Preclinical PET Imaging and Toxicity Study of a 68Ga-Functionalized Polymeric Cardiac Blood Pool Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
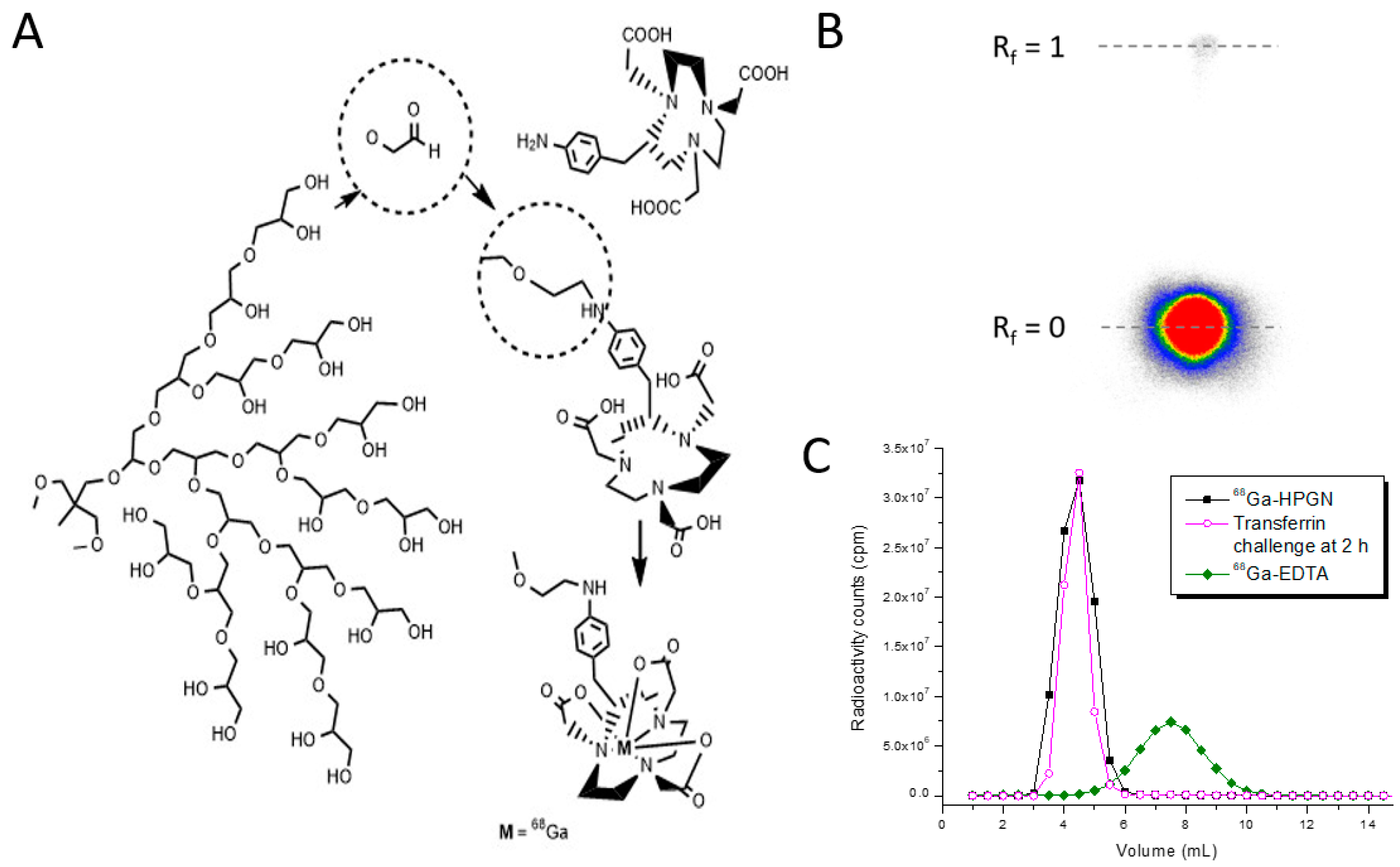
2.2. Synthesis of NOTA-HPG and Radiolabeling
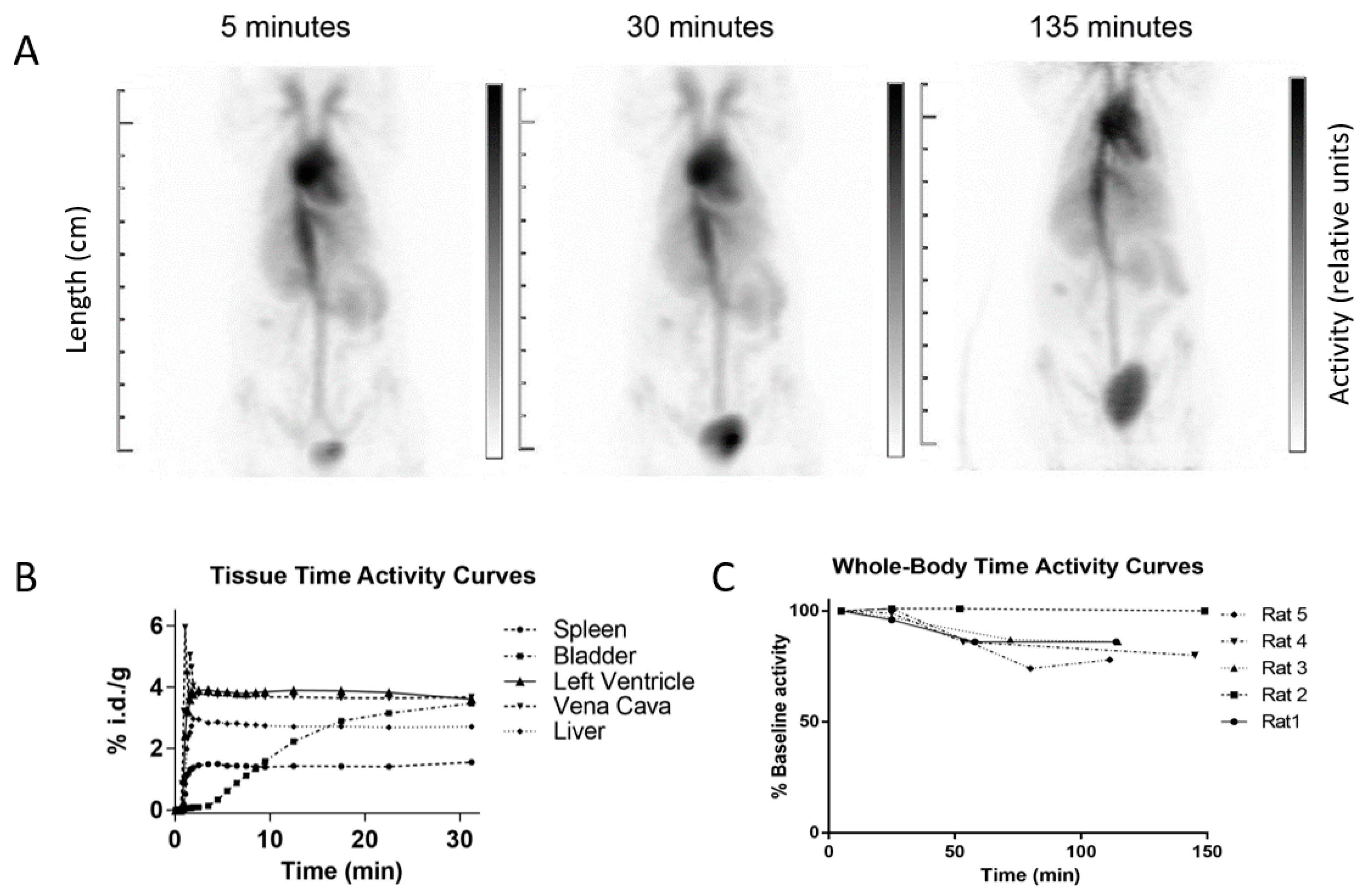
2.3. Cardiac Blood Pool Imaging
2.4. Image Reconstruction and Analysis
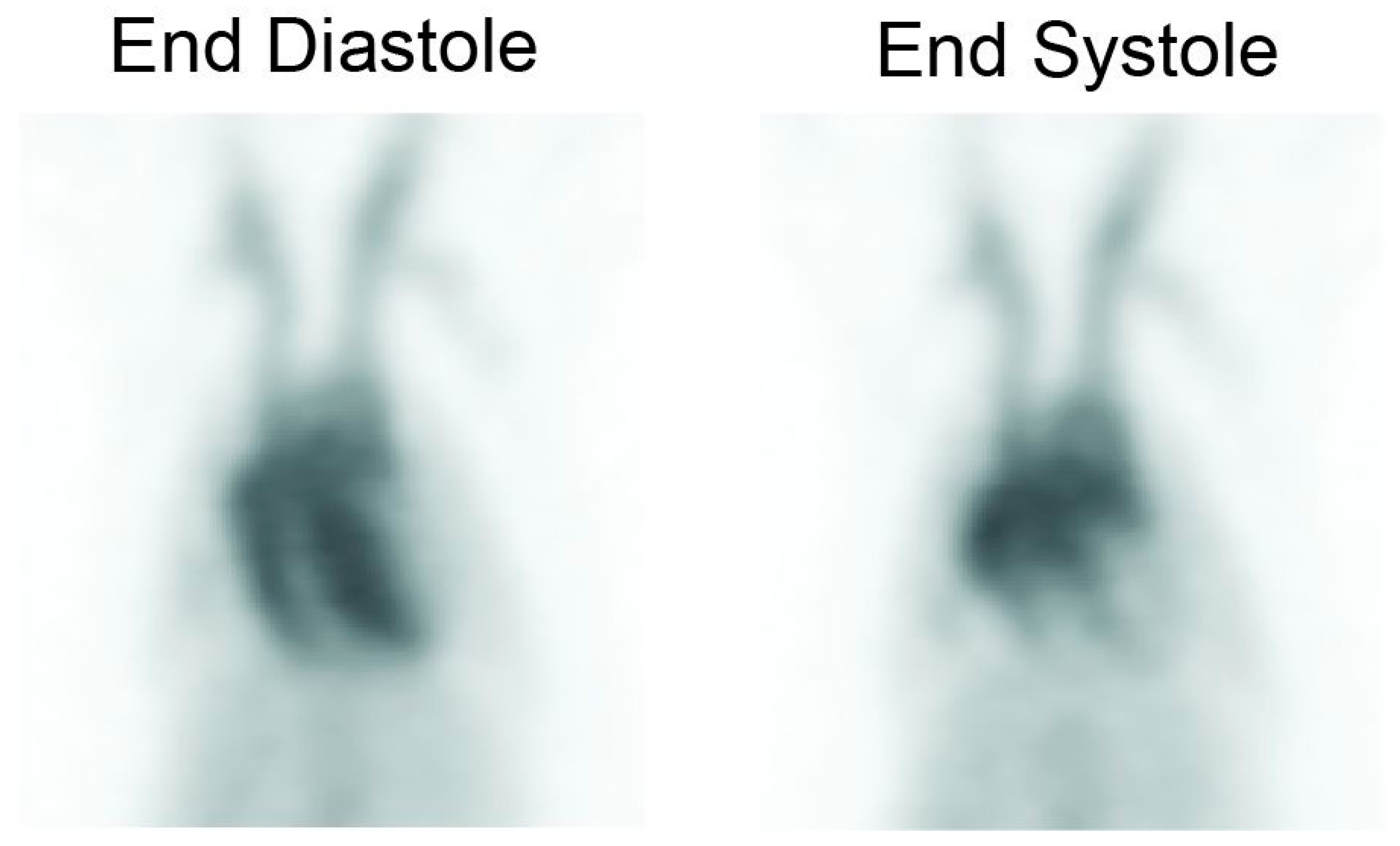
2.5. Gating and Ejection Fraction Calculations
2.6. Radiation Dosimetry
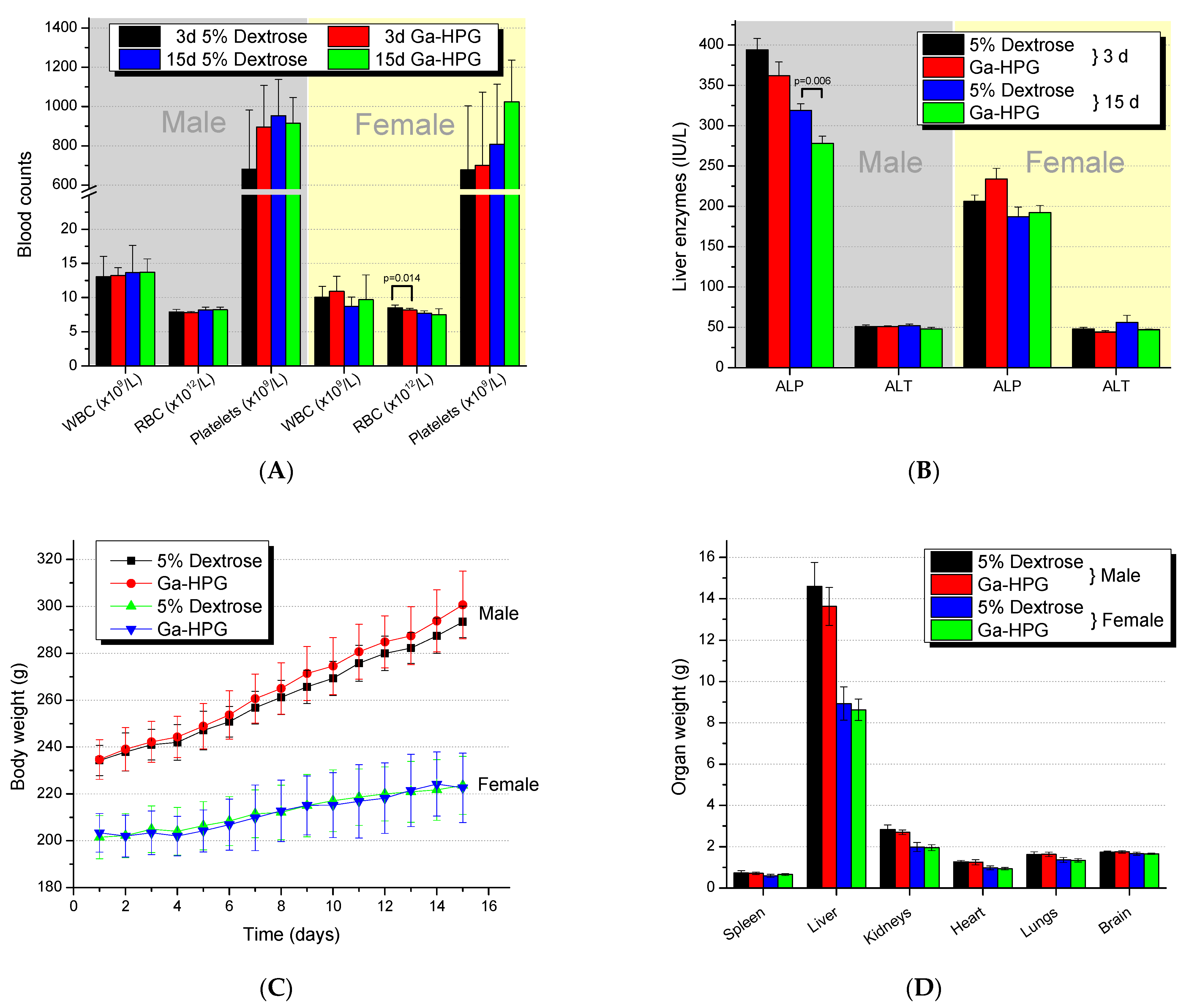
2.7. Comprehensive Toxicology Studies
2.7.1. Hematology
2.7.2. Blood Chemistry
2.7.3. Necropsy/Gross Pathology
2.7.4. Histopathology
2.7.5. Statistical Methods
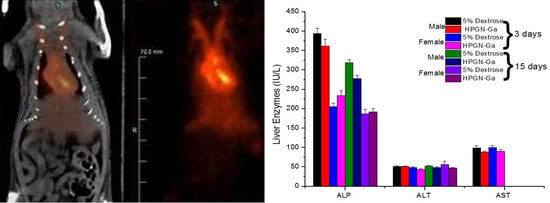
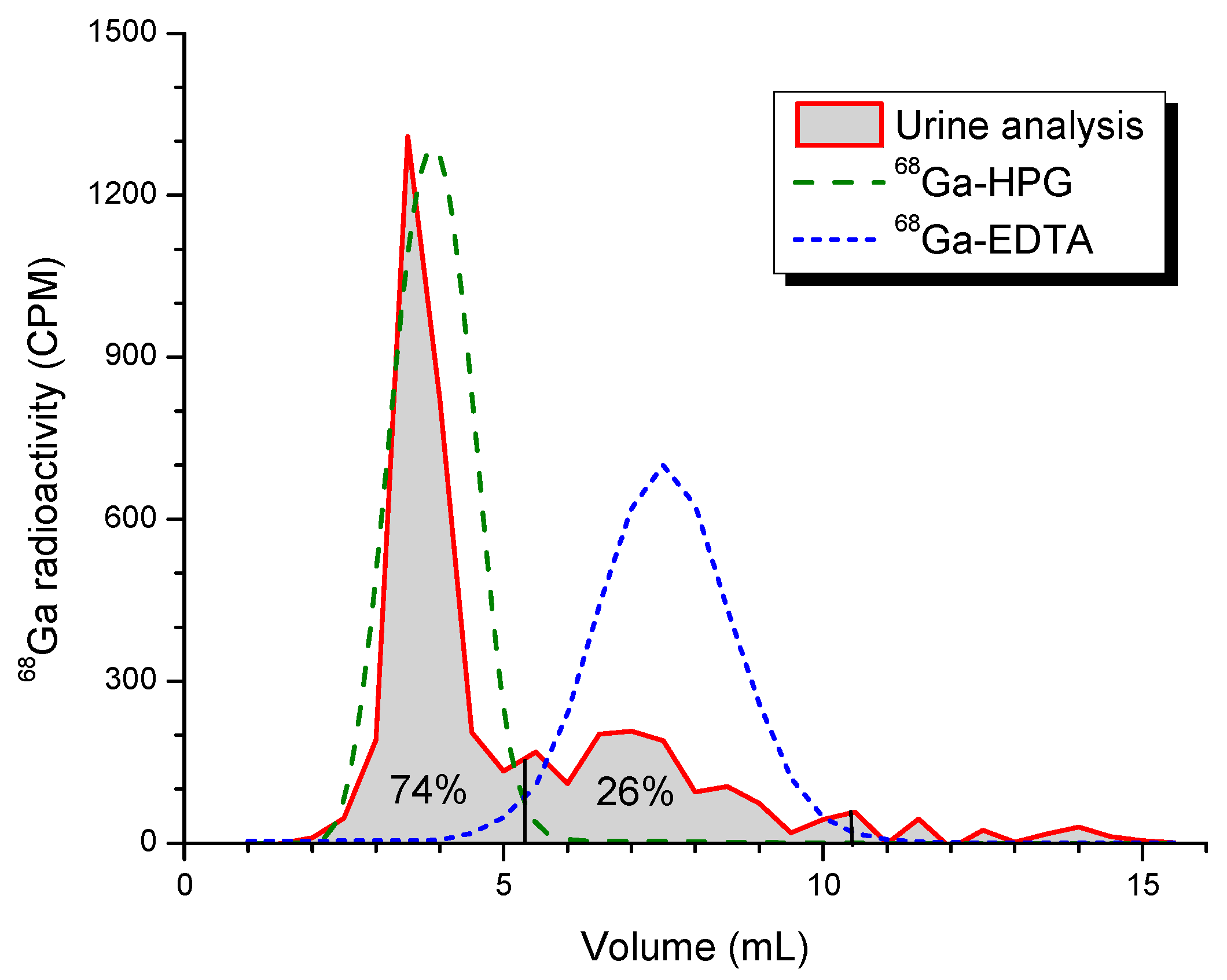
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartholoma, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and gallium derived radiopharmaceuticals: Comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef]
- Dilworth, J.R.; Parrott, S.J. The biomedical chemistry of technetium and rhenium. Chem. Soc. Rev. 1998, 27, 43–55. [Google Scholar] [CrossRef]
- Partington, S.L.; Valente, A.M.; Bruyere, J., Jr.; Rosica, D.; Shafer, K.M.; Landzberg, M.J.; Taqueti, V.R.; Blankstein, R.; Skali, H.; Kwatra, N.; et al. Reducing radiation dose from myocardial perfusion imaging in subjects with complex congenital heart disease. J. Nucl. Cardiol. 2021, 28, 1395–1408. [Google Scholar] [CrossRef]
- Al Badarin, F.J.; Spertus, J.A.; Bateman, T.M.; Patel, K.K.; Burgett, E.V.; Kennedy, K.F.; Thompson, R.C. Drivers of radiation dose reduction with myocardial perfusion imaging: A large health system experience. J. Nucl. Cardiol. 2020, 27, 785–794. [Google Scholar] [CrossRef]
- Khalil, M.M.; Tremoleda, J.L.; Bayomy, T.B.; Gsell, W. Molecular SPECT Imaging: An Overview. Int. J. Mol. Imaging 2011, 2011, 796025. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S. Production of a broad palette of positron emitting radioisotopes using a low-energy cyclotron: Towards a new success story in cancer imaging? Appl. Radiat. Isot. 2021, 176, 109860. [Google Scholar] [CrossRef]
- Dash, A.; Chakravarty, R. Radionuclide generators: The prospect of availing PET radiotracers to meet current clinical needs and future research demands. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 30–66. [Google Scholar]
- Benard, F.; Buckley, K.R.; Ruth, T.J.; Zeisler, S.K.; Klug, J.; Hanemaayer, V.; Vuckovic, M.; Hou, X.; Celler, A.; Appiah, J.P.; et al. Implementation of Multi-Curie Production of (99m)Tc by Conventional Medical Cyclotrons. J. Nucl. Med. 2014, 55, 1017–1022. [Google Scholar] [CrossRef]
- Kidane, B.; Zabel, P.L.; Gupta, V.; Whiston, C.; Wright, F.; Brackstone, M. Cysteine rhenium colloid: A novel radiocolloid for identifying sentinel lymph nodes in breast cancer surgery. Clin. Breast. Cancer 2015, 15, e41–e45. [Google Scholar] [CrossRef]
- Wei, L.; Bensimon, C.; Lockwood, J.; Yan, X.; Fernando, P.; Wells, R.G.; Duan, Y.; Chen, Y.X.; Redshaw, J.R.; Covitz, P.A.; et al. Synthesis and characterization of 123I-CMICE-013: A potential SPECT myocardial perfusion imaging agent. Bioorganic Med. Chem. 2013, 21, 2903–2911. [Google Scholar] [CrossRef]
- Lekx, K.S.; deKemp, R.A.; Beanlands, R.S.; Wisenberg, G.; Wells, R.G.; Stodilka, R.Z.; Lortie, M.; Klein, R.; Zabel, P.; Kovacs, M.S.; et al. Quantification of regional myocardial blood flow in a canine model of stunned and infarcted myocardium: Comparison of rubidium-82 positron emission tomography with microspheres. Nucl. Med. Commun. 2010, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lekx, K.S.; de Kemp, R.A.; Beanlands, R.S.; Wisenberg, G.; Wells, G.; Stodilka, R.Z.; Lortie, M.; Klein, R.; Zabel, P.; Kovacs, M.S.; et al. 3D versus 2D dynamic 82Rb myocardial blood flow imaging in a canine model of stunned and infarcted myocardium. Nucl. Med. Commun. 2010, 31, 75–81. [Google Scholar] [CrossRef]
- Williams, K.A. Measurement of ventricular function with scintigraphic techniques: Part I-imaging hardware, radiopharmaceuticals, and first-pass radionuclide angiography. J. Nucl. Cardiol. 2005, 12, 86–95. [Google Scholar] [CrossRef]
- Williams, K.A. A historical perspective on measurement of ventricular function with scintigraphic techniques: Part II--Ventricular function with gated techniques for blood pool and perfusion imaging. J. Nucl. Cardiol. 2005, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Winzelberg, G.G.; Castronovo, F.P.; Callahan, R.J.; McKusick, K.A.; Strauss, H.W. 111In oxine labeled red cells for detection of simulated lower gastrointestinal bleeding in an animal model. Radiology 1980, 135, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.C.; Chervu, L.R. Radionuclide-labeled red blood cells: Current status and future prospects. Semin. Nucl. Med. 1984, 14, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, B.A.; Weinreb, J.C.; Megibow, A.J.; Sanger, J.J.; Lubat, E.; Kanamuller, H.; Noz, M.E.; Bosniak, M.A. Definitive diagnosis of hepatic hemangiomas: MR imaging versus Tc-99m-labeled red blood cell SPECT. Radiology 1990, 176, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D. Recent Breakthrough in (68)Ga-Radiopharmaceuticals Cold Kits for Convenient PET Radiopharmacy. Bioconjugate Chem. 2021, 32, 430–447. [Google Scholar] [CrossRef]
- Knesaurek, K.; Machac, J.; Krynyckyi, B.R.; Almeida, O.D. Comparison of 2-dimensional and 3-dimensional 82Rb myocardial perfusion PET imaging. J. Nucl. Med. 2003, 44, 1350–1356. [Google Scholar]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F.; Spreckelmeyer, S. Good practices for (68)Ga radiopharmaceutical production. EJNMMI Radiopharm. Chem. 2022, 7, 27. [Google Scholar] [CrossRef]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Hambye, A.S.; Vandermeiren, R.; Vervaet, A.; Vandevivere, J. Failure to label red blood cells adequately in daily practice using an in vivo method: Methodological and clinical considerations. Eur. J. Nucl. Med. 1995, 22, 61–67. [Google Scholar] [CrossRef]
- Wangler, B.; Quandt, G.; Iovkova, L.; Schirrmacher, E.; Wangler, C.; Boening, G.; Hacker, M.; Schmoeckel, M.; Jurkschat, K.; Bartenstein, P.; et al. Kit-like 18F-labeling of proteins: Synthesis of 4-(di-tert-butyl [18F]fluorosilyl)benzenethiol (Si [18F]FA-SH) labeled rat serum albumin for blood pool imaging with PET. Bioconjugate Chem. 2009, 20, 317–321. [Google Scholar] [CrossRef]
- Basuli, F.; Li, C.; Xu, B.; Williams, M.; Wong, K.; Coble, V.L.; Vasalatiy, O.; Seidel, J.; Green, M.V.; Griffiths, G.L.; et al. Synthesis of fluorine-18 radio-labeled serum albumins for PET blood pool imaging. Nucl. Med. Biol. 2015, 42, 219–225. [Google Scholar] [CrossRef]
- Wängler, C.; Wängler, B.; Lehner, S.; Elsner, A.; Todica, A.; Bartenstein, P.; Hacker, M.; Schirrmacher, R. A universally applicable 68Ga-labeling technique for proteins. J. Nucl. Med. 2011, 52, 586–591. [Google Scholar] [CrossRef]
- Feng, L.; Fang, J.; Zeng, X.; Liu, H.; Zhang, J.; Huang, L.; Guo, Z.; Zhuang, R.; Zhang, X. (68)Ga-Labeled Maleimide for Blood Pool and Lymph PET Imaging through Covalent Bonding to Serum Albumin In Vivo. ACS Omega 2022, 7, 28597–28604. [Google Scholar] [CrossRef]
- Zhang, J.; Lang, L.; Zhu, Z.; Li, F.; Niu, G.; Chen, X. Clinical Translation of an Albumin-Binding PET Radiotracer 68Ga-NEB. J. Nucl. Med. 2015, 56, 1609–1614. [Google Scholar] [CrossRef]
- Niu, G.; Lang, L.; Kiesewetter, D.O.; Ma, Y.; Sun, Z.; Guo, N.; Guo, J.; Wu, C.; Chen, X. In Vivo Labeling of Serum Albumin for PET. J. Nucl. Med. 2014, 55, 1150–1156. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible-material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Gnanamani, M.; Ganguli, M.; Ghosh, T.; Brooks, D.E.; Maiti, S.; Kizhakkedathu, J.N. Blood compatibility of novel water soluble hyperbranched polyglycerol-based multivalent cationic polymers and their interaction with DNA. Biomaterials 2006, 27, 5377–5390. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Janzen, J.; Kizhakkedathu, J.N.; Devine, D.V.; Brooks, D.E. Hydrophobically derivatized hyperbranched polyglycerol as a human serum albumin substitute. Biomaterials 2008, 29, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, V.; Rodriguez-Rodriguez, C.; Hamilton, J.L.; Shenoi, R.A.; Schaffer, P.; Sossi, V.; Kizhakkedathu, J.N.; Saatchi, K.; Häfeli, U.O. Quantitative SPECT Imaging and Biodistribution Point to Molecular Weight Independent Tumor Uptake For Some Long-Circulating Polymer Nanocarriers. RSC Adv. 2018, 8, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Muliawan, E.B.; Hatzikiriakos, S.G.; Brooks, D.E. Synthesis, characterization, and viscoelastic properties of high molecular weight hyperbranched polyglycerols. Macromolecules 2006, 39, 7708–7717. [Google Scholar] [CrossRef]
- Saatchi, K.; Gelder, N.; Gershkovich, P.; Sivak, O.; Wasan, K.M.; Kainthan, R.K.; Brooks, D.E.; Häfeli, U.O. Long-circulating nontoxic cardiac blood pool imaging agent based on hyperbranched polyglycerols. Int. J. Pharm. 2012, 422, 418–427. [Google Scholar] [CrossRef]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Thomas, S.R.; Stubbs, J.B.; Stabin, M.G.; Hays, M.T.; Koral, K.F.; Robertson, J.S.; Howell, R.W.; Wessels, B.W.; Fisher, D.R.; et al. MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J. Nucl. Med. 1999, 40, 37S–61S. [Google Scholar]
- Vanhove, C.; Lahoutte, T.; Defrise, M.; Bossuyt, A.; Franken, P.R. Reproducibility of left ventricular volume and ejection fraction measurements in rat using pinhole gated SPECT. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 211–220. [Google Scholar] [CrossRef]
- Croteau, E.; Benard, F.; Cadorette, J.; Gauthier, M.E.; Aliaga, A.; Bentourkia, M.; Lecomte, R. Quantitative gated PET for the assessment of left ventricular function in small animals. J. Nucl. Med. 2003, 44, 1655–1661. [Google Scholar]
- Stegger, L.; Heijman, E.; Schafers, K.P.; Nicolay, K.; Schafers, M.A.; Strijkers, G.J. Quantification of left ventricular volumes and ejection fraction in mice using PET, compared with MRI. J. Nucl. Med. 2009, 50, 132–138. [Google Scholar] [CrossRef]
- Hurford, W.E.; Crosby, G.; Strauss, H.W.; Jones, R.; Lowenstein, E. Ventricular performance and glucose uptake in rats during chronic hypobaric hypoxia. J. Nucl. Med. 1990, 31, 1344–1351. [Google Scholar]
- Redfors, B.; Shao, Y.; Omerovic, E. Influence of anesthetic agent, depth of anesthesia and body temperature on cardiovascular functional parameters in the rat. Lab. Anim. 2014, 48, 6–14. [Google Scholar] [CrossRef]
- Cicone, F.; Viertl, D.; Quintela Pousa, A.M.; Denoël, T.; Gnesin, S.; Scopinaro, F.; Vozenin, M.-C.; Prior, J.O. Cardiac Radionuclide Imaging in Rodents: A Review of Methods, Results, and Factors at Play. Front. Med. 2017, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M.G. Radiopharmaceuticals for nuclear cardiology: Radiation dosimetry, uncertainties, and risk. J. Nucl. Med. 2008, 49, 1555–1563. [Google Scholar] [CrossRef]
- Corbett, R.H. Ethical issues, justification, referral criteria for budget limited and high-dose procedures. Radiat. Prot. Dosim. 2008, 130, 125–132. [Google Scholar] [CrossRef]
- Dam, H.Q.; Brandon, D.C.; Grantham, V.V.; Hilson, A.J.; Howarth, D.M.; Maurer, A.H.; Stabin, M.G.; Tulchinsky, M.; Ziessman, H.A.; Zuckier, L.S. The SNMMI Procedure Standard/EANM Practice Guideline for Gastrointestinal Bleeding Scintigraphy 2.0. J. Nucl. Med. Technol. 2014, 42, 308. [Google Scholar] [CrossRef]
- Kalender, W.A. Dose in x-ray computed tomography. Phys. Med. Biol. 2014, 59, R129–R150. [Google Scholar] [CrossRef]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Thrall, J.H.; Freitas, J.E.; Swanson, D.; Rogers, W.L.; Clare, J.M.; Brown, M.L.; Pitt, B. Clinical comparison of cardiac blood pool visualization with technetium-99m red blood cells labeled in vivo and with technetium-99m human serum albumin. J. Nucl. Med. 1978, 19, 796–803. [Google Scholar]
- Boros, E.; Ferreira, C.L.; Cawthray, J.F.; Price, E.W.; Patrick, B.O.; Wester, D.W.; Adam, M.J.; Orvig, C. Acyclic chelate with ideal properties for 68Ga PET imaging agent elaboration. J. Am. Chem. Soc. 2010, 132, 15726–15733. [Google Scholar] [CrossRef]
- Hoffend, J.; Mier, W.; Schuhmacher, J.; Schmidt, K.; Dimitrakopoulou-Strauss, A.; Strauss, L.G.; Eisenhut, M.; Kinscherf, R.; Haberkorn, U. Gallium-68-DOTA-albumin as a PET blood-pool marker: Experimental evaluation in vivo. Nucl. Med. Biol. 2005, 32, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.J.; Lee, H.S.; Metcalfe, M.J.; Norton, M.Y.; Evans, N.T.; Walton, S. Assessment of left ventricular regional wall motion with blood pool tomography: Comparison of 11CO PET with 99Tcm SPECT. Nucl. Med. Commun. 1994, 15, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Hofman, H.A.; Knaapen, P.; Boellaard, R.; Bondarenko, O.; Gotte, M.J.; van Dockum, W.G.; Visser, C.A.; van Rossum, A.C.; Lammertsma, A.A.; Visser, F.C. Measurement of left ventricular volumes and function with O-15-labeled carbon monoxide gated positron emission tomography: Comparison with magnetic resonance imaging. J. Nucl. Cardiol. 2005, 12, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Cardinal Health. Ga-68 Generator. Available online: https://www.cardinalhealth.com/en/product-solutions/pharmaceutical-products/nuclear-medicine/radiopharmaceuticals/pet/ga-68.html (accessed on 17 December 2022).
- RadioMedix. Gallium-68 Generator. Available online: https://radiomedix.com/products/gallium-68-generator (accessed on 17 December 2022).
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed]
| Tracer | T1/2 (min) | β+ (%) | β+ Max. Energy (keV) | β+ Mean Energy (keV) | β+ Max Range (mm) | β+ Mean Range (mm) | Production Routes |
|---|---|---|---|---|---|---|---|
| 13N | 9.97 | 99.8 | 1198.5 | 491.8 | 5.5 | 1.2 | 16O(p,α) |
| 15O | 2.03 | 99.9 | 1732.0 | 735.3 | 8.4 | 3.0 | 15N(p,n) |
| 18F | 110 | 97 | 633.5 | 249.8 | 2.4 | 0.6 | 18O(p,n) |
| 38K | 7.6 | 99.3 | 2724.4 * | 1211.4 | 12.1 | 5.5 | 40Ar(p,3n) |
| 82Rb | 1.27 | 95.2 | 3378 | 1479 | 17.0 | 7.1 | 82Sr/82Rb generator |
| 94mTc | 52 | 72 | 2439 | 1072 | 11.1 | 4.8 | 94Mo(p,n) |
| 68Ga | 68 | 89 | 1899.1 | 836.0 | 9.2 | 3.5 | 68Ge/68Ga generator |
| Current Methods | New Method | |||
|---|---|---|---|---|
| In Vivo | Modified In Vivo/In Vitro | In Vitro 1 | In Vitro 2 | Long-Circulating Polymer |
| Reconstitute Ultratag® kit with saline | Reconstitute Ultratag® kit with saline | Withdraw 3 mL of blood and place in empty vial | Withdraw 3 mL of blood | Add 68Ga solution to kit |
| Inject measured dose into patient’s vein | Inject measured dose into patient’s vein | Add freshly made tin and EDTA solution and saline | Add blood to Ultratag® kit | Incubate at RT for 2–5 min |
| Wait 20–30 min | Wait 20 min | Centrifuge | Incubate for 5 min | Perform quality control, ~10 min |
| Inject 99mTc pertechnetate solution into patient | Place i.v. catheter into patient and draw 5 mL blood | Wait 2 min | Add contents of syringes 1 and 2 from the kit to the vial; mix by inversion | If labeling > 95%, inject into patient within the next 1 h |
| Image | Add 99mTc pertechnetate through stopcock and cover with syringe shield | Collect RBCs—not plasma—and add to vial with 99mTc pertechnetate | Place in lead shield and add 99mTc pertechnetate, mix | Image |
| Wait 5 min | Wait 5 min | Wait 20 min | ||
| Reinject into patient | Perform quality control, ~10 min | Perform quality control, ~10 min | ||
| Image | If labeling > 95%, inject into patient within the next 2 h | If labeling > 95%, inject into patient within 30 min | ||
| Image | Image | |||
| ≪80% * | 90% * | >95% * | >95% * | >95% |
| 68Ga-HPG (mSv/148 MBq) | 99mTc-RBCs (mSv/740 MBq *) | ||||
|---|---|---|---|---|---|
| Target Organ | Average | S.D. | Min | Max | |
| Adrenals | 1.9 | 0.1 | 1.7 | 2.0 | 4.0 |
| Brain | 1.5 | 0.2 | 1.2 | 1.7 | 2.0 |
| Breasts | 1.4 | 0.1 | 1.3 | 1.6 | 2.4 |
| Gallbladder wall | 2.0 | 0.1 | 1.9 | 2.2 | 3.8 |
| LLI wall | 1.7 | 0.1 | 1.5 | 1.8 | 3.4 |
| Small intestine | 1.8 | 0.2 | 1.6 | 1.9 | 3.3 |
| Stomach wall | 1.7 | 0.1 | 1.5 | 1.9 | 3.4 |
| ULI wall | 1.8 | 0.2 | 1.6 | 1.9 | 3.3 |
| Heart wall | 8.0 | 0.5 | 7.3 | 8.5 | 11.8 |
| Kidneys | 6.3 | 0.6 | 5.8 | 7.1 | 5.1 |
| Liver | 6.1 | 1.0 | 4.8 | 7.1 | 4.8 |
| Lungs | 1.7 | 0.1 | 1.5 | 1.8 | 8.9 |
| Muscle | 1.4 | 0.3 | 1.1 | 1.8 | 2.7 |
| Ovaries | 1.7 | 0.1 | 1.5 | 1.9 | 3.5 |
| Pancreas | 1.9 | 0.1 | 1.7 | 2.0 | 4.2 |
| Red marrow | 1.3 | 0.1 | 1.2 | 1.5 | 3.0 |
| Osteogenic cells | 2.1 | 0.2 | 1.9 | 2.4 | 4.9 |
| Skin | 1.3 | 0.1 | 1.1 | 1.4 | 1.7 |
| Spleen | 1.7 | 0.1 | 1.5 | 1.8 | 8.9 |
| Testes | 1.4 | 0.1 | 1.3 | 1.6 | 2.4 |
| Thymus | 1.7 | 0.1 | 1.6 | 1.9 | 4.2 |
| Thyroid | 1.5 | 0.1 | 1.3 | 1.7 | 2.7 |
| Urinary bladder wall | 1.6 | 0.1 | 1.5 | 1.8 | 11.1 |
| Uterus | 1.7 | 0.1 | 1.5 | 1.9 | 4.0 |
| Effective Dose | 1.8 | 0.1 | 1.6 | 1.9 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saatchi, K.; Bénard, F.; Hundal, N.; Grimes, J.; Shcherbinin, S.; Pourghiasian, M.; Brooks, D.E.; Celler, A.; Häfeli, U.O. Preclinical PET Imaging and Toxicity Study of a 68Ga-Functionalized Polymeric Cardiac Blood Pool Agent. Pharmaceutics 2023, 15, 767. https://doi.org/10.3390/pharmaceutics15030767
Saatchi K, Bénard F, Hundal N, Grimes J, Shcherbinin S, Pourghiasian M, Brooks DE, Celler A, Häfeli UO. Preclinical PET Imaging and Toxicity Study of a 68Ga-Functionalized Polymeric Cardiac Blood Pool Agent. Pharmaceutics. 2023; 15(3):767. https://doi.org/10.3390/pharmaceutics15030767
Chicago/Turabian StyleSaatchi, Katayoun, François Bénard, Navjit Hundal, Joshua Grimes, Sergey Shcherbinin, Maral Pourghiasian, Donald E. Brooks, Anna Celler, and Urs O. Häfeli. 2023. "Preclinical PET Imaging and Toxicity Study of a 68Ga-Functionalized Polymeric Cardiac Blood Pool Agent" Pharmaceutics 15, no. 3: 767. https://doi.org/10.3390/pharmaceutics15030767
APA StyleSaatchi, K., Bénard, F., Hundal, N., Grimes, J., Shcherbinin, S., Pourghiasian, M., Brooks, D. E., Celler, A., & Häfeli, U. O. (2023). Preclinical PET Imaging and Toxicity Study of a 68Ga-Functionalized Polymeric Cardiac Blood Pool Agent. Pharmaceutics, 15(3), 767. https://doi.org/10.3390/pharmaceutics15030767