Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Pharmacokinetic Study
2.2.1. Animals
2.2.2. Study Design
2.2.3. LC-MS/MS Conditions
- Pyronaridine
- Artesunate and dihydroartemisinin
2.2.4. Sample Preparation
- Pyronaridine
- Artesunate and dihydroartemisinin
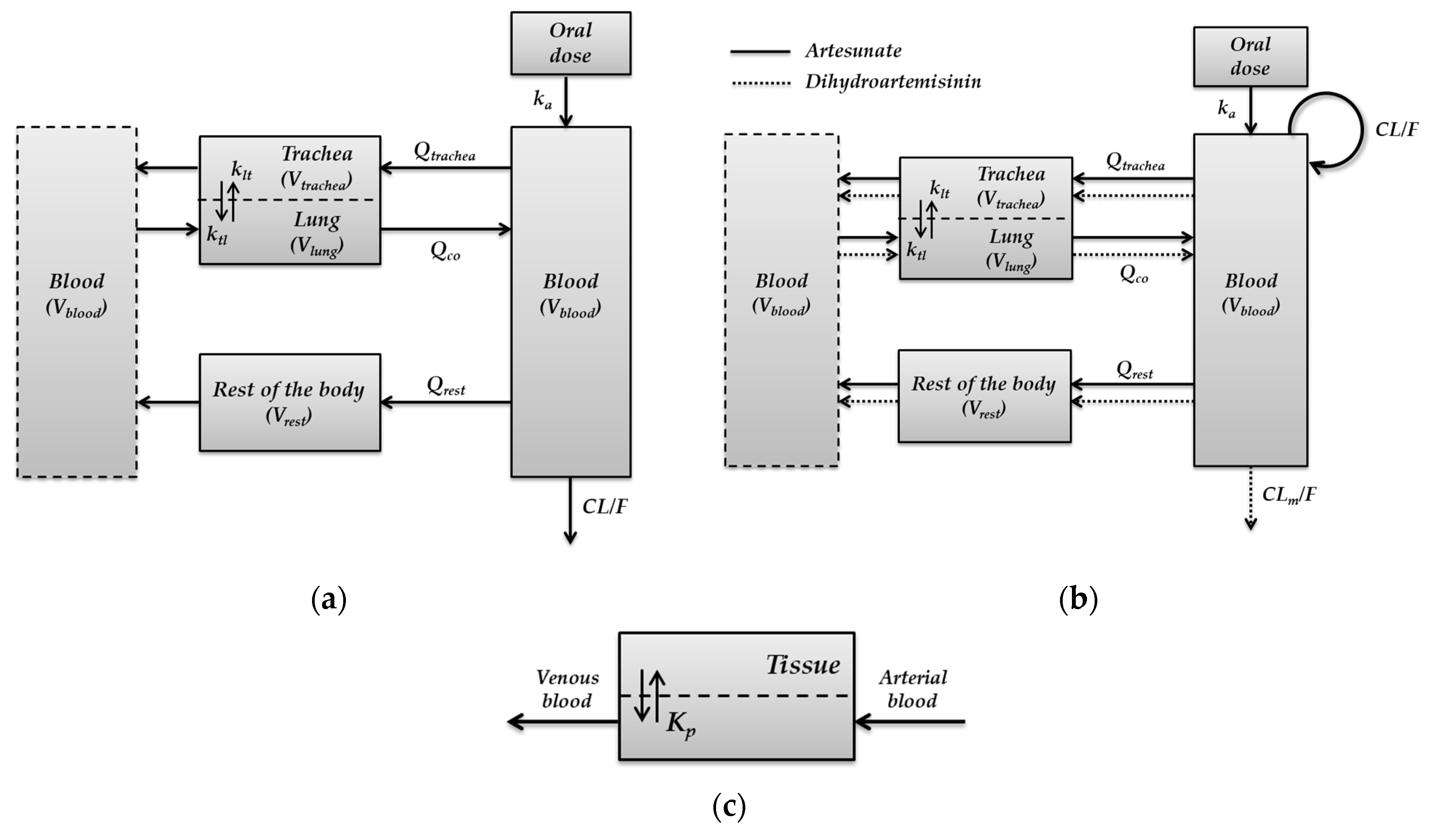
2.3. Development of Minimal Physiologically-Based Pharmacokinetic Models
- Minimal PBPK model for pyronaridine
- Parent-metabolite PBPK model for artesunate
- Parent-metabolite PBPK model for dihydroartemisinin
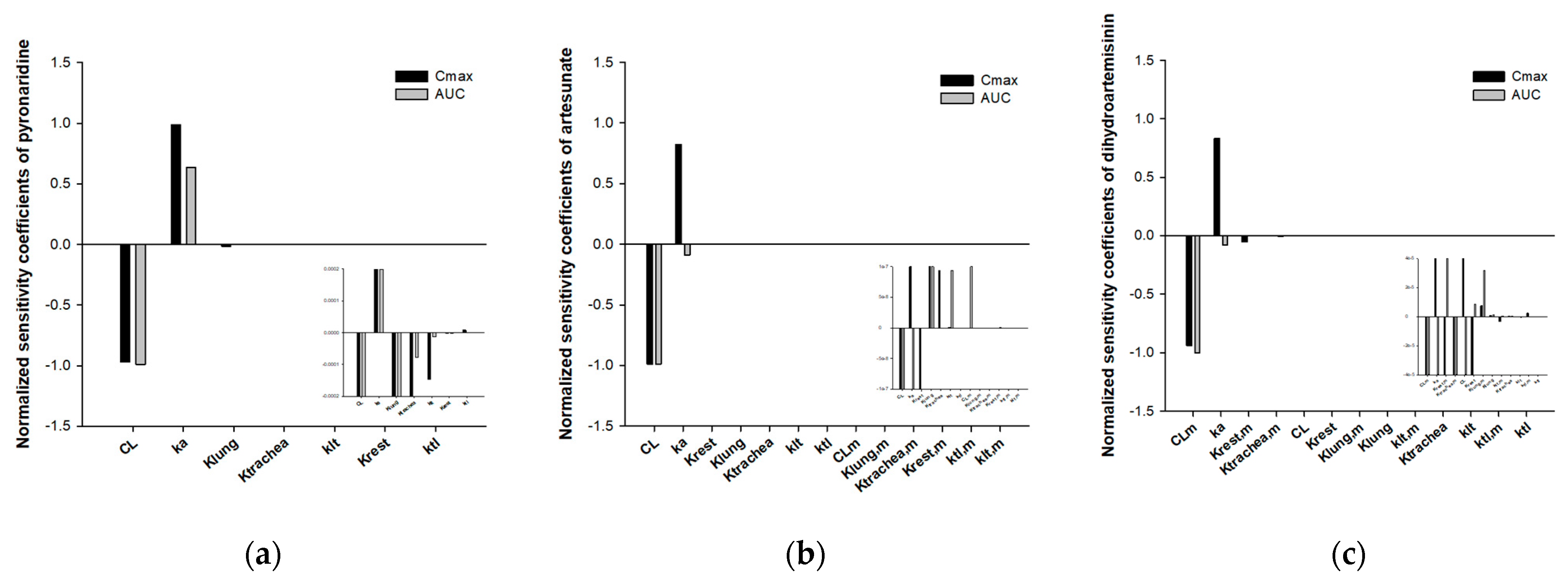
2.4. Parameters Estimation
3. Results
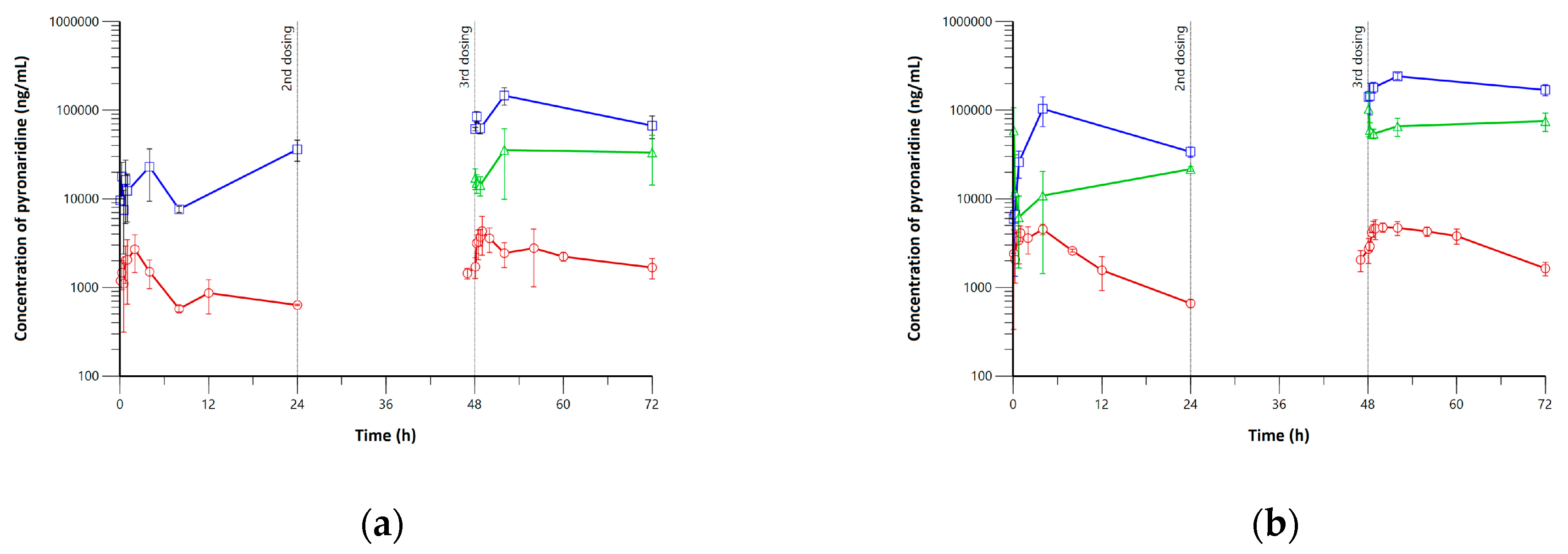
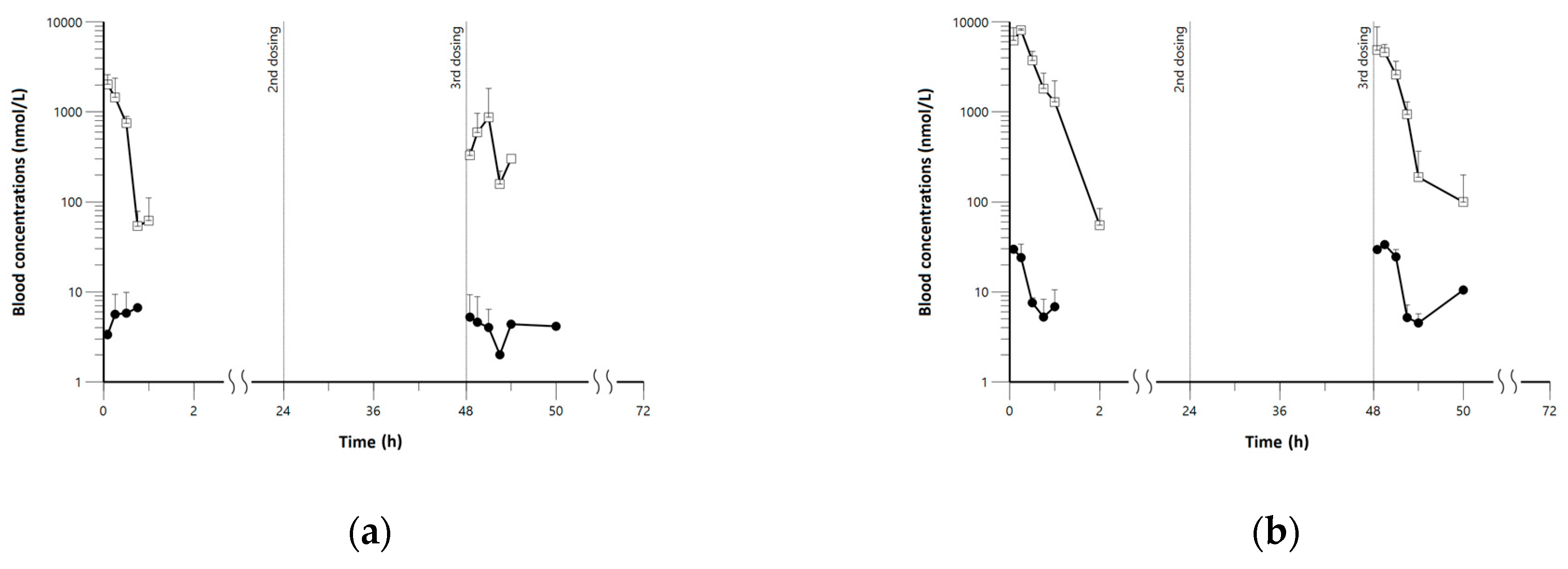
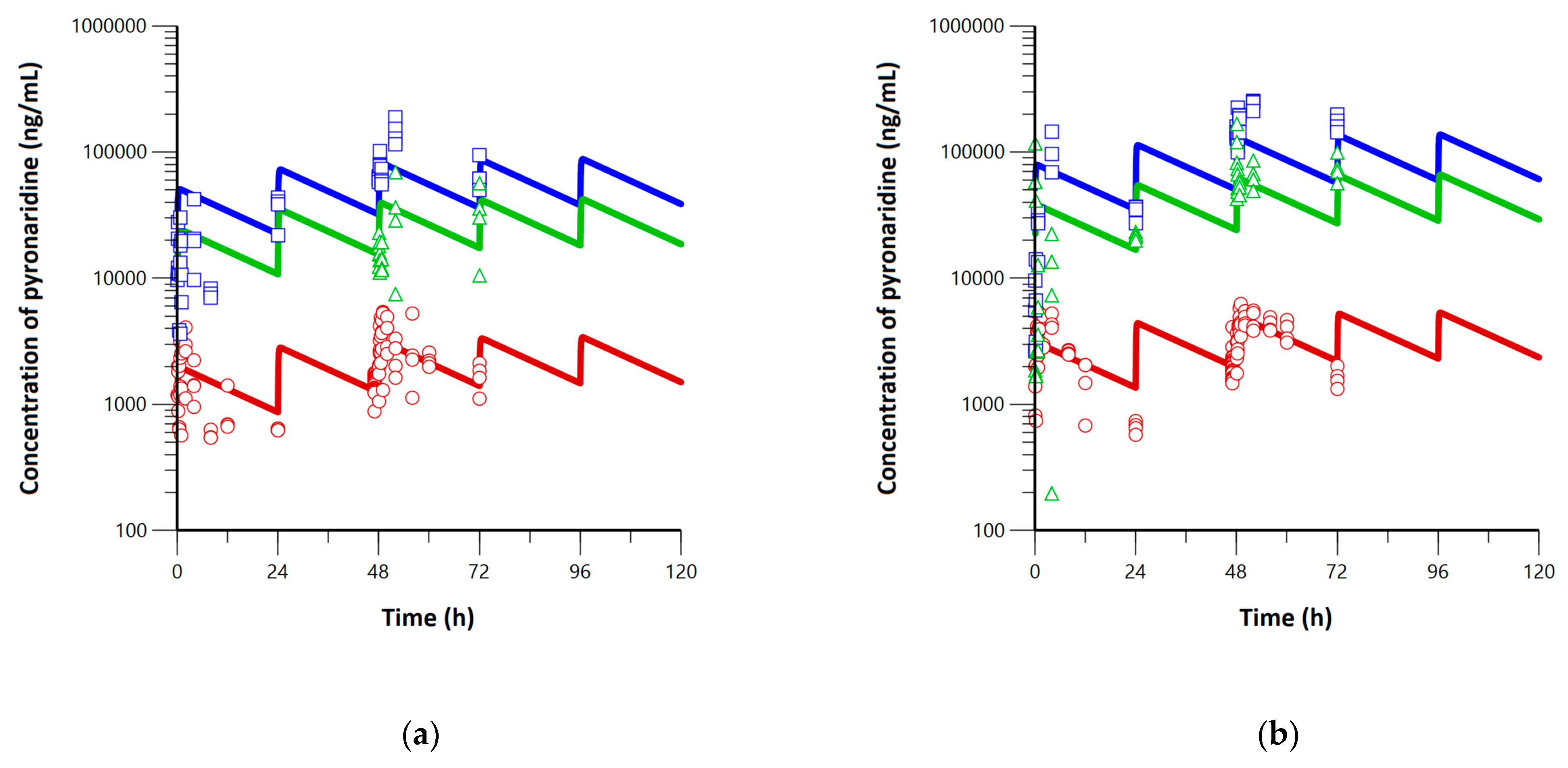
3.1. Pharmacokinetics, Lung, and Tissue Distribution of Pyronaridine and Artesunate
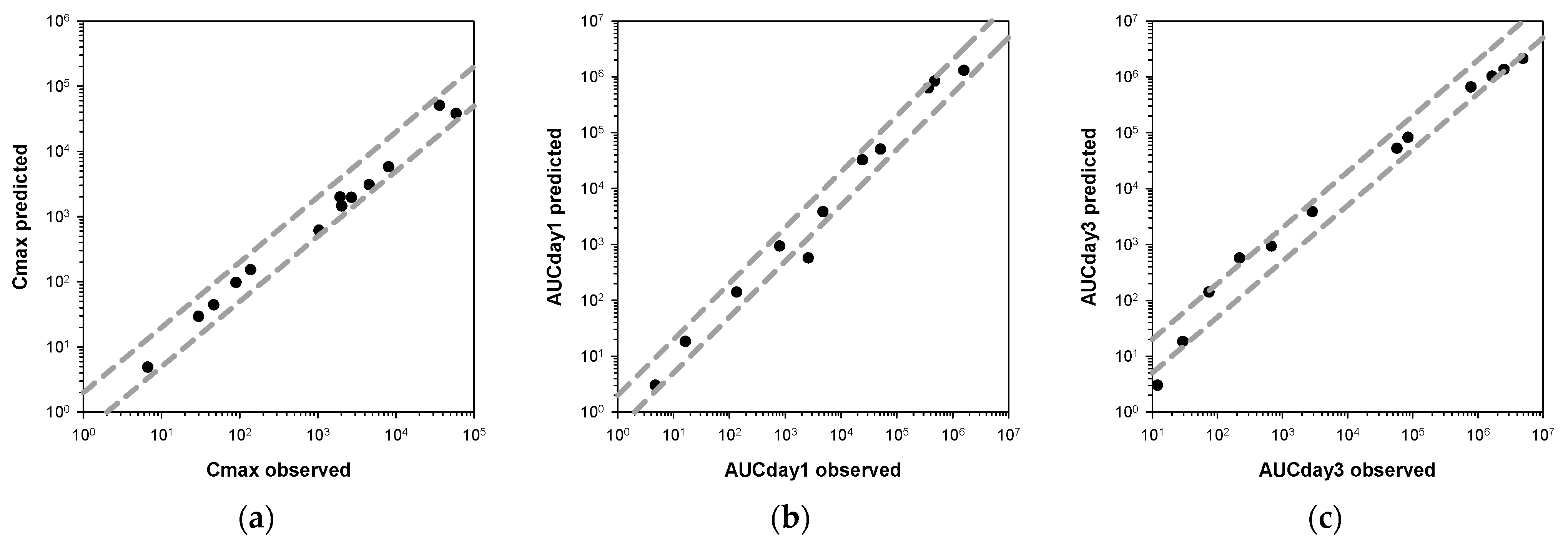
3.2. Development of Minimal Physiologically-Based Pharmacokinetic Models
3.3. Application of Minimal Physiologically-Based Pharmacokinetic Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Agoramoorthy, G.; Lee, S.-S. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: Lessons learned from major clinical studies. Front. Pharmacol. 2021, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Bento Cunha, R.; Vassilevskaia, T.; Viveiros, M.; Cunha, C. Drug Repurposing for COVID-19: A Review and a Novel Strategy to Identify New Targets and Potential Drug Candidates. Molecules 2022, 27, 2723. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, V.; Setlur, A.S.; Karunakaran, C.; Uttarkar, A.; Kumar, K.M.; Skariyachan, S. Scope of repurposed drugs against the potential targets of the latest variants of SARS-CoV-2. Struct. Chem. 2022, 33, 1585–1608. [Google Scholar] [CrossRef]
- Eastman, R.T.; Fidock, D.A. Artemisinin-based combination therapies: A vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 2009, 7, 864–874. [Google Scholar] [CrossRef]
- Puhl, A.C.; Fritch, E.J.; Lane, T.R.; Tse, L.V.; Yount, B.L.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Tavella, T.A.; Maranhão Costa, F.T.; Weston, S. Repurposing the ebola and marburg virus inhibitors tilorone, quinacrine, and pyronaridine: In vitro activity against SARS-CoV-2 and potential mechanisms. ACS Omega 2021, 6, 7454–7468. [Google Scholar] [CrossRef]
- Gendrot, M.; Andreani, J.; Boxberger, M.; Jardot, P.; Fonta, I.; Le Bideau, M.; Duflot, I.; Mosnier, J.; Rolland, C.; Bogreau, H. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Travel Med. Infect. Dis. 2020, 37, 101873. [Google Scholar] [CrossRef] [PubMed]
- Puhl, A.C.; Gomes, G.F.; Damasceno, S.; de Godoy, A.S.; Noske, G.D.; Nakamura, A.M.; Gawrijuk, V.O.; Fernandes, R.S.; Monakhova, N.; Riabova, O. Pyronaridine Protects Against SARS-CoV-2 in Mouse. ACS Infect. Dis. 2022, 8, 1147–1160. [Google Scholar] [CrossRef]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 2020, 6, 2524–2531. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Lee, G.E.; Park, H.; Cho, J.; Kim, Y.-E.; Lee, J.-Y.; Ju, C.; Kim, W.-K.; Kim, J.I.; Park, M.-S. Pyronaridine and artesunate are potential antiviral drugs against COVID-19 and influenza. bioRxiv 2020. bioRxiv: 2028.225102. [Google Scholar]
- Calvo-Alvarez, E.; Dolci, M.; Perego, F.; Signorini, L.; Parapini, S.; D’Alessandro, S.; Denti, L.; Basilico, N.; Taramelli, D.; Ferrante, P. Antiparasitic Drugs against SARS-CoV-2: A Comprehensive Literature Survey. Microorganisms 2022, 10, 1284. [Google Scholar] [CrossRef]
- Jermain, B.; Hanafin, P.O.; Cao, Y.; Lifschitz, A.; Lanusse, C.; Rao, G.G. Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 drug repurposing. J. Pharm. Sci. 2020, 109, 3574–3578. [Google Scholar] [CrossRef]
- Djokovic, N.; Ruzic, D.; Djikic, T.; Cvijic, S.; Ignjatovic, J.; Ibric, S.; Baralic, K.; Buha Djordjevic, A.; Curcic, M.; Djukic-Cosic, D. An Integrative in silico drug repurposing approach for identification of potential inhibitors of SARS-CoV-2 main protease. Mol. Inform. 2021, 40, 2000187. [Google Scholar] [CrossRef]
- Dodds, M.; Xiong, Y.; Mouksassi, S.; Kirkpatrick, C.M.; Hui, K.; Doyle, E.; Patel, K.; Cox, E.; Wesche, D.; Brown, F. Model-informed drug repurposing: A pharmacometric approach to novel pathogen preparedness, response and retrospection. Br. J. Clin. Pharmacol. 2021, 87, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Pilla Reddy, V.; El-Khateeb, E.; Jo, H.; Giovino, N.; Lythgoe, E.; Sharma, S.; Tang, W.; Jamei, M.; Rastomi-Hodjegan, A. Pharmacokinetics under the COVID-19 storm. Br. J. Clin. Pharmacol. 2023, 89, 158–186. [Google Scholar] [CrossRef] [PubMed]
- Idkaidek, N.; Hawari, F.; Dodin, Y.; Obeidat, N. Development of a physiologically-based pharmacokinetic (PBPK) model of nebulized hydroxychloroquine for pulmonary delivery to COVID-19 patients. Drug Res. 2021, 71, 250–256. [Google Scholar] [CrossRef] [PubMed]
- EPA, U. Approaches for the application of physiologically based pharmacokinetic (PBPK) models and supporting data in risk assessment. J. Toxicol. Environ. Health Part B 2008, 11, 519–547. [Google Scholar]
- Committee for Medicinal Products for Human Use. Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation; European Medicines Agency: London, UK, 2018. [Google Scholar]
- Reitz, R.; McDougal, J.; Himmelstein, M.; Nolan, R.; Schumann, A. Physiologically based pharmacokinetic modeling with methylchloroform: Implications for interspecies, high dose/low dose, and dose route extrapolations. Toxicol. Appl. Pharmacol. 1988, 95, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jusko, W.J. Applications of minimal physiologically-based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2012, 39, 711–723. [Google Scholar] [CrossRef]
- Lindegardh, N.; Hanpithakpong, W.; Kamanikom, B.; Pattayaso, J.; Singhasivanon, P.; White, N.J.; Day, N.P. Quantification of dihydroartemisinin, artesunate and artemisinin in human blood: Overcoming the technical challenge of protecting the peroxide bridge. Bioanalysis 2011, 3, 1613–1624. [Google Scholar] [CrossRef]
- House, E.; Pansky, B.; Raphaely, R.; Palmer, J.; Jacobs, M. Blood volume, total protein and total cholesterol in normal and diabetic hamsters. Anat. Rec. 1962, 144, 25–30. [Google Scholar] [CrossRef]
- Gulati, O.; Ponard, G. Cardiac output and regional blood flow studies in golden hamsters. Experientia 1980, 36, 984–985. [Google Scholar] [CrossRef]
- Boger, E.; Evans, N.; Chappell, M.; Lundqvist, A.; Ewing, P.; Wigenborg, A.; Fridén, M. Systems pharmacology approach for prediction of pulmonary and systemic pharmacokinetics and receptor occupancy of inhaled drugs. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 201–210. [Google Scholar] [CrossRef]
- Campbell, J.; Van Landingham, C.; Crowell, S.; Gentry, R.; Kaden, D.; Fiebelkorn, S.; Loccisano, A.; Clewell, H. A preliminary regional PBPK model of lung metabolism for improving species dependent descriptions of 1,3-butadiene and its metabolites. Chem.-Biol. Interact. 2015, 238, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, E.-J.; Choi, G.-W.; Lee, Y.-B.; Cho, H.-Y. Exploring sex differences in human health risk assessment for PFNA and PFDA using a PBPK model. Arch. Toxicol. 2019, 93, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Loccisano, A.E.; Longnecker, M.P.; Campbell, J.L., Jr.; Andersen, M.E.; Clewell, H.J., III. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J. Toxicol. Environ. Health Part A 2013, 76, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.C.; Covington, T.R.; Clewell, H.J. Investigation of the impact of pharmacokinetic variability and uncertainty on risks predicted with a pharmacokinetic model for chloroform. Toxicology 1996, 111, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Grandoni, S.; Cesari, N.; Brogin, G.; Puccini, P.; Magni, P. Building in-house PBPK modelling tools for oral drug administration from literature information. Admet Dmpk 2019, 7, 4–21. [Google Scholar] [CrossRef]
- Mayumi, K.; Ohnishi, S.; Hasegawa, H. Successful prediction of human pharmacokinetics by improving calculation processes of physiologically based pharmacokinetic approach. J. Pharm. Sci. 2019, 108, 2718–2727. [Google Scholar] [CrossRef]
- Maharaj, A.R.; Wu, H.; Hornik, C.P.; Arrieta, A.; James, L.; Bhatt-Mehta, V.; Bradley, J.; Muller, W.J.; Al-Uzri, A.; Downes, K.J. Use of normalized prediction distribution errors for assessing population physiologically-based pharmacokinetic model adequacy. J. Pharmacokinet. Pharmacodyn. 2020, 47, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A. Evaluation of a generic physiologically based pharmacokinetic model for lineshape analysis. Clin. Pharmacokinet. 2008, 47, 261–275. [Google Scholar] [CrossRef]
- Morris, C.A.; Lopez-Lazaro, L.; Jung, D.; Methaneethorn, J.; Duparc, S.; Borghini-Fuhrer, I.; Pokorny, R.; Shin, C.-S.; Fleckenstein, L. Drug-drug interaction analysis of pyronaridine/artesunate and ritonavir in healthy volunteers. Am. J. Trop. Med. Hyg. 2012, 86, 489. [Google Scholar] [CrossRef] [PubMed]
- Sahakijpijarn, S.; Moon, C.; Warnken, Z.N.; Maier, E.Y.; DeVore, J.E.; Christensen, D.J.; Koleng, J.J.; Williams, R.O., III. In vivo pharmacokinetic study of remdesivir dry powder for inhalation in hamsters. Int. J. Pharm. X 2021, 3, 100073. [Google Scholar] [CrossRef]
- EMA. Artesunate Amivas: Assessment Report; European Medicines Agency: London, UK, 2021. [Google Scholar]
- Khalil, F.; Läer, S. Physiologically based pharmacokinetic modeling: Methodology, applications, and limitations with a focus on its role in pediatric drug development. J. Biomed. Biotechnol. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Rowland-Yeo, K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, 1–12. [Google Scholar] [CrossRef]
- EMA. Pyramax: Public Assessment Report; European Medicines Agency: London, UK, 2021. [Google Scholar]
- Ayyoub, A.; Methaneethorn, J.; Ramharter, M.; Djimde, A.A.; Tekete, M.; Duparc, S.; Borghini-Fuhrer, I.; Shin, J.-S.; Fleckenstein, L. Population pharmacokinetics of pyronaridine in pediatric malaria patients. Antimicrob. Agents Chemother. 2016, 60, 1450–1458. [Google Scholar] [CrossRef]
- Newton, P.N.; van Vugt, M.; Teja-Isavadharm, P.; Siriyanonda, D.; Rasameesoroj, M.; Teerapong, P.; Ruangveerayuth, R.; Slight, T.; Nosten, F.; Suputtamongkol, Y. Comparison of oral artesunate and dihydroartemisinin antimalarial bioavailabilities in acute falciparum malaria. Antimicrob. Agents Chemother. 2002, 46, 1125–1127. [Google Scholar] [CrossRef]
- Greenblatt, D.J. Elimination half-life of drugs: Value and limitations. Annu. Rev. Med. 1985, 36, 421–427. [Google Scholar] [CrossRef]
- Dhillon, S.; Gill, K. Basic Pharmacokinetics; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–44. Available online: https://globex.coe.pku.edu.cn/file/upload/201807/05/124636651065.pdf (accessed on 1 March 2023).
- Holford, N. Absorption and half-life. Transl. Clin. Pharmacol. 2016, 24, 157–160. [Google Scholar] [CrossRef]
- Toutain, P.-L.; Bousquet-mélou, A. Plasma terminal half-life. J. Vet. Pharmacol. Ther. 2004, 27, 427–439. [Google Scholar] [CrossRef]
- Wadhwa, R.R.; Cascella, M. Steady State Concentration; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Xie, L.H.; Li, Q.; Zhang, J.; Weina, P.J. Pharmacokinetics, tissue distribution and mass balance of radiolabeled dihydroartemisinin in male rats. Malar. J. 2009, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Gendrot, M.; Andreani, J.; Duflot, I.; Boxberger, M.; Le Bideau, M.; Mosnier, J.; Jardot, P.; Fonta, I.; Rolland, C.; Bogreau, H. Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents 2020, 56, 106202. [Google Scholar] [CrossRef] [PubMed]
- Aherfi, S.; Pradines, B.; Devaux, C.; Honore, S.; Colson, P.; Scola, B.L.; Raoult, D. Drug repurposing against SARS-CoV-1, SARS-CoV-2 and MERS-CoV. Future Microbiol. 2021, 16, 1341–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnøe, U.; Feng, S.; Offersgaard, A.; Trimpert, J. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Unit | Description | Value | Reference |
|---|---|---|---|---|
| Vtotal | mL | Total body volume | 102.01 | Experimental data |
| Vblood | mL | Blood volume | 7.20 | [22] |
| Vlung | mL | Lung volume | 0.48 | Experimental data |
| Vtrachea | mL | Trachea volume | 0.06 | Experimental data |
| Vrest of body | mL | Volume of the rest of the body | 94.27 | Calculated 1 |
| Qco | mL/hr | Cardiac output | 1181.28 | [23] |
| Qtrachea | mL/hr | Blood flow rate for the trachea | 24.81 | [24,25,26] |
| Qrest | mL/hr | Blood flow rate for the rest of the body | 1156.47 | Calculated 2 |
| Kb:p | - | Blood-to-plasma partition coefficient (artesunate) | 0.75 | [21] |
| Kb:p,m | - | Blood-to-plasma partition coefficient (dihydroartemisinin) | 0.75 | [21] |
| Parameter | Unit | Value |
|---|---|---|
| Pyronaridine | ||
| ka | 1/h | 0.03 |
| CL/F | L/h | 0.21 |
| Klung | - | 26.06 |
| Ktrachea | - | 8.67 |
| Krest | - | 5.25 × 10−7 |
| ktl | 1/h | 1.01 |
| klt | 1/h | 0.92 |
| Artesunate and dihydroartemisinin | ||
| ka | 1/h | 1.74 |
| CL/F | L/h | 2517.70 |
| Klung | - | 10.33 |
| Ktrachea | - | 1.48 |
| Krest | - | 1.32 |
| ktl | 1/h | 1.50 |
| klt | 1/h | 0.34 |
| CLm/F | L/h | 10.33 |
| Klung,m | - | 0.34 |
| Ktrachea,m | - | 1.08 |
| Krest,m | - | 1.21 |
| ktl,m | 1/h | 6.98 |
| klt,m | 1/h | 0.35 |
| Parameters | Pyronaridine | Artesunate | Dihydroartemisinin | |||
|---|---|---|---|---|---|---|
| Day 1 | Steady-State | Day 1 | Steady-State | Day 1 | Steady-State | |
| Low-dose group | ||||||
| Cavg,blood 1 | 1354.5 | 2382.9 | 0.1 | 0.1 | 38.8 | 38.8 |
| Cavg,lung 1 | 34,978.8 | 61,547.4 | 0.4 | 0.4 | 13.4 | 13.4 |
| Cavg,trachea 1 | 16,806.7 | 29,579.9 | 0.2 | 0.2 | 5.9 | 5.9 |
| AUCblood 2 | 32,508.2 | 57,189.7 | 3.0 | 3.0 | 931.4 | 931.4 |
| AUClung 2 | 839,491.5 | 1,477,137.6 | 10.1 | 10.1 | 320.5 | 320.5 |
| AUCtrachea 2 | 403,360.1 | 709,917.6 | 4.6 | 4.6 | 140.5 | 140.5 |
| t1/2 (h) | 19.7 | 0.4 | 0.4 | |||
| Accumulation ratio | 1.8 | 1 | 1 | |||
| High-dose group | ||||||
| Cavg,blood 1 | 2111.3 | 3735.0 | 0.8 | 0.8 | 160.4 | 160.4 |
| Cavg,lung 1 | 54,525.7 | 96,472.7 | 2.5 | 2.5 | 55.2 | 55.2 |
| Cavg,trachea 1 | 26,199.6 | 46,366.1 | 1.2 | 1.2 | 23.8 | 23.8 |
| AUCblood 2 | 50,672.1 | 89,639.9 | 18.3 | 18.3 | 3849.7 | 3849.7 |
| AUClung 2 | 1,308,615.7 | 2,315,344.4 | 61.2 | 61.2 | 1324.9 | 1324.9 |
| AUCtrachea 2 | 628,789.5 | 1,112,786.7 | 27.6 | 27.6 | 570.5 | 570.5 |
| t1/2 (h) | 19.9 | 0.4 | 0.4 | |||
| Accumulation ratio | 1.8 | 1 | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.W.; Kim, K.M.; Kim, J.H.; Cho, H.-Y. Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters. Pharmaceutics 2023, 15, 838. https://doi.org/10.3390/pharmaceutics15030838
Kang DW, Kim KM, Kim JH, Cho H-Y. Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters. Pharmaceutics. 2023; 15(3):838. https://doi.org/10.3390/pharmaceutics15030838
Chicago/Turabian StyleKang, Dong Wook, Kyung Min Kim, Ju Hee Kim, and Hea-Young Cho. 2023. "Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters" Pharmaceutics 15, no. 3: 838. https://doi.org/10.3390/pharmaceutics15030838
APA StyleKang, D. W., Kim, K. M., Kim, J. H., & Cho, H.-Y. (2023). Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters. Pharmaceutics, 15(3), 838. https://doi.org/10.3390/pharmaceutics15030838





