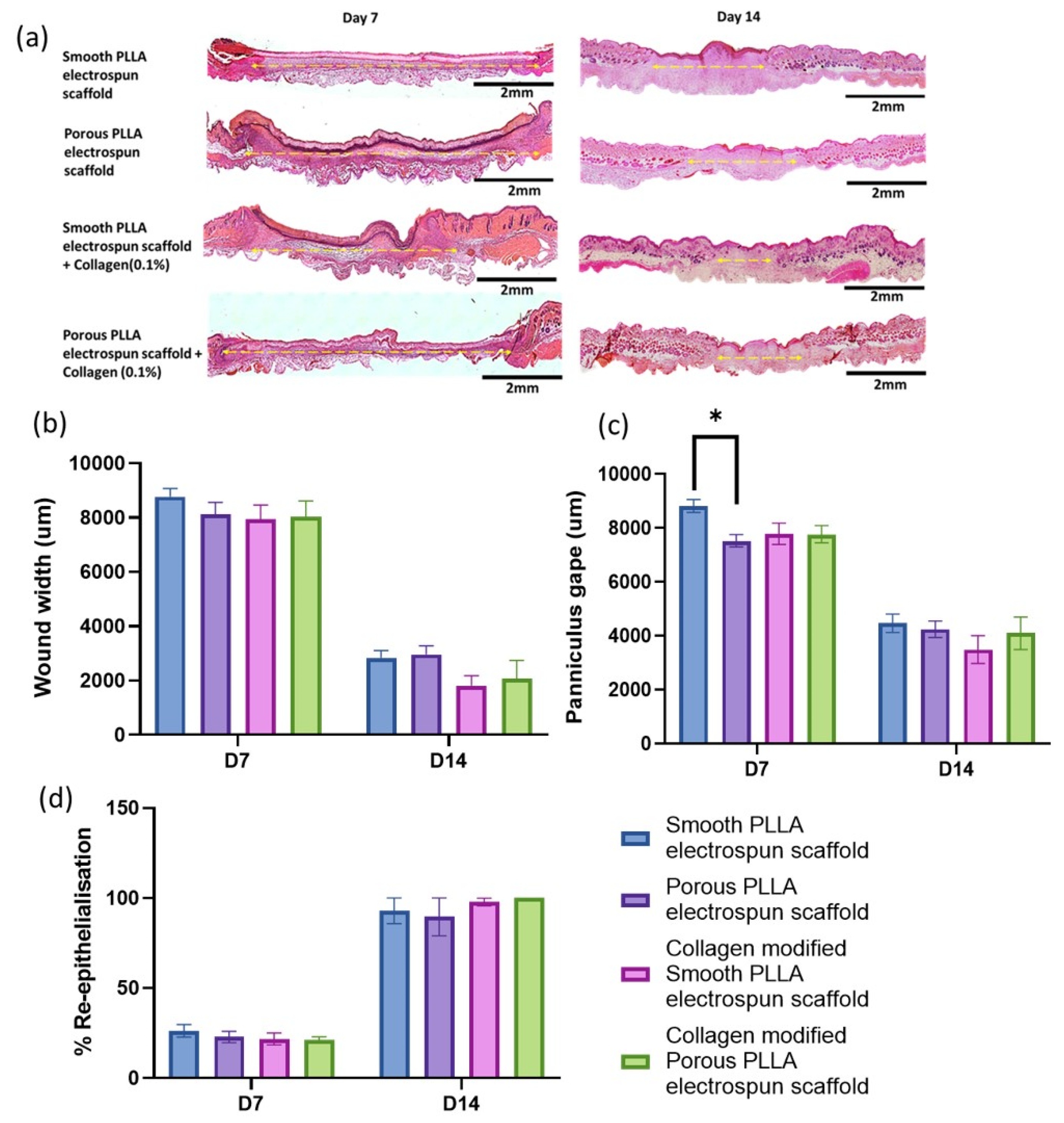
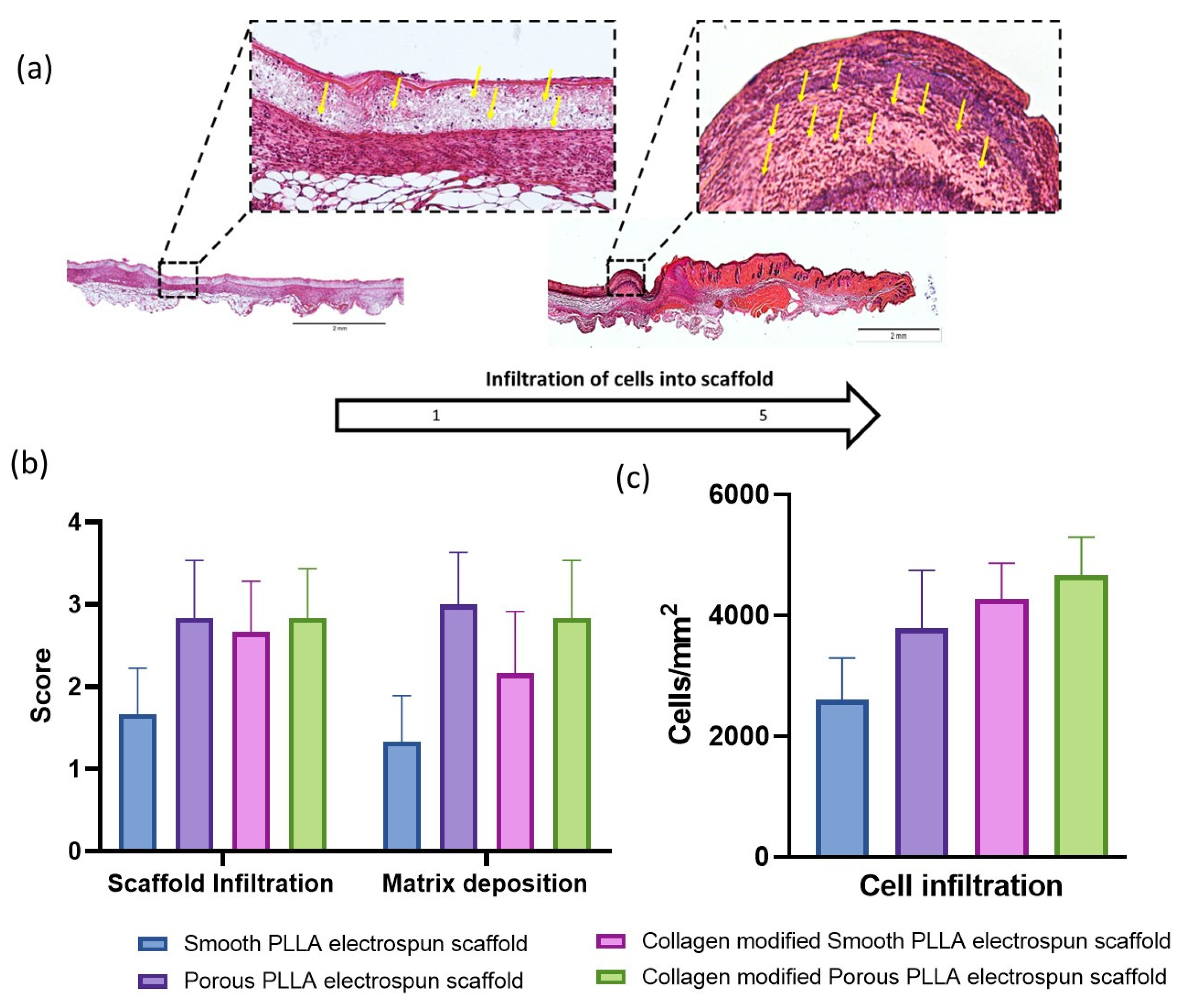
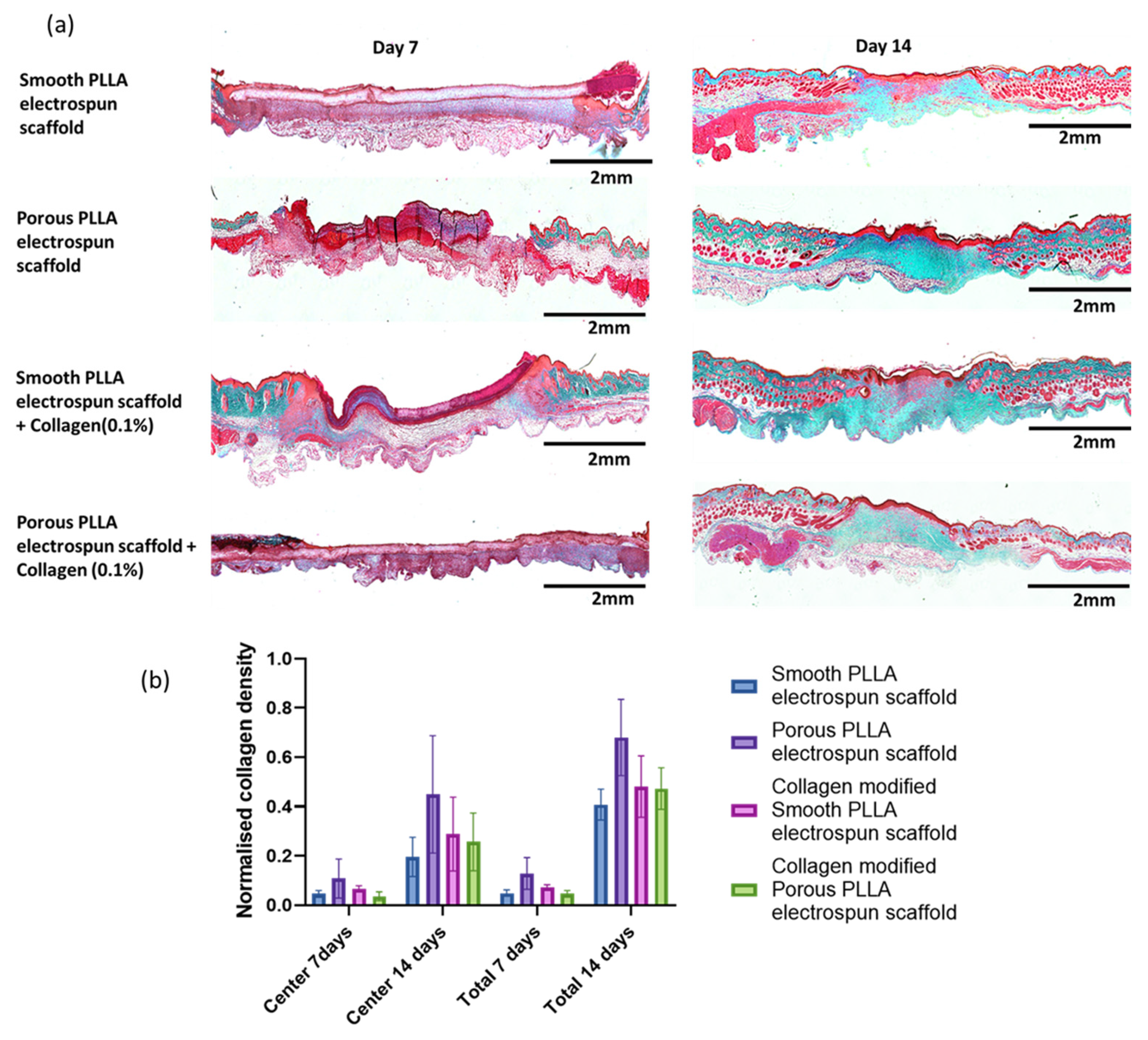
2.4. Histological Assessment of Healing
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.globenewswire.com/news-release/2020/03/04/1995036/0/en/Global-Skin-and-Wound-Care-Market-Is-Expected-to-Reach-USD-25-98-Billion-by-2025-Fior-Markets.html (accessed on 23 November 2022).
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymeric wound healing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Ghaee, A.; Bagheri-khoulenjani, S.; Amir, H.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCLnanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. Part B 2019, 177, 107339. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.K.; Seifalian, A.M. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Subia, B.; Kundu, J.; Kundu, C.S. Biomaterial Scaffold fabrication techniques for potential tissue engineering applications. In Tissue Engineering; Eberli, D., Ed.; IntechOpen: London, UK, 2010; pp. 141–157. [Google Scholar]
- Sachlos, E.; Czernuszka, J.T.; Gogolewski, S.; Dalby, M. Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of nanofibers for tissue engineering applications. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Chouhan, D.; Mandal, B.B. 3D functional scaffolds for skin tissue engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Elsevier: Rapid City, SD, USA, 2018; pp. 345–365. [Google Scholar]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Ghorbani, S.; Eyni, H.; Tiraihi, T.; Asl, L.S.; Soleimani, M.; Atashi, A.; Beiranvand, S.P.; Warkiani, M.E. Combined effects of 3D bone marrow stem cell-seeded wet-electrospun poly lactic acid scaffolds on full-thickness skin wound healing. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 905–912. [Google Scholar] [CrossRef]
- Gould, L.J. Topical Collagen-Based Biomaterials for Chronic Wounds: Rationale and Clinical Application. Adv. Wound Care 2016, 5, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Brett, D. A Review of Collagen and Collagen-based Wound Dressings. Wounds Repair Regen 2018, 20, 347–356. [Google Scholar]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Farhanulhakim, M.; Razip, M.; Tabata, Y.; Bt, R.; Idrus, H.; Nordin, A.; Fauzi, M.B. Antibacterial-Integrated CollagenWound Dressing for Diabetes-Related Foot Ulcers: An Evidence-Based Review of Clinical Studies. Polymers 2020, 12, 2168. [Google Scholar] [CrossRef]
- Agrawal, P.; Soni, S.; Mittal, G.; Bhatnagar, A. Role of polymeric biomaterials as wound healing agents. Int. J. Low Extrem. Wounds 2014, 13, 180–190. [Google Scholar] [CrossRef]
- Gaspar-pintiliescu, A.; Stanciuc, A.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. 2021, 109, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef]
- Aktürk, Ö.; Keskin, D. Collagen/PEO/gold nanofibrous matrices for skin tissue engineering. Turk. J. Biol. 2016, 40, 380–398. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Vaez, A.; Samadian, H.; Sahrapeyma, H.; Mirzaii, M.; Ghorbani, S.; Goodarzi, A. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018, 117, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Z.; Jiang, P.; Lin, T.; Li, X.; Sun, D. Characterization and cell response of electrospun R ana chensinensis skin collagen/poly(l-lactide) scaffolds with different fiber orientations. J. Appl. Polym. Sci. 2017, 134, 45109. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Jahir Hussain, F.S.; Abdull Rasad, M.S.; Mohd Yusoff, M. Improved cellular response of chemically crosslinked collagen incorporated hydroxyethyl cellulose/poly(vinyl) alcohol nanofibers scaffold. J. Biomater. Appl. 2015, 29, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Jiménez Vázquez, J.; San Martín Martínez, E. Collagen and elastin scaffold by electrospinning for skin tissue engineering applications. J. Mater. Res. 2019, 34, 2819–2827. [Google Scholar] [CrossRef]
- Lin, H.; Kuo, Y.; Chang, S.; Ni, T. Characterization of electrospun nanofiber matrices made of collagen blends as potential skin substitutes. Biomed. Mater. 2013, 8, 025009. [Google Scholar] [CrossRef] [PubMed]
- Shuxiang, C.; Chuanxiang, W.; Wenguang, Y.; Wenfeng, L.; Haibo, Y.; Lianqing, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- Iruthayapandi, S.R.; Seok, H.L.; Moon, S.K.; Suong-Hyu, H.; Aravindha, R.S.; Kandasamy, P.; Dong-Wook, H. The predominant factor influencing cellular behavior on electrospun nanofibrous scaffolds: Wettability or surface morphology? Mater. Des. 2022, 216, 110580. [Google Scholar]
- Girija, A.R.; Palaninathan, V.; Strudwick, X.; Balasubramanian, S.; Nair, S.D.; Cowin, A.J. Collagen-functionalized electrospun smooth and porous polymeric scaffolds for the development of human skin-equivalent. RSC Adv. 2020, 10, 26594–26603. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Vetteth, S.; Periyasamy, S.M.; Kanj, M.; Fedorova, L.; Khouri, S.; Kahaleh, M.B.; Xie, Z.; Malhotra, D.; Kolodkin, N.I.; et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 2006, 47, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, P.; Palaninathan, V.; Veeranarayanan, S.; Ukai, T.; Maekawa, T.; Hanajiri, T.; Mohamed, M.S. ECM Mimetic Electrospun Porous Poly (L-lactic acid) (PLLA) Scaffolds as Potential Substrates for Cardiac Tissue Engineering. Polymers 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Pavlova, E.; Polyanskaya, A.; Volkova, M.; Biryukova, E.; Filkov, G.; Trofimenko, A.; Durymanov, M.; Klinov, D.; Bagrov, D. Acceleration of Electrospun PLA Degradation by Addition of Gelatin. Int. J. Mol. Sci. 2023, 24, 3535. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Markowski, J.; Pilch, J.; Blazewicz, S. Degradation Behavior of Electrospun PLA and PLA/CNT Nanofibres in Aqueous Environment. J. Nanomater. 2018, 2018, 8796583. [Google Scholar] [CrossRef]
- Carlos, A.; Rodrigues, A.C.; Tofanello, A.; Nantes, I.L.; Rosa, D.S. Biological Oxidative Mechanisms for Degradation of Poly(lactic acid) Blended with Thermoplastic Starch. ACS Sustain. Chem. Eng. 2015, 3, 2756–2766. [Google Scholar]
- Ishii, D.; Ying, T.H.; Mahara, A.; Murakami, S.; Yamaoka, T.; Lee, W.K.; Iwata, T. In vivo tissue response and degradation behavior of PLLA and stereocomplexed PLA nanofibers. Biomacromolecules 2009, 10, 237–242. [Google Scholar] [CrossRef]
- Behere, I.; Ingavle, G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J. Biomed. Mater. Res. A 2022, 110, 443–461. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindran Girija, A.; Strudwick, X.; Balasubramanian, S.; Palaninathan, V.; Nair, S.D.; Cowin, A.J. Collagen Functionalization of Polymeric Electrospun Scaffolds to Improve Integration into Full-Thickness Wounds. Pharmaceutics 2023, 15, 880. https://doi.org/10.3390/pharmaceutics15030880
Ravindran Girija A, Strudwick X, Balasubramanian S, Palaninathan V, Nair SD, Cowin AJ. Collagen Functionalization of Polymeric Electrospun Scaffolds to Improve Integration into Full-Thickness Wounds. Pharmaceutics. 2023; 15(3):880. https://doi.org/10.3390/pharmaceutics15030880
Chicago/Turabian StyleRavindran Girija, Aswathy, Xanthe Strudwick, Sivakumar Balasubramanian, Vivekanandan Palaninathan, Sakthikumar Dasappan Nair, and Allison J. Cowin. 2023. "Collagen Functionalization of Polymeric Electrospun Scaffolds to Improve Integration into Full-Thickness Wounds" Pharmaceutics 15, no. 3: 880. https://doi.org/10.3390/pharmaceutics15030880
APA StyleRavindran Girija, A., Strudwick, X., Balasubramanian, S., Palaninathan, V., Nair, S. D., & Cowin, A. J. (2023). Collagen Functionalization of Polymeric Electrospun Scaffolds to Improve Integration into Full-Thickness Wounds. Pharmaceutics, 15(3), 880. https://doi.org/10.3390/pharmaceutics15030880







