Supramolecular Organization in Salts of Riluzole with Dihydroxybenzoic Acids—The Key Role of the Mutual Arrangement of OH Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.1.1. Mechanochemical Experiments
2.1.2. Single Crystal XRD
2.2. Computational Details
2.2.1. Periodic DFT Calculations
2.2.2. Enthalpy of the Intermolecular H-Bonds
2.2.3. Estimation of the Crystal Lattice Energy
2.2.4. Molecular Electrostatic Potential
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunawardana, C.A.; Aakeröy, C.B. Co-crystal synthesis: Fact, fancy, and great expectations. Chem. Commun. 2018, 54, 14047–14060. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef] [PubMed]
- Shattock, T.R.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Carboxylic Acid·Pyridine Hydrogen Bonds in Cocrystals That also Contain a Hydroxyl Moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Strategy and Methodology in the Synthesis of Multicomponent Molecular Solids: The Quest for Higher Cocrystals. Acc. Chem. Res. 2019, 52, 2210–2220. [Google Scholar] [CrossRef]
- Topić, F.; Rissanen, K. Systematic Construction of Ternary Cocrystals by Orthogonal and Robust Hydrogen and Halogen Bonds. J. Am. Chem. Soc. 2016, 138, 6610–6616. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen Bridges in Crystal Engineering: Interactions without Borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Etter, M.C. Hydrogen bonds as design elements in organic chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Seddon, K.R. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 1993, 22, 397–407. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Karas, L.J.; Wu, C.-H.; Das, R.; Wu, J.I.C. Hydrogen bond design principles. WIREs Comput. Mol. Sci. 2020, 10, e1477. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Crystal Engineering: A Holistic View. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L. On the significance of weak hydrogen bonds in crystal packing: A large databank comparison of polymorphic structures. CrystEngComm 2018, 20, 5976–5989. [Google Scholar] [CrossRef]
- Vologzhanina, A.V. Intermolecular Interactions in Functional Crystalline Materials: From Data to Knowledge. Crystals 2019, 9, 478. [Google Scholar] [CrossRef]
- Moragues-Bartolome, A.M.; Jones, W.; Cruz-Cabeza, A.J. Synthon preferences in cocrystals of cis-carboxamides:carboxylic acids. CrystEngComm 2012, 14, 2552–2559. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Baldrighi, M.; Desper, J.; Metrangolo, P.; Resnati, G. Supramolecular Hierarchy among Halogen-Bond Donors. Chem. A Eur. J. 2013, 19, 16240–16247. [Google Scholar] [CrossRef]
- Otero-de-la-Roza, A.; Johnson, E.R. A benchmark for non-covalent interactions in solids. J. Chem. Phys. 2012, 137, 054103. [Google Scholar] [CrossRef]
- Vener, M.V.; Levina, E.O.; Koloskov, O.A.; Rykounov, A.A.; Voronin, A.P.; Tsirelson, V.G. Evaluation of the Lattice Energy of the Two-Component Molecular Crystals Using Solid-State Density Functional Theory. Cryst. Growth Des. 2014, 14, 4997–5003. [Google Scholar] [CrossRef]
- Taylor, R. Which intermolecular interactions have a significant influence on crystal packing? CrystEngComm 2014, 16, 6852–6865. [Google Scholar] [CrossRef]
- Miller, D.K.; Chernyshov, I.Y.; Torubaev, Y.V.; Rosokha, S.V. From weak to strong interactions: Structural and electron topology analysis of the continuum from the supramolecular chalcogen bonding to covalent bonds. Phys. Chem. Chem. Phys. 2022, 24, 8251–8259. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Fang, T.; De Marchi, F.; Neel, D.; Van Weehaeghe, D.; Berry, J.D.; Paganoni, S. Pharmacotherapy for Amyotrophic Lateral Sclerosis: A Review of Approved and Upcoming Agents. Drugs 2022, 82, 1367–1388. [Google Scholar] [CrossRef]
- Bensimon, G.; Lacomblez, L.; Meininger, V. A Controlled Trial of Riluzole in Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lacomblez, L.; Bensimon, G.; Leigh, P.N.; Guillet, P.; Meininger, V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996, 347, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.P.; Hudson, J.I.; Jensen, J.E.; McCarthy, J.; Roberts, J.L.; Prescot, A.P.; Cohen, B.M.; Pope, H.G.; Renshaw, P.F.; Öngür, D. Rapid Enhancement of Glutamatergic Neurotransmission in Bipolar Depression Following Treatment with Riluzole. Neuropsychopharmacology 2010, 35, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Belluzzi, O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur. J. Neurosci. 2000, 12, 3567–3574. [Google Scholar] [CrossRef]
- Benoit, E.; Escande, D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflügers Arch. 1991, 419, 603–609. [Google Scholar] [CrossRef]
- Centonze, D.; Calabresi, P.; Pisani, A.; Marinelli, S.; Marfia, G.A.; Bernardi, G. Electrophysiology of the neuroprotective agent riluzole on striatal spiny neurons. Neuropharmacology 1998, 37, 1063–1070. [Google Scholar] [CrossRef]
- Mizuta, I.; Ohta, M.; Ohta, K.; Nishimura, M.; Mizuta, E.; Kuno, S. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neurosci. Lett. 2001, 310, 117–120. [Google Scholar] [CrossRef]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2006, 1762, 1068–1082. [Google Scholar] [CrossRef]
- Cheah, B.C.; Vucic, S.; Krishnan, A.V.; Kiernan, M.C. Riluzole, Neuroprotection and Amyotrophic Lateral Sclerosis. Curr. Med. Chem. 2010, 17, 1942–1959. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Manji, H.K. Riluzole in psychiatry: A systematic review of the literature. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Coric, V.; Banasr, M.; Bloch, M.; Krystal, J.H.; Sanacora, G. Riluzole in the Treatment of Mood and Anxiety Disorders. CNS Drugs 2008, 22, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Blyufer, A.; Lhamo, S.; Tam, C.; Tariq, I.; Thavornwatanayong, T.; Mahajan, S.S. Riluzole: A neuroprotective drug with potential as a novel anti-cancer agent (Review). Int. J. Oncol. 2021, 59, 95. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.J.; LaCava, S.; Mehta, M.; Schiff, D.; Thandoni, A.; Jhawar, S.; Danish, S.; Haffty, B.G.; Chen, S. The glutamate release inhibitor riluzole increases DNA damage and enhances cytotoxicity in human glioma cells, in vitro and in vivo. Oncotarget 2019, 10, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Serrato, A.; Saunders, J.T.; Holmes, B.; Nishimura, R.N.; Lichtenstein, A.; Gera, J. Repurposing Potential of Riluzole as an ITAF Inhibitor in mTOR Therapy Resistant Glioblastoma. Int. J. Mol. Sci. 2020, 21, 344. [Google Scholar] [CrossRef]
- Yu, L.J.; Wall, B.A.; Chen, S. The current management of brain metastasis in melanoma: A focus on riluzole. Expert Rev. Neurother. 2015, 15, 779–792. [Google Scholar] [CrossRef]
- Liboux, A.L.; Lefebvre, P.; Roux, Y.L.; Truffinet, P.; Aubeneau, M.; Kirkesseli, S.; Montay, G. Single- and Multiple-Dose Pharmacokinetics of Riluzole in White Subjects. J. Clin. Pharmacol. 1997, 37, 820–827. [Google Scholar] [CrossRef]
- Bryson, H.M.; Fulton, B.; Benfield, P. Riluzole. Drugs 1996, 52, 549–563. [Google Scholar] [CrossRef]
- Mondal, P.K.; Rao, V.; Mittapalli, S.; Chopra, D. Exploring Solid State Diversity and Solution Characteristics in a Fluorine-Containing Drug Riluzole. Cryst. Growth Des. 2017, 17, 1938–1946. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Balasubramanian, S.; Chavan, R.B.; Thipparaboina, R.; Naidu, V.G.M.; Shastri, N.R. Hepatoprotective Cocrystals and Salts of Riluzole: Prediction, Synthesis, Solid State Characterization, and Evaluation. Cryst. Growth Des. 2018, 18, 1047–1061. [Google Scholar] [CrossRef]
- Thomas, S.P.; Kumar, V.; Alhameedi, K.; Guru Row, T.N. Non-Classical Synthons: Supramolecular Recognition by S⋅⋅⋅O Chalcogen Bonding in Molecular Complexes of Riluzole. Chem. A Eur. J. 2019, 25, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Arora, I.; Javed, K.; Akhtar, M.; Samim, M. Enhancement in the Neuroprotective Power of Riluzole Against Cerebral Ischemia Using a Brain Targeted Drug Delivery Vehicle. ACS Appl. Mater. Interfaces 2016, 8, 19716–19723. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.J.; Patel, A.A.; Patel, H.P. Stabilized amorphous state of riluzole by immersion-rotavapor method with synthesized mesoporous SBA-15 carrier to augment in-vitro dissolution. J. Drug Deliv. Sci. Technol. 2021, 61, 102270. [Google Scholar] [CrossRef]
- Povedano Panades, M.; Couratier, P.; Sidle, K.; Sorarù, G.; Tsivgoulis, G.; Ludolph, A.C. Administration of Riluzole Oral Suspension During the Different Stages of Amyotrophic Lateral Sclerosis. Front. Neurol. 2021, 12, 633854. [Google Scholar] [CrossRef]
- Wymer, J.; Apple, S.; Harrison, A.; Hill, B.A. Pharmacokinetics, Bioavailability, and Swallowing Safety With Riluzole Oral Film. Clin. Pharmacol. Drug Dev. 2023, 12, 57–64. [Google Scholar] [CrossRef]
- Parikh, R.H.; Patel, R.J. Nanoemulsions for Intranasal Delivery of Riluzole to Improve Brain Bioavailability: Formulation Development and Pharmacokinetic Studies. Curr. Drug Deliv. 2016, 13, 1130–1143. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Reid, J.; Watson, R.D.; Cochran, J.B.; Sproull, D.H.; Clayton, B.E.; Prunty, F.T. Sodium gamma-resorcylate in rheumatic fever. Br. Med. J. 1951, 2, 321–326. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Surov, A.O.; Vasilev, N.A.; Churakov, A.V.; Parashchuk, O.D.; Artobolevskii, S.V.; Alatortsev, O.A.; Makhrov, D.E.; Vener, M.V. Two Faces of Water in the Formation and Stabilization of Multicomponent Crystals of Zwitterionic Drug-Like Compounds. Symmetry 2021, 13, 425. [Google Scholar] [CrossRef]
- Karamertzanis, P.G.; Day, G.M.; Welch, G.W.A.; Kendrick, J.; Leusen, F.J.J.; Neumann, M.A.; Price, S.L. Modeling the interplay of inter- and intramolecular hydrogen bonding in conformational polymorphs. J. Chem. Phys. 2008, 128, 244708. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SADABS, Program for Scaling and Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Deringer, V.L.; George, J.; Dronskowski, R.; Englert, U. Plane-Wave Density Functional Theory Meets Molecular Crystals: Thermal Ellipsoids and Intermolecular Interactions. Acc. Chem. Res. 2017, 50, 1231–1239. [Google Scholar] [CrossRef]
- Cutini, M.; Civalleri, B.; Corno, M.; Orlando, R.; Brandenburg, J.G.; Maschio, L.; Ugliengo, P. Assessment of Different Quantum Mechanical Methods for the Prediction of Structure and Cohesive Energy of Molecular Crystals. J. Chem. Theory Comput. 2016, 12, 3340–3352. [Google Scholar] [CrossRef] [PubMed]
- Mohaček-Grošev, V.; Grdadolnik, J.; Stare, J.; Hadži, D. Identification of hydrogen bond modes in polarized Raman spectra of single crystals of α-oxalic acid dihydrate. J. Raman Spectrosc. 2009, 40, 1605–1614. [Google Scholar] [CrossRef]
- Surov, A.O.; Manin, A.N.; Voronin, A.P.; Churakov, A.V.; Perlovich, G.L.; Vener, M.V. Weak Interactions Cause Packing Polymorphism in Pharmaceutical Two-Component Crystals. The Case Study of the Salicylamide Cocrystal. Cryst. Growth Des. 2017, 17, 1425–1437. [Google Scholar] [CrossRef]
- Zhang, F.; Kambara, O.; Tominaga, K.; Nishizawa, J.-I.; Sasaki, T.; Wang, H.-W.; Hayashi, M. Analysis of vibrational spectra of solid-state adenine and adenosine in the terahertz region. RSC Adv. 2014, 4, 269–278. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Vener, M.V.; Churakov, A.V.; Perlovich, G.L. Specific features of supramolecular organisation and hydrogen bonding in proline cocrystals: A case study of fenamates and diclofenac. CrystEngComm 2018, 20, 6970–6981. [Google Scholar] [CrossRef]
- Masunov, A.E.; Torres, K.; Dyakov, A.A.; Yushina, I.D.; Bartashevich, E.V. First-Principles Crystal Engineering of Nonlinear Optical Materials. II. Effect of Halogen Bonds on the Structure and Properties of Triiodobenzenes. J. Phys. Chem. C 2018, 122, 22622–22631. [Google Scholar] [CrossRef]
- Vener, M.V.; Chernyshov, I.Y.; Rykounov, A.A.; Filarowski, A. Structural and spectroscopic features of proton hydrates in the crystalline state. Solid-state DFT study on HCl and triflic acid hydrates. Mol. Phys. 2018, 116, 251–262. [Google Scholar] [CrossRef]
- Vener, M.V.; Kharlanov, O.G.; Sosorev, A.Y. High-Mobility Naphthalene Diimide Derivatives Revealed by Raman-Based In Silico Screening. Int. J. Mol. Sci. 2022, 23, 13305. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Gruzdev, M.S.; Perlovich, G.L.; Prashanth, J.; Balasubramanian, S. Polymorphic forms of antiandrogenic drug nilutamide: Structural and thermodynamic aspects. Phys. Chem. Chem. Phys. 2021, 23, 9695–9708. [Google Scholar] [CrossRef]
- Rogers, F.J.M.; Radhanpura, K.; Horvat, J.; Farrant, D. On the use of a volume constraint to account for thermal expansion effects on the low-frequency vibrations of molecular crystals. Phys. Chem. Chem. Phys. 2022, 24, 10408–10419. [Google Scholar] [CrossRef]
- Rozenberg, M.; Loewenschuss, A.; Marcus, Y. An empirical correlation between stretching vibration redshift and hydrogen bond length. Phys. Chem. Chem. Phys. 2000, 2, 2699–2702. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Melikova, S.M.; Voronin, A.P.; Panek, J.; Frolov, N.E.; Shishkina, A.V.; Rykounov, A.A.; Tretyakov, P.Y.; Vener, M.V. Interplay of π-stacking and inter-stacking interactions in two-component crystals of neutral closed-shell aromatic compounds: Periodic DFT study. RSC Adv. 2020, 10, 27899–27910. [Google Scholar] [CrossRef]
- Gatti, C.; Casassa, S.M. TOPOND14 User’s Manual; CNR-ISTM Milano: Milano, Italy, 2013. [Google Scholar]
- Medvedev, A.G.; Churakov, A.V.; Navasardyan, M.A.; Prikhodchenko, P.V.; Lev, O.; Vener, M.V. Fast Quantum Approach for Evaluating the Energy of Non-Covalent Interactions in Molecular Crystals: The Case Study of Intermolecular H-Bonds in Crystalline Peroxosolvates. Molecules 2022, 27, 4082. [Google Scholar] [CrossRef]
- Vener, M.V.; Makhrov, D.E.; Voronin, A.P.; Shalafan, D.R. Molecular Dynamics Simulation of Association Processes in Aqueous Solutions of Maleate Salts of Drug-like Compounds: The Role of Counterion. Int. J. Mol. Sci. 2022, 23, 6302. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Hydrogen Bonding: A Coulombic σ-Hole Interaction. J. Indian Inst. Sci. 2020, 100, 21–30. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Bis, J.A.; Zaworotko, M.J. The 2-Aminopyridinium-carboxylate Supramolecular Heterosynthon: A Robust Motif for Generation of Multiple-Component Crystals. Cryst. Growth Des. 2005, 5, 1169–1179. [Google Scholar] [CrossRef]
- Ebenezer, S.; Muthiah, P.T. Design of Co-crystals/Salts of Aminopyrimidines and Carboxylic Acids through Recurrently Occurring Synthons. Cryst. Growth Des. 2012, 12, 3766–3785. [Google Scholar] [CrossRef]
- da Silva, C.C.; Cirqueira, M.d.L.; Martins, F.T. Lamivudine salts with 1,2-dicarboxylic acids: A new and a rare synthon with double pairing motif fine-tuning their solubility. CrystEngComm 2013, 15, 6311–6317. [Google Scholar] [CrossRef]
- Voronin, A.P.; Surov, A.O.; Churakov, A.V.; Parashchuk, O.D.; Rykounov, A.A.; Vener, M.V. Combined X-ray Crystallographic, IR/Raman Spectroscopic, and Periodic DFT Investigations of New Multicomponent Crystalline Forms of Anthelmintic Drugs: A Case Study of Carbendazim Maleate. Molecules 2020, 25, 2386. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Sadeghi, F.; Molčanov, K.; Zarȩba, J.K.; Gomila, R.M.; Frontera, A. Recurrent Supramolecular Motifs in a Series of Acid–Base Adducts Based on Pyridine-2,5-Dicarboxylic Acid N-Oxide and Organic Bases: Inter- and Intramolecular Hydrogen Bonding. Cryst. Growth Des. 2020, 20, 1738–1751. [Google Scholar] [CrossRef]
- Vener, M.V.; Churakov, A.V.; Voronin, A.P.; Parashchuk, O.D.; Artobolevskii, S.V.; Alatortsev, O.A.; Makhrov, D.E.; Medvedev, A.G.; Filarowski, A. Comparison of Proton Acceptor and Proton Donor Properties of H2O and H2O2 in Organic Crystals of Drug-like Compounds: Peroxosolvates vs. Crystallohydrates. Molecules 2022, 27, 717. [Google Scholar] [CrossRef]
- Garg, U.; Azim, Y.; Kar, A.; Pradeep, C.P. Cocrystals/salt of 1-naphthaleneacetic acid and utilizing Hirshfeld surface calculations for acid–aminopyrimidine synthons. CrystEngComm 2020, 22, 2978–2989. [Google Scholar] [CrossRef]
- Gelbrich, T.; Hursthouse, M.B. A versatile procedure for the identification, description and quantification of structural similarity in molecular crystals. CrystEngComm 2005, 7, 324–336. [Google Scholar] [CrossRef]
- Gelbrich, T.; Hursthouse, M.B. Systematic investigation of the relationships between 25 crystal structures containing the carbamazepine molecule or a close analogue: A case study of the XPac method. CrystEngComm 2006, 8, 448–460. [Google Scholar] [CrossRef]
- Shan, N.; Bond, A.D.; Jones, W. Supramolecular synthons in the co-crystal structures of 2-aminopyrimidine with diols and carboxylic acids. Tetrahedron Lett. 2002, 43, 3101–3104. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Tao, Q.; Hao, Q.-Q.; Voronin, A.P.; Dai, X.-L.; Huang, Y.; Perlovich, G.L.; Lu, T.-B.; Chen, J.-M. Polymorphic Forms of a Molecular Salt of Phenazopyridine with 3,5-Dihydroxybenzoic Acid: Crystal Structures, Theoretical Calculations, Thermodynamic Stability, and Solubility Aspects. Cryst. Growth Des. 2019, 19, 5636–5647. [Google Scholar] [CrossRef]
- Voronin, A.P.; Perlovich, G.L.; Vener, M.V. Effects of the crystal structure and thermodynamic stability on solubility of bioactive compounds: DFT study of isoniazid cocrystals. Comput. Theor. Chem. 2016, 1092, 1–11. [Google Scholar] [CrossRef]
- Jain, N.; Yalkowsky, S.H. Estimation of the aqueous solubility I: Application to organic nonelectrolytes. J. Pharm. Sci. 2001, 90, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.S.; O’Boyle, N.M.; Glen, R.C.; Mitchell, J.B.O. Random Forest Models To Predict Aqueous Solubility. J. Chem. Inf. Model. 2007, 47, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, T. Recent Advances on Aqueous Solubility Prediction. Comb. Chem. High Throughput Screen. 2011, 14, 328–338. [Google Scholar] [CrossRef]
- Sacchi, P.; Loconte, L.; Macetti, G.; Rizzato, S.; Lo Presti, L. Correlations of Crystal Structure and Solubility in Organic Salts: The Case of the Antiplasmodial Drug Piperaquine. Cryst. Growth Des. 2019, 19, 1399–1410. [Google Scholar] [CrossRef]
- de Moraes, L.S.; Edwards, D.; Florence, A.J.; Johnston, A.; Johnston, B.F.; Morrison, C.A.; Kennedy, A.R. Aqueous Solubility of Organic Salts. Investigating Trends in a Systematic Series of 51 Crystalline Salt Forms of Methylephedrine. Cryst. Growth Des. 2017, 17, 3277–3286. [Google Scholar] [CrossRef]
- Arlin, J.-B.; Florence, A.J.; Johnston, A.; Kennedy, A.R.; Miller, G.J.; Patterson, K. Systematic Data Set for Structure−Property Investigations: Solubility and Solid-State Structure of Alkaline Earth Metal Salts of Benzoates. Cryst. Growth Des. 2011, 11, 1318–1327. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, T.; Yang, S.; Li, X.; Chi, Y.; Yang, Y.; Gu, J.; Hu, C. Synthesis and Pharmacokinetic Study of Three Gemfibrozil Salts: An Exploration of the Structure–Property Relationship. Cryst. Growth Des. 2016, 16, 6060–6068. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Perlovich, G.L. Thermodynamic Approaches to the Challenges of Solubility in Drug Discovery and Development. Mol. Pharm. 2014, 11, 1–11. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Trivedi, D.R. Pharmaceutical salts of ethionamide with GRAS counter ion donors to enhance the solubility. Eur. J. Pharm. Sci. 2017, 96, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Vecchio, S.; Brunetti, B. Thermochemical study of 2,4-, 2,6- and 3,4-dihydroxybenzoic acids in the liquid phase using a TG apparatus. Thermochim. Acta 2011, 515, 84–90. [Google Scholar] [CrossRef]
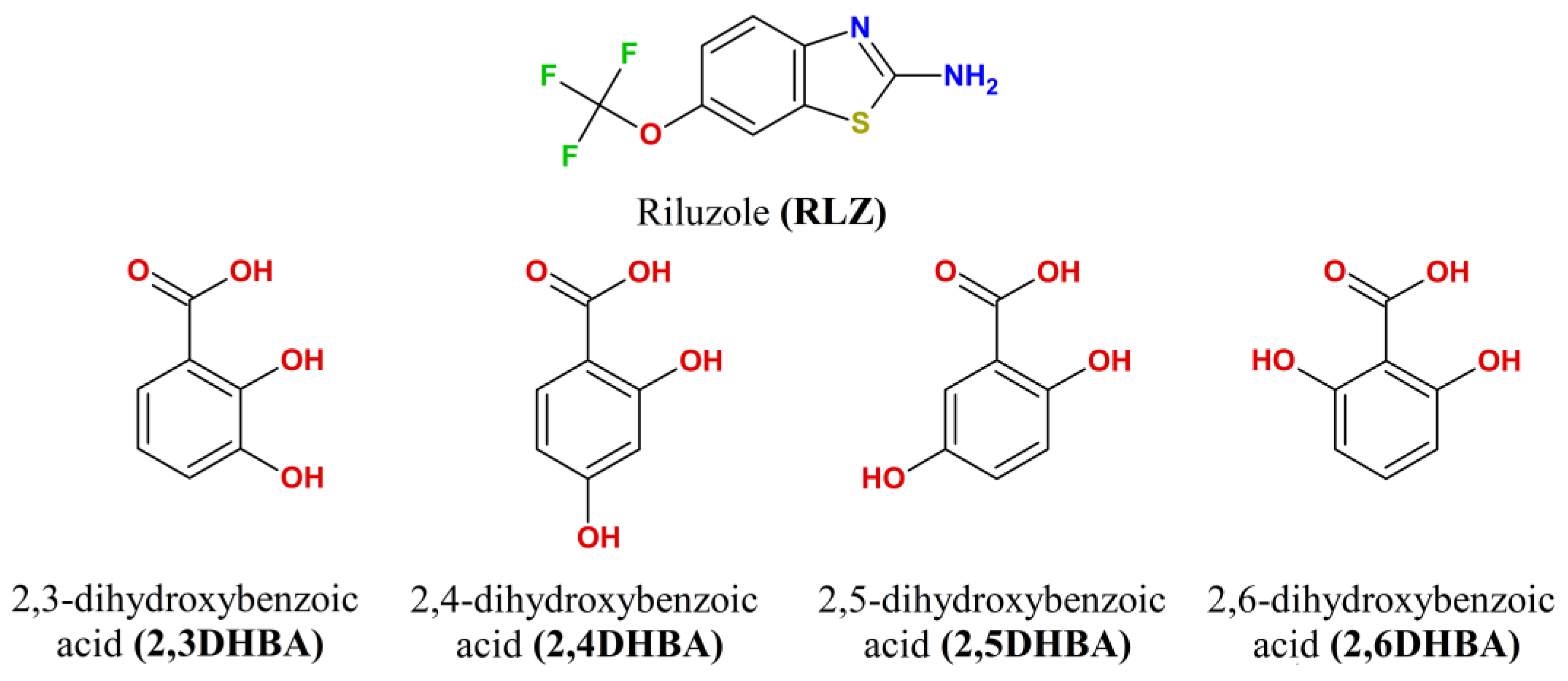
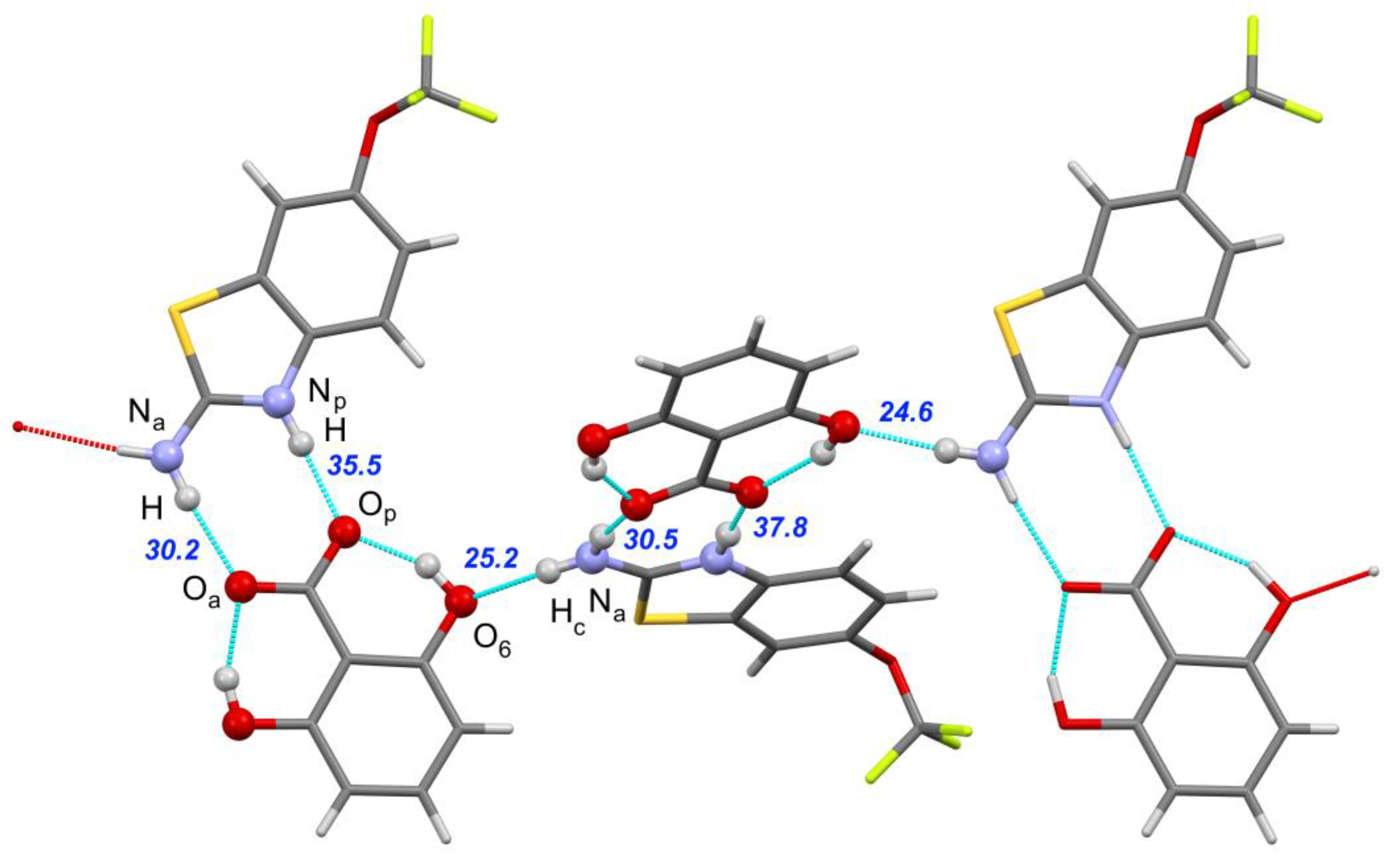
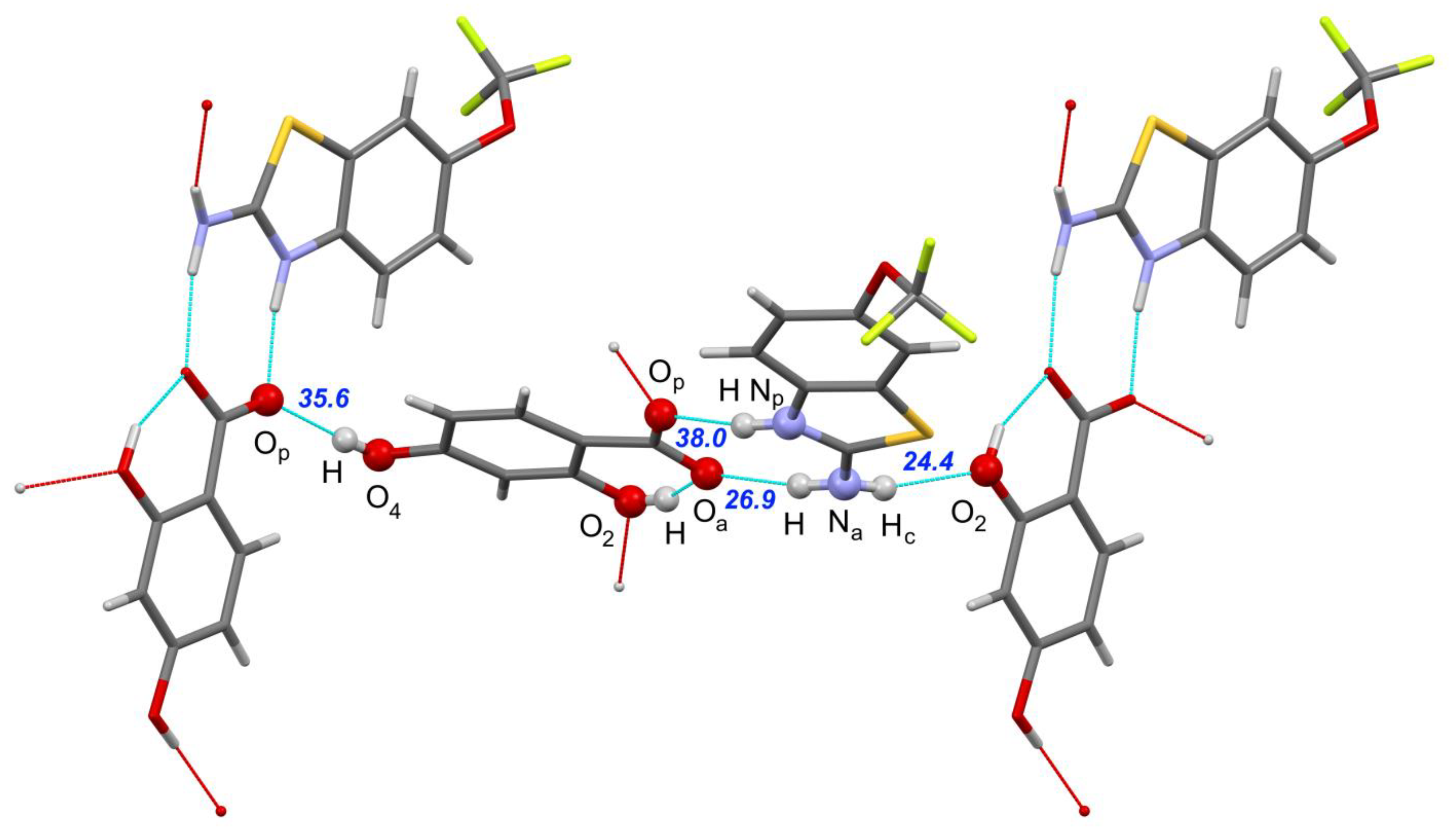

| [RLZ+2HBA] | [RLZ+2,6DHBA] (b) | [RLZ+2,5DHBA] | [RLZ+2,4DHBA] | |||||
|---|---|---|---|---|---|---|---|---|
| Fragment (a) | ΔD, Å | −ΔHHB | ΔD, Å | −ΔHHB | ΔD, Å | −ΔHHB | ΔD, Å | −ΔHHB |
| Np–H∙∙∙Op | 0.046 0.003 | 32.1 36.3 | −0.026 −0.024 | 35.8 36.6 | 0.001 −0.038 | 34.4 38.7 | −0.001 −0.016 | 35.4 38.0 |
| Na–H∙∙∙Oa | 0.009 −0.017 | 29.3 30.3 | −0.008 −0.028 | 28.5 30.4 | 0.004 −0.005 | 30.1 31.1 | 0.014 −0.002 | 25.6 26.9 |
| Na–Hc∙∙∙O’p | 0.002 −0.017 | 27.4 28.8 | - | - | - | - | - | - |
| Na–Hc∙∙∙O’6 | - | - | 0.082 0.030 | 22.5 24.6 | - | - | - | - |
| Na–Hc∙∙∙O’2 | - | - | - | - | −0.022 −0.051 | 26.9 28.8 | −0.019 −0.042 | 22.7 24.4 |
| O’5–H∙∙∙Op | - | - | - | - | −0.021 −0.042 | 29.7 31.9 | - | - |
| O’4–H∙∙∙Op | - | - | - | - | - | - | −0.019 −0.051 | 32.6 35.6 |
| Crystal | [RLZ+2HBA] | [RLZ+2,4DHBA] | [RLZ+2,5DHBA] | [RLZ+2,6DHBA] |
|---|---|---|---|---|
| ΣEHB | 95.4 | 124.9 | 130.7 | 91.6 |
| Elatt | 229.0 | 281.9 | 266.0 | 209.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronin, A.P.; Surov, A.O.; Churakov, A.V.; Vener, M.V. Supramolecular Organization in Salts of Riluzole with Dihydroxybenzoic Acids—The Key Role of the Mutual Arrangement of OH Groups. Pharmaceutics 2023, 15, 878. https://doi.org/10.3390/pharmaceutics15030878
Voronin AP, Surov AO, Churakov AV, Vener MV. Supramolecular Organization in Salts of Riluzole with Dihydroxybenzoic Acids—The Key Role of the Mutual Arrangement of OH Groups. Pharmaceutics. 2023; 15(3):878. https://doi.org/10.3390/pharmaceutics15030878
Chicago/Turabian StyleVoronin, Alexander P., Artem O. Surov, Andrei V. Churakov, and Mikhail V. Vener. 2023. "Supramolecular Organization in Salts of Riluzole with Dihydroxybenzoic Acids—The Key Role of the Mutual Arrangement of OH Groups" Pharmaceutics 15, no. 3: 878. https://doi.org/10.3390/pharmaceutics15030878
APA StyleVoronin, A. P., Surov, A. O., Churakov, A. V., & Vener, M. V. (2023). Supramolecular Organization in Salts of Riluzole with Dihydroxybenzoic Acids—The Key Role of the Mutual Arrangement of OH Groups. Pharmaceutics, 15(3), 878. https://doi.org/10.3390/pharmaceutics15030878







