A Novel Approach for the Fabrication of 3D-Printed Dental Membrane Scaffolds including Antimicrobial Pomegranate Extract
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of the Pomegranate Peel and Seed Extracts
2.3. Preparation of the Solutions
2.4. Design and Fabrication of the 3D-Printed Scaffolds
2.5. Preparation of the Crosslinked 3D Scaffolds
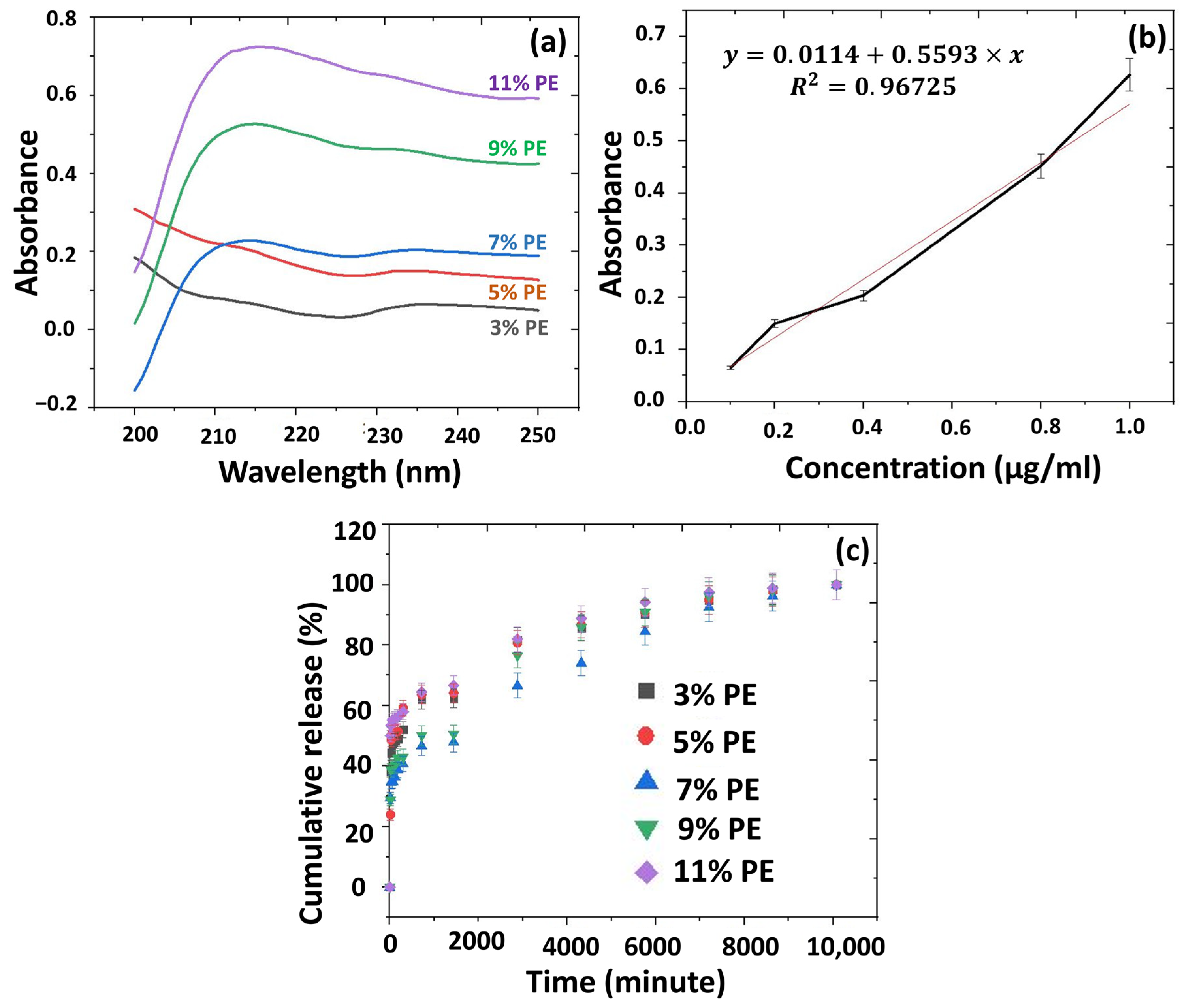
2.6. Characterisation of the Solutions and 3D-Printed Scaffolds
2.6.1. Physical Characterisations of the Solutions
2.6.2. Morphological Characterisations of the Scaffolds
2.6.3. Chemical Characterisations of the Scaffolds
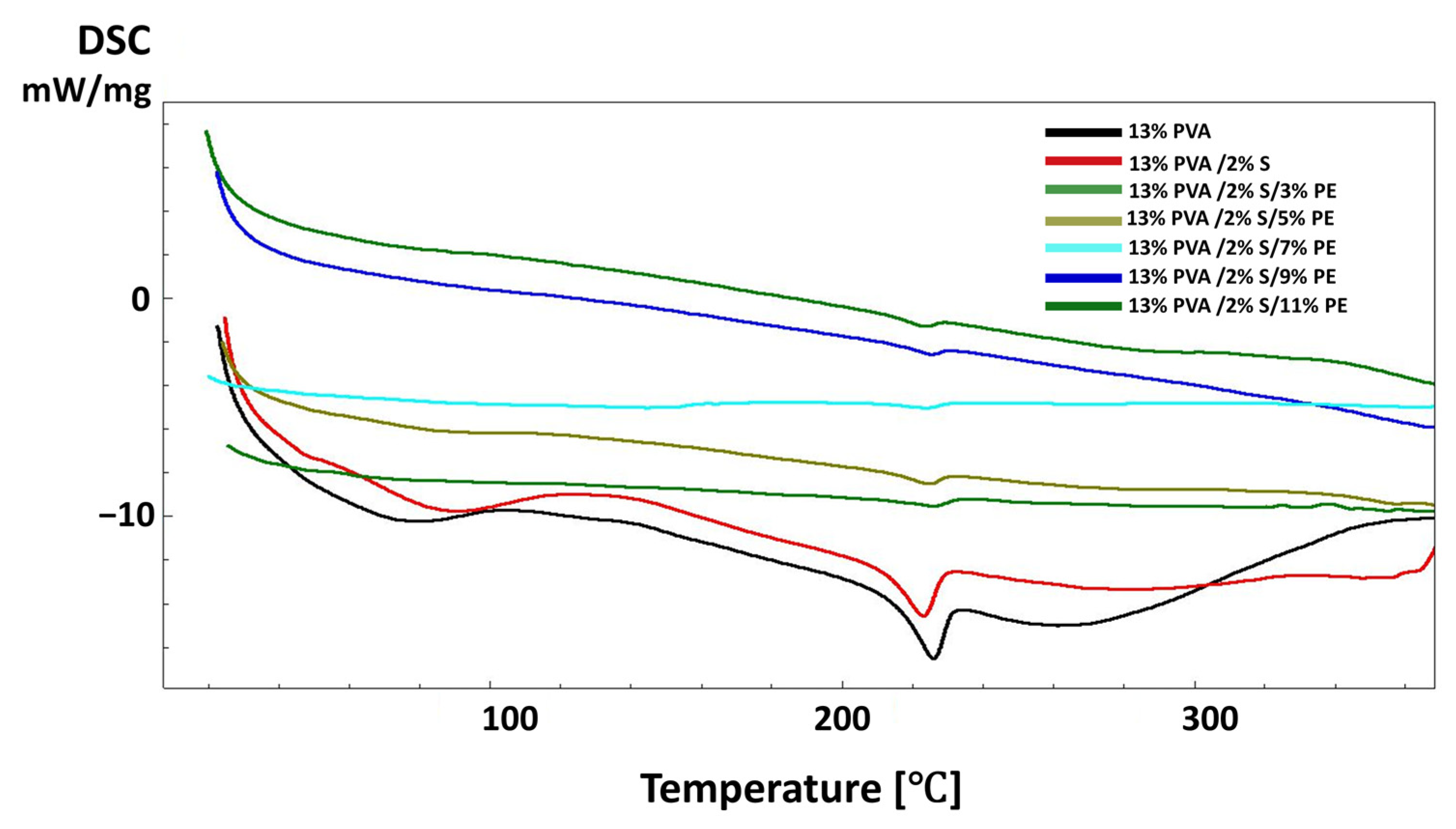
2.6.4. Thermal Characterisations of the Scaffolds
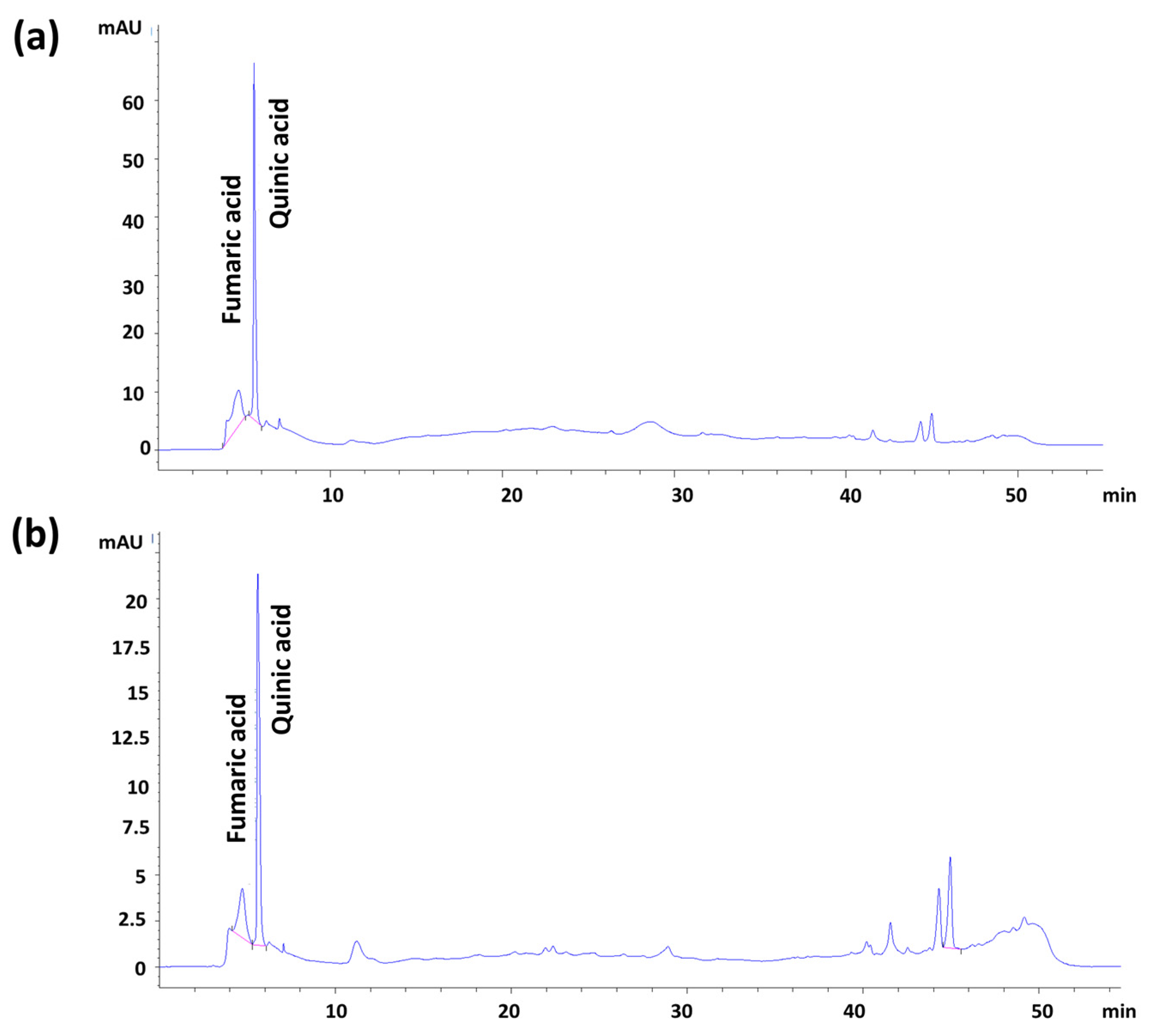
2.6.5. Pomegranate Extract Content Analysis by HPLC-DAD
2.6.6. Mechanical Properties of the Scaffolds
2.6.7. In Vitro Release Studies from the 3D-Printed Scaffolds
2.6.8. Cell Culture Assays
Cell Culture
Cell Viability-MTT Assay
Fluorescence Imaging
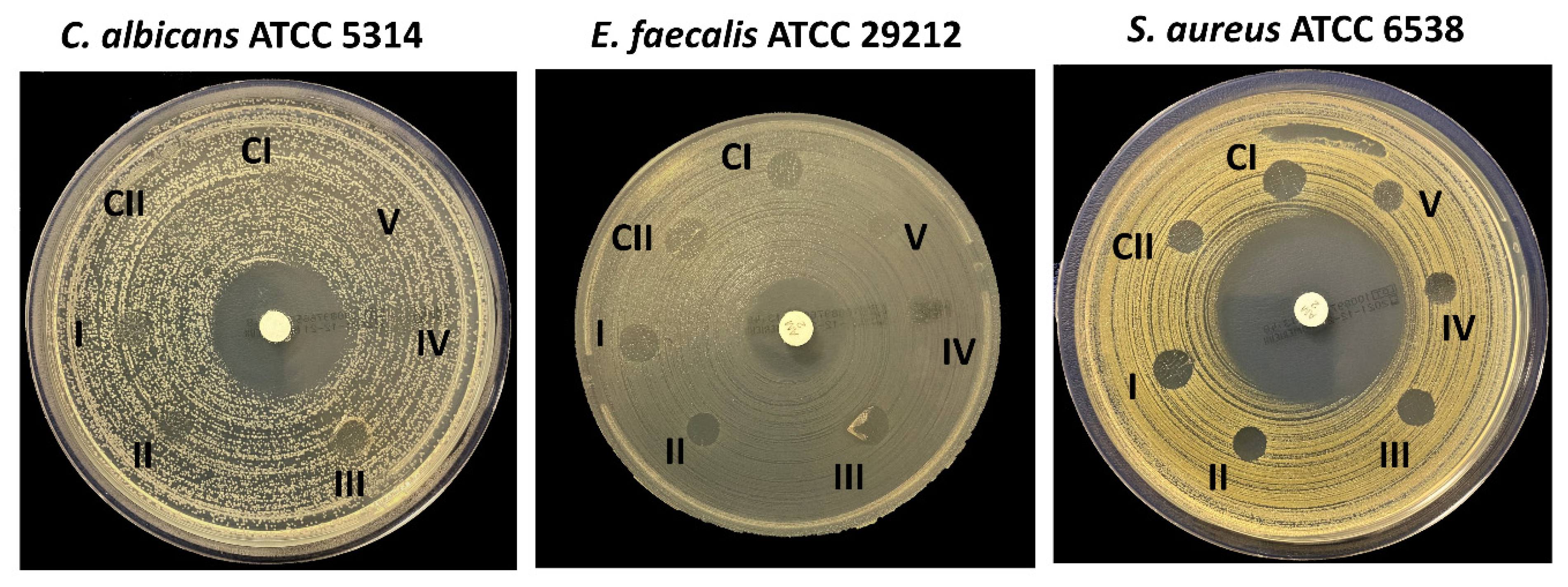
2.6.9. Antimicrobial Activities of the 3D Scaffolds
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, I.A.; Selders, G.S.; Fetz, A.E.; Gehrmann, C.J.; Stein, S.H.; Evensky, J.A.; Bowlin, G.L. Barrier membranes for dental applications: A review and sweet advancement in membrane developments. Mouth Teeth 2018, 2, 1–9. [Google Scholar]
- Nemati, E.; Gholami, A. Nano bacterial cellulose for biomedical applications: A mini review focus on tissue engineering. Adv. Appl. NanoBio-Technol. 2021, 2, 93–101. [Google Scholar]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on preva-lence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000 1994, 5, 78–111. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu TM, G.; Kowolik, M.J.; Janowski, G.M. Recent advanc-es in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Brägger, U.; Lang, N.P.; Nyman, S. Regeneration and enlargement of jaw bone using guided tis-sue regeneration. Clin. Oral Implant. Res. 1990, 1, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun Fibers for Dental and Craniofacial Applications. Curr. Stem Cell Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982–1992: A Decade of Technology Development for Guided Tissue Regeneration. J. Periodontol. 1993, 64, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implant. Res. 2009, 20, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Lin, C.; Chang, J.; Xiao, Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012, 8, 3805–3815. [Google Scholar] [CrossRef]
- Lundgren, D.; Lundgren, A.K.; Sennerby, L.; Nyman, S. Augmentation of intramembraneous bone beyond the skeletal envelope using an occlusive titanium barrier. An experimental study in the rabbit. Clin. Oral Implant. Res. 1995, 6, 67–72. [Google Scholar] [CrossRef]
- Lundgren, A.; Sennerby, L.; Lundgren, D. Guided jaw-bone regeneration using an experimental rabbit model. Int. J. Oral Maxillofac. Surg. 1998, 27, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J. A space-maintaining resorbable membrane for guided tissue regeneration. In Proceedings of the Annual Conference of the International Association of Dental Research, Honolulu, HI, USA, March 2004. [Google Scholar]
- Kostopoulos, L.; Karring, T. Augmentation of the rat mandible using guided tissue regeneration. Clin. Oral Implant. Res. 1994, 5, 75–82. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Jiang, H.B.; Ryu, J.-H.; Kang, H.; Kim, K.-M.; Kwon, J.-S. Comparing Properties of Variable Pore-Sized 3D-Printed PLA Membrane with Conventional PLA Membrane for Guided Bone/Tissue Regeneration. Materials 2019, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. dell’Istituto Super. Sanita 2015, 51, 229–235. [Google Scholar]
- Pilipchuk, S.P.; Fretwurst, T.; Yu, N.; Larsson, L.; Kavanagh, N.; Asa’Ad, F.; Cheng, K.C.K.; Lahann, J.; Giannobile, W.V. Micropatterned Scaffolds with Immobilized Growth Factor Genes Regenerate Bone and Periodontal Ligament-Like Tissues. Adv. Health Mater. 2018, 7, e1800750. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Tai, W.-C.; Ho, M.-H.; Chang, P.-C. Combination of a biomolecule-aided biphasic cryogel scaffold with a barrier membrane adhering PDGF-encapsulated nanofibers to promote periodontal regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration—A Materials Perspective. Biomater. Oral Craniomaxillofacial Appl. 2015, 17, 90–100. [Google Scholar]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and Characterization of Collagen/PVA Dual-Layer Membranes for Periodontal Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.-R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021, 187, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2019, 12, 7. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Hemamalini, T.; Dev, V.R.G. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Rodrigues, S.; Fornazier, M.; Magalhães, D.; Ruggiero, R. Potential utilization of glycerol as crosslinker in starch films for application in Regenerative Dentistry. Res. Soc. Dev. 2021, 10, e148101623640. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Licciardello, F.; Kharchoufi, S.; Muratore, G.; Restuccia, C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life 2018, 17, 114–119. [Google Scholar] [CrossRef]
- Widyarman, A.S.; Suhalim, O.P.; Nandary, D.; Theodorea, C.F. Pomegranate juice inhibits periodontal pathogens biofilm In Vitro. Sci. Dent. J. 2018, 2, 101–108. [Google Scholar] [CrossRef]
- Zekry, S.S.A.; Abdellatif, A.; Azzazy, H.M. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Tezcan, F.; Gültekin-Özgüven, M.; Diken, T.; Özçelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Dommati, H.; Ray, S.S.; Wang, J.-C.; Chen, S.-S. A comprehensive review of recent developments in 3D printing technique for ceramic membrane fabrication for water purification. RSC Adv. 2019, 9, 16869–16883. [Google Scholar] [CrossRef]
- Soo, A.; Ali, S.M.; Shon, H.K. 3D printing for membrane desalination: Challenges and future prospects. Desalination 2021, 520, 115366. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Meng, L.; Wang, C.; Song, Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Ulag, S.; Ilhan, E.; Sahin, A.; Yilmaz, B.K.; Kalaskar, D.M.; Ekren, N.; Kilic, O.; Oktar, F.N.; Gunduz, O. 3D printed artificial cornea for corneal stromal transplantation. Eur. Polym. J. 2020, 133, 109744. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel ex-tracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- De Sousa, B.G.B.; Pedrotti, G.; Sponchiado, A.P.; Cunali, R.S.; Aragones, Á.; Sarot, J.R.; Zielak, J.C.; Ornahgi, B.P.; Leão, M.P. Analysis of tensile strength of poly (lactic-co-glycolic acid)(PLGA) membranes used for guided tissue regeneration. RSBO Rev. Sul-Bras. De Odontol. 2014, 11, 59–65. [Google Scholar]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Biocompatibility of materials. In Biomaterials and Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2004; pp. 83–143. [Google Scholar]
- Bartee, B.K.; Carr, J.A. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J. Oral Implant. 1995, 21, 88–95. [Google Scholar]
- Murphy, C.M.; Haugh, M.G.; O’brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus WM, M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Kavitha, A.S.; Kiruthika, R.; Kalaivani, A.; Synthesis, S. Characterization and Antimicrobial Studies of Tosyl Esters of Carboxylic Acid. Int. J. Sci. Res. Publ. 2014, 4, 1–4. [Google Scholar]
- Awada, H.; Daneault, C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Appl. Sci. 2015, 5, 840–850. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nate, M.M.; Kurup, M.B.; Bambole, V.A.; Sabharwal, S. Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 237, 585–592. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2019, 23, 100439. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N. Characterization of active nanochitosan film containing natural preservative agents. Nanomed. Res. J. 2018, 3, 109–116. [Google Scholar]
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yılmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094. [Google Scholar] [CrossRef]
- Siddaiah, T.; Ojha, P.; Kumar, N.O.G.V.R.; Ramu, C. Structural, Optical and Thermal Characterizations of PVA/MAA:EA Polyblend Films. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Saeed, A.A.; Abdu, O.H.; Salem TA, B.F. HPLC Analysis and DPPH Assay of Some Bioactive Compounds in Pomegranate Peel Extracts. Res. Rev. J. Med. Chem. 2020, 2. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Ulag, S.; Dena, A.S.A.; Sahin, A.; Grinholc, M.; Gunduz, O.; El-Sherbiny, I.; Megahed, M. Chitosan/Gold Hybrid Nanoparticles Enriched Electrospun PVA Nanofibrous Mats for the Topical Delivery of Punica granatum L. Extract: Synthesis, Characterization, Biocompatibility and Antibacterial Properties. Int. J. Nanomed. 2021, 16, 5133–5151. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Mamdouh, W.; Habib, S.I.; El Deftar, M.; Habib, A.N.A. In Vitro Biological Evaluation of a Fabricated Polycaprolactone/Pomegranate Electrospun Scaffold for Bone Regeneration. ACS Omega 2021, 6, 34447–34459. [Google Scholar] [CrossRef]
- Yang, X.; Yang, K.; Wu, S.; Chen, X.; Yu, F.; Li, J.; Ma, M.; Zhu, Z. Cytotoxicity and wound healing properties of PVA/ws-chitosan/glycerol hydrogels made by irradiation followed by freeze–thawing. Radiat. Phys. Chem. 2010, 79, 606–611. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Gholami, Z.; Hasanpour, S.; Sadigh, S.; Johari, S.; Shahveghar, Z.; Ataei, K.; Javari, E.; Amani, M.; Kia, L.J.; Akbari, Z.D.; et al. Antibacterial agent-releasing scaffolds in dental tissue engineering. J. Adv. Periodontol. Implant. Dent. 2021, 13, 43–47. [Google Scholar] [CrossRef]
- Kichler, V.; Teixeira, L.S.; Prado, M.M.; Colla, G.; Schuldt, D.P.; Coelho, B.S.; Porto, L.M.; de Almeida, J. A novel antimicrobial-containing nanocellulose scaffold for regenerative endodontics. Restor. Dent. Endod. 2021, 46, e20. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Carvalho, C.; Lang, M.; Follo, M.; Braun, G.; Wittmer, A.; Mülhaupt, R.; Hellwig, E. Bacterial Candida Albicans adhesion on rapid prototyping-produced 3D-scaffolds manufactured as bone replacement materials. J. Biomed. Mater. Res. Part A 2008, 87, 933–943. [Google Scholar] [CrossRef] [PubMed]
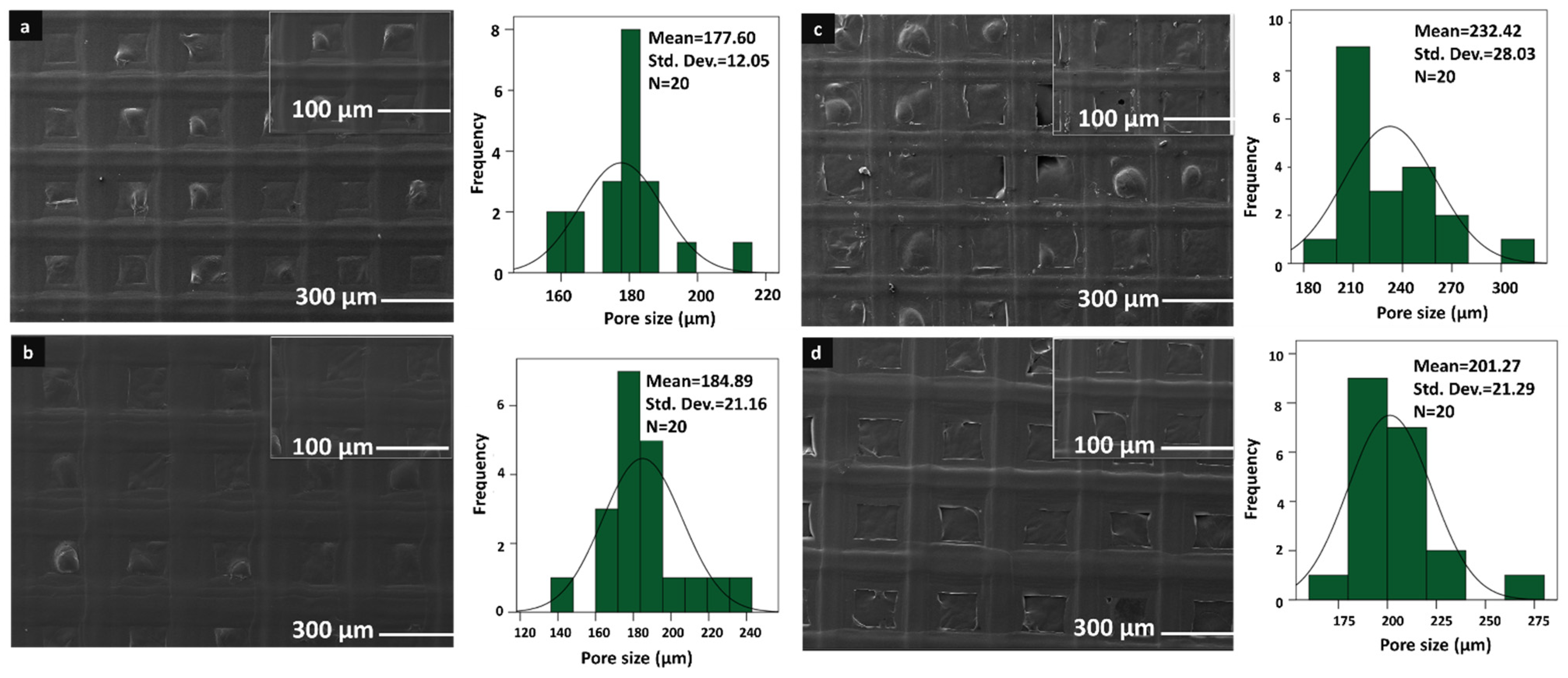
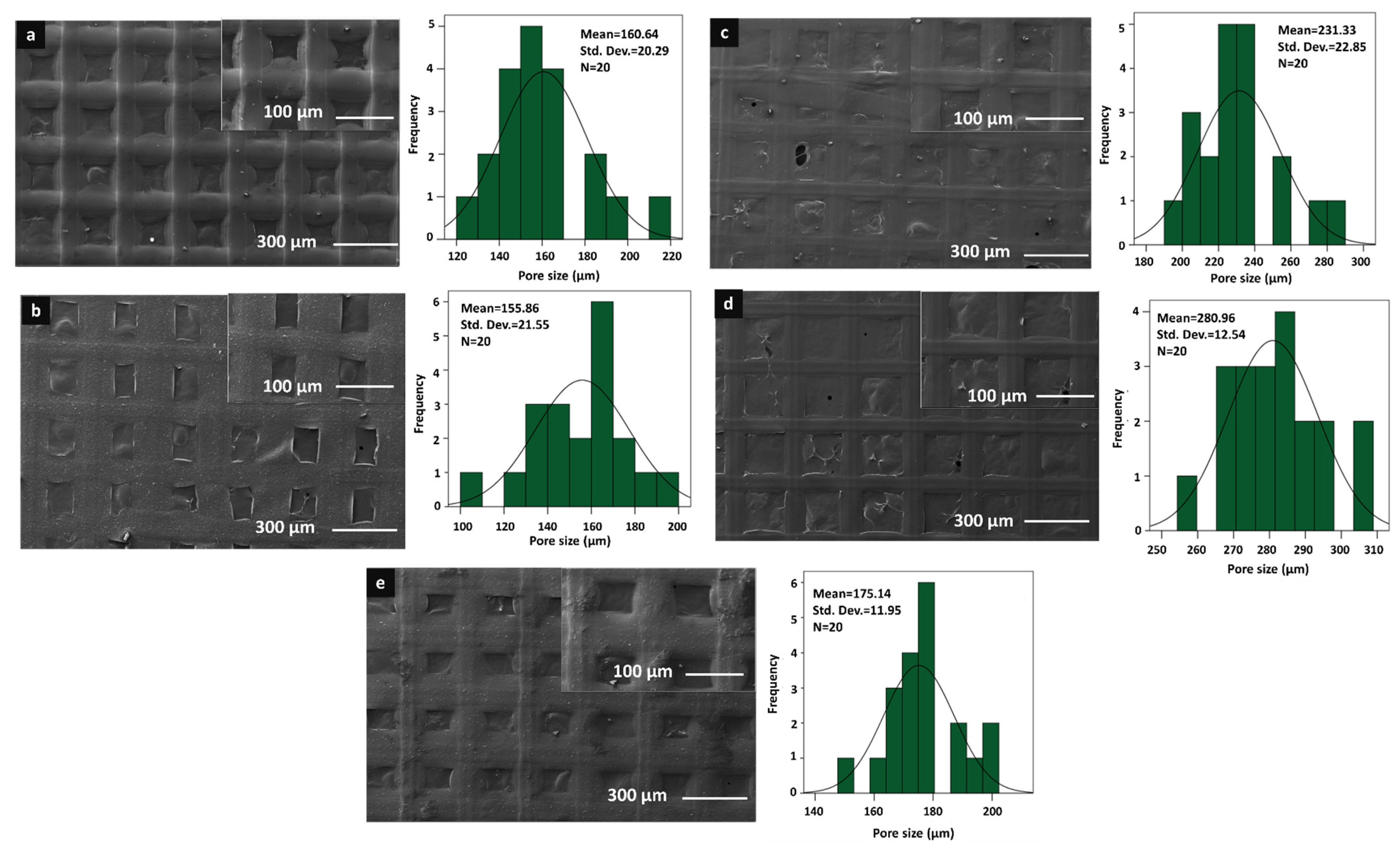
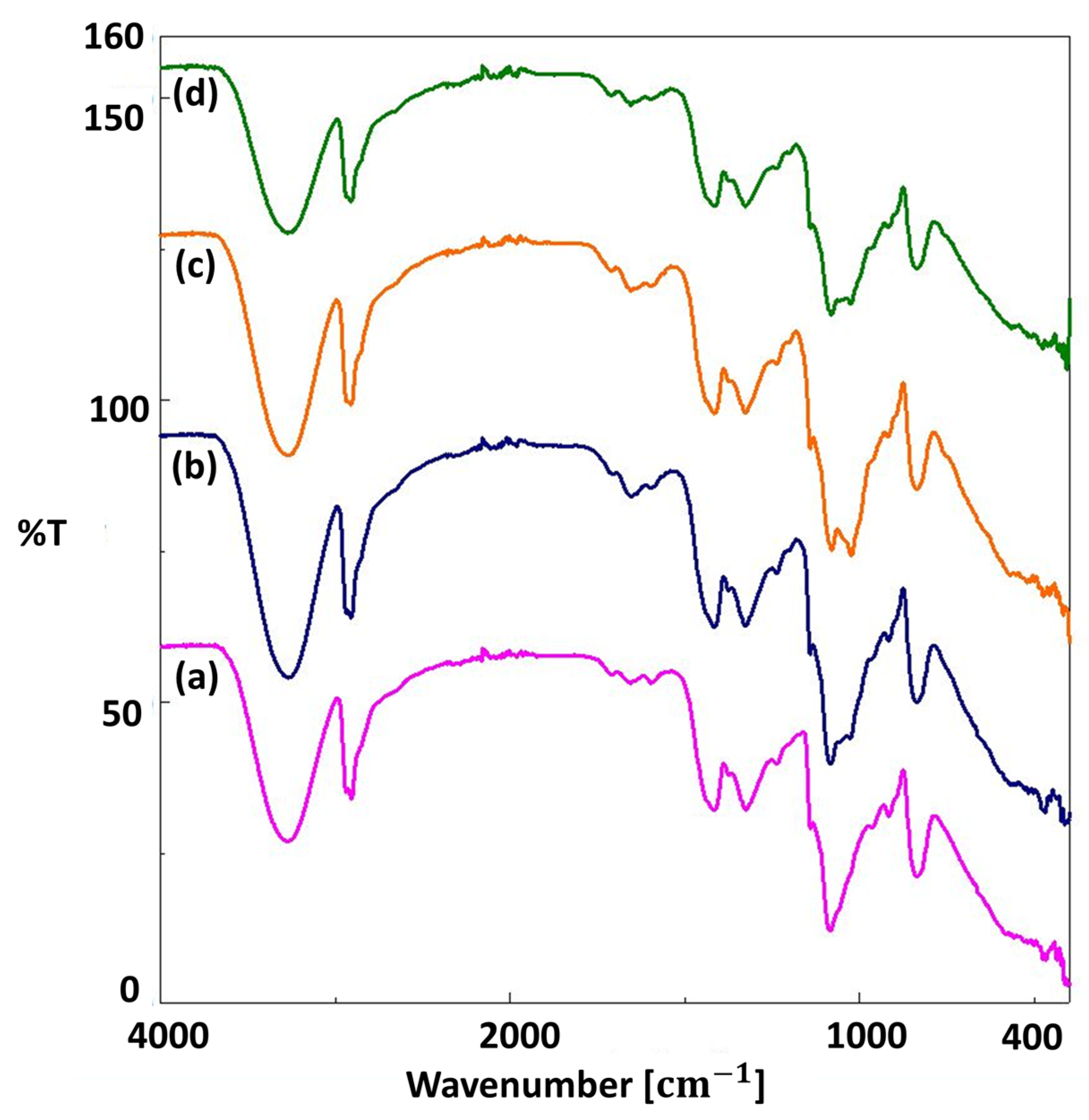

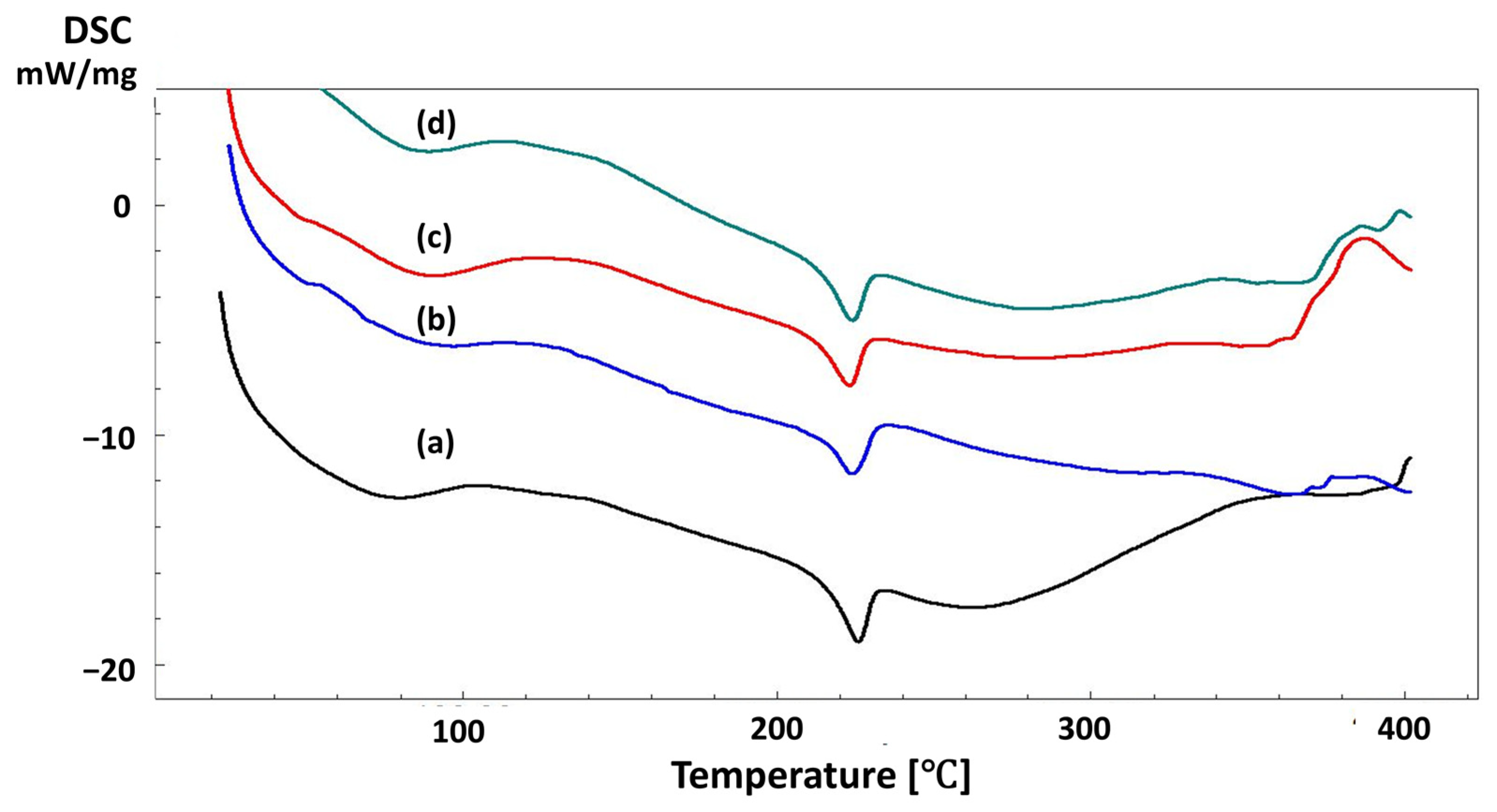


| Solutions | Contents | pH Value | Density (g/mL) |
|---|---|---|---|
| 13% PVA | 13% PVA (w/v) | 6.83 | 1.038 |
| 13% PVA/1% S | 13% PVA (w/v)/1% S (w/v) | 6.22 | 1.030 |
| 13% PVA/2% S | 13% PVA (w/v)/2% S (w/v) | 5.72 | 1.028 |
| 13% PVA/3% S | 13% PVA (w/v)/3% S (w/v) | 5.49 | 1.021 |
| 3% PE | 13% PVA (w/v)/1% S (w/v)/3% PPE:PSE (v/v) | 5.34 | 1.015 |
| 5 % PE | 13% PVA (w/v)/1% S (w/v)/5% PPE:PSE (v/v) | 5.21 | 1.028 |
| 7% PE | 13% PVA (w/v)/1% S (w/v)/7% PPE:PSE (v/v) | 4.98 | 1.025 |
| 9% PE | 13% PVA (w/v)/1% S (w/v)/9% PPE:PSE (v/v) | 4.84 | 1.021 |
| 11% PE | 13% PVA (w/v)/1% S (w/v)/11% PPE:PSE (v/v) | 4.77 | 1.011 |
| PPE:PSE | Pomegranate peel extract:Pomegranate seed extract (1:1) | 3.53 | 1.019 |
| PSE | Pomegranate seed extract (PSE) | 3.48 | 1.024 |
| PPE | Pomegranate peel extract (PPE) | 3.63 | 1.001 |
| Compounds | Regression Equation | R2 | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Fumaric acid | y = 27.52x − 43.96 | 0.9821 | 20–100 | 4.25 | 14.60 |
| Quinic acid | y = 28.79x + 98.34 | 0.9762 | 20–100 | 7.15 | 10.36 |
| Sample Groups | Tensile Strength (MPa) | Strain at Break (%) |
|---|---|---|
| 13% PVA | 10.5 ± 2.3 | 15.2 ± 11.9 |
| 13% PVA/1% S | 15.1 ± 3.4 | 6.5 ± 4.1 |
| 13% PVA/2% S | 23.9 ± 4.1 | 8.4 ± 5.9 |
| 13% PVA/3% S | 19.4 ± 2.8 | 4.5 ± 0.1 |
| 13% PVA/2% S/3% PE | 10.7 ± 2.8 | 5.5 ± 0.3 |
| 13% PVA/2% S/5% PE | 15.8 ± 1.3 | 5.8 ± 0.2 |
| 13% PVA/2% S/7% PE | 13.6 ± 0.8 | 7.8 ± 0.01 |
| 13% PVA/2% S/9% PE | 12.7 ± 3.7 | 1.8 ± 3.3 |
| 13% PVA/2% S/11% PE | 8.7 ± 2.5 | 6.6 ± 2.5 |
| Group Number | Scaffolds | S. aureus (mm) | E. faecalis (mm) | C. albicans (mm) |
|---|---|---|---|---|
| CI | 13% PVA | 9 | 7 | 0 |
| CII | 13% PVA/2% S | 7 | 8 | 0 |
| I | 13% PVA/2% S/3% PE | 8 | 7 | 0 |
| II | 13% PVA/2% S/5% PE | 8 | 5 | 0 |
| III | 13% PVA/2% S/7% PE | 8 | 7 | 0 |
| IV | 13% PVA/2% S/9% PE | 6 | 0 | 0 |
| V | 13% PVA/2% S/11% PE | 6 | 0 | 0 |
| AMP-Ampicillin (2 µg) | 39 | 20 | - | |
| Chlorhexidine gluconate (20%) | - | - | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabulut, H.; Ulag, S.; Dalbayrak, B.; Arisan, E.D.; Taskin, T.; Guncu, M.M.; Aksu, B.; Valanezhad, A.; Gunduz, O. A Novel Approach for the Fabrication of 3D-Printed Dental Membrane Scaffolds including Antimicrobial Pomegranate Extract. Pharmaceutics 2023, 15, 737. https://doi.org/10.3390/pharmaceutics15030737
Karabulut H, Ulag S, Dalbayrak B, Arisan ED, Taskin T, Guncu MM, Aksu B, Valanezhad A, Gunduz O. A Novel Approach for the Fabrication of 3D-Printed Dental Membrane Scaffolds including Antimicrobial Pomegranate Extract. Pharmaceutics. 2023; 15(3):737. https://doi.org/10.3390/pharmaceutics15030737
Chicago/Turabian StyleKarabulut, Hatice, Songul Ulag, Basak Dalbayrak, Elif Damla Arisan, Turgut Taskin, Mehmet Mucahit Guncu, Burak Aksu, Alireza Valanezhad, and Oguzhan Gunduz. 2023. "A Novel Approach for the Fabrication of 3D-Printed Dental Membrane Scaffolds including Antimicrobial Pomegranate Extract" Pharmaceutics 15, no. 3: 737. https://doi.org/10.3390/pharmaceutics15030737
APA StyleKarabulut, H., Ulag, S., Dalbayrak, B., Arisan, E. D., Taskin, T., Guncu, M. M., Aksu, B., Valanezhad, A., & Gunduz, O. (2023). A Novel Approach for the Fabrication of 3D-Printed Dental Membrane Scaffolds including Antimicrobial Pomegranate Extract. Pharmaceutics, 15(3), 737. https://doi.org/10.3390/pharmaceutics15030737








