Implementation of Systematic Bioanalysis of Antibody–Drug Conjugates for Preclinical Pharmacokinetic Study of Ado-Trastuzumab Emtansine (T-DM1) in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. ELISA Methods for DM1-Conjugated Trastuzumab and Total Trastuzumab
2.4. LC–MS/MS Analysis for DM1
2.5. Analysis of Immunogenicity
2.6. Statistics
3. Results
3.1. Validation of ELISA for DM1-Conjugated Trastuzumab and Total Trastuzumab
3.2. Validation of LC–MS/MS for DM1
3.3. Characterization of Assay for ADC
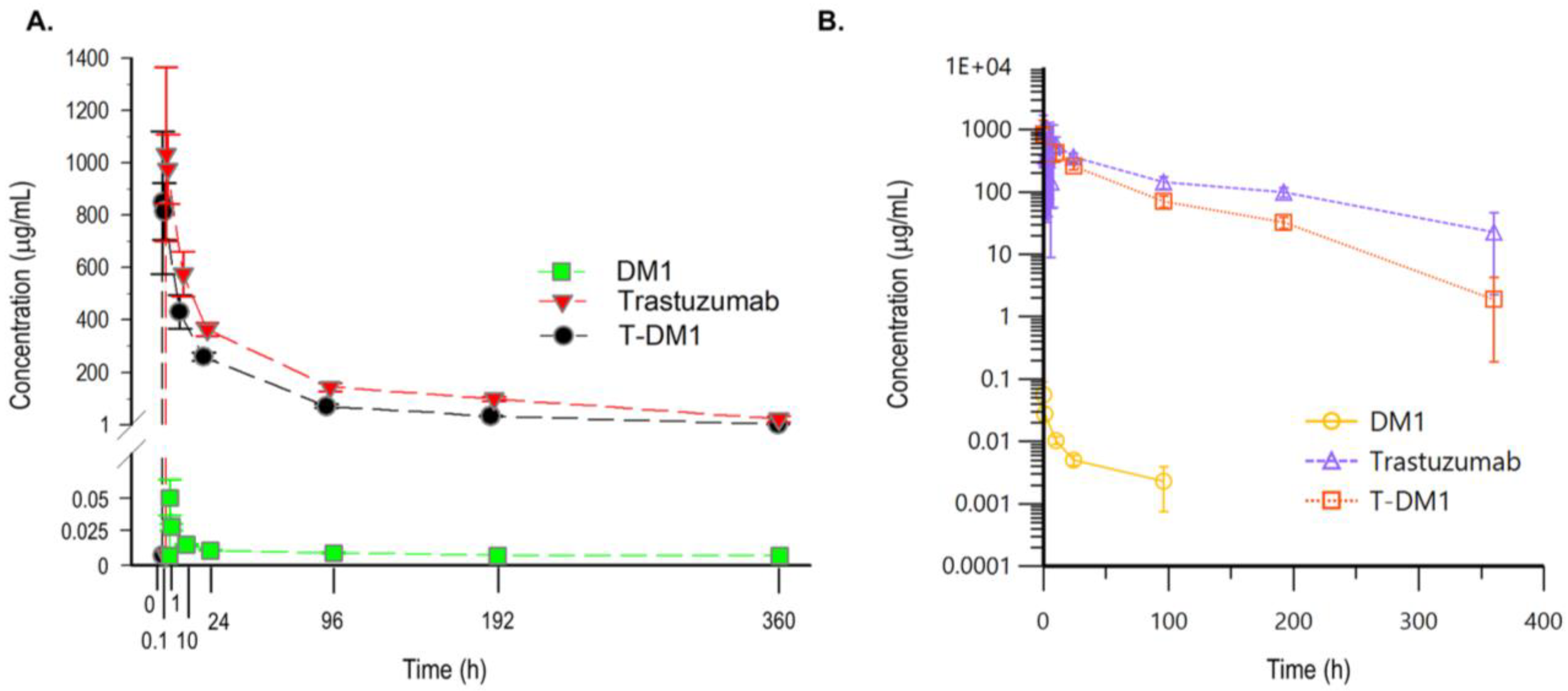
3.4. PK Study of T-DM1 in Rats
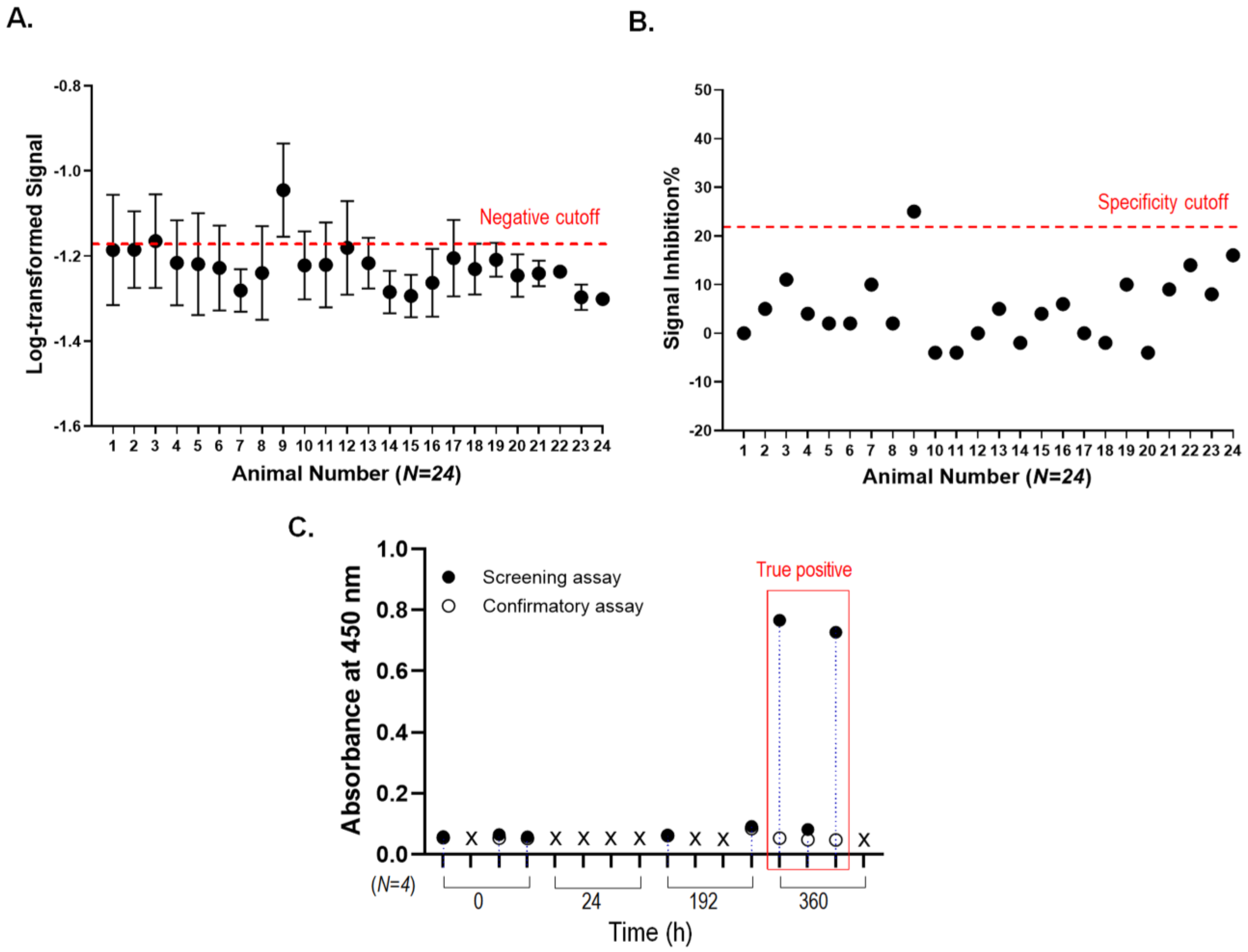
3.5. Immunogenicity Assessment for T-DM1 Administered Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody–drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef]
- Baah, S.; Laws, M.; Rahman, K. Antibody–Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Chari, R.V. Antibody Conjugate Therapeutics: Challenges and Potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Glennie, M.J.; van de Winkel, J.G. Renaissance of cancer therapeutic antibodies. Drug Discov. Today 2003, 8, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.T.W.; Harris, P.W.R.; Brimble, M.A.; Kavianinia, I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef] [PubMed]
- Schwach, J.; Abdellatif, M.; Stengl, A. More than Toxins—Current Prospects in Designing the Next Generation of Antibody Drug Conjugates. Front. Biosci. (Landmark Ed.) 2022, 27, 240. [Google Scholar] [CrossRef]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. Mabs 2021, 13, 1951427. [Google Scholar] [CrossRef]
- Kovtun, Y.V.; Audette, C.A.; Ye, Y.; Xie, H.; Ruberti, M.F.; Phinney, S.J.; Leece, B.A.; Chittenden, T.; Blattler, W.A.; Goldmacher, V.S. Antibody-Drug Conjugates Designed to Eradicate Tumors with Homogeneous and Heterogeneous Expression of the Target Antigen. Cancer Res. 2006, 66, 3214–3221. [Google Scholar] [CrossRef]
- Sievers, E.L.; Senter, P.D. Antibody-Drug Conjugates in Cancer Therapy. Annu. Rev. Med. 2013, 64, 15–29. [Google Scholar] [CrossRef]
- Kovtun, Y.V.; Goldmacher, V.S. Cell killing by antibody–drug conjugates. Cancer Lett. 2007, 255, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Zhang, X.; Okeley, N.M.; Anderson, M.; Law, C.-L.; Senter, P.D.; Benjamin, D.R. The Pharmacologic Basis for Antibody-Auristatin Conjugate Activity. J. Pharmacol. Exp. Ther. 2009, 330, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Dere, R.; Yi, J.-H.; Lei, C.; Saad, O.M.; Huang, C.; Li, Y.; Baudys, J.; Kaur, S. PK assays for antibody–drug conjugates: Case study with ado-trastuzumab emtansine. Bioanalysis 2013, 5, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, G.; Gampenrieder, S.P.; Greil, R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: State of the art and future directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Junttila, T.T.; Li, G.; Parsons, K.; Phillips, G.L.; Sliwkowski, M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 2011, 128, 347–356. [Google Scholar] [CrossRef]
- Remillard, S.; Rebhun, L.I.; Howie, G.A.; Kupchan, S.M. Antimitotic Activity of the Potent Tumor Inhibitor Maytansine. Science 1975, 189, 1002–1005. [Google Scholar] [CrossRef]
- Chari, R.V.; Martell, B.; Gross, J.L.; Cook, S.B.; Shah, S.; Blättler, W.; McKenzie, S.J.; Goldmacher, V.S. Immunoconjugates containing novel maytansinoids: Promising anticancer drugs. Cancer Res. 1992, 52, 127–131. [Google Scholar]
- Issell, B.F.; Crooke, S.T. Maytansine. Cancer Treat. Rev. 1978, 5, 199–207. [Google Scholar] [CrossRef]
- Lopus, M. Antibody-DM1 conjugates as cancer therapeutics. Cancer Lett. 2011, 307, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Tibbitts, J. Pharmacokinetic Considerations for Antibody Drug Conjugates. Pharm. Res. 2012, 29, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Xu, K.; Saad, O.M.; Dere, R.C.; Carrasco-Triguero, M. Bioanalytical assay strategies for the development of antibody–drug conjugate biotherapeutics. Bioanalysis 2013, 5, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Yuan, J.; Huang, Y.; Meissen, J.K.; Mou, S.; Liang, M.; Rosenbaum, A.I. Bioanalytical Methods and Strategic Perspectives Addressing the Rising Complexity of Novel Bioconjugates and Delivery Routes for Biotherapeutics. Biodrugs 2022, 36, 181–196. [Google Scholar] [CrossRef]
- Fiorotti, C.K. Immunogenicity considerations for antibody–drug conjugates: A focus on neutralizing antibody assays. Bioanalysis 2018, 10, 65–70. [Google Scholar] [CrossRef]
- Carrasco-Triguero, M.; Yi, J.-H.; Dere, R.; Qiu, Z.J.; Lei, C.; Li, Y.; Mahood, C.; Wang, B.; Leipold, D.; Poon, K.A.; et al. Immunogenicity assays for antibody–drug conjugates: Case study with ado-trastuzumab emtansine. Bioanalysis 2013, 5, 1007–1023. [Google Scholar] [CrossRef]
- Younes, A.; Kim, S.; Romaguera, J.; Copeland, A.; Farial, S.D.C.; Kwak, L.W.; Fayad, L.; Hagemeister, F.; Fanale, M.; Neelapu, S.; et al. Phase I Multidose-Escalation Study of the Anti-CD19 Maytansinoid Immunoconjugate SAR3419 Administered by Intravenous Infusion Every 3 Weeks to Patients with Relapsed/Refractory B-Cell Lymphoma. J. Clin. Oncol. 2012, 30, 2776–2782. [Google Scholar] [CrossRef]
- Lapusan, S.; Vidriales, M.-B.; Thomas, X.; De Botton, S.; Vekhoff, A.; Tang, R.; Dumontet, C.; Morariu-Zamfir, R.; Lambert, J.M.; Ozoux, M.-L.; et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Investig. New Drugs 2012, 30, 1121–1131. [Google Scholar] [CrossRef]
- Fayad, L.; Offner, F.; Smith, M.; Verhoef, G.; Johnson, P.; Kaufman, J.L.; Rohatiner, A.; Advani, A.; Foran, J.; Hess, G.; et al. Safety and Clinical Activity of a Combination Therapy Comprising Two Antibody-Based Targeting Agents for the Treatment of Non-Hodgkin Lymphoma: Results of a Phase I/II Study Evaluating the Immunoconjugate Inotuzumab Ozogamicin with Rituximab. J. Clin. Oncol. 2013, 31, 573–583. [Google Scholar] [CrossRef]
- EMA; Committee for Medicinal Products for Human Use (CHMP). Guideline on Bioanalytical Method Validation; CHMP: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Girish, S.; Gupta, M.; Wang, B.; Lu, D.; Krop, I.E.; Vogel, C.L.; Iii, H.A.B.; LoRusso, P.M.; Yi, J.-H.; Saad, O.; et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): An antibody–drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother. Pharmacol. 2012, 69, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.A.; Flagella, K.; Beyer, J.; Tibbitts, J.; Kaur, S.; Saad, O.; Yi, J.-H.; Girish, S.; Dybdal, N.; Reynolds, T. Preclinical safety profile of trastuzumab emtansine (T-DM1): Mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol. Appl. Pharmacol. 2013, 273, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Indelicato, S.R.; Jethwa, V.; Kawabata, T.; Kelley, M.; Mire-Sluis, A.R.; Richards, S.M.; Rup, B.; Shores, E.; Swanson, S.J.; et al. Recommendations for the design, optimization, and qualification of cell-based assays used for the detection of neutralizing antibody responses elicited to biological therapeutics. J. Immunol. Methods 2007, 321, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.P.; Kozak, K.R.; Wong, W.L.T. Challenges in developing bioanalytical assays for characterization of antibody–drug conjugates. Bioanalysis 2011, 3, 677–700. [Google Scholar] [CrossRef] [PubMed]
- ICH. M10 Bioanalytical Method Validation and Study Sample Analysis; ICH: Geneva, Switzerland, 2022. [Google Scholar]
- US FDA; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM). Bioanalytical Method Validation Guidance for Industry; US FDA: White Oak, MD, USA, 2018.
- Gorovits, B.; Alley, S.C.; Bilic, S.; Booth, B.; Kaur, S.; Oldfield, P.; Purushothama, S.; Rao, C.; Shord, S.; Siguenza, P. Bioanalysis of antibody–drug conjugates: American Association of Pharmaceutical Scientists Antibody–Drug Conjugate Working Group position paper. Bioanalysis 2013, 5, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.-P.; Chan, P.; Lee, C.; Nelson, C.; Elliott, J.M.; Bechtel, C.; Raab, H.; Xie, D.; Akutagawa, J.; Baudys, J.; et al. Anti-CD22-MCC-DM1 and MC-MMAF Conjugates: Impact of Assay Format on Pharmacokinetic Parameters Determination. Bioconjug. Chem. 2008, 19, 1673–1683. [Google Scholar] [CrossRef]
- Lo, C.-F.; Chiu, T.-Y.; Liu, Y.-T.; Huang, L.-R.; Yeh, T.-K.; Huang, K.-H.; Liu, K.-L.; Hsu, C.-Y.; Fang, M.-Y.; Huang, Y.-C.; et al. Synthesis and Evaluation of Small Molecule Drug Conjugates Harnessing Thioester-Linked Maytansinoids. Pharmaceutics 2022, 14, 1316. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.C.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef]
- Leelawattanachai, J.; Kwon, K.-W.; Michael, P.; Ting, R.; Kim, J.-Y.; Jin, M.M. Side-by-Side Comparison of Commonly Used Biomolecules That Differ in Size and Affinity on Tumor Uptake and Internalization. PLoS ONE 2015, 10, e0124440. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II Study of the Antibody Drug Conjugate Trastuzumab-DM1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Breast Cancer After Prior HER2-Directed Therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef]
- Baselga, J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 2001, 12 (Suppl. 1), S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Advani, A.; Coiffier, B.; Czuczman, M.S.; Dreyling, M.; Foran, J.; Gine, E.; Gisselbrecht, C.; Ketterer, N.; Nasta, S.; Rohatiner, A.; et al. Safety, Pharmacokinetics, and Preliminary Clinical Activity of Inotuzumab Ozogamicin, a Novel Immunoconjugate for the Treatment of B-Cell Non-Hodgkin’s Lymphoma: Results of a Phase I Study. J. Clin. Oncol. 2010, 28, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Bütikofer, L.; Lemaillet, G.; Faust, H. Strategies to estimate and improve drug tolerance in anti-drug antibody assays. Bioanalysis 2012, 4, 1999–2012. [Google Scholar] [CrossRef]
- Mire-Sluis, A.R.; Barrett, Y.C.; Devanarayan, V.; Koren, E.; Liu, H.; Maia, M.; Parish, T.; Scott, G.; Shankar, G.; Shores, E.; et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J. Immunol. Methods 2004, 289, 1–16. [Google Scholar] [CrossRef]
- Swanson, S.J.; Bussiere, J. Immunogenicity assessment in non-clinical studies. Curr. Opin. Microbiol. 2012, 15, 337–347. [Google Scholar] [CrossRef] [PubMed]
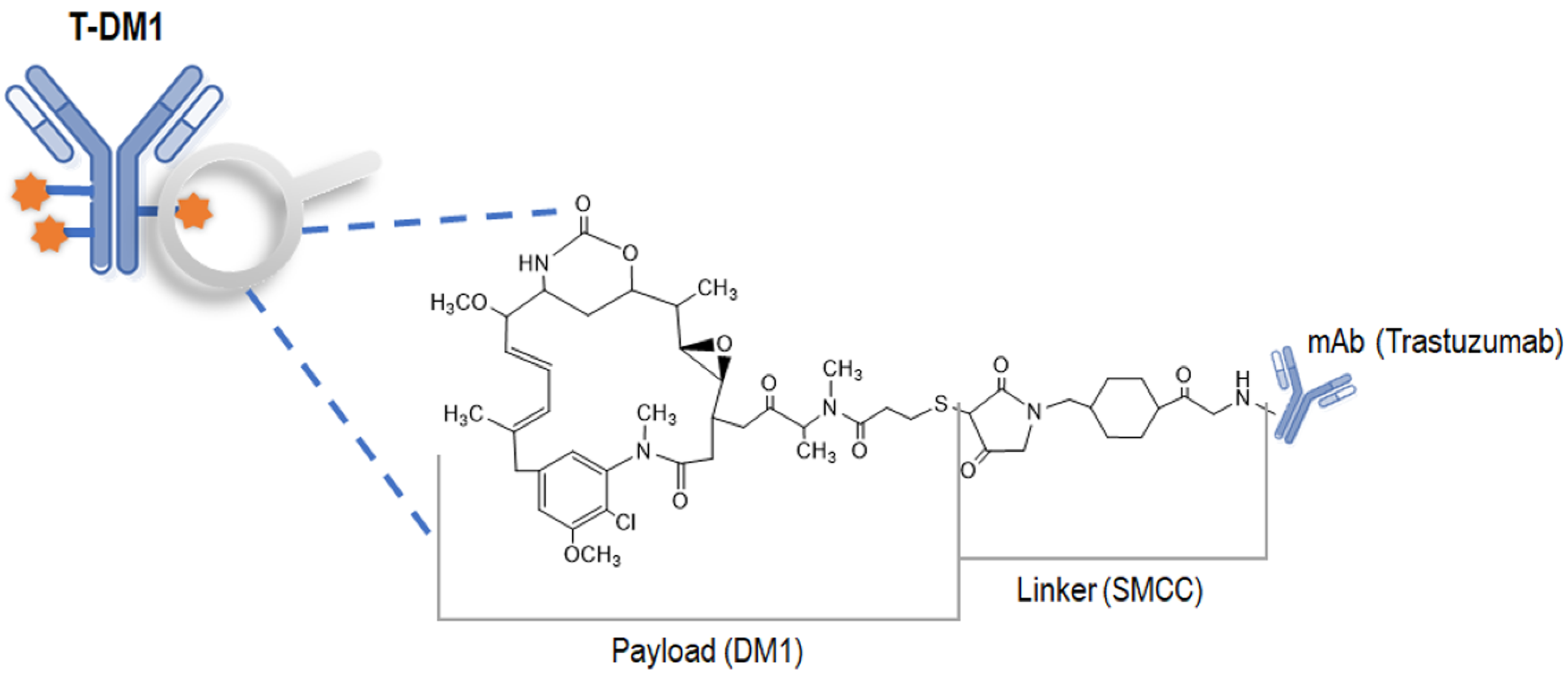
| Parameters | DM1-Conjugated Trastuzumab | Total Trastuzumab | DM1 |
|---|---|---|---|
| Analytical method | ELISA | ELISA | LC–MS/MS |
| Matrix | Serum | Serum | Plasma |
| Target molecule | DM1 | trastuzumab | DM1 |
| Minimum required dilution | 1:1000 | 1:100 | NA |
| Lower limit of quantitation (LLOQ) | 100 ng/mL | 100 ng/mL | 2 ng/mL |
| Upper limit of quantitation (ULOQ) | 6000 ng/mL | 6000 ng/mL | 400 ng/mL |
| Quantitative analysis model | 4-parameter logistic regression model | 1/x2-weighted linear regression model | |
| Between-run accuracy (%RE) | −9.2–−4.4 | −3.0–−0.8 | −2.8–6.5 |
| Between-run precision (%CV) | 8.4–16.8 | 6.7–12.0 | 6.8–10.6 |
| Ratio (T-DM1: Trastuzumab) | |||||
|---|---|---|---|---|---|
| 5:0 | 4:1 | 1:1 | 1:4 | 0:5 | |
| N. Con. (ng/mL) | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Mean ± SD | 1045.5 ± 23.6 | 1036.5 ± 13.1 | 1159.6 ± 18.7 | 1189.9 ± 16.2 | 1315.2 ± 25.4 |
| %CV | 2.3 | 1.3 | 1.6 | 1.4 | 1.9 |
| %RE | 4.5 | 3.6 | 16.0 | 19.0 | 31.5 |
| Trastuzumab with Trastuzumab Standard Curve | Trastuzumab with T-DM1 Standard Curve | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LLOQ | LQC | MQC | HQC | ULOQ | LLOQ | LQC | MQC | HQC | ULOQ | |
| N. Con. (ng/mL) | 100.0 | 300.0 | 1000.0 | 3000.0 | 6000.0 | 100.0 | 300.0 | 1000.0 | 3000.0 | 6000.0 |
| Mean ± SD | 102.0 ± 2.2 | 306.9 ± 10.9 | 1092.7 ± 31.4 | 3244.8 ± 85.3 | 7289.7 ± 271.7 | 126.4 ± 2.7 | 369.8 ± 12.8 | 1274.3 ± 36.1 | 3830.4 ± 105.8 | 9367.0 ± 410.8 |
| %CV | 2.1 | 3.6 | 2.9 | 2.6 | 3.7 | 2.1 | 3.4 | 2.8 | 2.8 | 4.4 |
| %RE | 2.0 | 2.3 | 9.3 | 8.2 | 21.5 | 26.4 | 23.3 | 27.4 | 27.7 | 56.1 |
| Parameters | DM1 | Total Trastuzumab | T-DM1 |
|---|---|---|---|
| Tmax (min) | 5 | 5 | 5 |
| T1/2 terminal (h) | 1.89 | 3.69 | 1.96 |
| Cmax (μg/mL) * | 56.40 ± 17.53 a | 1032.72 ± 331.63 | 848.03 ± 271.89 |
| AUC0-360 (day·μg/mL) * | 24.49 ± 1.74 b | 2280.29 ± 87.67 | 1291.40 ± 52.07 |
| AUC0-inf (day·μg/mL) | 30.82 b | 2401.55 | 1296.76 |
| Cl (mL/day/kg) | 1126.65 | 8.40 | 15.36 |
| Vss (mL/kg) | 1430091.52 | 37.53 | 37.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, E.-J.; Han, J.-H.; Seo, Y.; Koh, E.M.; Han, K.-H.; Hwang, K.; Jung, K.J. Implementation of Systematic Bioanalysis of Antibody–Drug Conjugates for Preclinical Pharmacokinetic Study of Ado-Trastuzumab Emtansine (T-DM1) in Rats. Pharmaceutics 2023, 15, 756. https://doi.org/10.3390/pharmaceutics15030756
Jeon E-J, Han J-H, Seo Y, Koh EM, Han K-H, Hwang K, Jung KJ. Implementation of Systematic Bioanalysis of Antibody–Drug Conjugates for Preclinical Pharmacokinetic Study of Ado-Trastuzumab Emtansine (T-DM1) in Rats. Pharmaceutics. 2023; 15(3):756. https://doi.org/10.3390/pharmaceutics15030756
Chicago/Turabian StyleJeon, Eun-Jeong, Ju-Hee Han, Youjin Seo, Eun Mi Koh, Kang-Hyun Han, Kyunghwa Hwang, and Kyung Jin Jung. 2023. "Implementation of Systematic Bioanalysis of Antibody–Drug Conjugates for Preclinical Pharmacokinetic Study of Ado-Trastuzumab Emtansine (T-DM1) in Rats" Pharmaceutics 15, no. 3: 756. https://doi.org/10.3390/pharmaceutics15030756
APA StyleJeon, E.-J., Han, J.-H., Seo, Y., Koh, E. M., Han, K.-H., Hwang, K., & Jung, K. J. (2023). Implementation of Systematic Bioanalysis of Antibody–Drug Conjugates for Preclinical Pharmacokinetic Study of Ado-Trastuzumab Emtansine (T-DM1) in Rats. Pharmaceutics, 15(3), 756. https://doi.org/10.3390/pharmaceutics15030756






