Effects of Tocilizumab on Inflammation and Iron Metabolism in Critically Ill Patients with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
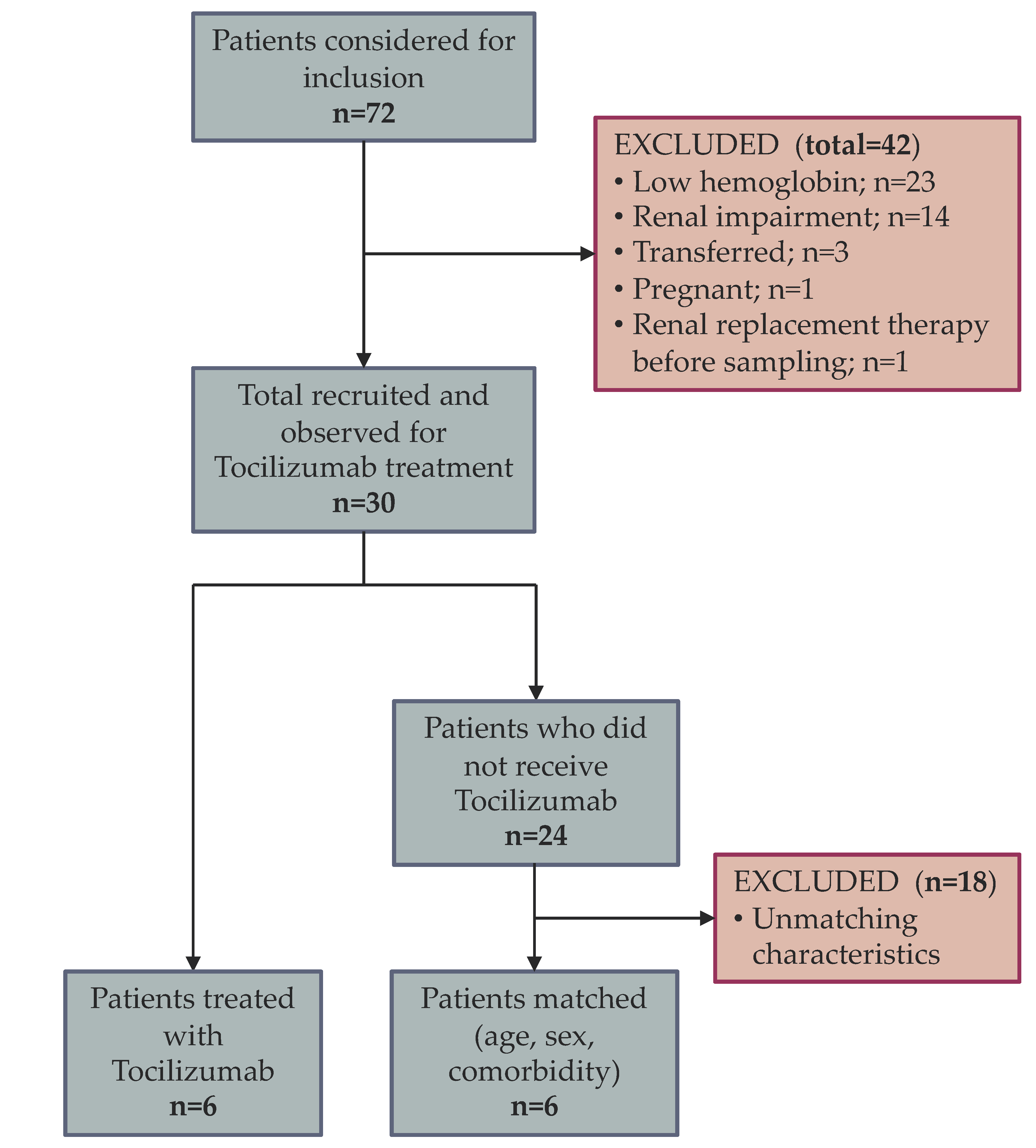
2.2. Participants
2.3. Variables and Data Source
2.4. Statistical Methods
3. Results
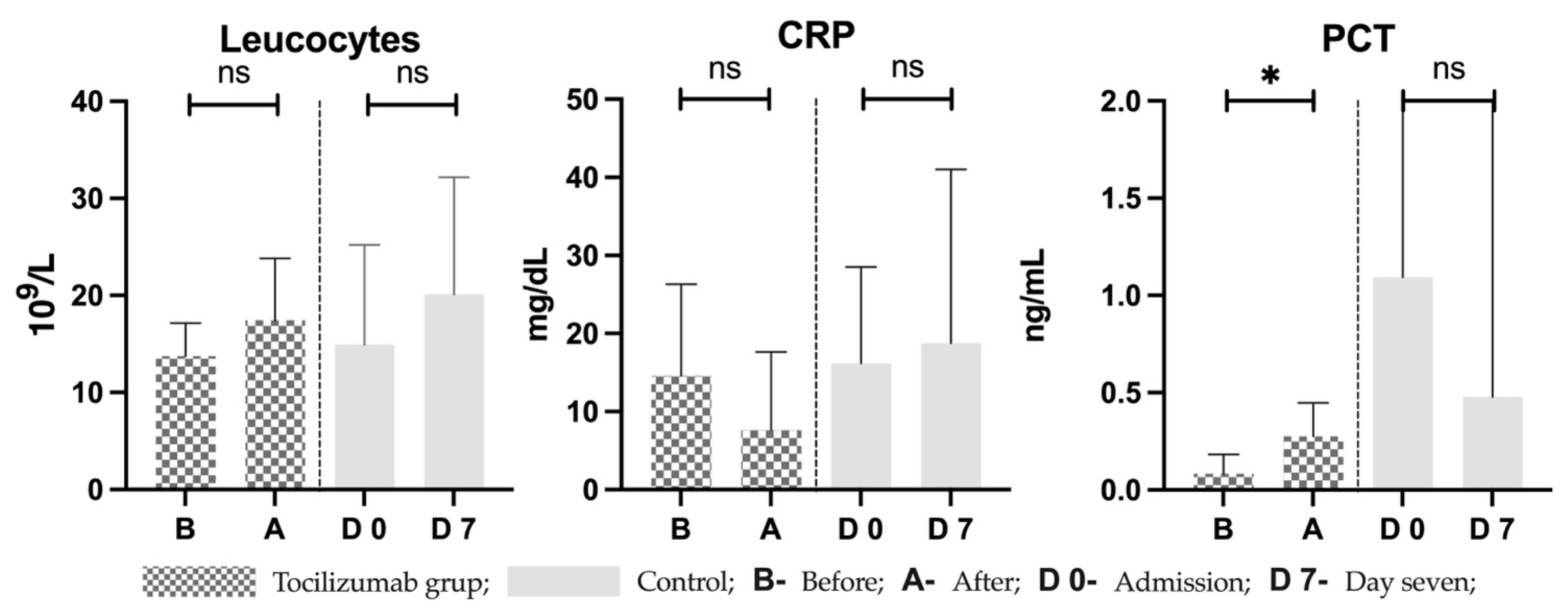
4. Discussion
4.1. COVID-19 May Produce Cytokine Storm and Persistent Inflammation
4.2. Anemia Is Multifactorial
4.3. Limitations of Tocilizumab and Potential Alternative Therapies
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Criteria for Defining Severe and Critical Illness
| Severe Cases |
| pulse oximetry reading < 94% (with supplemental oxygen) OR arterial oxygen pressure to fraction of inspired oxygen ratio (P/F) below 300 |
| elevated respiratory rate (>30 breaths per minute) and dyspnea |
| >50% lung infiltrate |
| Critically ill |
| respiratory insuficiency |
| cardio-circulatory instability requiring pressor support OR multiple organ failure |
References
- Roth, M.P.; Meynard, D.; Coppin, H. Regulators of hepcidin expression. Vitam. Horm. 2019, 110, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pastora, J.; Weigand, M.; Kim, J.; Wu, X.; Strayer, J.; Palmer, A.F.; Zborowski, M.; Yazer, M.; Chalmers, J.J. Hyperferritinemia in critically ill COVID-19 patients—Is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta 2020, 509, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, V.; Andras, D.; Amarinei, G.; Seicean, R.I.; Bintintan, V.V.; Dindelegan, G.C.; Cainap, C.I. Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study. Medicina 2022, 58, 1202. [Google Scholar] [CrossRef] [PubMed]
- Layoun, A.; Samba-Mondonga, M.; Fragoso, G.; Calvé, A.; Santos, M.M. MyD88 Adaptor Protein Is Required for Appropriate Hepcidin Induction in Response to Dietary Iron Overload in Mice. Front. Physiol. 2018, 9, 159. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Wisniewski, O.W.; Kolodziejski, P.; Krauss, H. Role of hepcidin in physiology and pathophysiology. Emerging experimental and clinical evidence. J. Physiol. Pharmacol. 2021, 72, 23–33. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef]
- Banchini, F.; Vallisa, D.; Maniscalco, P.; Capelli, P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020, 91, e2020013. [Google Scholar] [CrossRef]
- Bode, J.G.; Albrecht, U.; Haussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins--regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagunduz, P.; Tabak, F.; Atagunduz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti- inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Karaba, S.M.; Jones, G.; Helsel, T.; Smith, L.L.; Avery, R.; Dzintars, K.; Salinas, A.B.; Keller, S.C.; Townsend, J.L.; Klein, E.; et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open. Forum Infect. Dis. 2020, 8, ofaa578. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Szabo, R.; Petrisor, C.; Bodolea, C.; Simon, R.; Maries, I.; Tranca, S.; Mocan, T. Hyperferritinemia, Low Circulating Iron and Elevated Hepcidin May Negatively Impact Outcome in COVID-19 Patients: A Pilot Study. Antioxidants 2022, 11, 1364. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Andras, D.; Crisan, D.; Craciun, R.; Nemes, A.; Caziuc, A.; Drasovean, R.; Seicean, R.; Scurtu, R.; Bintintan, V.; Eniu, D.; et al. Neutrophil-to-lymphocyte ratio: A hidden gem in predicting neoadjuvant treatment response in locally advanced rectal cancer? J. BUON 2020, 25, 1436–1442. [Google Scholar]
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Shen, A.; Chen, Y.; Qi, Z. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin. Drug Investig. 2020, 40, 511–518. [Google Scholar] [CrossRef]
- Wang, D.; Fu, B.; Peng, Z.; Yang, D.; Han, M.; Li, M.; Yang, Y.; Yang, T.; Sun, L.; Li, W.; et al. Tocilizumab in patients with moderate or severe COVID-19: A randomized, controlled, open-label, multicenter trial. Front. Med. 2021, 15, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Lin, H.; Wei, R.G.; Chen, N.; He, F.; Zou, D.H.; Wei, J.R. Tocilizumab treatment for COVID-19 patients: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Snow, T.A.C.; Saleem, N.; Ambler, G.; Nastouli, E.; Singer, M.; Arulkumaran, N. Tocilizumab in COVID-19: A meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials. Intensive Care Med. 2021, 47, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Barciszewska, A.M. Elucidating of oxidative distress in COVID-19 and methods of its prevention. Chem. Biol. Interact. 2021, 344, 109501. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Ong, E.Z.; Chan, Y.F.Z.; Leong, W.Y.; Lee, N.M.Y.; Kalimuddin, S.; Haja Mohideen, S.M.; Chan, K.S.; Tan, A.T.; Bertoletti, A.; Ooi, E.E.; et al. A Dynamic Immune Response Shapes COVID-19 Progression. Cell Host Microbe 2020, 27, 879–882.e2. [Google Scholar] [CrossRef]
- Shanmugam, N.K.; Chen, K.; Cherayil, B.J. Commensal Bacteria-induced Interleukin 1beta (IL-1beta) Secreted by Macrophages Up-regulates Hepcidin Expression in Hepatocytes by Activating the Bone Morphogenetic Protein Signaling Pathway. J. Biol. Chem. 2015, 290, 30637–30647. [Google Scholar] [CrossRef]
- Sukhomlin, T. Hepcidin is a friend rather than a foe in COVID19-induced complications. Acta Biomed. 2020, 91, e2020138. [Google Scholar] [CrossRef]
- Peng, D.; Gao, Y.; Zhang, L.; Liu, Z.; Wang, H.; Liu, Y. The Relationship Between Hepcidin-Mediated Iron Dysmetabolism and COVID-19 Severity: A Meta-Analysis. Front. Public Health 2022, 10, 881412. [Google Scholar] [CrossRef]
- Guz, D.; Gafter-Gvili, A.; Lev, N.; Sahaf Levin, G.; Lev, S. Tocilizumab Treatment Effect on Iron Homeostasis in Severe COVID-19 Patients. Acta Haematol. 2022, 145, 440–447. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Harari, O.; Kobold, U.; Lee, J.S.; Bernasconi, C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R204. [Google Scholar] [CrossRef]
- Kawabata, H.; Tomosugi, N.; Kanda, J.; Tanaka, Y.; Yoshizaki, K.; Uchiyama, T. Anti-interleukin 6 receptor antibody tocilizumab reduces the level of serum hepcidin in patients with multicentric Castleman’s disease. Haematologica 2007, 92, 857–858. [Google Scholar] [CrossRef]
- Tothova, Z.; Tomc, J.; Debeljak, N.; Solar, P. STAT5 as a Key Protein of Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7109. [Google Scholar] [CrossRef]
- Sasaki, A.; Yasukawa, H.; Shouda, T.; Kitamura, T.; Dikic, I.; Yoshimura, A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J. Biol. Chem. 2000, 275, 29338–29347. [Google Scholar] [CrossRef]
- Shouda, T.; Yoshida, T.; Hanada, T.; Wakioka, T.; Oishi, M.; Miyoshi, K.; Komiya, S.; Kosai, K.; Hanakawa, Y.; Hashimoto, K.; et al. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Investig. 2001, 108, 1781–1788. [Google Scholar] [CrossRef]
- Wang, C.Y.; Babitt, J.L. Hepcidin regulation in the anemia of inflammation. Curr. Opin. Hematol. 2016, 23, 189–197. [Google Scholar] [CrossRef]
- Litton, E.; Xiao, J.; Allen, C.T.; Ho, K.M. Iron-restricted erythropoiesis and risk of red blood cell transfusion in the intensive care unit: A prospective observational study. Anaesth. Intensive Care 2015, 43, 612–616. [Google Scholar] [CrossRef]
- Filipescu, D.; Bănăţeanu, R.; Beuran, M.; Burcoş, T.; Corneci, D.; Cristian, D.; Diculescu, M.; Dobrotă, A.; Droc, G.; Isacoff, D.; et al. Perioperative Patient Blood Management Programme. Multidisciplinary recommendations from the Patient Blood Management Initiative Group. Rom. J. Anaesth. Intensive Care 2017, 24, 139–157. [Google Scholar] [CrossRef]
- Song, S.N.; Tomosugi, N.; Kawabata, H.; Ishikawa, T.; Nishikawa, T.; Yoshizaki, K. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood 2010, 116, 3627–3634. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ippolito, M.; Greco, M.; Granone, V.; Protti, A.; Gregoretti, C.; Giarratano, A.; Einav, S.; Cecconi, M. Rationale and evidence on the use of tocilizumab in COVID-19: A systematic review. Pulmonology 2021, 27, 52–66. [Google Scholar] [CrossRef]
- Mendoza, V.M.M. Interleukin-17: A potential therapeutic target in COVID-19. J. Infect. 2020, 81, e136–e138. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-alpha for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Colardyn, F.A.; Philippe, J.J.; Vanholder, R.C.; De Sutter, J.H.; Lameire, N.H. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol. 1999, 10, 846–853. [Google Scholar] [CrossRef]
- Szabo, R.; Bodolea, C.; Mocan, T. Iron, Copper, and Zinc Homeostasis: Physiology, Physiopathology, and Nanomediated Applications. Nanomaterials 2021, 11, 2958. [Google Scholar] [CrossRef]
- Antonoglou, O.; Lafazanis, K.; Mourdikoudis, S.; Vourlias, G.; Lialiaris, T.; Pantazaki, A.; Dendrinou-Samara, C. Biological relevance of CuFeO2 nanoparticles: Antibacterial and anti- inflammatory activity, genotoxicity, DNA and protein interactions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 264–274. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Khaniabadi, P.M.; Aziz, A.A.; Jameel, M.S.; Mehrdel, B.; Oglat, A.A.; Khaleel, H.A. Focused role of nanoparticles against COVID-19: Diagnosis and treatment. Photodiagn. Photodyn. Ther. 2021, 34, 102287. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Toudjarska, I.; Sendamarai, A.K.; Racie, T.; Milstein, S.; Bettencourt, B.R.; Hettinger, J.; Bumcrot, D.; Fleming, M.D. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood 2013, 121, 1200–1208. [Google Scholar] [CrossRef]
- Masjedi, A.; Ahmadi, A.; Atyabi, F.; Farhadi, S.; Irandoust, M.; Khazaei-Poul, Y.; Chaleshtari, M.G.; Fathabad, M.E.; Baghaei, M.; Haghnavaz, N. Silencing of IL-6 and STAT3 by siRNA loaded hyaluronate-N, N, N-trimethyl chitosan nanoparticles potently reduces cancer cell progression. Int. J. Biol. Macromol. 2020, 149, 487–500. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.; Bhang, S.H.; Kim, B.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate–gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. Acs Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef]
- Wang, S.; Reinhard, S.; Li, C.; Qian, M.; Jiang, H.; Du, Y.; Lächelt, U.; Lu, W.; Wagner, E.; Huang, R. Antitumoral cascade-targeting ligand for IL-6 receptor-mediated gene delivery to glioma. Mol. Ther. 2017, 25, 1556–1566. [Google Scholar] [CrossRef]
- Rao, L.; Xia, S.; Xu, W.; Tian, R.; Yu, G.; Gu, C.; Pan, P.; Meng, Q.; Cai, X.; Qu, D. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2020, 117, 27141–27147. [Google Scholar] [CrossRef]
- Qin, M.; Landriscina, A.; Rosen, J.M.; Wei, G.; Kao, S.; Olcott, W.; Agak, G.W.; Paz, K.B.; Bonventre, J.; Clendaniel, A. Nitric oxide–releasing nanoparticles prevent Propionibacterium acnes–induced inflammation by both clearing the organism and inhibiting microbial stimulation of the innate immune response. J. Investig. Dermatol. 2015, 135, 2723–2731. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, S.; Kim, Y.J.; Perumalsamy, H.; Lee, S.; Hwang, E.; Yi, T.H. Green synthesis of gold nanoparticles using Euphrasia officinalisleaf extract to inhibit lipopolysaccharide-induced inflammation through NF-kappaB and JAK/STAT pathways in RAW 264.7 macrophages. Int. J. Nanomed. 2019, 14, 2945–2959. [Google Scholar] [CrossRef]



| General | Control (C) | Treatment (T) | Normal Value | p-Value (C and T) | |
|---|---|---|---|---|---|
| Patients | |||||
| Total | 12 | 6 | 6 | - | - |
| Female n (%) | 8 (67) | 4 (67) | 4 (67) | - | 0.99 |
| Male n (%) | 4 (33) | 2 (33) | 2 (33) | - | |
| Mean (±SD) or Median (range) | |||||
| Age (years) | 67 (±13) | 67.83 (±11.82) | 66.17 (±14.77) | - | 0.8335 |
| Charlson | 3 (±1) | 3.500 (±1.38) | 3.333 (±1.97) | - | 0.8684 |
| APACHE II score | 13 (±8) | 11.33 (±5.72) | 15.50 (±10.23) | - | 0.4043 |
| Hemoglobin (g/dL) | 13.37 (±0.6) | 13.47 (±0.62) | 13.27 (±0.62) | Female: 12–15.5 | 0.5868 |
| Male: 13–17 | |||||
| MCV (fL) | 87.85 (±2.5) | 87.35 (±2.29) | 88.35 (±2.8) | 80–95 | 0.5139 |
| TS (%) | 17.67 (±10.63) | 17.00 (±9.84) | 18.33 (±12.27) | 16–45 | 0.8397 |
| Transferrin (mg/dL) | 139.6 (±48.22) | 154.3 (±65.77) | 125.0 (±16.49) | 200–360 | 0.3143 |
| Ferritin (ng/mL) | 969.5 (650.8–2199) | 1145 (595–2587) | 913 (675.8–1695) | 15–200 | 0.9372 |
| Iron (µg/dL) | 31.67 (±18.65) | 29.83 (±12.94) | 33.50 (±24.28) | 65–180 | 0.7508 |
| Hepcidin (pg/mL) | 247.7 (±69.72) | 258.7 (±103.5) | 238.6 (±30.55) | * | 0.6580 |
| IL-6 (pg/mL) | 147.5 (36.15–372.8) | 83.40 (15.10–884.3) | 178.3 (103.5–295.6) | * | 0.4500 |
| Leucocytes (109/L) | 13.56 (±7.19) | 14.94 (±10.29) | 12.18 (±1.83) | <14 | 0.5332 |
| Neutrophiles (109/L) | 12.13 (±7.61) | 13.52 (±10.87) | 10.74 (±2.15) | 1.5–7.4 | 0.5532 |
| Lymphocytes (109/L) | 0.87 (0.37–1.23) | 0.5850 (0.3–1.46) | 1.130 (0.66–14.19) | 1.5–3.5 | 0.3095 |
| Thrombocytes (109/L) | 303.9 (±92.41) | 254.7 (±54.69) | 353.2 (±99.87) | 150–380 | 0.0601 |
| CRP (mg/dL) | 15.17 (±11.60) | 16.18 (±12.35) | 14.15 (±11.88) | <0.5 | 0.7780 |
| PCT (ng/mL) | 0.15 (0.05–1.58) | 1.095 (0.11–12.73) | 0.11 (0.05–0.18) | <0.5 | 0.2597 |
| ALAT (U/L) | 34 (±8.79) | 30.50 (±3.017) | 37.50 (±11.47) | <35 | 0.1788 |
| Albumin (g/dL) | 2.82 (±0.55) | 2.8 (±0.79) | 2.84 (±0.19) | 3.5–5 | 0.9067 |
| Fibrinogen (mg/dL) | 524.6 (±124.0) | 494.3 (±147.3) | 555.0 (±99.44) | 200–400 | 0.4223 |
| Bilirubin (mg/dL) | 0.55 (±0.18) | 0.58 (±0.21) | 0.53 (±0.16) | <1.2 | 0.6683 |
| Creatinine (mg/dL) | 1.0 (±0.561) | 1.21 (±0.7041) | 0.79 (±0.3022) | <1.2 | 0.2090 |
| LDH (U/L) | 483.6 (±188.8) | 385.0 (±57.82) | 582.2 (±227.4) | <250 | 0.0666 |
| Lipase (U/L) | 32.42 (±15.60) | 34.0 (±18.34) | 30.83 (±13.89) | <67 | 0.7430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabo, R.; Petrișor, C.; Bodolea, C.; Dobre, V.; Tranca, S.; Clichici, S.; Szabo, I.; Melinte, R.M.; Mocan, T. Effects of Tocilizumab on Inflammation and Iron Metabolism in Critically Ill Patients with COVID-19. Pharmaceutics 2023, 15, 646. https://doi.org/10.3390/pharmaceutics15020646
Szabo R, Petrișor C, Bodolea C, Dobre V, Tranca S, Clichici S, Szabo I, Melinte RM, Mocan T. Effects of Tocilizumab on Inflammation and Iron Metabolism in Critically Ill Patients with COVID-19. Pharmaceutics. 2023; 15(2):646. https://doi.org/10.3390/pharmaceutics15020646
Chicago/Turabian StyleSzabo, Robert, Cristina Petrișor, Constantin Bodolea, Vlad Dobre, Sebastian Tranca, Simona Clichici, Iulia Szabo, Razvan Marian Melinte, and Teodora Mocan. 2023. "Effects of Tocilizumab on Inflammation and Iron Metabolism in Critically Ill Patients with COVID-19" Pharmaceutics 15, no. 2: 646. https://doi.org/10.3390/pharmaceutics15020646
APA StyleSzabo, R., Petrișor, C., Bodolea, C., Dobre, V., Tranca, S., Clichici, S., Szabo, I., Melinte, R. M., & Mocan, T. (2023). Effects of Tocilizumab on Inflammation and Iron Metabolism in Critically Ill Patients with COVID-19. Pharmaceutics, 15(2), 646. https://doi.org/10.3390/pharmaceutics15020646








