Low-Density Lipoprotein Pathway Is a Ubiquitous Metabolic Vulnerability in High Grade Glioma Amenable for Nanotherapeutic Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples and Cell Lines
2.2. Immunohistochemistry (IHC)
2.3. Cell Culture and Reagents
2.4. Immunofluorescence (IF)
2.5. Statistical Analysis
3. Results
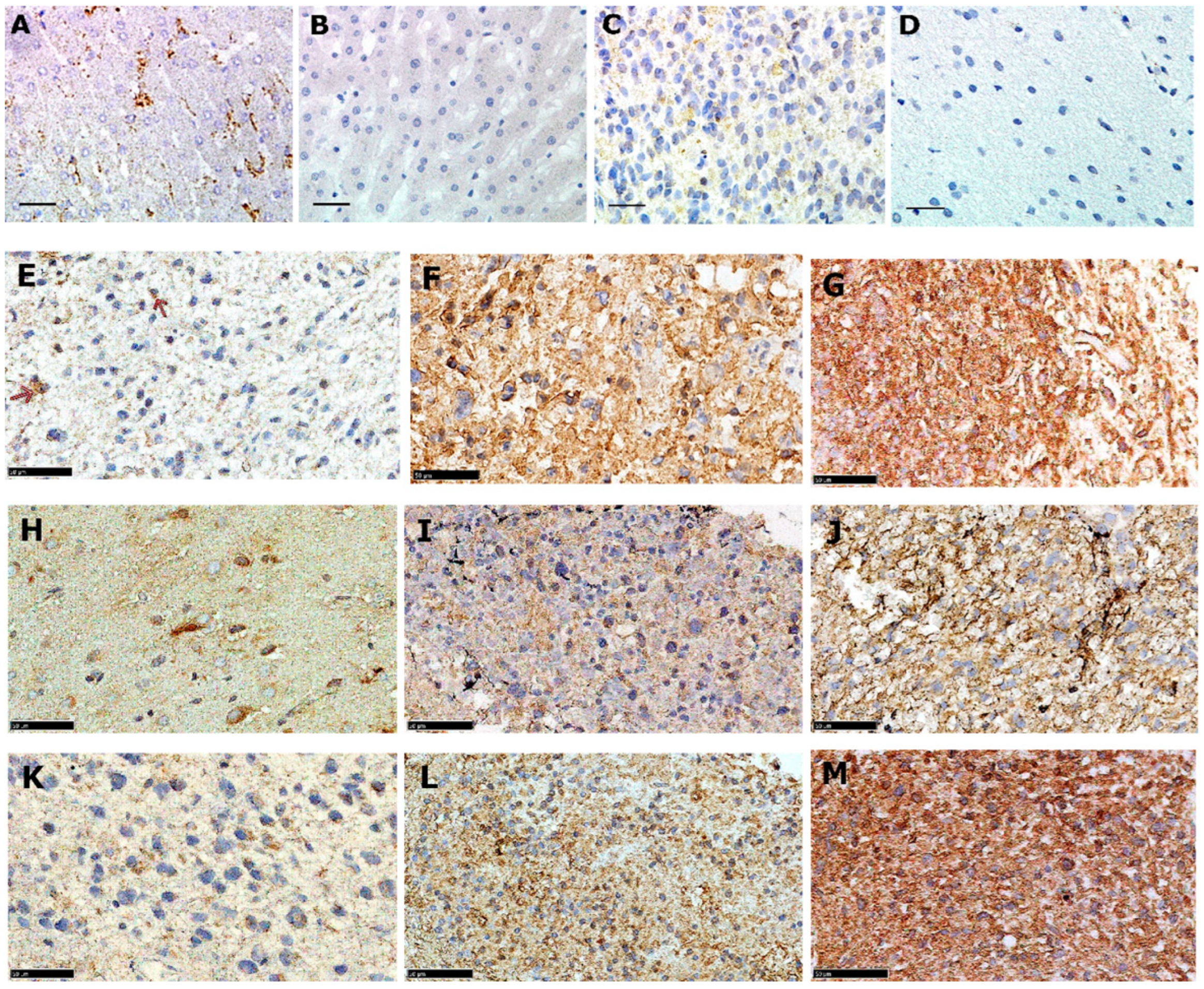
3.1. LDLR Protein Is Expressed in a Majority of Adult GBMs with Intra-Tumour Variation
3.2. LDLR Is Expressed in Paediatric Malignant Gliomas Irrespective of Tumour Grade
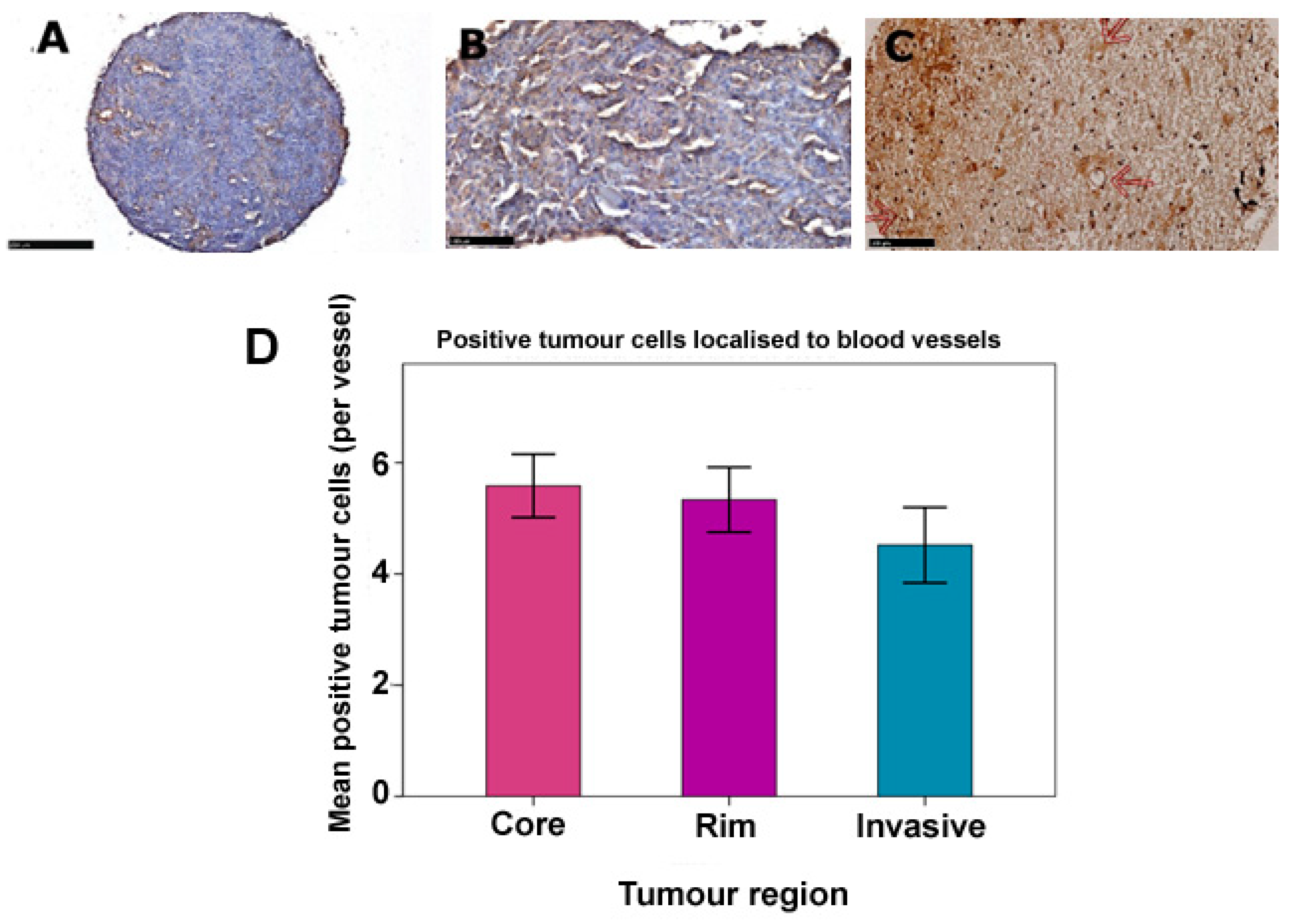
3.3. Glioma LDLR Expression Is Localized within Peri-Vascular Niches
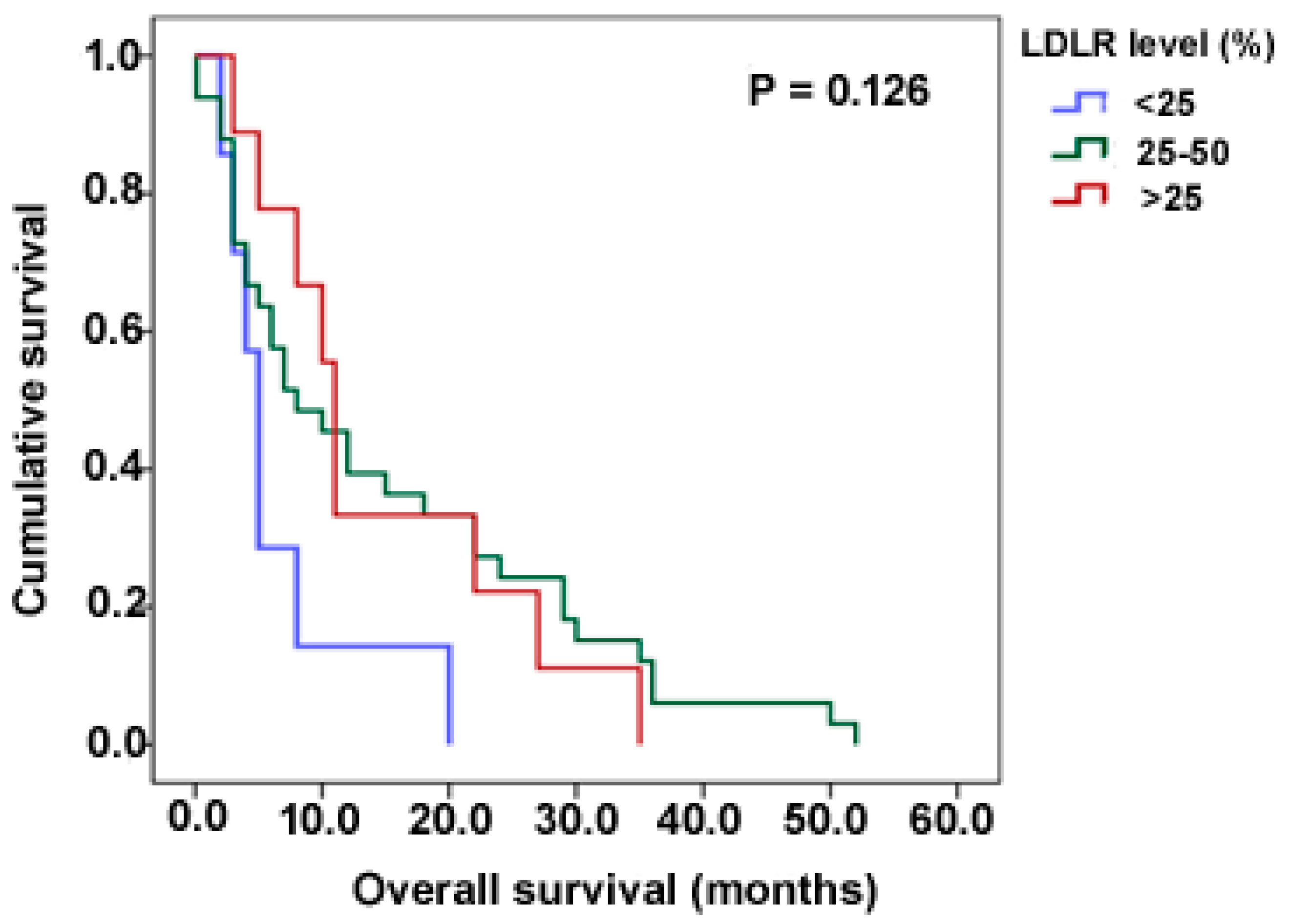
3.4. LDLR Expression Is Not Dependent on Clinicopathological Variables
3.5. Adult and Paediatric GBM Cell Lines Express Variable Levels of LDLR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; Curschmann, J.; Janzer, R. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2005, 1, 19–28. [Google Scholar] [CrossRef]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavare, S. Intratumor Heterogeneity in Human Glioblastoma Reflects Cancer Evolutionary Dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef]
- Rao, J.S. Molecular Mechanisms of Glioma Invasiveness: The Role of Proteases. Nat. Rev. Cancer 2003, 3, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Cloughesy, T.F.; Mischel, P.S. Molecular Pathology in Adult High-grade Gliomas: From Molecular Diagnostics to Target Therapies. Neuropathol. Appl. Neurobiol. 2012, 38, 271–291. [Google Scholar] [CrossRef]
- Maher, E.A.; Furnari, F.B.; Bachoo, R.M.; Rowitch, D.H.; Louis, D.N.; Cavenee, W.K.; DePinho, R.A. Malignant Glioma: Genetics and Biology of a Grave Matter. Genes Dev. 2001, 15, 1311–1333. [Google Scholar] [CrossRef]
- Burnet, N.G.; Jefferies, S.J.; Benson, R.J.; Hunt, D.P.; Treasure, F.P. Years of Life Lost (YLL) from Cancer Is an Important Measure of Population Burden—And Should Be Considered When Allocating Research Funds. Br. J. Cancer 2005, 92, 241–245. [Google Scholar] [CrossRef]
- Walker, M.D.; Green, S.B.; Byar, D.P.; Alexander, E.; Batzdorf, U.; Brooks, W.H.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; et al. Randomized Comparisons of Radiotherapy and Nitrosoureas for the Treatment of Malignant Glioma after Surgery. N. Engl. J. Med. 1980, 303, 1323–1329. [Google Scholar] [CrossRef]
- Brada, M.; Hoang-Xuan, K.; Rampling, R.; Dietrich, P.-Y.; Dirix, L.Y.; Macdonald, D.; Heimans, J.J.; Zonnenberg, B.A.; Bravo-Marques, J.M.; Henriksson, R.; et al. Multicenter Phase II Trial of Temozolomide in Patients with Glioblastoma Multiforme at First Relapse. Ann. Oncol. 2001, 12, 259–266. [Google Scholar] [CrossRef]
- Germano, I.; Swiss, V.; Casaccia, P. Primary Brain Tumors, Neural Stem Cell, and Brain Tumor Cancer Cells: Where Is the Link? Neuropharmacology 2010, 58, 903–910. [Google Scholar] [CrossRef]
- Needham, D.; Arslanagic, A.; Glud, K.; Hervella, P.; Karimi, L.; Høeilund-Carlsen, P.-F.; Kinoshita, K.; Mollenhauer, J.; Parra, E.; Utoft, A.; et al. Bottom up Design of Nanoparticles for Anti-Cancer Diapeutics: “Put the Drug in the Cancer’s Food”. J. Drug Target. 2016, 24, 836–856. [Google Scholar] [CrossRef] [PubMed]
- Paugh, B.S.; Qu, C.; Jones, C.; Liu, Z.; Adamowicz-Brice, M.; Zhang, J.; Bax, D.A.; Coyle, B.; Barrow, J.; Hargrave, D.; et al. Integrated Molecular Genetic Profiling of Pediatric High-Grade Gliomas Reveals Key Differences with the Adult Disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumor Heterogeneity: Causes and Consequences. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Wilson, M.; Ward, J.H.; Rahman, C.V.; Peet, A.C.; Macarthur, D.C.; Rose, F.R.A.J.; Grundy, R.G.; Rahman, R. Recapitulation of Tumor Heterogeneity and Molecular Signatures in a 3D Brain Cancer Model with Decreased Sensitivity to Histone Deacetylase Inhibition. PLoS ONE 2012, 7, e52335. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Miyazaki, H.; Vaidyan, L.K.; Kagawa, Y.; Ebrahimi, M.; Yamamoto, Y.; Ogata, M.; Katsuyama, Y.; Sadahiro, H.; Suzuki, M.; et al. Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS ONE 2016, 11, e0147717. [Google Scholar] [CrossRef]
- Rudling, M.J.; Angelin, B.; Peterson, C.O.; Collins, V.P. Low Density Lipoprotein Receptor Activity in Human Intracranial Tumors and Its Relation to the Cholesterol Requirement. Cancer Res. 1990, 50, 483–487. [Google Scholar]
- Maletínská, L.; Blakely, E.A.; Bjornstad, K.A.; Deen, D.F.; Knoff, L.J.; Forte, T.M. Human Glioblastoma Cell Lines: Levels of Low-Density Lipoprotein Receptor and Low-Density Lipoprotein Receptor-Related Protein. Cancer Res. 2000, 60, 2300–2303. [Google Scholar]
- Havel, R.J. The Formation of LDL: Mechanisms and Regulation. J. Lipid Res. 1984, 25, 1570–1576. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. M58 A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Nikanjam, M.; Gibbs, A.R.; Hunt, C.A.; Budinger, T.F.; Forte, T.M. Synthetic Nano-LDL with Paclitaxel Oleate as a Targeted Drug Delivery Vehicle for Glioblastoma Multiforme. J. Control. Release 2007, 124, 163–171. [Google Scholar] [CrossRef]
- Teerlink, T.; Scheffer, P.G.; Bakker, S.J.L.; Heine, R.J. Combined Data from LDL Composition and Size Measurement Are Compatible with a Discoid Particle Shape. J. Lipid Res. 2004, 45, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhan, J.; Fang, W.; Zhao, Y.; Yang, Y.; Hou, X.; Zhang, Z.; He, X.; Zhang, Y.; Huang, Y.; et al. Serum Low-Density Lipoprotein and Low-Density Lipoprotein Expression Level at Diagnosis Are Favorable Prognostic Factors in Patients with Small-Cell Lung Cancer (SCLC). BMC Cancer 2017, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues dos Santos, C.; Fonseca, I.; Dias, S.; de Almeida, J.C.M. Plasma Level of LDL-Cholesterol at Diagnosis Is a Predictor Factor of Breast Tumor Progression. BMC Cancer 2014, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Lacko, A.G.; Nair, M.; Prokai, L.; McConathy, W.J. Prospects and Challenges of the Development of Lipoprotein-Based Formulations for Anti-Cancer Drugs. Expert Opin. Drug Deliv. 2007, 4, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, N.; Lacko, A.G. Drug Delivery via Lipoprotein-Based Carriers: Answering the Challenges in Systemic Therapeutics. Ther. Deliv. 2012, 3, 599–608. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-Mediated Peptide-22-Conjugated Nanoparticles for Dual-Targeting Therapy of Brain Glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef]
- Huntosova, V.; Buzova, D.; Petrovajova, D.; Kasak, P.; Nadova, Z.; Jancura, D.; Sureau, F.; Miskovsky, P. Development of a New LDL-Based Transport System for Hydrophobic/Amphiphilic Drug Delivery to Cancer Cells. Int. J. Pharm. 2012, 436, 463–471. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, 189. [Google Scholar] [CrossRef]
- dos Santos, C.R.; Domingues, G.; Matias, I.; Matos, J.; Fonseca, I.; de Almeida, J.M.; Dias, S. LDL-Cholesterol Signaling Induces Breast Cancer Proliferation and Invasion. Lipids Health Dis. 2014, 13, 16. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Zelenko, Z.; Neel, B.A.; Antoniou, I.M.; Rajan, L.; Kase, N.; LeRoith, D. Elevated Tumor LDLR Expression Accelerates LDL Cholesterol-Mediated Breast Cancer Growth in Mouse Models of Hyperlipidemia. Oncogene 2017, 36, 6462–6471. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Diksin, M.; Chhaya, S.; Sairam, S.; Estevez-cebrero, M.A. The Invasive Region of Glioblastoma Defined by 5ALA Guided Surgery Has an Altered Cancer Stem Cell Marker Profile Compared to Central Tumour. Int. J. Mol. Sci. 2017, 18, 2452. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.; Loo, M.A.; Tran, N.; Haskett, D.; Coons, S.W.; Berens, M.E. Dichotomy of Astrocytoma Migration and Proliferation. Int. J. Cancer 1996, 67, 275–282. [Google Scholar] [CrossRef]
- Xie, Q.; Mittal, S.; Berens, M.E. Targeting Adaptive Glioblastoma: An Overview of Proliferation and Invasion. Neuro-Oncology 2014, 16, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Kopeček, J.; Kopečková, P.; Minko, T.; Lu, Z.-R. HPMA Copolymer–Anticancer Drug Conjugates: Design, Activity, and Mechanism of Action. Eur. J. Pharm. Biopharm. 2000, 50, 61–81. [Google Scholar] [CrossRef]
- Duncan, R. The Dawning Era of Polymer Therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J. Natl. Cancer Inst. 2006, 98, 335–344. [Google Scholar] [CrossRef]
- Pinzõn-Daza, M.L.; Garzõn, R.; Couraud, P.O.; Romero, I.; Weksler, B.; Ghigo, D.; Bosia, A.; Riganti, C. The Association of Statins plus LDL Receptor-Targeted Liposome-Encapsulated Doxorubicin Increases in Vitro Drug Delivery across Blood-Brain Barrier Cells. Br. J. Pharmacol. 2012, 167, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, P.P.; Cascante, A.; Vilà, P.B.; Vallejo, V.G.; Llop, J.; Borrós, S. Peptide-Functionalized and High Drug Loaded Novel Nanoparticles as Dual-Targeting Drug Delivery System for Modulated and Controlled Release of Paclitaxel to Brain Glioma. Int. J. Pharm. 2018, 553, 169–185. [Google Scholar] [CrossRef]
- Seo, S.; Kim, E.H.; Chang, W.-S.; Lee, W.-S.; Kim, K.-H.; Kim, J.-K. Enhanced Proton Treatment with a LDLR-Ligand Peptide-Conjugated Gold Nanoparticles Targeting the Tumor Microenvironment in an Infiltrative Brain Tumor Model. Am. J. Cancer Res. 2022, 12, 198–209. [Google Scholar] [PubMed]


| Variable | Adult a Frequency (%) | Paediatric b Frequency (%) |
|---|---|---|
| Age (years) c | ||
| paediatric d | 29 (39.2) | |
| <6 | 30 (40.5) | |
| 6–12 | 15 (20.3) | |
| >12 | ||
| Adult | 7 (19.4) | |
| <50 | 16 (44.4) | |
| 50–65 | 13 (36.1) | |
| >65 | ||
| Gender d | ||
| Male | 18 (50) | 53 (64.6) |
| Female | 18 (50) | 29 (35.4) |
| Tumour grade d | ||
| III (AA) | - | 13 (19.1) |
| IV (GBM) | 36 (100) | 55 (80.9) |
| Tumour location d | ||
| Supratentorial | ||
| Infratentorial | 36 (100) | 57 (73.1) |
| Treatment (post-surgery) d | - | 21 (26.9) |
| Radiotherapy | ||
| Chemotherapy | 2 (12.5) | 9 (18.8) |
| Chemotherapy + Radiotherapy | 1 (6.3) | 15 (31.3) |
| None | 12 (75) | 23 (47.9) |
| Survival time (months) d,e | 1 (6.3) | 1 (2.1) |
| <12 | ||
| 12–36 | 5 (100) | 29 (63) |
| >36 | - | 15 (32.6) |
| Tumour type d | - | 2 (4.3) |
| Primary | ||
| Recurrent | ||
| progressive | N/A | 66 (84.6) |
| N/A | 10 (12.8) | |
| N/A | 2 (2.6) |
| % Expression | Core | Frequency (%) Rim | Invasive |
|---|---|---|---|
| Negative | 0 | 0 | 0 |
| <25 | 3 (8.3) | 8 (22.2) | 8 (25.8) |
| 25–50 | 14 (38.9) | 19 (52.8) | 15 (48.4) |
| >50 | 19 (52.8) | 9 (25) | 8 (25.8) |
| Total | 36 (100) | 36 (100) | 31 (100) * |
| % LDLR Expression | Frequency (%) |
|---|---|
| Negative | 5 (3.8) |
| <25 | 16 (12) |
| 25–50 | 77 (57.9) |
| >50 | 35 (26.3) |
| Total | 133 (100%) |
| LDLR Expression a | Core | Frequency (%) Adult Rim | Invasive | Paediatric b |
|---|---|---|---|---|
| Low | 1 (2.8) | 4 (11.1) | 8 (27.6) | 22 (18.0) |
| Moderate | 24 (66.7) | 23 (63.9) | 17 (58.6) | 86 (70.5) |
| High | 11 (30.6) | 9 (25.0) | 4 (13.8) | 14 (11.5) |
| Total | 36 (100) | 36 (100) | 29 (100) | 122 (100) |
| Variable | Core | Adult Rim | Invasive | Paediatric |
|---|---|---|---|---|
| Age | 0.047 | 0.653 | 0.578 | 0.445 |
| Gender | 0.106 | 0.223 | 0.623 | 0.270 |
| Tumour site a | 0.382 | 0.173 | 0.462 | 0.846 |
| Treatment | 0.186 | 0.173 | 0.362 | 0.431 |
| Tumour type b | - | - | - | 0.958 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekeye, A.O.; Needham, D.; Rahman, R. Low-Density Lipoprotein Pathway Is a Ubiquitous Metabolic Vulnerability in High Grade Glioma Amenable for Nanotherapeutic Delivery. Pharmaceutics 2023, 15, 599. https://doi.org/10.3390/pharmaceutics15020599
Adekeye AO, Needham D, Rahman R. Low-Density Lipoprotein Pathway Is a Ubiquitous Metabolic Vulnerability in High Grade Glioma Amenable for Nanotherapeutic Delivery. Pharmaceutics. 2023; 15(2):599. https://doi.org/10.3390/pharmaceutics15020599
Chicago/Turabian StyleAdekeye, Adenike O., David Needham, and Ruman Rahman. 2023. "Low-Density Lipoprotein Pathway Is a Ubiquitous Metabolic Vulnerability in High Grade Glioma Amenable for Nanotherapeutic Delivery" Pharmaceutics 15, no. 2: 599. https://doi.org/10.3390/pharmaceutics15020599
APA StyleAdekeye, A. O., Needham, D., & Rahman, R. (2023). Low-Density Lipoprotein Pathway Is a Ubiquitous Metabolic Vulnerability in High Grade Glioma Amenable for Nanotherapeutic Delivery. Pharmaceutics, 15(2), 599. https://doi.org/10.3390/pharmaceutics15020599






