Abstract
Despite all the advances seen in recent years, the severe adverse effects and low specificity of conventional chemotherapy are still challenging problems regarding cancer treatment. Nanotechnology has helped to address these questions, making important contributions in the oncological field. The use of nanoparticles has allowed the improvement of the therapeutic index of several conventional drugs and facilitates the tumoral accumulation and intracellular delivery of complex biomolecules, such as genetic material. Among the wide range of nanotechnology-based drug delivery systems (nanoDDS), solid lipid nanoparticles (SLNs) have emerged as promising systems for delivering different types of cargo. Their solid lipid core, at room and body temperature, provides SLNs with higher stability than other formulations. Moreover, SLNs offer other important features, namely the possibility to perform active targeting, sustained and controlled release, and multifunctional therapy. Furthermore, with the possibility to use biocompatible and physiologic materials and easy scale-up and low-cost production methods, SLNs meet the principal requirements of an ideal nanoDDS. The present work aims to summarize the main aspects related to SLNs, including composition, production methods, and administration routes, as well as to show the most recent studies about the use of SLNs for cancer treatment.
1. Introduction
Cancer treatment, especially in advanced stages, is a high-priority unmet clinical need since, until today, the high toxicity of chemotherapeutic treatment, their lack of specificity to cancer cells, and the acquired drug resistance hamper a successful cancer treatment. The oncology research field has experienced great benefits from nanotechnology. Nanotechnology-based drug delivery systems (nanoDDS) brought solutions to the major drawbacks related to conventional chemotherapy, namely: (i) drug protection from degradation, (ii) improving the solubility of hydrophobic drugs, (iii) increase of circulation time, (iv) reducing toxicity, (v) decreasing the required dose, and (vi) facilitating the overcoming of biological barriers. In summary, by encapsulating the chemotherapeutic drugs into nanoDDS, it is possible to improve its therapeutic index. Moreover, nanoDDS allow the safe and efficient delivery of genetic material and other biomolecules that could not reach the cells on their own [1].
A wide range of nanoDDS types have been reported in the last decades with countless different compositions and preparation methods. Among them, solid lipid nanoparticles (SLNs), constituted by a solid lipidic core at body temperature, called the attention of multiple research groups due to their high versatility regarding different cargos and administration routes, high stability in physiological conditions, low cost of materials, and easy production [2]. SLNs were first developed in the early 1990s as an alternative colloidal delivery system to overcome the limitations of the most common formulations at the time: emulsions, liposomes, and polymeric nanoparticles [3]. The traditional colloidal carriers deal with problems such as poor stability, polymer toxicity and degradation, high cost, and difficulties in scaling-up the production [4]. The solid lipid core of SLNs was a great improvement in the nanoDDS stability issues. Moreover, the possibility of using physiologically biocompatible products in SLN composition and their scalable production methods are highly important parameters in the development of a new nanoDDS.
2. SLN Properties
SLNs comprise a lipid core and are solid at room and body temperatures, surrounded by a surfactant or emulsifier, stabilizing the core by lowering the interfacial tension with the aqueous media [5]. Sometimes co-surfactants are also included in the formulation to further reduce the interfacial tension and increase the stability. The main components of SLNs used as lipids, surfactants, and co-surfactants and some examples and commercial names are described in Table 1 [4,5,6]. When SLNs are applied to gene therapy, cationic lipids are used, including, among others, n-[1-(2,3-dioleoyloxy)propyl]-n,n,n-trimethylammonium chloride (DOTAP), cetrimide (CTAB), cetylpyridinium chloride (CPC), or benzalkonium chloride [7].

Table 1.
Commonly used components for SLNs preparation.
The components of the formulation must be chosen based on the desired application (Figure 1). For example, only excipients approved for parenteral administration must be used for intravenous SLNs. Also, the compatibility between the drugs and the lipids/surfactants must be considered. Lipids that promote a sustained release of the drugs for days must not be chosen for applications that require a small permanence of SLNs in the body, such as oral administration.
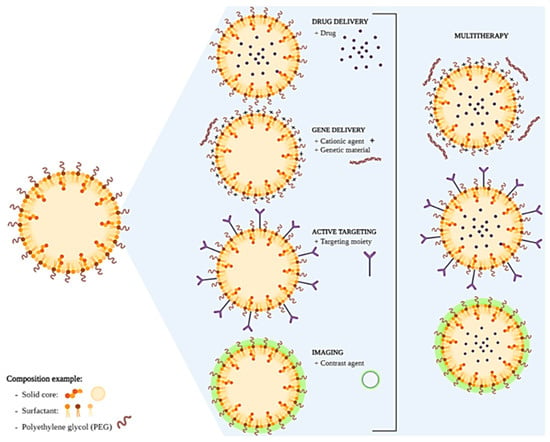
Figure 1.
Schematic representation of SLNs development and final applications. Created in BioRender.com, accessed on 24 February 2023.
Coating materials, viscosity enhancers, antioxidants, absorption-enhancing agents, preservatives, adhesives, and other excipients can also be added to the final formulation based on the drugs and the desired application [7].
The size of the SLNs can be adapted accordingly to the formulation components and production method, varying from 50–1000 nm. In addition, the loading capacity of hydrophobic and hydrophilic drugs and their release profile depends on SLNs composition and manufacturing [4,8]. The different techniques that can be applied for SLNs characterization were previously reviewed elsewhere [7].
SLNs’ surfaces can be functionalized with polyethylene glycol (PEG) coating to increase the efficiency of drug and gene delivery to target cells and tissues, improve the systemic circulation time, and decrease immunogenicity. The PEG coating shields the surface from aggregation, opsonization, and phagocytosis, prolonging systemic circulation time [9]. Moreover, the therapeutic effect is potentially more efficient when the SLNs selectively deliver the drug to its specific site of action with active targeting. For this, the SLN surface is functionalized with ligands (targeting moieties) that can selectively recognize overexpressed receptors on the surface of cancer cells and, ultimately, be translocated inside the cells. Consequently, the selective delivery of the pharmacologically active compounds to the tumor can reduce the toxicity and harmful side effects on other healthy cells [2]. This is being explored as a multifunctional/multitasking platform for efficient drug or gene delivery and as a diagnostic tool.
SLNs present some advantages, such as the capacity to load both hydrophobic and hydrophilic drugs, biocompatibility, low susceptibility to erosion, and slow water absorption. Also, the main advantages of SLNs over liposomes rely on the higher stability and loading capacity of hydrophobic drugs [7,10]. Importantly, some of the manufacturing processes used for SLNs production can be applied to large-scale production, with excellent reproducibility being important for downstream commercial and clinical applications [11].
SLNs are the first generation of this type of lipidic particles that present some limitations, including possible polymorphic transitions, particle growth and gelling, limited cargo (although higher than liposomes), and stability of some drugs since, during storage, lipid crystallization leads to drug expulsion and premature release [10,12]. Also, like other nanoparticle-based drug delivery systems, SLNs also present high manufacturing costs compared to conventional formulations. In order to overcome the limitations of SLNs, a second-generation, nanostructured lipid carrier (NLC) was developed. NLCs differentiate from SLNs by having a blend of solid and liquid (oils) lipids in the composition. This allows a reduction in the melting point of the lipidic core and a higher loading capacity and storage stability of the system [13]. Moreover, in the last years, many lipidic nanoparticles (LNPs), some of them with mixed/hybrid properties, have been proposed, and their categorization is not always consensual. For example, Moderna’s and BioNTech/Pfizer’s COVID-19 vaccines are referred to as LNPs or SLNs, depending on the source of information [14,15]. This makes difficult the proper identification and classification of the formulations. Despite the similitudes among the SLNs, LNPs, and NLCs, in this review, we will focus on the use of classic SLNs for cancer treatment.
3. SLNs Production Methods
Different methods for producing SLNs have been developed and proposed over the years [16,17]. Each of them produces particles with specific characteristics and may be applied to different drugs. Thus, the production method must be chosen based on the available equipment, the properties of the drugs to be encapsulated, and the desired application. For example, methods that comprise a step at high temperatures cannot be used to formulate thermosensitive drugs. In the same way, drugs sensitive to organic solvents or sonication cannot be incorporated into SLNs using methods that require the use of organic solvents and sonication, respectively. Also, the encapsulation of hydrophilic or hydrophobic drugs requires different methods. For example, while hydrophobic drugs can be loaded using a single emulsion step, hydrophilic drugs require a double emulsion process. Also, formulations intended for parenteral administration require a more controlled size and low polydispersity of the particles and methods able to produce sterile formulations in comparison with particles designed for oral administration. Thus, to successfully develop an SLN formulation able to be translated from the bench to the bedside, it is of utmost importance to choose the proper production method based on the components of the formulations and the desired application. A brief description of the most common ones is presented below, and an overall comparison of their advantages and disadvantages is presented in Table 2. A more detailed and schematic representation of the different methods is presented elsewhere [10,16,17]. Note that new methods and variations of the presented methods are constantly being proposed.

Table 2.
Advantages and disadvantages of the different SLN production techniques.
3.1. Hot High-Pressure Homogenization
The hot, high-pressure homogenization (HHPH) technique is based on high-pressure homogenization (HPH). Firstly, the lipid is melted 5–10 °C above the melting point, and the drug or active component is dissolved. Then, the lipid phase is dispersed into the aqueous phase, where the surfactant was previously dissolved at the same temperature under high-speed stirring. This pre-emulsion is then homogenized by passing the liquid at high pressures (100–2000 bars) through a narrow gap (micron dimensions), achieving very high velocity. Very high shear stress and cavitation forces disrupt the particles down to the submicron range. Finally, the obtained nanoemulsion is cooled to room temperature to allow the nanoparticles to crystalize and form the SLNs [18].
3.2. Cold High-Pressure Homogenization
Cold-high pressure homogenization (CHPH) is similar to HHPH. The lipids with the active compound are melted 5–10 °C above the melting temperature, and it is cooled quickly with liquid nitrogen or dried ice to homogeneously distribute the drug in the solid lipid matrix. Then, the lipid phase is milled to obtain microparticles of around 50–100 μm. These solid microparticles are then dispersed in the aqueous phase at a cold temperature. Finally, as in the HHPH, the pre-suspension is homogenized at high-pressure to obtain SLNs in the range of 50–100 nm [19,20].
3.3. Microemulsion
This technique is based on the dilution of microemulsions. Microemulsions are thermodynamically stable, optically isotropic, and transparent. Firstly, the hydrophobic drug is dissolved in the warm lipid (55–85 °C) with the surfactant. At the same time, the water containing the co-surfactant is heated at the same temperature and dispersed into the lipid mixture. Then, the warm microemulsion is dispersed into cold water (2–3 °C) at a ratio varying from 1:25–1:50, under mechanical stirring. The SLNs dispersion is often purified by tangential ultrafiltration to remove the remaining surfactant and co-surfactant [6].
3.4. Double Microemulsion
The double microemulsion is similar to the microemulsion and is widely used to entrap hydrophilic drugs into the SLN. The hydrophilic drug is dissolved in water and dispersed in the melted lipid (55–85 °C), forming a water-in-oil dispersion. Then, the first microemulsion is dispersed in warm water with the surfactants and co-surfactants (as microemulsions), followed by the dispersion in cold water [5,21].
3.5. High Shear Homogenization
High shear homogenization (HSH) is a technique, like the ones referred to previously, performed in the absence of organic solvent. Briefly, the lipid is melted 5–10 °C above the melting point, and the drug is dissolved. The aqueous phase, with the surfactant, is heated at the same temperature and homogenized with the lipid phase by a rotor-stator homogenizer. The nanoemulsion is let to cool down to obtain the SLNs. In HSH, the amount of surfactant, the stirring, and the cooling time are very important to optimize the particle size [18].
An alternative to HSH is high-speed sonication (HSS). It has the same protocol as the HSH, but the formulation is sonicated instead of using a rotor-stator homogenizer. However, this change also presents some disadvantages, such as the possible metal contamination and damage to sensitive biomolecules [5,22].
3.6. Solvent Emulsification-Evaporation
The solvent emulsification-evaporation (SEE) starts with dissolving the drug and the lipid matrix in an organic solvent. Using a high-speed homogenizer, this solution is then emulsified with water containing the surfactant. Finally, the emulsion is passed through the high-pressure homogenizer to obtain the nanoemulsion and kept overnight in stirring to eliminate the organic solvent. The SLN is formed when the lipid precipitates in the aqueous solution [23].
3.7. Solvent Emulsification-Diffusion
The solvent emulsification-diffusion method (SED) consists of saturating a partially water-soluble solvent such as benzyl alcohol or butyl lactate with water to obtain a thermodynamic equilibrium between both liquids. The lipid is dissolved in the organic phase (water-saturated solvent) and emulsified with the aqueous phase (solvent-saturated water) containing the surfactant. Then, the emulsion is quickly diluted in water and stirred to extract the solvent into the external water phase by diffusion, thus, precipitating the lipid and generating the SLNs [24].
3.8. Solvent Injection
The solvent injection (SI) is a modification of the SED technique. As in SED, the lipid and drug are dissolved in a partially water-soluble solvent or a mixture of solvents. Then, the organic phase is rapidly injected through a needle into the aqueous phase containing the surfactant in constant stirring. The SLNs are formed when the solvent diffuses to the external water phase and precipitates [25].
3.9. Solvent Injection Lyophilization
The solvent injection lyophilization (SIL) is a modification of the SI. The procedure is the same, but instead of an aqueous phase containing surfactant, it contains lyoprotectants. Then, the system is lyophilized to obtain the dry SLNs, that, once rehydrated, forms a dispersion [26].
3.10. Coacervation
The coacervation method is also known as fatty acid coacervation due to the use of fatty acid alkaline salts. Firstly, a solution with a polymeric stabilizer is prepared. Then, the fatty acid sodium salt is dispersed homogenously and heated under stirring above its Kraft point. The drug is then dissolved in an organic solvent added to the solution and stirred until a unique phase, and a clear solution is obtained. Finally, an acidic solution is added drop by drop to form a suspension with a pH of around 4. The suspension is then cooled down in a water bath under stirring at a low temperature (15 °C), and the SLNs are formed [27].
3.11. Microwave-Assisted Microemulsion Technique
The microwave-assisted microemulsion technique (MAMT) is characterized by single-pot production. The drug, lipids, and surfactants are heated in a highly temperature-controlled microwave above the lipid melting point. The melted solution is stirred in order to obtain a hot microemulsion that is finally dispersed in cold water (2–4 °C), originating the SLNs [28].
One of the main difficulties in developing nanomedicines relies on the production scale-up to an industrial level. To enter the market, nanomedicines must be produced with reproducibility from batch-to-batch the more affordable way possible at a large scale by techniques that comply with the regulatory authorities [29]. High-pressure homogenization (HPH) is already qualified for parenteral nutrition applications, thus having scale-up potential for SLN production [16]. Also, some publications have published scale-up of SLNs production using methods such as microfluidic [30] or supercritical fluid [31]. Moreover, the SLNs that are under clinical evaluation are being produced at a large scale. Additionally, considering that various SLN formulations are used in the cosmetic field, the step of scale-up is already performed and only must be adapted for regulatory qualification, which may speed-up their entrance into the pharmaceutical market.
4. Solid Lipid Nanoparticles Administration Routes
One of the major benefits of the SLNs is that by manipulating their composition and physicochemical features, they can be adapted to numerous applications and administration routes. Despite being highly applied for dermal applications, SLNs have the potential to be used in other clinical applications, which is the reason why different groups have been exploring many administration routes. In fact, the majority of the works/publications regarding SLNs in recent years have been intended for parenteral administration [32].
4.1. Topical and Dermal Administration
SLNs are widely used for the dermal administration and treatment of skin disorders. In fact, SLNs were first used in the cosmetic field prior to dermopharmacy/medicine. Topical administration usually presents some limitations related to the poor skin penetration of drugs or skin irritation caused by the drug. SLN-based formulations, generally consisting of SLNs incorporated into creams or gels, form a thin film over the skin that provides the required hydration to the stratum corneum and enables penetration of small-size particles through the epidermis. Moreover, the presence of SLNs increases the transport of active compounds through the skin by improving drug solubilization in the formulation, drug partitioning into the skin, and fluidizing skin lipids; it also protects the active compound from light, oxidation, and hydrolysis and reduces the possible irritation caused by the direct contact of drugs with skin [4,5,12]. Therefore, several SLN formulations have been successfully developed to load glucocorticoids, retinoids, anti-inflammatories, and antimycotics, among other drugs [33,34].
Interestingly, due to their solid lipid core, SLNs present the capacity to reflect ultraviolet (UV) radiation. Therefore they have been widely applied in producing several sunscreens to improve UV protection [33]. SLNs also give a smoother texture to the cream compared to conventional cosmetics [35].
4.2. Pulmonary Administration
The pulmonary administration route allows a non-invasive local and systemic drug administration. Contrary to the oral route, the administered drug is absorbed in the alveolar epithelium with a large absorption area, highly permeable alveolar epithelial membrane, and high vascularization with limited hepatic first-pass metabolism. In this type of administration, the formulation should be nebulized through an inhaler device. For that, all of the formulation’s physicochemical features should be controlled to obtain nebulized particles with the perfect size [12,36,37].
There are several studies where SLNs are administered through the pulmonary route for the treatment of diverse pulmonary pathologies, namely tuberculosis [38,39], asthma, chronic obstructive pulmonary disease (COPD) [40], and lung cancer [41,42,43]. Furthermore, there are SLN treatments administered through the pulmonary route for systemic diseases such as diabetes [44] and hypertension [45].
4.3. Oral Administration
The oral route is non-invasive, not requiring medical assistance for drug administration. Therefore, is the preferred route of administration by patients and the most desired in clinical practice. Several authors have studied the stability of SLNs in dried or suspension form for oral administration [4,12]. There are some examples of SLNs orally administered in order to improve the bioavailability of the loaded drugs; this is the case of lopinavir (for HIV treatment) [46], risperidone (schizophrenia treatment) [47], rifampicin (tuberculosis) [48], and hydrochlorothiazide (pediatric hypertension) [49].
4.4. Ocular Administration
Ocular delivery is a complex route of administration due to the sensitive inner structure of the eye and the poor penetration of drugs in this tissue. Therefore, the drugs are usually locally administrated using eyedrops. In this type of administration, SLNs benefit from their small size, meaning they do not obstruct vision, which happens with other formulations. Additionally, SLNs can reduce the clearance by the eye’s protective mechanisms due to adhesive properties related to nanometric size. Some formulations have been tested in animal models for treating diverse ocular diseases, like retinitis pigmentosa [50], glaucoma [51], and ocular bacterial infections [52].
4.5. Intravenous Administration
Intravenous administration is the most common administration route for chemotherapeutic drugs. Nanomedicine has contributed to increasing the stability and bioavailability of drugs when intravenously administrated. The formulations should be deeply characterized in order to be in accordance with the requirements for this administration route, mainly the small size, serum stability, and hemocompatible components. Accordingly, to their compositions, SLNs are perfectly able to fulfill all the requirements for adequate intravenous administration. The majority of the developed intravenous SLNs are intended for cancer therapy (deeply discussed in the next sections of the present review). However, there are also studies using intravenously administered SLNs for neurodegenerative diseases [53] as well as for the administration of contrast agents for imaging and diagnosis [54,55].
4.6. Intranasal Administration
Managing central nervous system (CNS) disorders is challenging due to the need for drugs to cross the blood-brain barrier (BBB) and reach the brain. The lipid nature of lipid-based nanocarriers such as SLNs can facilitate the transition across the BBB and translocate them into the brain through passive diffusion [56]. Few studies using SLNs have been proposed for the nose-to-brain delivery of drugs [57]. Although the exact mechanism of drug transport from the nose to the brain is not fully understood, and its effectiveness in humans is unclear, intranasally administered SLNs have been shown to be more effective in crossing the BBB than other formulations and administration routes.
5. SLNs’ Advantages for Cancer Treatment
The higher specificity of nanoDDS to the tumor site is commonly explained by the well-known permeation and retention effect (EPR effect) (Figure 2A). Accordingly, due to their nanometric size, nanoDDS can profit from the weak vessel structure and the small fenestras (300–900 nm) present in the tumor vasculature while suppressed lymphatic drainage causes retention within the tissue, thus easily reaching the tumor site by passive targeting. After tissue accumulation, nanoDDS are more prone to suffer cell internalization. The mechanism for SLN internalization into cells usually occurs through clathrin- or caveolin-mediated endocytosis (Figure 2B). Briefly, the plasmatic membrane surrounds the SLNs, forming a vesicle. This vesicle will suffer different stages of maturation, beginning with the early endosome and finishing in the lysosome. Depending on the SLN’s superficial charge and composition, it can be degraded in the endosome or lysosome, releasing its content into the cytosol [58]. Moreover, by modulating the composition of the nanoDDS, they can be easily functionalized with different targeting moieties to favor active targeting against the type of cell of interest, which allows a highly specific treatment compared with the usual unspecific conventional therapy.
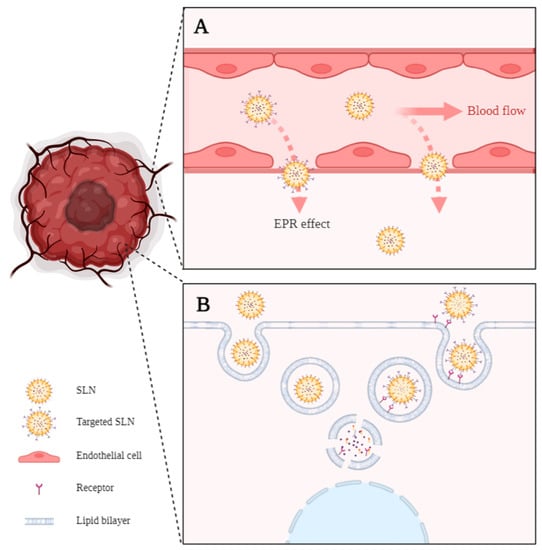
Figure 2.
(A) Enhanced permeability and retention effect (EPR effect). Passive targeting is explained by the ability of small SLNs to pass through the gaps in the leaky tumor vasculature. (B) Passive and active internalization of SLNs by endocytosis. The active internalization implies SLNs functionalized with an antigen and receptor-mediated internalization of the nanoparticle. Created in BioRender.com, accessed on 24 February 2023.
Regarding SLN formulations under clinical evaluation, when searching for lipid nanoparticles, despite the difficulties in identifying the type of formulation used, some lipidic nanoparticles, including SLNs, appear as enrolled in clinical trials for the treatment of different diseases. The majority are intended for topical [59,60] and oral [61] administration; however, to our knowledge, no clinical trials are ongoing to study the use of SLNs for cancer therapy. Despite the absence of an actual clinical evaluation, based on the number of works published and formulations under development for cancer treatment, it is expected the translation of some of them from the bench to clinical trials in the near future. In fact, boosted by the success of COVID-19 vaccines, the interest in lipidic nanoparticles exponentially increased, and the pharmaceutical economic studies from Fortune Business Insights predict an increase in the global market share of lipidic nanoparticles, including SLNs [62].
As referred to, SLN development began in the 1990s, with the first formulations reaching the market in the cosmetic field. With the increasing interest in this type of system, many patents related to the composition and techniques used have been presented. An example is an SLN formulation to improve sorafenib bioavailability [63]. Also, another patent relates to silymarin-loaded SLNs targeting tumor cells through folic acid surface modification [64]. The authors claimed an active lung tumor targeting effect, with improved bioavailability and reduced toxic effects. Oral delivery of docetaxel by means of SLNs was also claimed in a patent for the improvement of solubility, and sustained release effect of the drug [65]. An extended list of patents is reviewed elsewhere [66].
6. SLNs for Drug Delivery
Over the years, several researchers have developed SLN-based formulations for the encapsulation of different anti-cancer drugs (Table 3). The ultimate goal of all these studies was to reach an efficacious and non-toxic formulation that could be used as a clinical alternative to improve the therapeutic index of conventional drugs. Due to the high number of reported studies, we will focus on some of the most recent publications in this review. For example, M.C. Leiva et al. [67] used glyceryl tripalmitate SLNs loaded with paclitaxel (PTX) for breast and lung cancer treatment. They observed that the encapsulated PTX was more effective in inhibiting cell proliferation than the free drug, thus improving the drug’s therapeutic index. Another example of drug efficacy improvement was demonstrated by N. Clemente et al. encapsulating temozolomide (TMZ) for melanoma treatment. Their SLN-TMZ was more efficient than free TMZ in cell cultures, and in vivo, they reduced the tumor growth and increased mouse survival by administrating a lower dose of the drug [68].
One of the major challenges regarding cancer therapy relies on the development of drug resistance by cancer cells. Usually, after several cycles of chemotherapy, cancer cells start to activate multi-drug resistance (MDR) mechanisms [2,69]. Most commonly, MDR is caused by the overexpression of drug efflux pumps, such as the P-glycoprotein (P-gp), which utilizes ATP-derived energy to pump chemotherapy drugs out of tumor cells and protect tumor tissues from chemical toxicity [70,71]. This problem was studied by G. Guney Eskiler et al. [72] and B. Stella et al. [73], who were able to avoid drug resistance using SLNs. In the work of G. Guney Eskiler et al. [72], stearic acid-based SLNs encapsulating tamoxifen (TMX) were able to reduce the cell viability of TMX-resistant breast cancer cell line (MCF7-TamR). In the case of B. Stella et al. [73], a sodium behenate SLN encapsulating squalenoyl doxorubicin (SQ-Dox) increased the inhibition of Dox-resistant ovarian cancer (A2780-DoxR) cell growth and the capacity of colony formation when compared with the cells treated with the free drugs. In another study, to overcome MDR in breast cancer, Wenrui Wang et al. [71] studied the in vitro and in vivo efficacy of a resveratrol (Res)-loaded SLNs formulation with and without D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS), a derivative of natural vitamin E (α-tocopherol) that inhibits the activity of ATP-dependent P-gp. It was found that SKBR3/PR cells treated with TPGS containing SLNs (TPGS-Res-SLNs) exhibited significant inhibition of cell migration and invasion, as compared with free Res and SLNs without TPGS (Res-SLNs). In addition, TPGS-Res-SLNs promoted more apoptosis of tumor cells and induced higher tumor reduction in SKBR3/PR xenografts that the free Res or the Res-SLNs, owning a better therapeutic outcome (Figure 3).
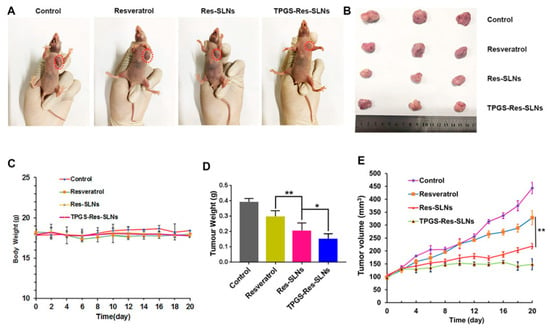
Figure 3.
The in vivo efficacies of resveratrol, Res-SLNs, and TPGS-Res-SLNs on mice bearing SKBR3/PR xenografts. (A) Representative images of mice on the 16th day in the different treatment groups. (B) Digital images of tumors excised from representative mice after the indicated treatments. (C) Body weight vs. time curves for mice treated with the indicated formulations. (D) Tumor weight of mice in the different treatment groups. (E) Tumor volume vs. time curves for mice treated with the indicated formulations. The data represented mean ± SD (n = 4). * p < 0.05, ** p < 0.01. Reprinted with permission of Frontiers Media S.A. under Creative Commons Attribution License (CC BY 4.0) from [71].
Once the encapsulation of a single drug in an SLN with a certain composition becomes trivial, several authors innovate by creating stimuli-responsive formulations or systems for dual therapy, diagnostic, or even theragnostic. For example, A. Grillone et al. [74] and T. Liu et al. [75] produced SLNs with superparamagnetic iron oxide nanoparticles (SPIONs) and Nutlin-3 or Dox, respectively. A. Grillone et al. [74] successfully delivered the nanoformulation through the BBB for the treatment of glioblastoma. T. Liu et al. [75] developed a pH-sensitive SLN with Dox and SPIONs to reduce tumor growth more efficiently due to the combination of magnetic nanoparticle-driven thermal therapy and chemotherapy in vivo.
The use of stimuli-responsive lipids can also be a crucial strategy in the context of controlled and sustained drug release since it allows an accurate manipulation of the drug release profile. For example, through the thermal properties of the lipids, it is possible to increase the drug release at a specific body region, as demonstrated by M. Rehman et al. [76]. They developed a thermosensitive SLN (mixture of lauric acid with oleic or linoleic acid) loaded with 5-fluoracil (5-FU) for breast cancer. This SLN releases quickly and higher doses of 5-FU at high temperatures, such as the tumor temperature, thus increasing the drug availability in the tumor and its therapeutic index.
Another example is the pH-sensitive SLN developed by G. Zheng et al. [77] which added adipic acid dihryzacide to the glycerin monoestereate (GMS) matrix. With this modification, the presented nanoformulation loaded with Dox had a preferential release in the tumor acidic environment (pH = 5), being possible to observe an initial burst release of the drug (within 8 h) followed by a more sustained release in time (until 96 h). This work is a representative example of the high versatility of SLNs since, by the simple modification of SLNs composition, it is possible to manipulate the release of chemotherapeutic drugs and adapt such release to the desired application.
While several authors are dedicated to improving the therapeutic index of conventional drugs, other researchers are more focused on substituting such drugs for more natural compounds or at least reducing the dose of the chemotherapeutic by combination with natural substances. This is the case of the works by J.S. Baek et al. [78] and W. Wang et al. [79], where stearic acid-based formulations were used for the encapsulation of wogonin and curcumin, respectively. These two natural substances are very active in vitro but, due to their unfavorable pharmacokinetics, require advanced delivery systems such as nanoDDS for in vivo administration. Guorgui et al. [80] also loaded curcumin in stearic acid-based SLNs and TPGS nanoparticles. They obtained higher curcumin plasma levels in mice using the SLNs and observed tumor growth regression in Hodgkin’s Lymphoma mice models. Moreover, when given in combination with bleomycin, Dox, and vinblastine, curcumin showed an additive growth inhibitory effect. The authors concluded that once adequately formulated, curcumin can be used as an adjuvant agent for the treatment of Hodgkin’s Lymphoma.

Table 3.
Examples of SLNs formulation for cancer treatment in different stages of development.
Table 3.
Examples of SLNs formulation for cancer treatment in different stages of development.
| Lipid | Method | Drug | Cancer | Phase | Ref |
|---|---|---|---|---|---|
| SA | Hot sonication | Wogonin | BC | In vitro | [78] |
| SA | Emulsification-solidification | Curcumin | BC | In vitro | [79] |
| SA & lecithin & TPGS | Emulsification-solidification | Resveratrol | BC | In vivo | [71] |
| CP | SEE | Nutlin3 & SPIONS | GBM | In vitro (BBB model) | [74] |
| Trilaurin | Microemulsion cold dilution | Curcumin | PC | In vivo | [81] |
| Gliceryl tripalmitate | HSH | PTX | BC and LC | In vitro | [67] |
| Resveratrol-stearated | Microemulsion | Omg-3 | CRC | In vitro | [82] |
| SA | HSH | TMX | BC | In vitro | [72] |
| Lauric acid & (linoleic acid/oleic acid) | Hot melt encapsulation | 5-FU | BC | In vitro | [76] |
| Sodium behenate | Coacervation | TMZ | Melanoma | In vivo | [68] |
| Behenic acid sodium salt | Coacervation | DOX | OC | In vitro | [73] |
| SA | SEE | Curcumin | HL | In vivo | [80] |
| Trilauriun & TPGS | Microemulsion | DOX and SPIONS | PTC (murine) | In vivo | [75] |
| CP | HPH | Indirubin | GBM | In vitro | [83] |
| CP | SEE | Topotecan hydrocloride | CC | In vitro | [84] |
| GMS | HSH | Talazoparib (BMN673) | BC | In vitro | [85] |
| Myristyl myristate | Hot sonication | Linalool | HC and LC | In vitro | [86] |
| GMS | Hot sonication | PTX and ascorbyl palmitate | Melanoma (murine) | In vivo | [87] |
| SA & lecithin | SIL | 5-FU | Melanoma | In vivo | [88] |
| Compritol® | Microemulsion | AP9-cd (ligand) | Leukaemia | In vivo | [89] |
| 1-tetradecanol | HHPH and ultrasonication | Temoporfin | BC | In vivo | [90] |
| GMS, AAD, and RGD | Emulsification-solidification | DOX | BC | In vivo | [77] |
| SA | SED | PTA | OC | In vitro | [91] |
| Precirol® ATO5 | Emulsification-solidification | PTX | BC | In vitro | [92] |
| Imwitor® 308 and Dynasan® 114 | Ultrasonic melt-emulsification | Celecoxib | CRC | In vitro | [93] |
| Lecithin & DSPE-PEG2000 | Film dispersion method | PTX, Curcumin | LC | In vivo | [94] |
| GMS | modified emulsion/solvent evaporation method | Abiraterone Acetate | PC | Ex vivo/In vivo | [95] |
Abbreviations: Lipid: Stearic Acid (SA), DL-α-Tocopherol methoxypolyethylene glycol succinate (TPGS), Cetyl Palmitate (CP), Glycerol Monostearate (GMS), Adipic acid dihydrazide (AAD), Arginine-Glycine-Aspartic (RDG). Method: Microwave-Assisted Microemulsion Technique (MAMT), Solvent Emulsification-Evaporation (SEE), High Shear Homogenization (HSH), Solvent Injection-Lyophilization (SIL), Hot High-Pressure Homogenization (HHPH), Solvent Emulsification-Diffusion (SED). Drug: Paclitaxel (PTX), Tamoxifen (TMX), 5-fluorouracil (5-FU), Temozolomide (TMZ), Superparamagnetic iron oxide nanoparticles (SPIONs), Doxorubicin hydrochloride (DOX), 1,3,5-triaza-7-phosphaadamantane (PTA). Cancer: Breast Cancer (BC), Glioblastoma (GBM), Prostate Cancer (PC), Lung Cancer (LC), Colorectal Cancer (CRC), Ovarian Cancer (OC), Hodgkin’s Lymphoma (HL), Papillary Thyroid Cancer (PTC), Prostate Carcinoma (PC), Cervical Cancer (CC), Hepatocarcinoma (HC).
7. Targeted SLNs
One of the challenges of cancer therapy is the management of adverse side effects caused by conventional chemotherapy that affect not only the cancer cells but also the healthy cells. In order to solve this problem, different approaches to specifically target tumor cells have been strongly explored in recent years. NanoDDS are one of the adopted strategies since their surface can be functionalized with several targeting moieties to increase the uptake of the delivery system by the target cells. In the case of SLNs, different molecules have been used to introduce active targeting moieties, and a summary of the most recent and relevant works is shown in Table 4.
It is widely known that some cancer cells overexpress certain receptors in comparison to healthy cells; thus, by targeting these receptors, it is expected to obtain a higher uptake by cancerous cells, as demonstrated by E. Souto et al. [96] and S. Shi et al. [97]. E. Souto et al. [96] developed a Compritol®-based SLN with active targeting against HER2 receptor by conjugating the compact antibody CAB51. The SLNs showed higher cellular uptake in BT-474 (HER2 positive cells) cells than in MCF-7 (HER2 negative cells), proving the active targeting of the HER2 receptor. S. Shi et al. [97] proposed dual-targeting by functionalizing the SLN’s surface with hyaluronic acid and tetraiodothyoacetic acid as ligands of CD44 and αvβ3 receptors, respectively. Functionalized SLNs had a higher cellular uptake in cells expressing those receptors and increased the therapeutic effect of docetaxel in vivo. Another example is the SLNs conjugated with the antibody against the receptor for advanced glycation end products (RAGE) developed by V. T. Siddhartha et al. [98]. These SLNs loaded with di-allyl-disulfide were taken up more efficiently and induced higher cytotoxicity than the unconjugated SLNs in triple-negative breast cancer cells. The higher cytotoxicity was due to a combined effect of intracellular accumulation of di-allyl-disulfide and the promotion of pro-apoptotic proteins by the inhibition of the RAGE receptor.
Targeting the folate receptor is also a widely used approach for increasing the tumoral accumulation/internalization of SLNs, as demonstrated by R. Rosière et al. [99], J.S. Baek et al. [100], and K. Rajpoot et al. [101], among others. In the case of R. Rosière et al. [100], they developed an SLN coated with a folate-grafted copolymer of PEG and chitosan for lung tumors. They observed that this nanoformulation loaded with PTX had a higher penetrating capacity into lung tumors when administrated by inhalation rather than the conventional treatment. Due to folate functionalization, the SLN does not have to rely on tumor vascularization to reach the tumor site. K. Rajpoot et al. [101] encapsulated oxaliplatin into SLNs conjugated with folic acid for colorectal cancer treatment and observed that the SLNs functionalized had a higher cytotoxicity effect in the HT29 cell line compared to the unfunctionalized SLNs. They believed this higher cytotoxicity was due to the higher cellular uptake of the nanoformulation mediated by the folate receptor. SLNs are also useful for transporting plant essential oils (fatty nature) due to their ability to encapsulate hydrophobic compounds. Based on this, S.F. Tabatabaeain et al. [102] successfully loaded Satureja khuzistanica essential oil into SLNs modified using a chitosan coating attached to folic acid to improve drug accumulation into breast cancer cells (MCF-7 cells). J.S. Baek et al. [100] developed SLNs coated with folic acid and conjugated to stearic acid for the treatment of MDR breast cancer. These SLNs loaded with curcumin and PTX showed a higher cellular internalization and synergic cytotoxicity partially due to the capacity of curcumin to inhibit P-gp, thus increasing the PTX intracellular accumulation.
The use of sugars has also been widely studied for targeting cancer cells due to their higher energetic metabolism. For that, N. Soni et al. [103] and N.K. Garg et al. [104] functionalized their SLN’s surface with mannose and fucose. N. Soni et al. [103] loaded gemcitabine in a stearyl amide-based SLNs for lung cancer. Adding mannose to the SLN’s surface increased the cellular uptake through the mannose receptors overexpressed in the macrophages. Also, in biodistribution studies, the SLNs functionalized showed more accumulation in the lungs than the basal SLNs. N. K. Garg et al. [104] used fucose-coated SLNs loaded with methotrexate for breast cancer treatment, achieving approximately 70% of tumor reduction.
Several treatments and targeting actives can be combined in the same SLNs. M.Y. Shen et al. [105] developed SLNs loaded with Dox and SPIONs with a double coating of folic acid and dextran for colon cancer treatment. The folic acid increased the cellular uptake through the folate receptors, and the dextran increased the tissue-specificity of SLNs since the enzyme capable of degrading dextran is only present in the colon. Besides, the coating also allowed the oral administration of SLNs by protecting them from degradation at harsh gastric conditions. This nanoformulation showed promising results in vitro and in vivo, not only by the high tumor cytotoxicity but by the lack of side effects. This is an example of a multifunctional nanoparticle that is expected to be the future of nanomedicine by combining different components and functions in the same vehicle for improved biological behavior and clinical outcome.
Another widely used approach to improve cancer treatment is the functionalization of nanoDDS with cell-penetrating peptides (CPP), as reported by B. Liu et al. [106]. They have modified the SLNs surface with a trans-activating transcriptional activator (TAT) peptide and studied how the functionalization can increase the accumulation of SLNs loaded with PTX and α-tocopherol succinate-cisplatin prodrug. They report a higher accumulation of the SLNs in the tumorous cells, increasing the drugs’ therapeutic index. They discuss that this higher accumulation is due to the presence of TAT. However, since the function of CPP is to translocate through the plasmatic membrane, not only to the tumor cells but also the healthy cells, the use of CPP should not be considered active targeting, contrary to what has been described in some literature. The use of CPP as moieties for nanoDDS surface functionalization relies on the improvement of cellular uptake and consequent improvement of drug effectiveness without tumor specificity. Even without this cell-specificity, the use of CPP is of major interest in brain tumors that require crossing the BBB. The use of SLNs modified with moieties that increase the permeability of drugs through the BBB has been proposed by Y.C. Kuo et al. [107,108] and A. Kadari et al. [109], among others. Y.C. Kuo et al. [107,108] developed different Compritol®-based SLNs formulations for the encapsulation of etoposide as a chemotherapeutic drug. One of the formulations was composed of a double surface conjugation with an 83–14 monoclonal antibody (83–14 MAb) and the anti-epithelial growth factor receptor (AEGFR) [107]. The double targeting facilitated the BBB crossing due to the recognition of the α-subunit insulin receptor by the 83–14 MAb, and the active targeting of cancerous cells was achieved by the AEGFR. Another formulation developed by the same authors included an anti-melanotransferrin antibody attached to the surface of SLNs to increase the BBB crossing [108]. Both studies were tested in an in vitro model of BBB and glioblastoma and successfully decreased the glioblastoma cells proliferation without causing cytotoxicity to the BBB. A. Kadari et al. [109] have developed an SLN loaded with docetaxel with an angiopep-2 linked to its surface. Angiopep-2 is a specific ligand for lipoprotein receptor-related protein 1 (LRP1), a receptor overexpressed in the BBB and the glioma cells. The in vivo results demonstrated a higher accumulation of the SLNs in the brain and an increase in the survival rate of the animals compared with the current treatment, thus arising as a promising alternative for the treatment of glioblastoma.

Table 4.
Summary of targeted SLNs for different cancer types in different stages of development. N.A.—not applicable.
Table 4.
Summary of targeted SLNs for different cancer types in different stages of development. N.A.—not applicable.
| Lipid | Method | Drug | Targeting | Cancer | Phase | Ref |
|---|---|---|---|---|---|---|
| Compritol® | HSH | N.A. | Antibody against HR2 | BC | In vitro | [96] |
| Glyceryl stearate & Chol | Nanoprecipitation | PTX | Folic acid coated | LC | In vivo | [99] |
| SA and lecithin | HHPH | Satureja khuzistanica Essential Oil & folate-bound chitosan | Folic acid | BC | In vitro | [102] |
| Behenic acid | Coacervation | Methotrexate | Apoemimkin Chimera | GBM | In vivo | [110] |
| GMS and SA | SEE | Docetaxel | Angiopep-2 | GBM | In vivo | [109] |
| PA | SED | di-allyl-disulfide | RAGE antibody | BC | In vitro | [98] |
| DSPE | SED | Oxaliplatin | Folic acid | CRC | In vitro | [101] |
| GMS and TPGS | SEE | PTX and Curcumin | SA-folate | BC | In vitro | [100] |
| GMS, SPC, and Oda | Film-ultrasonic method | Docetaxel | HA-Te | BC | In vivo | [97] |
| Tristearin | SED | Irinotecan | Folic acid | CRC | In vivo | [111] |
| GMS | SEE | PTX and TSC | TAT | CC | In vivo | [106] |
| Trilaurin and TPGS | Microemulsion | DOX and SPIONs | Folic acid | CRC | In vivo | [105] |
| Glyceryl palmitostearate | Emulsification-solidification | PTX | Anti CD44v6 antibody | BC | In vitro | [112] |
| Stearyl amine | SEE | Gemcitabine | Mannose | LC | In vivo | [103] |
| Gelucire® 50/13 | Microemulsion | Methotrexate | Fucose | BC | In vivo | [104] |
| GMS | HHPH | PTX | Hyaluronic acid | CC & BC | In vivo | [113] |
| SA | SI (modified) | Resveratrol and Ferulic acid | Folic acid | CRC | In vitro | [114] |
| SA | Hot melted sonication | PTX | HP-β-CD | BC | In vivo | [115] |
| GMS and Compritol® | Hot melt-emulsification | Dox and Curcumin | Folic acid | BC | In vivo | [116] |
| Compritol®, tripalmitin, SA, and Chol | SEE | ETP | Melanotransferr-in antibody and Tamoxifen | GBM | In vitro | [108] |
| GMS | SEE | PTX | Wheat germ agglutinin | LC | In vivo | [117] |
| Compritol®, CL, and SA | Microemulsion | ETP | 83–14 MAb and AEGFR | GBM | In vitro | [107] |
| Behenic acid, tripalmitin, and cacao butter | SEE | Carmustine | Tamoxifen and Lectoferrin | GBM | In vitro | [118] |
| Tristearin & HSPC | SI | PTX | Lactoferrin | LC | In vitro | [39] |
| PA and Dynasan® | N.A. | Saquinavir | 83–14 MAb | GBM | In vitro | [119] |
| Compritol® 888 ATO and Precirol® ATO | Sonication of pre-emulsion | DTX | AS1411 anti-nucleolin aptamers | CRC | In vivo | [120] |
| Cetyl palmitate | Hot ultrasonication method | Mitoxantrone | Folate receptor | BC | In vitro | [121] |
Abbreviations: Lipid: Stearic Acid (SA), Glycerol Monostearate (GMS), Palmitic acid (PA), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), DL-α-Tocopherol methoxypolyethylene glycol succinate (TPGS), Soy phosphatidylcholine (SPC), Octadecylamine (Oda), Cardiolipin (CL), Hydrogenated soya phosphatidylcholine (HSPC). Method: High Shear Homogenization (HSH), Hot High-Pressure Homogenization (HHPH), Solvent Emulsification-Evaporation (SEE), Solvent Emulsification-Diffusion (SED), Solvent Injection (SI). Drug: Paclitaxel (PTX), α-tocopherol succinate-cisplatin (TSC), Doxorubicin hydrochloride (DOX), Superparamagnetic iron oxide nanoparticles (SPIONs), Etoposide (ETP), Docetaxel (DTX). Cancer: Breast Cancer (BC), Lung Cancer (LC), Glioblastoma (GBM), Colorectal Cancer (CRC), Cervical Cancer (CC), Prostate Cancer (PC), Ovarian Cancer (OC).
8. SLNs for Gene Delivery
Gene therapy gained a huge interest in the field of cancer treatment to overcome the high toxicity and the low specificity associated with conventional drugs. Gene therapy allows the expression of an exogenous oligonucleotide, encoding for a missing or defective gene or achieving the silence of a particular gene [122,123]. The exogenous expression is usually mediated by plasmid DNA (pDNA) which consists of a small circular sequence of DNA that can replicate after entering the nucleus [124]. Gene silencing, on the other hand, is usually performed by technologies of RNA interference (RNAi). There are three types of RNAi: short hairpin RNA (shRNA), small interfering RNA (siRNA), and microRNA (miRNA). The shRNA, like the pDNA, has its function in the nucleus, where it is transcribed to small RNA and binds to the complementary mRNA, thus inhibiting the expression of the protein [125]. On the other hand, siRNA and miRNA do not have to enter the nucleus since they exert activity in the cytoplasm [126]. In the case of the siRNA, it is a 20–25 base nucleotide with a specific sequence that targets and degrades specific mRNAs [127]. Regarding miRNA is not as specific as siRNA since the same miRNA can bind to different mRNAs [128,129].
The major issue regarding gene therapy is the delivery of genetic material into the cells. The oligonucleotide’s physicochemical properties make its entrance into the cell difficult without a proper vector. Despite their low packaging capacity, high production costs, and immunogenicity, viral vectors are still the preferred vectors for gene delivery in the clinical setting. In order to overcome all the drawbacks associated with the viral vectors, considerable investment and efforts have been made to develop non-viral vectors [130,131], including some of the recent vaccines against the SARS-CoV-2 virus [14]. In this review, we are focused on the application of SLNs for gene therapy in oncological diseases.
Many formulations have been proposed with differences regarding the components and the physicochemical properties of the SLNs enjoying the lipidic nature of the particles to promote cell internalization. However, many of them have at least one cationic lipid and/or phospholipid, such as 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), in the composition to facilitate the complexation of genetic material. Table 5 summarizes different SLNs developed for cancer gene therapy, where the main components used for its formulation can be observed. J. Jin et al. [132] developed an SLN complexed with c-Met siRNA for glioblastoma. The c-Met complexation with SLNs increased its accumulation in the brains of mice, leading to a decrease in tumor growth. C. Botto et al. [133] have successfully loaded the shNUPR1 in a Precirol®-based SNL and delivery it to the cells. Moreover, SLNs/shNUPR1 downregulated the NUPR1 gene, which is known to cause chemoresistance and cancer proliferation in hepatocellular carcinoma. Several works describe the use of SLNs to downregulate the expression of signal transducer and activator of transcription 3 (STAT 3). M. Kotmakçi et al. [134] used shRNA against STAT3 to reduce STAT3 levels and re-sensitize resistant lung cancer cells (CR-Calu1) to cisplatin. Zhang et al. used decoy oligodeoxynucleotides (ODN) to target STAT-3. The results were promising, showing an inhibition of tumor growth activating the apoptotic cascade, regulating autophagy, and reversing the epithelial-mesenchymal transition program with no obvious toxicity on nude mice [135].
In many studies, gene therapy is combined with chemotherapeutic drugs within the same nanoparticle to potentiate their synergy, as reported by G. Büyükköroglu [136], Y.H. Yu et al. [137] and T. Li et al. [138], among many others. In the case of G. Büyükköroglu [136], they loaded SLNs with Bcl-2 siRNA and PTX for the treatment of cervical cancer. Their results show higher cytotoxicity with the combination of siRNA and PTX than with individual treatments. Also, they propose a local administration by encapsulating the SLNs in a PEG suppository to reduce the systemic exposure of the pharmacologic compounds and thus reduce their side effects [139]. Y.H. Yu et al. [137] developed cationic SLNs for breast cancer treatment loaded with PTX and MCL1-siRNA that successfully reduced tumor growth in vivo. T. Li et al. [138] studied the synergetic anticancer activity of sorafenib and all-trans retinoic acid (ATRA) combined with miRNA-542-3p loaded in SLNs. Again, the SLNs showed a much higher cell growth inhibition than individual treatments, such as free drugs or in SLNs. Moreover, in vivo, it also showed promising results by an increased reduction of tumor growth and increased blood circulation time compared to the free drugs. They believe this high antitumor efficacy is due to the combined therapeutic effects of drugs with the microRNA.
Targeted SLNs have also been developed for gene delivery. D.M. Yu 2016 et al. [140] loaded SLNs with PTX and pDNA and attached hyaluronic acid (HA) with a pH-sensitive linker to promote release at low pH. This nanoformulation not only efficiently released the chemotherapeutic drug and the pDNA in breast cancer cells but also increased the accumulation and preferential release of the compounds in the tumor tissue by actively targeting HA to CD44 receptors.
Finally, different nanoDDS can be successfully combined for improved therapy, as demonstrated by V. Juang et al. [141]. They developed an SLN and a liposome loaded with miRNA200 and irinotecan for colon cancer treatment. Both nanoformulations had their surface modified with three different peptides (including one cell-penetrating peptide, one peptide targeting tumor neovasculature undergoing angiogenesis, and one mitochondria-targeting peptide) that increased the cellular uptake and provided an active target to tumoral cells. Moreover, this nanoformulation was coated with a pH-sensitive PEG-lipid that enhanced the release of the miRNA in tumor sites due to the acidic environment. The combination therapies of different delivery systems promoted the synergetic activity between irinotecan and miR-200 since miR-200 increased the cancer cell sensitivity to irinotecan. The in vivo studies showed a highly effective inhibition of tumor growth caused by suppressing several proteins such as Rac-1, KRAS, and β-catenin, among others, being a promising study for further development.

Table 5.
SLNs with different genetic material for different cancer types in different stages of development. N.A.—not applicable.
Table 5.
SLNs with different genetic material for different cancer types in different stages of development. N.A.—not applicable.
| Lipid | Method | Drug | Genetic Material | Cancer | Phase | Ref |
|---|---|---|---|---|---|---|
| Gelucire® 50/13 | SEE | PTX | Bcl-2 siRNA | CC | In vitro | [136] |
| Precirol ATO5 and Compritol® | Microemulsion (modified) | N.A. | pDNA stat3 | LC | In vitro | [134] |
| Steryalmide | Microemulsion | Sorafenib/ATRA | miR-542-3p | GC | In vivo | [138] |
| DSPE, DOTAP, and αPC | SED | Irinotecan | miR200 | CRC | In vivo | [141] |
| DOPE, Chol, and DC-Chol | SEE | N.A. | c-Met siRNA | GBM | In vivo | [132] |
| DOPC | emulsification solidification methods | PTX | MCL-1 siRNA | BC | In vivo | [137] |
| GMS | film-ultrasonic dispersion method | PTX | pDNA | BC | In vivo | [140] |
| Precirol ATO5 | ethanolic precipitation technique & HPH | N.A. | shNUPR1 | HC | In vitro | [133] |
| DOTAP and GMS | SED | N.A. | miR200 | BC | In vitro | [142] |
| GMS, SPC, and Chol | film-ultrasonic method | N.A. | miR-34a | LC | In vivo | [143] |
| GMS and SPC | SED | N.A. | AMO | LC | In vitro | [144] |
| GMS and soya lecithin | solvent diffusion method | N.A. | STAT3 decoy oligodeoxynucleotides | OC | In vivo | [135] |
| Cetyl palmitate and Cremephor RH 40, Peceol, and propylene glycol | melt-emulsification technique | N.A. | siRNA-EGFR siRNA-PD-L1 | GBM | In vivo | [145] |
Abbreviations: Lipid: 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]-cholesterol (DC-Chol), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP), l-α-Phosphatidylcholine (αPC), 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), Glycerol Monostearate (GMS), Soy phosphatidylcholine (SPC). Stearic Acid (SA), Glycerol Monostearate (GMS), Palmitic acid (PA), DL-α-Tocopherol methoxypolyethylene glycol succinate (TPGS), Soy phosphatidylcholine (SPC), Octadecylamine (Oda), Cardiolipin (CL), Hydrogenated soya phosphatidylcholine (HSPC). Method: High Shear Homogenization (HSH), Hot High-Pressure Homogenization (HHPH), Solvent Emulsification-Evaporation (SEE), Solvent Emulsification-Diffusion (SED), Solvent Injection (SI). Drug: Paclitaxel (PTX), α-tocopherol succinate-cisplatin (TSC), Doxorubicin hydrochloride (DOX), Superparamagnetic iron oxide nanoparticles (SPIONs), Etoposide (ETP). Cancer: Cervical Cancer (CC), Lung Cancer (LC), Gastric Cancer (GC), Colorectal Cancer (CRC), Glioblastoma (GBM), Breast Cancer (BC), Hepatocarcinoma (HC), Ovarian Cancer (OC).
9. Other Applications
9.1. Immunotherapy
The idea of using a patient’s immune system to fight cancer lead to the concept of cancer immunotherapy. In the last years, this approach increased exponentially, becoming an important and promising alternative at the clinical level.
Cancer cells have the capacity to make themselves invisible to the immune system by selecting for certain genetic changes, by having proteins on their surface that turn off immune cells (e.g., PD-L1), or by inducing changes in the surrounding stroma. Different approaches have been proposed in this scenario to activate the immune response. This includes monoclonal antibodies, immune checkpoint inhibitors, adaptive T-cell transfer, and cancer vaccination [146]. Specifically, here are limited studies in the literature related to cancer immunotherapy with SLNs.
G. Erel-Akbaba et al. [145] developed an iRGD (CCRGDKGPDC)-conjugated SLN to deliver siRNA against both epidermal growth factor receptor (EGFR) and PD-L1 for glioblastoma treatment. Moreover, 5 Gy radiation pre-treatment led to enhanced transportation of SLNs to glioblastomas, yielding activation of an immune response, slower tumor growth, and prolonged mouse survival (Figure 4).
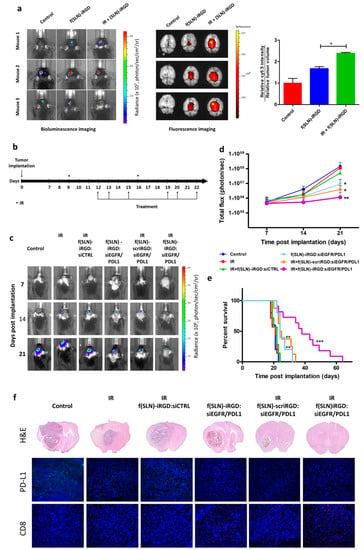
Figure 4.
Radiation primes glioblastoma for SLNs targeted delivery. (a) Mice bearing GL261-Fluc tumors were irradiated (or not as control) and, three days later, received retro-orbital administration of f(SLNs)-iRGD:Cy5.5 or PBS control. Twenty-four hours post-injection, tumor volume was first evaluated by Fluc imaging (left), and brains were removed and imaged ex vivo for Cy5.5 (middle). The mean fluorescence intensity was calculated and normalized to tumor volume (right; n = 3, * p < 0.05). (b–f) Mice bearing GL261-Fluc tumors were irradiated (or not as a control) and retro-orbitally injected with either f(SLNs)-iRGD:siCTRL, f(SLNs)-iRGD:siEGFR/PDL1, or f(SLNs)-scriRGD:siEGFR/PDL1 according to the scheme in (b). Tumor growth was monitored weekly by Fluc imaging, and survival was recorded. Images from a representative mouse from each group are shown over time (c). Quantification of tumor-associated Fluc radiance intensity with data presented as mean ± SD; * p < 0.05 control vs. f(SLNs)-iRGD:siRNA and control vs. IR + f(SLNs)-scriRGD:siRNA; ** p < 0.01 control vs. IR + f(SLNs)-iRGD:siRNA by ANOVA (d). Kaplan–Meier survival curves are shown (n = 5–12); ** p < 0.01 control vs. f(SLNs)-iRGD:siRNA; ** p < 0.01 control vs. IR + f(SLNs)-scriRGD:siRNA; *** p < 0.001 control vs. IR + f(SLNs)-iRGD:siRNA; ** p < 0.01 IR + f(SLNs)-scriRGD:siRNA vs. IR + f(SLNs)-iRGD:siRNA; by Mantel–Cox (log-rank) test (e). H&E staining and immunohistological analysis using anti-PD-L1 and anti-CD8 antibodies on brain sections of a representative mouse from each group (DAPI, blue; PD-L1, green; and CD8, red) (f). Reprinted with permission of American Chemical Society from [145].
9.2. Imaging
The advances in imaging techniques acquired major importance in the cancer field since they allow the achievement of an accurate early diagnosis and monitoring of the patient during and after the treatment. This can substantially impact the treatment efficacy and patient survival [147]. There are multiple techniques for imaging, and some of them need contrast agents for better differentiation between the tissues. The contrast agents are administered intravenously, being one of their major issues the lack of specificity to the site of interest. J. Sun et al. [55] have developed SLNs loaded with gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA), a widely used contrast agent in magnetic resonance imaging (MRI), for colon cancer imaging. Due to the encapsulation of the Gd-DTPA, they have been able to increase its cellular uptake and the tumor’s resolution and differentiation, and limits. Also, an in vivo biodistribution of SLNs-Gd-DTPA showed a high accumulation in the tumor site.
9.3. Theragnostic
Theragnostic consists of the combination of treatment and diagnosis in the same system. Cancer treatment is a promising new strategy that will contribute not only to early tumor diagnosis but also allow constant monitoring of the treatment’s effectiveness [148,149]. J. Kulbacka et al. [150] and Y. Kuang et al. [151] developed SLNs for cancer theragnostics. J. Kulbacka et al. [150] developed cetyl palmitate-based SLNs loaded with cyanine IR-780 and flavonoid derivates for colon cancer. The flavonoid was used as the drug in this nanoformulation and the cyanine IR-780 as a diagnostic agent and a photosensitizer. The encapsulation of cyanine IR-780 decreased its cytotoxicity and increased its efficacy. Y. Kuang et al. [151] also loaded IR-780 in SLNs for phototherapy and imaging. This nanoformulation enabled the specific treatment due to the guided imaging that allows light activation in the area of interest. Finally, K.H. Bae et al. [152] developed SLNs loaded with quantum dots PTX and siRNA-bcl2 for the synergistic treatment and in situ lung cancer imaging, paving the way for SLNss to be used as multifunctional and optically traceable nanocarriers for anticancer theragnostics.
10. Conclusions
SLNs have been reported to be effective multitasking nanoDDS for cancer treatment since they are demonstrated to be a promising approach to increasing the therapeutic index of the delivered cargo. Currently, multiple SLNs-based formulations are proposed for different cancer treatments with various loading cargos, such as chemotherapeutic drugs and genetic material. Also, modifying the SLNs surface enables effective active targeting against the cells/tissues of interest, increasing the specificity of the treatment and reducing the secondary adverse effects.
Apart from the treatment applications, SLNs also demonstrated great potential as diagnostic tools, openings the possibility of creating multifunctional nanoDDS for theragnostics.
One of the biggest advantages of using SLNs is the possibility of using biocompatible, non-immunogenic, low-cost materials and production methods easily scalable for industrial scale. On the other hand, some limitations related to SLNs, mainly cargo and stability issues, should be overcome. In this sense is expected that the research in the field of SLNs-based nanosystems will keep evolving and that, in the future, more formulations will reach the clinical phases.
Author Contributions
Conceptualization, D.R. and F.A.; writing—original draft preparation, J.G.-C., M.V.-H., D.R. and F.A.; writing—review and editing, D.R., F.A. and I.A.; funding acquisition, I.A. All authors have read and agreed to the published version of the manuscript.
Funding
The present work as supported by the SGR grant from the Catalan Government (2021 SGR 01173), the Spanish Ministry of Science and Innovation (MICINN, RTC2019-006809-1), and the Networking Research Centre on Bioengineering, Biomaterials, and Nanomedicine (CIBER-BBN) which is financed by the Instituto de Salud Carlos III (ISCIII) with assistance from the European Regional Development Fund (ERDF, “A way to make Europe”/“Investing in your future”). FA was supported by an investigator grant from Asociación Española Contra el Cáncer (AECC), Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, G.; Wang, Y.; Yang, G.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef] [PubMed]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Musicanti, C.; Gasco, P. Solid Lipid Nanoparticles–SLN. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2471–2487. [Google Scholar]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Esquivel, R.-A.; Navarro-Tovar, G.; Zárate-Hernández, E.; Aguirre-Bañuelos, P. Solid Lipid Nanoparticles (SLN). In Nanocomposite Materials for Biomedical and Energy Storage Applications; Sharma, A., Ed.; IntechOpen Limited: London, UK, 2022. [Google Scholar]
- Prabhakaran, E.; Hasan, A.; Karunanidhi, P. Solid lipid nanoparticles: A review. Sci. Revs. Chem. Commun. 2012, 2, 80–102. [Google Scholar]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Zieneldien, T.; Kim, J.; Cao, J.; Cao, C. COVID-19 Vaccines: Current Conditions and Future Prospects. Biology 2021, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Sastri, K.T.; Radha, G.V.; Pidikiti, S.; Vajjhala, P. Solid lipid nanoparticles: Preparation techniques, their characterization, and an update on recent studies. JAPS 2020, 10, 126–141. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.S.; Müller, R.H. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Pooja, D.; Tunki, L.; Kulhari, H.; Reddy, B.B.; Sistla, R. Optimization of solid lipid nanoparticles prepared by a single emulsification-solvent evaporation method. Data Brief 2016, 6, 15–19. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Sjöström, B.; Bergenståhl, B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions I. Model studies of the precipitation of cholesteryl acetate. Int. J. Pharm. 1992, 88, 53–62. [Google Scholar] [CrossRef]
- Hu, F.Q.; Yuan, H.; Zhang, H.H.; Fang, M. Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization. Int. J. Pharm. 2002, 239, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.A.; Müller-Goymann, C.C. Solvent injection as a new approach for manufacturing lipid nanoparticles--evaluation of the method and process parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, N.; Zhang, Y.; Shen, W.; Gao, X.; Li, T. Solvent Injection-Lyophilization of Tert-Butyl alcohol/water Cosolvent Systems for the Preparation of Drug-Loaded Solid Lipid Nanoparticles. Colloids Surf. B Biointerfaces 2010, 79, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Cavalli, R.; Trotta, M. Solid Lipid Nanoparticles Produced Through a Coacervation Method. J. Microencapsul. 2010, 27, 78–85. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Production Techniques. In Lipid Nanoparticles: Production, Characterization and Stability; Springer International Publishing: Cham, Switzerland, 2015; pp. 23–43. [Google Scholar]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. View 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Arduino, I.; Liu, Z.; Rahikkala, A.; Figueiredo, P.; Correia, A.; Cutrignelli, A.; Denora, N.; Santos, H.A. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater 2021, 121, 566–578. [Google Scholar] [CrossRef]
- Andrade, L.N.; Oliveira, D.M.L.; Chaud, M.V.; Alves, T.F.R.; Nery, M.; da Silva, C.F.; Gonsalves, J.K.C.; Nunes, R.S.; Corrêa, C.B.; Amaral, R.G.; et al. Praziquantel-Solid Lipid Nanoparticles Produced by Supercritical Carbon Dioxide Extraction: Physicochemical Characterization, Release Profile, and Cytotoxicity. Molecules 2019, 24, 3881. [Google Scholar] [CrossRef]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid Nanoparticles for Improved Topical Application of Drugs for Skin Diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Netto, G. Role of Solid Lipid Nanoparticles as Photoprotective Agents in Cosmetics. J. Cosmet. Dermatol. 2019, 18, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Beppu, S.; Mizoe, T.; Takashima, Y.; Yuasa, H.; Okada, H. Preparation of Two-Drug Composite Microparticles to Improve the Dissolution of Insoluble Drug in Water for Use With a 4-fluid Nozzle Spray Drier. J. Control. Release Off. J. Control. Release Soc. 2005, 107, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Üner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Jawahar, N.; Reddy, G. Nanoparticles: A novel pulmonary drug delivery system for tuberculosis. J. Pharm. Sci. Res. 2012, 4, 1901–1906. [Google Scholar]
- Pandey, V.; Gajbhiye, K.R.; Soni, V. Lactoferrin-appended Solid Lipid Nanoparticles of Paclitaxel for Effective Management of Bronchogenic Carcinoma. Drug Deliv. 2015, 22, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Pulmonary Application: A Review of the State of the Art. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2014, 86, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.; Almeida, A.J.; Fabra, A. Preclinical Evaluation of a Pulmonary Delivered Paclitaxel-Loaded Lipid Nanocarrier Antitumor Effect. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jia, Y.; WenDing. Preparation and Characterization of Solid Lipid Nanoparticles Loaded With Epirubicin for Pulmonary Delivery. Die Pharm. 2010, 65, 585–587. [Google Scholar] [CrossRef]
- Dharmala, K.; Yoo, J.W.; Lee, C.H. Development of chitosan-SLN Microparticles for Chemotherapy: In Vitro Approach Through Efflux-Transporter Modulation. J. Control. Release Off. J. Control. Release Soc. 2008, 131, 190–197. [Google Scholar] [CrossRef]
- Liu, J.; Gong, T.; Fu, H.; Wang, C.; Wang, X.; Chen, Q.; Zhang, Q.; He, Q.; Zhang, Z. Solid Lipid Nanoparticles for Pulmonary Delivery of Insulin. Int. J. Pharm. 2008, 356, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Makled, S.; Nafee, N.; Boraie, N. Nebulized Solid Lipid Nanoparticles for the Potential Treatment of Pulmonary Hypertension via Targeted Delivery of phosphodiesterase-5-inhibitor. Int. J. Pharm. 2017, 517, 312–321. [Google Scholar] [CrossRef]
- Negi, J.S.; Chattopadhyay, P.; Sharma, A.K.; Ram, V. Development of Solid Lipid Nanoparticles (SLNs) of Lopinavir Using Hot Self Nano-Emulsification (SNE) Technique. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Kumar, A.; Wild, W.; Ferreira, D.; Santos, D.; Forbes, B. Long-term Stability, Biocompatibility and Oral Delivery Potential of Risperidone-Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2012, 436, 798–805. [Google Scholar] [CrossRef]
- Singh, H.; Bhandari, R.; Kaur, I.P. Encapsulation of Rifampicin in a Solid Lipid Nanoparticulate System to Limit Its Degradation and Interaction With Isoniazid at Acidic pH. Int. J. Pharm. 2013, 446, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Development and in Vivo Evaluation of an Innovative "Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles" Formulation With Sustained Release and Enhanced Oral Bioavailability for Potential Hypertension Treatment in Pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Strettoi, E.; Gargini, C.; Novelli, E.; Sala, G.; Piano, I.; Gasco, P.; Ghidoni, R. Inhibition of Ceramide Biosynthesis Preserves Photoreceptor Structure and Function in a Mouse Model of Retinitis Pigmentosa. Proc. Natl. Acad. Sci. USA 2010, 107, 18706–18711. [Google Scholar] [CrossRef]
- Liu, S.; Han, X.; Liu, H.; Zhao, Y.; Li, H.; Rupenthal, I.; Lv, Z.; Chen, Y.; Yang, F.; Ping, Q.; et al. Incorporation of Ion Exchange Functionalized-Montmorillonite Into Solid Lipid Nanoparticles With Low Irritation Enhances Drug Bioavailability for Glaucoma Treatment. Drug Deliv. 2020, 27, 652–661. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid Lipid Nanoparticles as Promising Tool for Intraocular Tobramycin Delivery: Pharmacokinetic Studies on Rabbits. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2016, 109, 214–223. [Google Scholar] [CrossRef]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) Disorders: A Present and Future Prospective. Adv. Pharm. Bull. 2016, 6, 319–335. [Google Scholar] [CrossRef]
- Gasco, M.R.; Priano, L.; Zara, G.P. Chapter 10—Solid Lipid Nanoparticles and Microemulsions for Drug Delivery The CNS. Prog. Brain Res. 2009, 180, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, S.; Jiang, S.; Bai, W.; Liu, F.; Yuan, H.; Ji, J.; Luo, J.; Han, G.; Chen, L.; et al. Gadolinium-Loaded Solid Lipid Nanoparticles as a Tumor-Absorbable Contrast Agent for Early Diagnosis of Colorectal Tumors Using Magnetic Resonance Colonography. J. Biomed. Nanotechnol. 2016, 12, 1709–1723. [Google Scholar] [CrossRef]
- Amiri, M.; Jafari, S.; Kurd, M.; Mohamadpour, H.; Khayati, M.; Ghobadinezhad, F.; Tavallaei, O.; Derakhshankhah, H.; Sadegh Malvajerd, S.; Izadi, Z. Engineered Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as New Generations of Blood-Brain Barrier Transmitters. ACS Chem. Neurosci. 2021, 12, 4475–4490. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Shah, R.M.; Rajasekaran, D.; Ludford-Menting, M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells. Colloids Surf. B Biointerfaces 2016, 140, 204–212. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef] [PubMed]
- University, M. Clinical Assessment of Oxiconazole Nitrate Solid Lipid Nanoparticles Loaded Gel. NCT03823040. Available online: https://www.clinicaltrials.gov/ (accessed on 20 February 2023).
- Mistraletti, G.; Paroni, R.; Umbrello, M.; Moro Salihovic, B.; Coppola, S.; Froio, S.; Finati, E.; Gasco, P.; Savoca, A.; Manca, D.; et al. Different routes and formulations of melatonin in critically ill patients. A pharmacokinetic randomized study. Clin. Endocrinol. (Oxf.) 2019, 91, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Insights, F.B. Lipid Nanoparticles Market Research Report. Available online: https://www.fortunebusinessinsights.com/segmentation/lipid-nanoparticles-market-106960 (accessed on 19 February 2023).
- Xie, B.; Wang, H.; Wang, H.; Liu, Y.; Yu, M. Sorafenib Solid Lipid Nanoparticles and Preparation Method Thereof. CN105326812A, 28 October 2015. Available online: https://patents.google.com/patent/CN105326812A/en (accessed on 19 February 2023).
- Yu, L.; Tian, L.; Zhao, Y.; Yang, C.; Su, J.; Sun, W.; Du, Y.; Zhou, T. Folic Acid Targeting Silymarin Solid Lipid Nanosphere Preparation Method. CN105708803A, 4 December 2014. Available online: https://patents.google.com/patent/CN105708803A/en (accessed on 19 February 2023).
- Kim, J.; Kim, H.; Cha, J.; Jeb, A.; Quresh, O.S. Solid Lipid Nanoparticles Composition Comprising Docetaxel for Oral Formulation. KR101799539B1, 7 February 2014. Available online: https://patents.google.com/patent/KR101799539B1/en (accessed on 19 February 2023).
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Leiva, M.C.; Ortiz, R.; Contreras-Cáceres, R.; Perazzoli, G.; Mayevych, I.; López-Romero, J.M.; Sarabia, F.; Baeyens, J.M.; Melguizo, C.; Prados, J. Tripalmitin Nanoparticle Formulations Significantly Enhance Paclitaxel Antitumor Activity against Breast and Lung Cancer Cells in Vitro. Sci. Rep. 2017, 7, 13506. [Google Scholar] [CrossRef]
- Clemente, N.; Ferrara, B.; Gigliotti, C.L.; Boggio, E.; Capucchio, M.T.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 255. [Google Scholar] [CrossRef]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Kartal-Yandim, M.; Adan-Gokbulut, A.; Baran, Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit. Rev. Biotechnol. 2016, 36, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Cheng, S.; Wang, Y.; Wei, X.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef] [PubMed]
- Guney Eskiler, G.; Cecener, G.; Dikmen, G.; Egeli, U.; Tunca, B. Solid Lipid Nanoparticles: Reversal of Tamoxifen Resistance in Breast Cancer. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 120, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Peira, E.; Dianzani, C.; Gallarate, M.; Battaglia, L.; Gigliotti, C.L.; Boggio, E.; Dianzani, U.; Dosio, F. Development and Characterization of Solid Lipid Nanoparticles Loaded With a Highly Active Doxorubicin Derivative. Nanomaterials 2018, 8, 110. [Google Scholar] [CrossRef]
- Grillone, A.; Battaglini, M.; Moscato, S.; Mattii, L.; de Julián Fernández, C.; Scarpellini, A.; Giorgi, M.; Sinibaldi, E.; Ciofani, G. Nutlin-loaded Magnetic Solid Lipid Nanoparticles for Targeted Glioblastoma Treatment. Nanomedicine 2019, 14, 727–752. [Google Scholar] [CrossRef]
- Liu, T.I.; Lu, T.Y.; Chang, S.H.; Shen, M.Y.; Chiu, H.C. Dual Stimuli-Guided Lipid-Based Delivery System of Cancer Combination Therapy. J. Control. Release Off. J. Control. Release Soc. 2020, 318, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Ihsan, A.; Madni, A.; Bajwa, S.Z.; Shi, D.; Webster, T.J.; Khan, W.S. Solid Lipid Nanoparticles for Thermoresponsive Targeting: Evidence From Spectrophotometry, Electrochemical, and Cytotoxicity Studies. Int. J. Nanomed. 2017, 12, 8325–8336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving Breast Cancer Therapy Using Doxorubicin Loaded Solid Lipid Nanoparticles: Synthesis of a Novel Arginine-Glycine-Aspartic Tripeptide Conjugated, pH Sensitive Lipid and Evaluation of the Nanomedicine in Vitro and in Vivo. Biomed. Biomed. Pharm. 2019, 116, 109006. [Google Scholar] [CrossRef]
- Baek, J.S.; Na, Y.G.; Cho, C.W. Sustained Cytotoxicity of Wogonin on Breast Cancer Cells by Encapsulation in Solid Lipid Nanoparticles. Nanomaterials 2018, 8, 159. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [PubMed]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin Formulated in Solid Lipid Nanoparticles Has Enhanced Efficacy in Hodgkin’s Lymphoma in Mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Rahiminejad, A.; Dinarvand, R.; Johari, B.; Nodooshan, S.J.; Rashti, A.; Rismani, E.; Mahdaviani, P.; Saltanatpour, Z.; Rahiminejad, S.; Raigani, M.; et al. Preparation and Investigation of Indirubin-Loaded SLN Nanoparticles and Their Anti-Cancer Effects on Human Glioblastoma U87MG Cells. Cell Biol. Int. 2019, 43, 2–11. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, Z.; Xie, B.B.; Zhang, H.Y. Development and Evaluation of Topotecan Loaded Solid Lipid Nanoparticles: A Study in Cervical Cancer Cell Lines. J. Photochem. Photobiol. B Biol. 2016, 165, 182–188. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Cecener, G.; Egeli, U.; Tunca, B. Synthetically Lethal BMN 673 (Talazoparib) Loaded Solid Lipid Nanoparticles for BRCA1 Mutant Triple Negative Breast Cancer. Pharm. Res. 2018, 35, 218. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, Characterization and in Vitro Evaluation of Linalool-Loaded Solid Lipid Nanoparticles as Potent Tool in Cancer Therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Li, Y.; Yao, Q.; Ming, Y.; Li, Z.; Lu, L.; Shi, S. Ascorbyl Palmitate-Incorporated Paclitaxel-Loaded Composite Nanoparticles for Synergistic Anti-Tumoral Therapy. Drug Deliv. 2017, 24, 1230–1242. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil Shell-Enriched Solid Lipid Nanoparticles (SLN) for Effective Skin Carcinoma Treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef]
- Bhushan, S.; Kakkar, V.; Pal, H.C.; Mondhe, D.M.; Kaur, I.P. The Augmented Anticancer Potential of AP9-cd Loaded Solid Lipid Nanoparticles in Human Leukemia Molt-4 Cells and Experimental Tumor. Chem.-Biol. Interact. 2016, 244, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Brezaniova, I.; Hruby, M.; Kralova, J.; Kral, V.; Cernochova, Z.; Cernoch, P.; Slouf, M.; Kredatusova, J.; Stepanek, P. Temoporfin-loaded 1-tetradecanol-based Thermoresponsive Solid Lipid Nanoparticles for Photodynamic Therapy. J. Control. Release Off. J. Control. Release Soc. 2016, 241, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Cortesi, R.; Gallerani, E.; Drechsler, M.; Marvelli, L.; Mariani, P.; Carducci, F.; Gavioli, R.; Esposito, E.; Bergamini, P. Solid Lipid Nanoparticles for the Delivery of 1,3,5-triaza-7-phosphaadamantane (PTA) Platinum (II) Carboxylates. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Arranja, A.; Gouveia, L.F.; Gener, P.; Rafael, D.F.; Pereira, C.; Schwartz, S.; Videira, M.A. Self-assembly PEGylation Assists SLN-paclitaxel Delivery Inducing Cancer Cell Apoptosis Upon Internalization. Int. J. Pharm. 2016, 501, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Alajami, H.N.; Fouad, E.A.; Ashour, A.E.; Kumar, A.; Yassin, A.E.B. Celecoxib-Loaded Solid Lipid Nanoparticles for Colon Delivery: Formulation Optimization and In Vitro Assessment of Anti-Cancer Activity. Pharmaceutics 2022, 14, 131. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, W.; Zeng, M.; Yuan, J.; Shen, H.; Li, K.; Su, Z.; Liu, Z.; Wen, J.; Song, X.; et al. Anti-lung cancer effect of paclitaxel solid lipid nanoparticles delivery system with curcumin as co-loading partner in vitro and in vivo. Drug Deliv. 2022, 29, 1878–1891. [Google Scholar] [CrossRef]
- Beg, S.; Malik, A.K.; Ansari, M.J.; Malik, A.A.; Ali, A.M.A.; Theyab, A.; Algahtani, M.; Almalki, W.H.; Alharbi, K.S.; Alenezi, S.K.; et al. Systematic Development of Solid Lipid Nanoparticles of Abiraterone Acetate with Improved Oral Bioavailability and Anticancer Activity for Prostate Carcinoma Treatment. ACS Omega 2022, 7, 16968–16979. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Campos, J.R.; Martins-Lopes, P.; Silva, A.M. Surface-tailored anti-HER2/neu-solid Lipid Nanoparticles for Site-Specific Targeting MCF-7 and BT-474 Breast Cancer Cells. Eur. J. Pharm. Sci. 2019, 128, 27–35. [Google Scholar] [CrossRef]
- Shi, S.; Zhou, M.; Li, X.; Hu, M.; Li, C.; Li, M.; Sheng, F.; Li, Z.; Wu, G.; Luo, M.; et al. Synergistic Active Targeting of Dually Integrin αvβ3/CD44-targeted Nanoparticles to B16F10 Tumors Located at Different Sites of Mouse Bodies. J. Control. Release Off. J. Control. Release Soc. 2016, 235, 1–13. [Google Scholar] [CrossRef]
- Siddhartha, V.T.; Pindiprolu, S.K.S.S.; Chintamaneni, P.K.; Tummala, S.; Nandha Kumar, S. RAGE Receptor Targeted Bioconjuguate Lipid Nanoparticles of Diallyl Disulfide for Improved Apoptotic Activity in Triple Negative Breast Cancer: In Vitro Studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 387–397. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New Folate-Grafted Chitosan Derivative To Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Baek, J.S.; Cho, C.W. A Multifunctional Lipid Nanoparticle for Co-Delivery of Paclitaxel and Curcumin for Targeted Delivery and Enhanced Cytotoxicity in Multidrug Resistant Breast Cancer Cells. Oncotarget 2017, 8, 30369–30382. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, K.; Jain, S.K. Colorectal Cancer-Targeted Delivery of Oxaliplatin via Folic Acid-Grafted Solid Lipid Nanoparticles: Preparation, Optimization, and in Vitro Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeain, S.F.; Karimi, E.; Hashemi, M. Satureja khuzistanica Essential Oil-Loaded Solid Lipid Nanoparticles Modified With Chitosan-Folate: Evaluation of Encapsulation Efficiency, Cytotoxic and Pro-apoptotic Properties. Front. Chem. 2022, 10, 904973. [Google Scholar] [CrossRef]
- Soni, N.; Soni, N.; Pandey, H.; Maheshwari, R.; Kesharwani, P.; Tekade, R.K. Augmented Delivery of Gemcitabine in Lung Cancer Cells Exploring Mannose Anchored Solid Lipid Nanoparticles. J. Colloid Interface Sci. 2016, 481, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Singh, B.; Jain, A.; Nirbhavane, P.; Sharma, R.; Tyagi, R.K.; Kushwah, V.; Jain, S.; Katare, O.P. Fucose Decorated Solid-Lipid Nanocarriers Mediate Efficient Delivery of Methotrexate in Breast Cancer Therapeutics. Colloids Surf. B Biointerfaces 2016, 146, 114–126. [Google Scholar] [CrossRef]
- Shen, M.Y.; Liu, T.I.; Yu, T.W.; Kv, R.; Chiang, W.H.; Tsai, Y.C.; Chen, H.H.; Lin, S.C.; Chiu, H.C. Hierarchically Targetable Polysaccharide-Coated Solid Lipid Nanoparticles as an Oral Chemo/Thermotherapy Delivery System for Local Treatment of Colon Cancer. Biomaterials 2019, 197, 86–100. [Google Scholar] [CrossRef]
- Liu, B.; Han, L.; Liu, J.; Han, S.; Chen, Z.; Jiang, L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int. J. Nanomed. 2017, 12, 955–968. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lee, C.H. Dual Targeting of Solid Lipid Nanoparticles Grafted With 83-14 MAb and anti-EGF Receptor for Malignant Brain Tumor Therapy. Life Sci. 2016, 146, 222–231. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, I.H. Enhanced Delivery of Etoposide Across the Blood-Brain Barrier to Restrain Brain Tumor Growth Using Melanotransferrin Antibody—And Tamoxifen-Conjugated Solid Lipid Nanoparticles. J. Drug Target. 2016, 24, 645–654. [Google Scholar] [CrossRef]
- Kadari, A.; Pooja, D.; Gora, R.H.; Gudem, S.; Kolapalli, V.R.M.; Kulhari, H.; Sistla, R. Design of Multifunctional Peptide Collaborated and Docetaxel Loaded Lipid Nanoparticles for Antiglioma Therapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2018, 132, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Muntoni, E.; Chirio, D.; Peira, E.; Annovazzi, L.; Schiffer, D.; Mellai, M.; Riganti, C.; Salaroglio, I.C.; Lanotte, M.; et al. Solid Lipid Nanoparticles by Coacervation Loaded With a Methotrexate Prodrug: Preliminary Study for Glioma Treatment. Nanomedicine 2017, 12, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, K.; Jain, S.K. Oral Delivery of pH-responsive Alginate Microbeads Incorporating Folic Acid-Grafted Solid Lipid Nanoparticles Exhibits Enhanced Targeting Effect Against Colorectal Cancer: A Dual-Targeted Approach. Int. J. Biol. Macromol. 2020, 151, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, M.C.; Pereira, C.; Kreutzer, B.; Gouveia, L.F.; Silva-Lima, B.; Brito, A.M.; Videira, M. Evading P-glycoprotein Mediated-Efflux Chemoresistance Using Solid Lipid Nanoparticles. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2017, 110, 76–84. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Liu, B.; Chen, Z.; Li, C. Hyaluronic Acid Decorated Pluronic P85 Solid Lipid Nanoparticles as a Potential Carrier to Overcome Multidrug Resistance in Cervical and Breast Cancer. Biomed. Pharm. 2017, 86, 595–604. [Google Scholar] [CrossRef]
- Senthil Kumar, C.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted Delivery and Apoptosis Induction of Trans-Resveratrol-Ferulic Acid Loaded Chitosan Coated Folic Acid Conjugate Solid Lipid Nanoparticles in Colon Cancer Cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef]
- Baek, J.S.; Kim, J.H.; Park, J.S.; Cho, C.W. Modification of Paclitaxel-Loaded Solid Lipid Nanoparticles With 2-hydroxypropyl-β-cyclodextrin Enhances Absorption and Reduces Nephrotoxicity Associated With Intravenous Injection. Int. J. Nanomed. 2015, 10, 5397–5405. [Google Scholar] [CrossRef]
- Pawar, H.; Surapaneni, S.K.; Tikoo, K.; Singh, C.; Burman, R.; Gill, M.S.; Suresh, S. Folic Acid Functionalized Long-Circulating Co-Encapsulated Docetaxel and Curcumin Solid Lipid Nanoparticles: In Vitro Evaluation, Pharmacokinetic and Biodistribution in Rats. Drug Deliv. 2016, 23, 1453–1468. [Google Scholar] [CrossRef]
- Pooja, D.; Kulhari, H.; Kuncha, M.; Rachamalla, S.S.; Adams, D.J.; Bansal, V.; Sistla, R. Improving Efficacy, Oral Bioavailability, and Delivery of Paclitaxel Using Protein-Grafted Solid Lipid Nanoparticles. Mol. Pharm. 2016, 13, 3903–3912. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Cheng, S.J. Brain Targeted Delivery of Carmustine Using Solid Lipid Nanoparticles Modified With Tamoxifen and Lactoferrin for Antitumor Proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Ko, H.F. Targeting Delivery of Saquinavir to the Brain Using 83-14 Monoclonal Antibody-Grafted Solid Lipid Nanoparticles. Biomaterials 2013, 34, 4818–4830. [Google Scholar] [CrossRef] [PubMed]
- Shakib, Z.; Mahmoudi, A.; Moosavian, S.A.; Malaekeh-Nikouei, B. PEGylated solid lipid nanoparticles functionalized by aptamer for targeted delivery of docetaxel in mice bearing C26 tumor. Drug Dev. Ind. Pharm. 2022, 48, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Nunes, C.; Sousa, C.T.; Reis, S. Folate receptor-mediated delivery of mitoxantrone-loaded solid lipid nanoparticles to breast cancer cells. Biomed. Pharm. 2022, 154, 113525. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery Materials for siRNA Therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.E.; Smith, J.A.; Shamu, C.E.; Neumüller, R.A.; Perrimon, N. RNAi Screening Comes of Age: Improved Techniques and Complementary Approaches. Nat. Rev. Mol. Cell Biol. 2014, 15, 591–600. [Google Scholar] [CrossRef]
- Bathula, S.R.; Huang, L. Gene Therapy with Plasmid DNA. Burg. Med. Chem. Drug Discov. 2010, 457–499. [Google Scholar] [CrossRef]
- Moore, C.B.; Guthrie, E.H.; Huang, M.T.H.; Taxman, D.J. Short Hairpin RNA (shRNA): Design, Delivery, and Assessment of Gene Knockdown. Methods Mol. Biol. 2010, 629, 141–158. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kapoor, M.; Burgess, D.J.; Patil, S.D. Physicochemical Characterization Techniques for Lipid Based Delivery Systems for siRNA. Int. J. Pharm. 2012, 427, 35–57. [Google Scholar] [CrossRef]
- Pillai, R.S.; Bhattacharyya, S.N.; Filipowicz, W. Repression of Protein Synthesis by miRNAs: How Many Mechanisms? Trends Cell Biol. 2007, 17, 118–126. [Google Scholar] [CrossRef]
- Alvarez, J.P.; Pekker, I.; Goldshmidt, A.; Blum, E.; Amsellem, Z.; Eshed, Y. Endogenous and Synthetic microRNAs Stimulate Simultaneous, Efficient, and Localized Regulation of Multiple Targets in Diverse Species. Plant Cell 2006, 18, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Linsley, P.S. Recognizing and Avoiding siRNA Off-Target Effects for Target Identification and Therapeutic Application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking Down Barriers: Advances in siRNA Delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Jin, J.; Bae, K.H.; Yang, H.; Lee, S.J.; Kim, H.; Kim, Y.; Joo, K.M.; Seo, S.W.; Park, T.G.; Nam, D.H. In Vivo Specific Delivery of c-Met siRNA to Glioblastoma Using Cationic Solid Lipid Nanoparticles. Bioconjugate Chem. 2011, 22, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Botto, C.; Augello, G.; Amore, E.; Emma, M.R.; Azzolina, A.; Cavallaro, G.; Cervello, M.; Bondì, M.L. Cationic Solid Lipid Nanoparticles as Non Viral Vectors for the Inhibition of Hepatocellular Carcinoma Growth by RNA Interference. J. Biomed. Nanotechnol. 2018, 14, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kotmakçı, M.; Çetintaş, V.B.; Kantarcı, A.G. Preparation and Characterization of Lipid nanoparticle/pDNA Complexes for STAT3 Downregulation and Overcoming Chemotherapy Resistance in Lung Cancer Cells. Int. J. Pharm. 2017, 525, 101–111. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, T.; Ma, Y.; Li, R.; Pang, Y.; Mao, H.; Liu, P. Novel Nanocomplexes Targeting STAT3 Demonstrate Promising Anti-Ovarian Cancer Effects in vivo. Onco Targets 2020, 13, 5069–5082. [Google Scholar] [CrossRef]
- Büyükköroğlu, G.; Şenel, B.; Başaran, E.; Yenilmez, E.; Yazan, Y. Preparation and in Vitro Evaluation of Vaginal Formulations Including siRNA and Paclitaxel-Loaded SLNs for Cervical Cancer. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2016, 109, 174–183. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kim, E.; Park, D.E.; Shim, G.; Lee, S.; Kim, Y.B.; Kim, C.W.; Oh, Y.K. Cationic Solid Lipid Nanoparticles for Co-Delivery of Paclitaxel and siRNA. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2012, 80, 268–273. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Meng, Y.P.; Bo, L.S.; Ke, W.B. miR-542-3p Appended Sorafenib/All-trans Retinoic Acid (ATRA)-Loaded Lipid Nanoparticles to Enhance the Anticancer Efficacy in Gastric Cancers. Pharm. Res. 2017, 34, 2710–2719. [Google Scholar] [CrossRef]
- Büyükköroğlu, G.; Şenel, B.; Yenilmez, E. Vaginal Suppositories with siRNA and Paclitaxel-Incorporated Solid Lipid Nanoparticles for Cervical Cancer: Preparation and In Vitro Evaluation. Methods Mol. Biol. 2019, 1974, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, W.; Zhang, Y.; Zhang, B. Anti-tumor Efficiency of Paclitaxel and DNA When Co-Delivered by pH Responsive Ligand Modified Nanocarriers for Breast Cancer Treatment. Biomed. Pharm. 2016, 83, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Juang, V.; Chang, C.H.; Wang, C.S.; Wang, H.E.; Lo, Y.L. pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy. Small 2019, 15, e1903296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, T.; Yuan, M.; Wen, L.; Cheng, B.; Liu, N.; Huang, X.; Hong, Y.; Yuan, H.; Hu, F. MicroRNA-200c Delivered by Solid Lipid Nanoparticles Enhances the Effect of Paclitaxel on Breast Cancer Stem Cell. Int. J. Nanomed. 2016, 11, 6713–6725. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Han, L.; Deng, L.; Zhang, Y.; Shen, H.; Gong, T.; Zhang, Z.; Sun, X. Dual Drugs (microRNA-34a and Paclitaxel)-Loaded Functional Solid Lipid Nanoparticles for Synergistic Cancer Cell Suppression. J. Control. Release Off. J. Control. Release Soc. 2014, 194, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.J.; Zhong, Z.R.; Liu, J.; Zhang, Z.R.; Sun, X.; Gong, T. Solid Lipid Nanoparticles Loaded With anti-microRNA Oligonucleotides (AMOs) for Suppression of microRNA-21 Functions in Human Lung Cancer Cells. Pharm. Res. 2012, 29, 97–109. [Google Scholar] [CrossRef]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef]
- Qiu, H.; Min, Y.; Rodgers, Z.; Zhang, L.; Wang, A.Z. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 10. [Google Scholar] [CrossRef]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef]
- Chen, X.; Wong, S. (Eds.) Cancer Theranostics: An introduction. In Cancer Theranostics; Academic Press, Elsevier: Amesterdam, The Netherlands, 2014; pp. 3–8. [Google Scholar]
- Sudhakar, C.K.; Upadhyay, N.; Verma, A.; Jain, A.; Narayana Charyulu, R.; Jain, S. Nanomedicine and Tissue Engineering. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; Elsevier: Amesterdam, The Netherlands, 2015; pp. 1–19. [Google Scholar]
- Kulbacka, J.; Pucek, A.; Kotulska, M.; Dubińska-Magiera, M.; Rossowska, J.; Rols, M.P.; Wilk, K.A. Electroporation and Lipid Nanoparticles With Cyanine IR-780 and Flavonoids as Efficient Vectors to Enhanced Drug Delivery in Colon Cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 Dye Encapsulated in cRGD-Conjugated Solid Lipid Nanoparticles for NIR Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Lee, J.Y.; Lee, S.H.; Park, T.G.; Nam, Y.S. Optically Traceable Solid Lipid Nanoparticles Loaded With siRNA and Paclitaxel for Synergistic Chemotherapy With in Situ Imaging. Adv. Healthc. Mater. 2013, 2, 576–584. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).