Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials and Apparatus
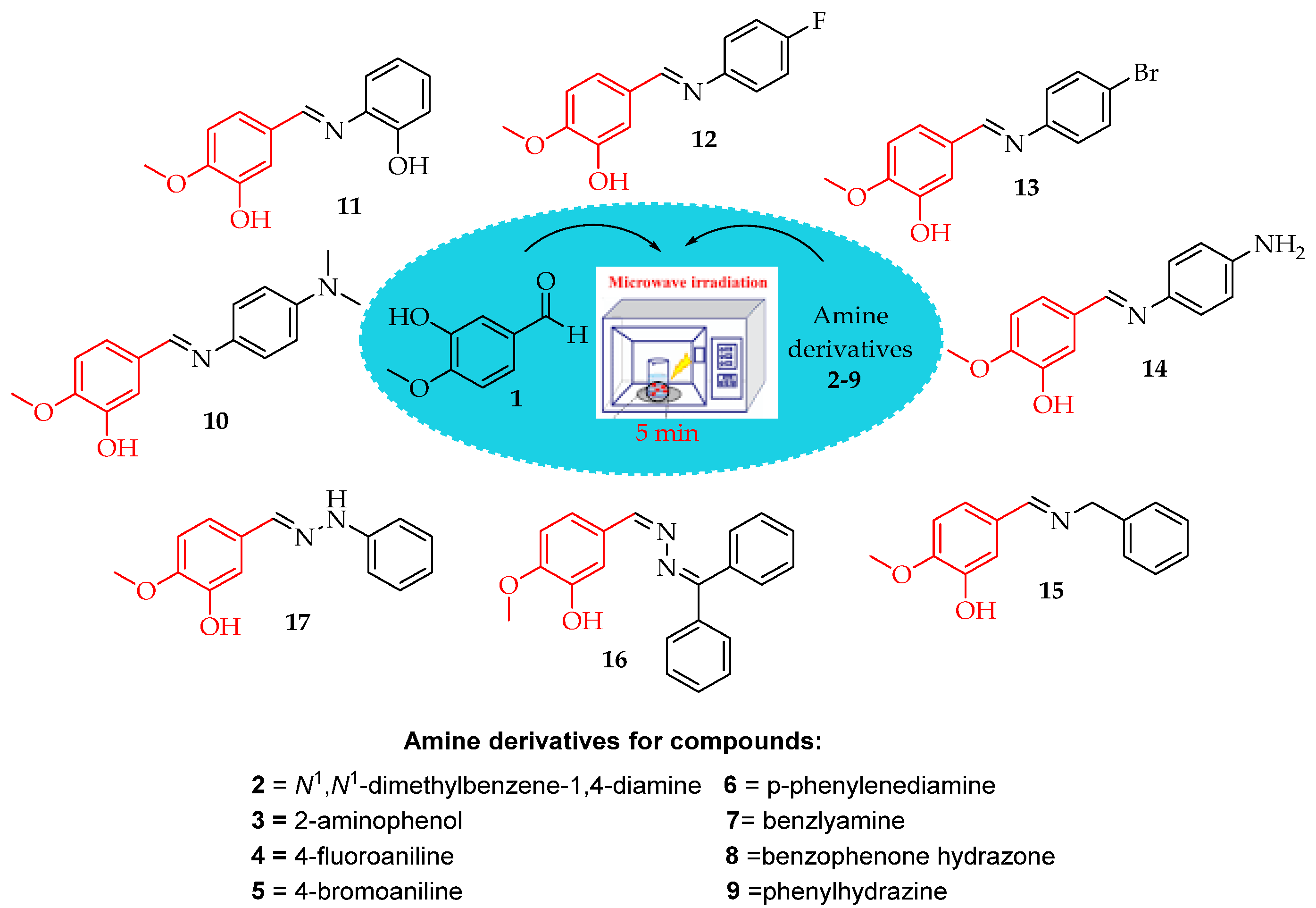
2.3. General Synthesis of Schiff Bases
2.4. Physical Properties and Spectral Data of Synthesized Compounds
2.4.1. (E)-5-(((4-(Dimethylamino)phenyl)imino)methyl)-2-methoxyphenol (10)
2.4.2. (E)-5-(((2-Hydroxyphenyl)imino)methyl)-2-methoxyphenol (11)
2.4.3. (E)-5-(((4-Fluorophenyl)imino)methyl)-2-methoxyphenol (12)
2.4.4. (E)-5-(((4-Bromophenyl)imino)methyl)-2-methoxyphenol (13)
2.4.5. (E/Z)-5-(((4-Aminophenyl)imino)methyl)-2-methoxyphenol (14)
2.4.6. (E)-5-((Benzylimino)methyl)-2-methoxyphenol (15)
2.4.7. (E)-5-(((Diphenylmethylene)hydrazineylidene)methyl)-2-methoxyphenol (16)
2.4.8. (E)-2-Methoxy-5-((2-phenylhydrazineylidene)methyl)phenol (17)
2.5. Reducing Ability Assays
2.6. Radical Scavenging Capacities
2.7. AChE and BChE Inhibition Assay
2.8. Carbonic Anhydrase Purification and Inhibition Studies
3. Results
3.1. Chemistry
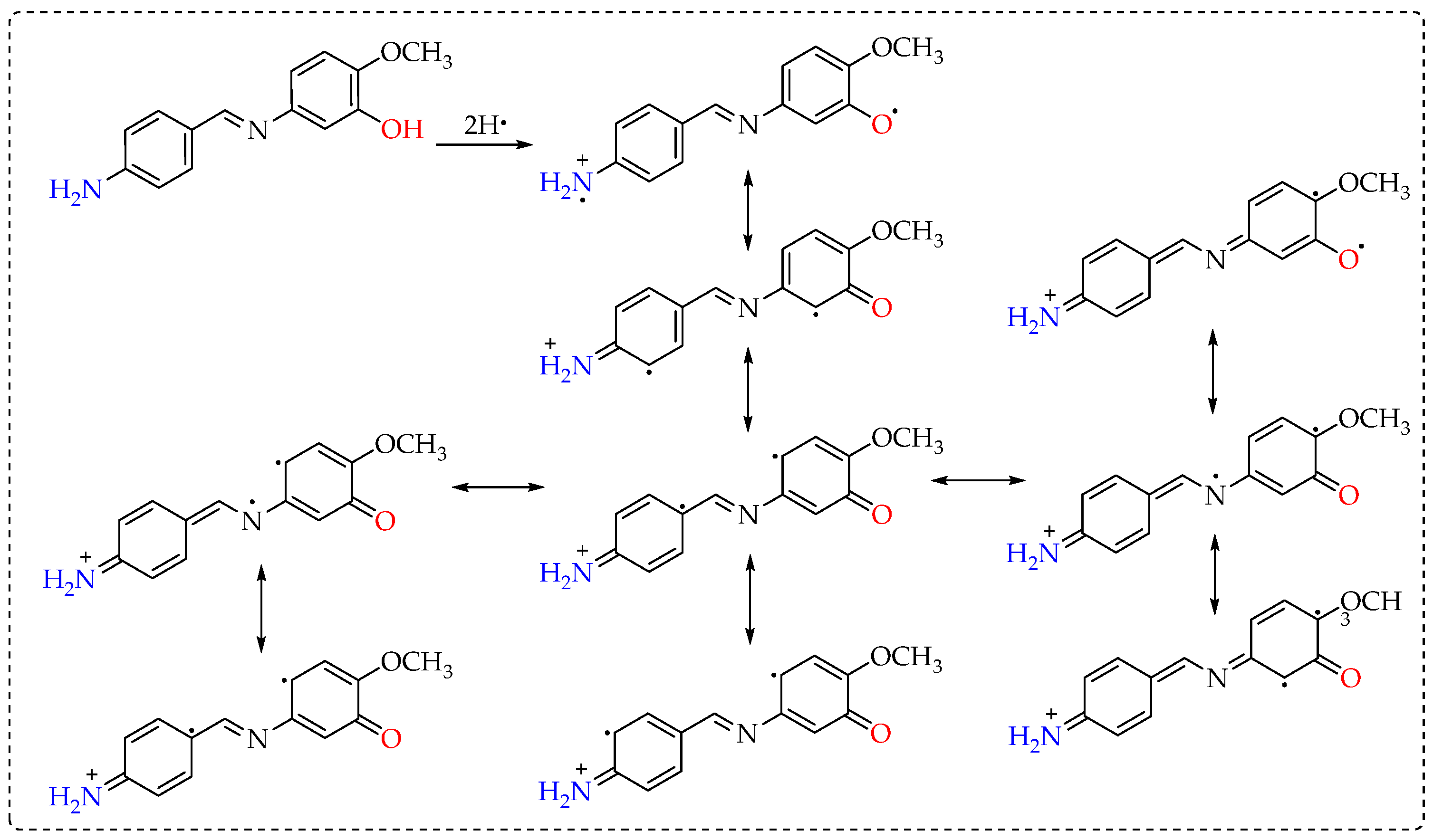
3.2. Antioxidant Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulcin, I. Antioxidants and antioxidant methods-An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, A.; Ikram, M.; Rehman, S.; Shah, M.; Nabi, H.U.; Ahuchaogu, A.A. In vitro antioxidant properties of novel Schiff base complexes. Asian J. Chem. Sci. 2017, 2, 32244. [Google Scholar] [CrossRef]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gulcin, I.; Cekic, S.D.; Guclu, K.; Ozyurek, M.; Celik, S.E.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species. Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Polat Kose, L.; Bingol, Z.; Kaya, R.; Goren, A.C.; Akincioglu, H.; Durmaz, L.; Koksal, E.; Alwasel, S.; Gulcin, I. Anticholinergic and antioxidant activities of avocado (Folium perseae) leaves—Phytochemical content by LC-MS/MS analysis. Int. J. Food Prop. 2020, 23, 878–893. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Ozmen Guler, G.; Cakmak, Y.S.; Duran, A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Padmini, T.; Ponnuvel, K. Synthesis, characterization and antioxidant activities of Schiff bases are of cholesterol. J. Saudi Chem. Soc. 2017, 21, 322–328. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of L-Adrenaline: An activity-structure insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Karagecili, H.; Yılmaz, M.A.; Erturk, A.; Kızıltas, H.; Guven, L.; Alwasel, S.H.; Gulcin, I. Comprehensive metabolite profiling of Berdav propolis using LC-MS/MS: Determination of antioxidant, anticholinergic, antiglaucoma, and antidiabetic effects. Molecules 2023, 28, 1739. [Google Scholar] [CrossRef]
- Ceyhan, G.; Celik, C.; Urus, S.; Demirtas, I.; Elmastas, M.; Tumer, M. Antioxidant, electrochemical, thermal, antimicrobial and alkane oxidation properties of tridentate Schiff base ligands and their metal complexes. Spectrochim. Acta A 2011, 81, 184–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, B.; Chen, Z.; Pan, Y.; Wang, H.; Liang, H.; Yi, X. Synthesis and antioxidant activities of novel 4-Schiff base-7-benzyloxy-coumarin derivatives. Bioorg. Med. Chem. Lett. 2021, 21, 6811–6815. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Mutlu, M.; Bingol, Z.; Uc, E.M.; Koksal, E.; Goren, A.C.; Alwasel, S.H.; Gulcin, I. Comprehensive metabolite profiling of cinnamon (Cinnamomum zeylanicum) leaf oil using LC-HR/MS, GC/MS, and GC-FID: Determination of antiglaucoma, antioxidant, anticholinergic, and antidiabetic profiles. Life 2023, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Oztaskin, N.; Goksu, S.; Demir, Y.; Maras, A.; Gulcin, I. Synthesis of novel bromophenol including diaryl Methanes-Determination of their inhibition effects on carbonic anhydrase and acetylcholinesterase. Molecules 2022, 27, 7426. [Google Scholar] [CrossRef] [PubMed]
- Kiziltas, H.; Goren, A.C.; Alwasel, S.; Gulcin, I. Comprehensive metabolic profiling of Acantholimon caryophyllaceum using LC-HRMS and evaluation of antioxidant activities, enzyme inhibition properties and molecular docking studies. S. Afr. J. Bot. 2022, 151, 743–751. [Google Scholar] [CrossRef]
- Pedrood, K.; Sherefati, M.; Taslimi, P.; Mohammadi-Khanaposhtani, M.; Asgari, M.S.; Hosseini, S.; Rastegar, H.; Larijani, B.; Mahdavi, M.; Taslimi, P.; et al. Design, synthesis, characterization, enzymatic inhibition evaluations, and docking study of novel quinazolinone derivatives. Int. J. Biol. Macromol. 2021, 170, 1–12. [Google Scholar] [CrossRef]
- Kaya, Y.; Ercag, A.; Zorlu, Y.; Demir, Y.; Gulcin, I. New Pd(II) complexes of the bisthiocarbohydrazones derived from isatin and disubstituted salicylaldehydes: Synthesis, characterization, crystal structures and inhibitory properties against some metabolic enzymes. J. Biol. Inorg. Chem. 2022, 27, 271–281. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Mason, P. Imaging free radicals in organelles, cells, tissue, and in vivo with immunospin trapping. Red. Biol. 2016, 8, 422–429. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncola, J.; Cronin, M.T.D.; Mazura, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mahmudov, I.; Demir, Y.; Sert, Y.; Abdullayev, Y.; Sujayev, E.; Alwasel, S.H.; Gulcin, I. Synthesis and inhibition profiles of N-benzyl- and N-allyl aniline derivatives against carbonic anhydrase and acetylcholinesterase—A molecular docking study. Arab. J. Chem. 2022, 15, 103645. [Google Scholar] [CrossRef]
- Nada, H.; Elkamhawy, A.; Abdellattif, M.H.; Angeli, A.; Lee, C.H.; Supuran, C.T.; Lee, K. 4-Anilinoquinazoline-based benzenesulfonamides as nanomolar inhibitors of carbonic anhydrase isoforms I, II, IX, and XII: Design, synthesis, in-vitro, and in-silico biological studies. J. Enzyme Inhib. Med. Chem. 2022, 37, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.; Barut Celepci, D.; Taslimi, P.; Yigit, B.; Cetinkaya, B.; Ozdemir, I.; Aygun, M.; Gulcin, I. Selenourea and thiourea derivatives of chiral and achiral enetetramines: Synthesis, characterization and enzyme inhibitory properties. Bioorg. Chem. 2022, 120, 105566. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- Annan, D.A.; Maishi, N.; Soga, T.; Dawood, R.; Li, C.; Kikuchi, H.; Hojo, T.; Morimoto, M.; Kitamura, T.; Alam, M.T.; et al. Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun. Signal. 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrıshnan, M.; Sureshkumar, P.; Kanagarajan, V.; Thanusu, J. New environmentally-friendly solvent-free synthesis of imines using calcium oxide under microwave irradiation. Res. Chem. Intermed. 2007, 33, 541–548. [Google Scholar] [CrossRef]
- Das, S.; Das, V.K.; Saikia, L.; Thakur, A.J. Environment-friendly and solvent-free synthesis of symmetrical bis-imines under microwave irradiation. Green Chem. Lett. Rev. 2012, 5, 457–474. [Google Scholar] [CrossRef]
- Karaca, E.O. Synthesis and structure elucidation of new Schiff base compounds. Politek. Derg. 2018, 21, 245–249. [Google Scholar]
- Altıner, S. Synthesis and Spectroscopic Analysis of Imine Compounds from Aminothiophene Compounds. Master’s Thesis, Hitit University Institute of Science and Technology, Çorum, Turkey, 2015. [Google Scholar]
- Shanty, A.A.; Mohanan, P.V. Heterocyclic Schiff bases as non toxic antioxidants: Solvent effect, structure activity relationship and mechanism of action. Spectrochim. Acta A Mol. Biomol. 2018, 192, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Yuldasheva, N.; Acikyildiz, N.; Akyuz, M.; Yabo-Dambagi, L.; Aydin, T.; Cakir, A.; Kazaz, C. The synthesis of Schiff bases and new secondary amine derivatives of p-vanillin and evaluation of their neuroprotective, antidiabetic, antidepressant and antioxidant potentials. J. Mol. Struct. 2022, 1270, 133883. [Google Scholar] [CrossRef]
- Bentoumi, H.; Tliba, S.; K’tir, H.; Chohra, D.; Aouf, Z.; Adjerou, Y.; Amira, A.; Zerrouki, R.; Ibrahim-Ouali, M.; Aouf, N.; et al. Experimental synthesis, biological evaluation, theoretical investigations of some novel benzoxazolinone based Schiff under eco-environmental conditions as potential antioxidant agents. J. Mol. Struct. 2022, 1270, 133986. [Google Scholar] [CrossRef]
- Chourasiya, S.S.; Kathuria, D.; Wani, A.A.; Prasad, V.; Bharatam, P.V. Azines: Synthesis, structure, electronic structure and their applications. Org. Biomol. Chem. 2019, 17, 8486–8521. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, G.; Pandey, A.; Mayar, P.; Thamaraichelvan, A. Microwave synthesis, crystal structure and spectroscopic investigations of 2-{[(2E)-(2-chlorobenzylidene) hydrazine] carbonyl} benzenesulfonamide and 2-({[(2E)-2-[4-(dimethylamino) benzylidene] hydrazine} carbonyl) benzenesulfonamide. Ind. J. Chem. 2014, 53, 200–207. [Google Scholar]
- Elgemeie, G.; Abd Elaziz, H. Microwave-assisted synthesis of azines and their condensed derivatives. Curr. Microw. Chem. 2015, 2, 90–128. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Zimmermann-Franco, D.C.; Esteves, B.; Lacerda, L.M.; de Oliveira Souza, I.; dos Santos, J.A.; de Castro CamposPinto, N.; Scio, E.; da Silva, A.D.; Macedo, G.C. In vitro and in vivo anti-inflammatory properties of imine resveratrol analogues. Bioorg. Med. Chem. 2018, 26, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Aytac, S. Re-synthesis of Schiff base compounds by an environmental method. J. Instit. Sci. Technol. 2021, 11, 2979–2991. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction prepared from glucoseamine. J. Acad. Nutr. Diet. 1986, 44, 307–314. [Google Scholar] [CrossRef]
- Gulcin, I. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J. Enzyme Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fuids and modifed version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bora, R.E.; Bilgicli, H.G.; Uc, E.M.; Alagöz, M.A.; Zengin, M.; Gulcin, I. Synthesis, characterization, evaluation of metabolic enzyme inhibitors and in silico studies of thymol based 2-amino thiol and sulfonic acid compounds. Chem. Biol. Interact. 2022, 366, 110134. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1967, 242, 4221–4229. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, D.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Tables of Spectral Data for Structure Determination of Organic Compounds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kiziltas, H.; Goren, A.C.; Alwasel, S.; Gulcin, I. Sahlep (Dactylorhiza osmanica): Phytochemical analyses by LC-HRMS, molecular docking, antioxidant activity and enzyme inhibition profiles. Molecules 2022, 27, 6907. [Google Scholar] [CrossRef]
- Eruygur, N.; Atas, M.; Tekin, M.; Taslimi, P.; Koçyigit, U.M.; Gulcin, I. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Topal, M. Secondary metabolites of ethanol extracts of Pinus sylvestris cones from eastern Anatolia and their antioxidant, cholinesterase and alpha-glucosidase activities. Rec. Nat. Prod. 2020, 14, 129–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Tang, K.; Rao, Z.; Chen, J. Effects of different aging methods on the phenolic compounds and antioxidant activity of red wine. Fermentation 2022, 8, 592. [Google Scholar] [CrossRef]
- Kanaan, N.C.; Peterson, A.L.; Pun, M.; Holck, P.S.; Starling, J.; Basyal, B.; Freeman, T.M.; Gehner, J.R.; Keyes, L.; Levin, D.R.; et al. Prophylactic acetaminophen or ibuprofen results in equivalent acute mountain sickness incidence at high altitude: A prospective randomized trial. Wild. Environ. Med. 2017, 28, 72–78. [Google Scholar] [CrossRef]
- Meier, D.; Collet, T.H.; Locatelli, I.; Cornuz, J.; Kayser, B.; Simel, D.L.; Sartori, C. Does this patient have acute mountain sickness? The rational clinical examination systematic review. JAMA 2017, 318, 1810–1819. [Google Scholar] [CrossRef]
- Kumar, A.; Siwach, K.; Rom, T.; Kumar, R.; Angeli, A.; Kumar Paul, A.K.; Supuran, C.T.; Pawan, K.; Sharma, P.K. Tail-approach based design and synthesis of arylthiazolylhydrazono-1,2,3-triazoles incorporating sulfanilamide and metanilamide as human carbonic anhydrase I, II, IV and IX inhibitors. Bioorg. Chem. 2022, 123, 105764. [Google Scholar] [CrossRef] [PubMed]
- Burmaoglu, S.; Yılmaz, A.O.; Polat, M.F.; Kaya, R.; Gulcin, I.; Algul, O. Synthesis and biological evaluation of novel tris-chalcones as potent carbonic anhydrase, acetylcholine esterase, butyrylcholinesterase and α-glycosidase inhibitors. Bioorg. Chem. 2019, 85, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Buldurun, K.; Turan, N.; Bursal, E.; Mantarcı, A.; Turkan, F.; Taslimi, P.; Gulcin, I. Synthesis, spectroscopic properties, crystal structures, antioxidant activities and enzyme inhibition determination of Co(II) and Fe(II) complexes of Schiff base. Res. Chem. Intermed. 2020, 46, 283–297. [Google Scholar] [CrossRef]
- Shuang, H.; Yuan, W. Synthesis, structural characterization and catalytic oxidation property of Schiff base copper(II) complexes. J. Chil. Chem. Soc. 2014, 59, 4. [Google Scholar]
- Yang, Z.Y.; Yang, R.D.; Li, F.S.; Yu, K.B. Crystal structure and antitumor activity of some rare earth metal complexes with Schiff base. Polyhedron 2000, 19, 2599. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 63. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264. [Google Scholar] [CrossRef]
- Shahid, M.; Salim, M.; Khalid, M.; Tahir, M.N.; Khan, M.U.; Braga, A.A.C. Synthetic, XRD, non-covalent interactions and solvent dependent nonlinear optical studies of sulfadiazine-ortho-vanillin Schiff base: (E)-4-((2-hydroxy-3-methoxy-benzylidene) amino)-N-(pyrimidin-2-yl)benzene-sulfonamide. J. Mol. Struct. 2018, 1161, 66–75. [Google Scholar] [CrossRef]
- Danyi, W.E.I.; Ning, L.I.; Gui, L.U.; Kemin, Y.A.O. Synthesis, catalytic and biological activity of novel dinuclear copper complex with Schiff base. Sci. China, Ser. B: Chem. 2006, 49, 225–229. [Google Scholar]
- Alves dos Santos, J.; Lima, R.M.; Pereira, T.V.; Resende do Carmo, A.M.; Raposo, N.R.B.; David da Silva, A. Antioxidant activity of thio-Schiff bases. Lett. Drug Des. Discov. 2013, 10, 557–560. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, L.; Tan, W.; Li, Q.; Guo, Z. The antioxidant and antibacterial activities of thepyridine-4-aldehyde Schiff bases grafted chloracetylchitosan oligosaccharide derivatives. Starch-Stärke 2023, 75, 2100268. [Google Scholar] [CrossRef]
- Revanna, R.H.; Raghavendra, K.P.; Babulal, D. Synthesis, characterization and antioxidant activity of new β-benzylselenated Schiff bases and their palladium complexes. J. Coord. Chem. 2022, 75, 1273–1288. [Google Scholar]
- Koksal, E.; Bursal, E.; Gulcin, I.; Korkmaz, M.; Caglayan, C.; Goren, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by LC-MS/MS. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Yigit, B.; Yigit, M.; Taslimi, P.; Gok, Y.; Gulcin, I. Schiff bases and their amines: Synthesis, and discovery of carbonic anhydrase and acetylcholinesterase enzymes inhibitors. Arch. Pharm. 2018, 351, e1800146. [Google Scholar] [CrossRef] [PubMed]

| Antioxidants | Fe3+ Reducing | Cu2+ Reducing | Fe3+-TPTZ Reducing | |||
|---|---|---|---|---|---|---|
| λ700 * | r2 | λ450 * | r2 | λ593 * | r2 | |
| BHA | 2.448 ± 0.021 | 0.9984 | 2.268 ± 0.011 | 0.9956 | 2.156 ± 0.005 | 0.9565 |
| BHT | 1.994 ± 0.033 | 0.9932 | 2.149 ± 0.019 | 0.9971 | 2.037 ± 0.027 | 0.9782 |
| Trolox | 1.570 ± 0.016 | 0.9915 | 1.174 ± 0.027 | 0.9738 | 2.051 ± 0.028 | 0.9931 |
| α-Tocopherol | 1.446 ± 0.009 | 0.9665 | 1.923 ± 0.032 | 0.9972 | 1.763 ± 0.026 | 0.9828 |
| 1 | 0.244 ± 0.005 | 0.9632 | 1.005 ± 0.009 | 0.9673 | 0.728 ± 0.020 | 0.9561 |
| 10 | 2.827 ± 0.016 | 0.9912 | 2.176 ± 0.044 | 0.9931 | 2.076 ± 0.004 | 0.9897 |
| 11 | 2.591 ± 0.010 | 0.9822 | 2.332 ± 0.018 | 0.9997 | 2.183 ± 0.016 | 0.9921 |
| 12 | 0.294 ± 0.008 | 0.9742 | 0.951 ± 0.010 | 0.9795 | 0.806 ± 0.003 | 0.9569 |
| 13 | 0.198 ± 0.004 | 0.9611 | 0.875 ± 0.020 | 0.9833 | 0.701 ± 0.003 | 0.9648 |
| 14 | 2.741 ± 0.026 | 0.9989 | 1.970 ± 0.018 | 0.9876 | 2.074 ± 0.017 | 0.9917 |
| 15 | 0.363 ± 0.021 | 0.9748 | 0.803 ± 0.013 | 0.9763 | 0.646 ± 0.034 | 0.9523 |
| 16 | 0.311 ± 0.018 | 0.9839 | 1.266 ± 0.029 | 0.9932 | 1.069 ± 0.057 | 0.9897 |
| 17 | 2.391 ± 0.037 | 0.9959 | 2.026 ± 0.016 | 0.9610 | 2.121 ± 0.014 | 0.9815 |
| Antioxidants | DPPH• Scavenging | ABTS•+ Scavenging | ||
|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | |
| BHA | 11.55 | 0.9690 | 4.47 | 0.9702 |
| BHT | 13.32 | 0.9734 | 4.95 | 0.9633 |
| Trolox | 12.15 | 0.9645 | 4.84 | 0.9769 |
| α-Tocopherol | 10.04 | 0.9760 | 9.49 | 0.9889 |
| 1 | 86.62 | 0.9989 | 12.15 | 0.9549 |
| 10 | 16.90 | 0.9687 | 14.43 | 0.9767 |
| 11 | 12.15 | 0.9636 | 4.38 | 0.9701 |
| 12 | 57.75 | 0.9998 | 27.72 | 0.9982 |
| 13 | 87.72 | 0.9922 | 34.65 | 0.9901 |
| 14 | 13.86 | 0.9629 | 5.33 | 0.9498 |
| 15 | 99.01 | 0.9980 | 23.89 | 0.9979 |
| 16 | 30.13 | 0.9723 | 11.74 | 0.9838 |
| 17 | 14.74 | 0.9874 | 4.30 | 0.9711 |
| Compounds | IC50 (nM) | Selectivity Index (hCA I/hCA II) | |||
|---|---|---|---|---|---|
| hCA I | r2 | hCA II | r2 | ||
| 1 | 138.60 | 0.9820 | 173.25 | 0.9770 | 0.340 |
| 10 | 66.00 | 0.9965 | 130.75 | 0.9691 | 0.372 |
| 11 | 86.62 | 0.9810 | 88.22 | 0.9766 | 0.730 |
| 12 | 61.87 | 0.9823 | 99.00 | 0.9794 | 0.424 |
| 13 | 63.00 | 0.9872 | 77.00 | 0.9930 | 0.483 |
| 14 | 53.30 | 0.9801 | 69.30 | 0.9883 | 0.306 |
| 15 | 99.00 | 0.9840 | 138.60 | 0.9732 | 0.521 |
| 16 | 69.30 | 0.9839 | 111.77 | 0.9738 | 0.326 |
| 17 | 96.25 | 0.9933 | 115.50 | 0.9689 | 0.247 |
| Acetazolamide | 46.20 | 0.9952 | 24.75 | 0.9878 | 1.167 |
| Compounds | IC50 (nM) | Selectivity Index (AChE/BChE) | |||
|---|---|---|---|---|---|
| AChE | r2 | BChE | r2 | ||
| 1 | 57.75 | 0.9823 | 53.31 | 0.9946 | 1.083 |
| 10 | 26.65 | 0.9947 | 23.89 | 26.653 | 1.115 |
| 11 | 22.35 | 0.9911 | 34.65 | 22.354 | 0.645 |
| 12 | 23.10 | 0.9868 | 33.00 | 23.100 | 0.700 |
| 13 | 17.76 | 0.9720 | 28.87 | 17.769 | 0.615 |
| 14 | 34.65 | 0.9980 | 38.50 | 34.650 | 0.900 |
| 15 | 25.66 | 0.9991 | 36.47 | 25.666 | 0.704 |
| 16 | 16.11 | 0.9801 | 25.66 | 16.116 | 0.627 |
| 17 | 23.89 | 0.9986 | 19.80 | 23.896 | 1.206 |
| Tacrine | 46.20 | 0.9952 | 24.75 | 0.9878 | 1.664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aytac, S.; Gundogdu, O.; Bingol, Z.; Gulcin, İ. Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics 2023, 15, 779. https://doi.org/10.3390/pharmaceutics15030779
Aytac S, Gundogdu O, Bingol Z, Gulcin İ. Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics. 2023; 15(3):779. https://doi.org/10.3390/pharmaceutics15030779
Chicago/Turabian StyleAytac, Sertan, Ozlem Gundogdu, Zeynebe Bingol, and İlhami Gulcin. 2023. "Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties" Pharmaceutics 15, no. 3: 779. https://doi.org/10.3390/pharmaceutics15030779
APA StyleAytac, S., Gundogdu, O., Bingol, Z., & Gulcin, İ. (2023). Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics, 15(3), 779. https://doi.org/10.3390/pharmaceutics15030779







