Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems
Abstract
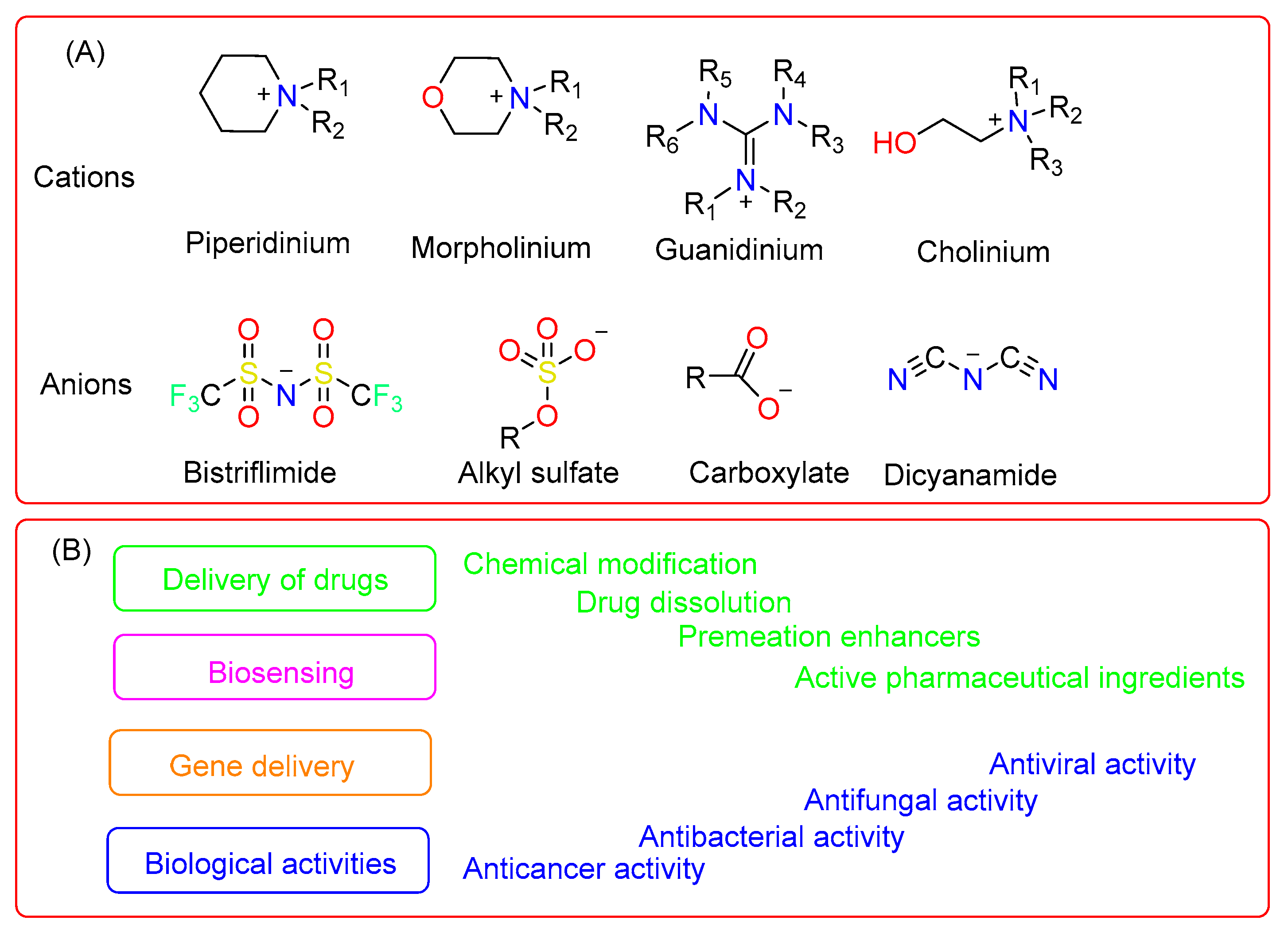
1. Introduction
2. Ionic Liquids (ILs) Used as Solvents
2.1. Pharmaceutical Drug Synthesis Using Ionic Liquids as an Alternative Medium
| S. No. | Cations/Anions | Applications | Ref. |
|---|---|---|---|
| 1. | Cations such as (C4MIM)in combination of anions (NTf2), (BF4), or (PF6) | Synthesis of APIs | [9] |
| 2. | 1-butyl-3-methylimidazolium hexafluorophosphate ((C4MIM)(PF6)) | Synthesize hybrids of pyrimidine nucleoside; thiazolini-4-one | [30] |
| 3. | (Emim)Cl | Synthesis of 5-hydroxymethylfurfural (HMF) from monosaccharides | [31] |
| 4. | (C4MIM)(X) (where X = BF4 or PF6) | Preparation of L-4-boronophenylalanine (L-BPA) | [32] |
| 5. | Pinacol borane protected p-iodophenylalanine with (C4MIM)(BF4) | Synthesis of L-BPA | [33] |
| 6. | Imidazolium-based ILs in combination of Friedel-Crafts reaction and nucleophilic displacement process | Synthesis of Pravadoline | [34] |
| 7. | Ru-BINAP catalyst and (C4MIM)(BF4) | Synthesis of (S)-naproxen | [36] |
| 8. | (C4MIM)(PF6) and (C4MIM)(BF4) | Synthesis of (R,S)-ibuprofen | [36] |
2.2. Drug Delivery Using Ionic Liquids
2.2.1. Enhancement of Permeability of Cell Membrane
| S. No. | Amphiphilic Nature | Applications | Ref. |
|---|---|---|---|
| 1. | 1,4-diazabicyclo[2.2.2]octane-, alicyclic pyrrolidinium-, and morpholinium-based ILs | As promoters of transdermal layers to administer the hypertension-treating agent diltiazem | [19] |
| 2. | Mono-cationic nature containing 1,4-diazabicyclo[2.2.2]octane | Beats the ineffective congener dicationic via more than two-folds during delivery of diltiazem hydrochloride salts | [19] |
| 3. | Morpholinium-containing IL | Promotes the penetration of diltiazem HCl salts into the skin faster as compared to pyrrolidinium-based IL | [19] |
| 4. | 1-octyl-3-methylimidazolium-based IL | Improved drug delivery | [44,45,46] |
| 5. | Imidazolium-based ILs | Cell membrane solvation to open channels to facilitate molecular passage | [46] |
| 6. | Cholinium cation | Readily permeate the cell membrane, is a developing bioinspired lead chemical. | [50] |
| 7. | Coupling of cholinium cation with geranic acid | Act as a pheromone in certain insects and also as a tyrosinase inhibitor in lemongrass | [50] |
| 8. | Choline geranate (CAGE) | Used for the delivery of drugs | [51] |
| 9. | Choline geranate (CAGE) | Ability to boost the transport of mannitol and cefadroxil by 5 and 16-fold, respectively | [52] |
| 10. | ILs such as tetraalkylphosphonium oleate, tetraalkylphosphonium hexanoate, choline oleate, and tetraalkylphosphoniumgeranate | Developed as effective permeation enhancers, allowing a fivefold increase in the transport of cefadroxil through dermis | [52] |
| 11. | Choline geranate (CAGE) | Promoters for fluorescein isothiocyanate-marked insulin, OVA, and BSA | [54] |
| 12. | Choline geranate (CAGE) | Better insulin transdermal delivery in male Wister rats | [54] |
| 13. | liquid salts generated from the carboxylic acid and aliphatic amines | Increase the drug’s penetration across physiological barriers | [55] |
2.2.2. Support for Low Solubility Drug Dissolution
2.2.3. Designing New Active Pharmaceutical Ingredients-Ionic Liquids
2.3. Remedies for Pharmaceutical Drug Polymorphism-Related Problems
3. Ionic Liquids with Metals Added to Detect Biomolecules
4. Ionic Liquid Toxicity
5. Ionic Liquid-Based New Active Pharmaceutical Ingredients
5.1. Pharmaceutical Salts Ionic Liquid (API-ILs) Synthesis
5.2. Salts of Protic Pharmaceuticals
5.3. Assessing Enhanced Properties of API-IL
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Procopio, D.; Siciliano, C.; Trombino, S.; Dumitrescu, D.E.; Suciu, F.; Di Gioia, M.L. Green solvents for the formation of amide linkages. Org. Biomol. Chem. 2022, 20, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 1800, 405–422. [Google Scholar]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Rao, R.V.; Patil, K.T.; Kumar, D.; Gupta, M.K.; Shin, D.-S. Mild and efficient one-pot synthesis of (E)-styrylperfluoroalkyl ketone from styrene. Results Chem. 2022, 4, 100260. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva, G.C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhong, R.; Hu, R.; Zhang, F. Applications of ionic liquids in biomedicine. Biophys. Rev. Lett. 2012, 7, 121–134. [Google Scholar] [CrossRef]
- Rao, V.R.; Patil, K.T.; Kumar, D.; Sebastian, S.; Gupta, M.K.; Shin, D.-S. Facile metal-free visible-light-mediated chlorotrifluoromethylation of terminal alkenes. Mon. Chem. 2022, 153, 495–500. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Moshikur, R.M.; Goto, M. Ionic Liquids as Active Pharmaceutical Ingredients (APIs). In Application of Ionic Liquids in Drug Delivery; Springer: Singapore, 2021; pp. 13–33. [Google Scholar]
- Shi, W.; Luebke, D.R. Enhanced gas absorption in the ionic liquid 1-n-hexyl-3-methylimidazolium bis (trifluoromethylsulfonyl) amide ([hmim][Tf2N]) confined in silica slit pores: A molecular simulation study. Langmuir 2013, 29, 5563–5572. [Google Scholar] [CrossRef] [PubMed]
- Greaves, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1709. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, D. Application and Perspective of Ionic Liquids on Rare Earths Green Separation. Sep. Sci. Technol. 2012, 47, 223–232. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco (cyto) activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Dinis, T.B.V.; e Silva, F.A.; Sousa, F.; Freire, M.G. Advances Brought by Hydrophilic Ionic Liquids in Fields Involving Pharmaceuticals. Materials 2021, 14, 6231. [Google Scholar] [CrossRef]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 3, 7–25. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Zandu, S.K.; Chopra, H.; Singh, I. Ionic Liquids for Therapeutic and Drug Delivery Applications. Curr. Drug Res. Rev. 2020, 12, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic liquids as potential enhancers for transdermal drug delivery. Int. J. Pharm. 2017, 516, 45–51. [Google Scholar] [CrossRef]
- Handa, M.; Almalki, W.H.; Shukla, R.; Afzal, O.; Altamimi, A.S.A.; Beg, S.; Rahman, M. Active pharmaceutical ingredients (APIs) in ionic liquids: An effective approach for API physiochemical parameter optimization. Drug Discov. Today 2022, 27, 2415–2424. [Google Scholar] [CrossRef]
- Ford, L.; Tay, E.; Nguyen, T.-H.; Williams, H.D.; Benameur, H.; Scammells, P.J.; Porter, C.J.H. API ionic liquids: Probing the effect of counterion structure on physical form and lipid solubility. RSC Adv. 2020, 10, 12788–12799. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Optimizing Oral Bioavailability in Drug Discovery: An Overview of Design and Testing Strategies and Formulation Options. J. Pharm. Sci. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. [Google Scholar]
- Pedro, S.N.; RFreire, C.S.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S.V. Synthesis of nucleoside-based antiviral drugs in ionic liquids. Bioorg. Med. Chem. Lett. 2008, 18, 5640–5642. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Li, D.; Qu, G.; Wang, J.; Loiseau, P.; Fan, X. Ionic liquid mediated and promoted eco-friendly preparation of thiazolidinone and pyrimidine nucleoside-thiazolidinone hybrids and their antiparasitic activities. Bioorg. Med. Chem. Lett. 2009, 19, 6280–6283. [Google Scholar] [CrossRef]
- Zunita, M.; Yuan, D.M.; Syafi’ Laksono, A. Glucose conversion into hydroxymethylfurfural via ionic liquid-based processes. Chem. Eng. J. Adv. 2022, 11, 100307. [Google Scholar] [CrossRef]
- Wolan, A.; Zaidlewicz, M. Synthesis of arylboronates by the palladium catalysed cross-coupling reaction in ionic liquids. Org. Biomol. Chem. 2003, 1, 3274–3276. [Google Scholar] [CrossRef] [PubMed]
- Kurata, A.; Kitamura, Y.; Irie, S.; Takemoto, S.; Akai, Y.; Hirota, Y.; Fujita, T.; Iwai, K.; Furusawa, M.; Kishimoto, N. Enzymatic synthesis of caffeic acid phenethyl ester analogues in ionic liquid. J. Biotechnol. 2010, 148, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Seddon, K.R.; McCormac, P.B. The first high yield green route to a pharmaceutical in a room temperature ionic liquid. Green Chem. 2000, 2, 261–262. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Monteiro, A.L.; Zinn, F.K.; de Souza, R.F.; Dupont, J. Asymmetric hydrogenation of 2-arylacrylic acids catalyzed by immobilized Ru-BINAP complex in 1-n-butyl-3-methylimidazolium tetrafluoroborate molten salt. Tetrahedron Asymmetry 1997, 8, 177–179. [Google Scholar] [CrossRef]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Carvalho, P.O.; Cass, Q.; Calafatti, S.A.; Contesini, F.; Bizaco, R. Review—Alternatives for the separation of drug enantiomers: Ibuprofen as a model compound. Braz. J. Chem. Eng. 2006, 23, 291–300. [Google Scholar] [CrossRef]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of Chemical Permeation Enhancers on Skin Permeability: In silico screening using Molecular Dynamics simulations. Sci. Rep. 2019, 9, 1456. [Google Scholar] [CrossRef]
- Lu, B.; Liu, T.; Wang, H.; Wu, C.; Chen, H.; Liu, Z.; Zhang, J. Ionic liquid transdermal delivery system: Progress, prospects, and challenges. J. Mol. Liq. 2022, 351, 118643. [Google Scholar] [CrossRef]
- Kumar, S.; Scheidt, H.A.; Kaur, N.; Kaur, A.; Kang, T.S.; Huster, D.; Mithu, V.S. Amphiphilic Ionic Liquid-Induced Membrane Permeabilization: Binding Is Not Enough. J. Phys. Chem. B 2018, 122, 6763–6770. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, A.; Bhakuni, K.; Sankaranarayanan, K.; Venkatesu, P. Implications of Imidazolium-Based Ionic Liquids as Refolding Additives for Urea-Induced Denatured Serum Albumins. ACS Sustain. Chem. Eng. 2020, 8, 604–612. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Gao, L.; Lu, C.; Ma, S.; Yan, X.; Jiang, X.; Wu, X.; He, G. Flexibly crosslinked and post-morpholinium-functionalized poly (2, 6-dimethyl-1, 4-phenylene oxide) anion exchange membranes. Int. J. Hydrog. Energy 2020, 45, 29681–29689. [Google Scholar] [CrossRef]
- Laksitorini, M.; Prasasty, V.D.; Kiptoo, P.K.; Siahaan, T.J. Pathways and progress in improving drug delivery through the intestinal mucosa and blood–brain barriers. Ther. Deliv. 2014, 5, 1143–1163. [Google Scholar] [CrossRef]
- Yu, A.S.L. Paracellular transport as a strategy for energy conservation by multicellular organisms? Tissue Barriers 2017, 5, e1301852. [Google Scholar] [CrossRef][Green Version]
- Boch, R.; Shearer, D.A. Identification of Nerolic and Geranic Acids in the Nassanoff Pheromone of the Honey Bee. Nature 1964, 202, 320–321. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, K.N.; Ma, H.; Banerjee, A.; Tanner, E.E.L.; Nangia, S.; Mitragotri, S. Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE). ACS Biomater. Sci. Eng. 2018, 4, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Shibata, A.; Yamaguchi, T. The molecular assembly of the ionic liquid/aliphatic carboxylic acid/aliphatic amine as effective and safety transdermal permeation enhancers. Eur. J. Pharm. Sci. 2016, 86, 75–83. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Cojocaru, O.A.; Kelley, S.P.; Bica, K.; Wallace, S.P.; Gurau, G.; Rogers, R.D. Acyclovir as an ionic liquid cation or anion can improve aqueous solubility. ACS Omega 2017, 2, 3483–3493. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250. [Google Scholar] [CrossRef]
- Porter, C.J.; Pouton, C.W.; Cuine, J.F.; Charman, W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Williams, H.D.; Sahbaz, Y.; Ford, L.; Nguyen, T.-H.; Scammells, P.J.; Porter, C.J.H. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem. Commun. 2014, 50, 1688–1690. [Google Scholar] [CrossRef]
- Neslihan Gursoy, R.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2014, 58, 173–182. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A.; Alruwaili, N.K.; Alotaibi, F.; Alanazi, A.; Shakeel, F. Potential of solid dispersions to enhance solubility, bioavailability, and therapeutic efficacy of poorly water-soluble drugs: Newer formulation techniques, current marketed scenario and patents. Drug Deliv. 2020, 27, 1625–1643. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, G.; Chen, G. Synthesis and biological activities of local anesthetics. RSC Adv. 2019, 9, 41173–41191. [Google Scholar] [CrossRef] [PubMed]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Araújo, J.M.; Florindo, C.; Pereiro, A.B.; Vieira, N.S.; Matias, A.A.; Duarte, C.M.; Rebelo, L.P.N.; Marrucho, I.M. Cholinium-based ionic liquids with pharmaceutically active anions. RSC Adv. 2014, 4, 28126–28132. [Google Scholar] [CrossRef]
- Lee, P.Y.; Wong, K.K. Nanomedicine: A new frontier in cancer therapeutics. Curr. Drug Deliv. 2011, 8, 245–253. [Google Scholar]
- McCord, J.; Lang, J.R.; Hill, D.; Strynar, M.; Chernoff, N. pH dependent octanol–water partitioning coefficients of microcystin congeners. J. Water Health 2018, 16, 340–345. [Google Scholar] [CrossRef]
- Sahbaz, Y.; Williams, H.D.; Nguyen, T.-H.; Saunders, J.; Ford, L.; Charman, S.A.; Scammells, P.J.; Porter, C.J.H. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid-based formulations. Mol. Pharm. 2015, 12, 1980–1991. [Google Scholar] [CrossRef]
- Florindo, C.; de Araújo, J.M.M.; Alves, F.; Matos, C.; Ferraz, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.; Rebelo, L.P.N.; et al. Evaluation of solubility and partition properties of ampicillin-based ionic liquids. Int. J. Pharm. 2013, 456, 553–559. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Dharamdasani, V.; Mandal, A.; Qi, Q.M.; Suzuki, I.; Bentley, M.V.L.B.; Mitragotri, S. Topical delivery of siRNA into skin using ionic liquids. J. Control. Release 2020, 323, 475–482. [Google Scholar] [CrossRef]
- Gruss, M. Aspects for Developing and Processing Solid Forms. In Solid State Development and Processing of Pharmaceutical Molecules: Salts Cocrystals, and Polymorphism; John Wiley & Sons: Hoboken, NJ, USA, 2021; Volume 79, pp. 1–43. [Google Scholar]
- Chistyakov, D.; Sergeev, G. The Polymorphism of Drugs: New Approaches to the Synthesis of Nanostructured Polymorphs. Pharmaceutics 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Grant, D.J. Crystal structures of drugs: Advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Kara, D.D.; Rathnanand, M. Cocrystals and Drug–Drug Cocrystals of Anticancer Drugs: A Perception towards Screening Techniques, Preparation, and Enhancement of Drug Properties. Crystals 2022, 12, 1337. [Google Scholar] [CrossRef]
- Waskewitz, P. Machine vision in manufacturing. In Handbook of Machine Vision; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 693–776. [Google Scholar]
- Pedro, S.N.; Freire, C.S.; Silvestre, A.J.; Freire, M.G. Deep Eutectic Solvents and Pharmaceuticals. Encyclopedia 2021, 1, 942–963. [Google Scholar] [CrossRef]
- Torimoto, T.; Tsuda, T.; Okazaki, K.I.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef]
- Prodius, D.; Mudring, A.-V. Rare earth metal-containing ionic liquids. Coord. Chem. Rev. 2018, 363, 1–16. [Google Scholar] [CrossRef]
- Kleitz, F. Ordered Microporous and Mesoporous Materials. In Nanoscale Materials in Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 243–329. [Google Scholar]
- Benjamin, M.; Manoj, D.; Thenmozhi, K.; Bhagat, P.R.; Saravanakumar, D.; Senthilkumar, S. A bioinspired ionic liquid tagged cobalt-salophen complex for nonenzymatic detection of glucose. Biosens. Bioelectron. 2017, 91, 380–387. [Google Scholar] [CrossRef]
- Gorle, D.B.; Ponnada, S.; Kiai, M.S.; Nair, K.K.; Nowduri, A.; Swart, H.C.; Ang, E.H.; Nanda, K.K. Review on recent progress in metal–organic framework-based materials for fabricating electrochemical glucose sensors. J. Mater. Chem. B 2021, 9, 7927–7954. [Google Scholar] [CrossRef]
- López, M.S.P.; Mecerreyes, D.; López-Cabarcos, E.; López-Ruiz, B. Amperometric glucose biosensor based on polymerized ionic liquid microparticles. Biosens. Bioelectron. 2006, 21, 2320–2328. [Google Scholar] [CrossRef]
- Peng, D.; Picchioni, F. Prediction of toxicity of Ionic Liquids based on GC-COSMO method. J. Hazard. Mater. 2020, 398, 122964. [Google Scholar] [CrossRef]
- Almutairi, S.M.; El-Sayed, W.S.; Sahu, P.K.; Thasneema, K.K. Some 4-dimethylaminopyridinium-based ionic liquids and/or salts. Part I: Efficient green ultrasound synthesis, characterization, in silico prediction analysis, toxicity and antimicrobial evaluation. Arab. J. Chem. Environ. Res. 2021, 8, 29–46. [Google Scholar]
- Kurnia, K.A.; Sintra, T.; Neves, C.S.; Shimizu, K.; Canongia Lopes, J.N.; Gonçalves, F.J.M.; Ventura, S.; Freire, M.; Santos, L.; Coutinho, J.A.P. The effect of the cation alkyl chain branching on mutual solubilities with water and toxicities. Phys. Chem. Chem. Phys. 2014, 16, 19952–19963. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Paredes, X.; Cristino, A.; Santos, F.; Queirós, C. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, A.; Feng, L.; Ruan, H.; Li, H.; Dong, S.; Hao, J. Tunable amphiphilicity and multifunctional applications of ionic-liquid-modified carbon quantum dots. ACS Appl. Mater. Interfaces 2015, 7, 6919–6925. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Wang, Y.Z.; Zhao, X.L.; He, X.W.; Li, W.Y.; Zhang, Y.K. Facile synthesis of ionic liquid functionalized silica-capped CdTe quantum dots for selective recognition and detection of hemoproteins. J. Mater. Chem. B 2014, 2, 5659–5665. [Google Scholar] [CrossRef]
- Nayl, A.A.; Arafa, W.A.A.; Ahmed, I.M.; Abd-Elhamid, A.I.; El-Fakharany, E.M.; Abdelgawad, M.A.; Gomha, S.M.; Ibrahim, H.M.; Aly, A.A.; Bräse, S.; et al. Novel pyridinium based ionic liquid promoter for aqueous knoevenagel condensation: Green and efficient synthesis of new derivatives with their anticancer evaluation. Molecules 2022, 27, 2940. [Google Scholar] [CrossRef]
- Wu, H.; Deng, Z.; Zhou, B.; Qi, M.; Hong, M.; Ren, G. Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. J. Mol. Liq. 2019, 283, 399–409. [Google Scholar] [CrossRef]
- Clark, K.D.; Emaus, M.N.; Varona, M.; Bowers, A.N.; Anderson, J.L. Ionic liquids: Solvents and sorbents in sample preparation. J. Sep. Sci. 2018, 41, 209–235. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Wang, H.; Zhou, X.; Gurau, G.; Rogers, R.D. Ionic liquids in pharmaceutical industry. In Green Techniques for Organic Synthesis and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 539–577. [Google Scholar]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: A position paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef]
- Santos, M.M.; Raposo, L.R.; Carrera, G.V.S.M.; Costa, A.; Dionísio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. ChemMedChem 2019, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Magina, S.; Barros-Timmons, A.; Ventura, S.P.; Evtuguin, D.V. Evaluating the hazardous impact of ionic liquids–challenges and opportunities. J. Hazard. Mater. 2021, 412, 125215. [Google Scholar] [CrossRef] [PubMed]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E. Recent advances in ionic liquids in biomedicine. Adv. Sci. 2021, 8, 2004819. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, M.K.; Tiwari, H.; Verma, R.; Dong, W.-L.; Azizov, S.; Kumar, B.; Pandey, S.; Kumar, D. Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems. Pharmaceutics 2023, 15, 702. https://doi.org/10.3390/pharmaceutics15020702
Shukla MK, Tiwari H, Verma R, Dong W-L, Azizov S, Kumar B, Pandey S, Kumar D. Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems. Pharmaceutics. 2023; 15(2):702. https://doi.org/10.3390/pharmaceutics15020702
Chicago/Turabian StyleShukla, Monu Kumar, Harshita Tiwari, Rachna Verma, Wen-Liang Dong, Shavkatjon Azizov, Brajesh Kumar, Sadanand Pandey, and Deepak Kumar. 2023. "Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems" Pharmaceutics 15, no. 2: 702. https://doi.org/10.3390/pharmaceutics15020702
APA StyleShukla, M. K., Tiwari, H., Verma, R., Dong, W.-L., Azizov, S., Kumar, B., Pandey, S., & Kumar, D. (2023). Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems. Pharmaceutics, 15(2), 702. https://doi.org/10.3390/pharmaceutics15020702










