A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare
Abstract
1. Introduction
2. Classification of Eudragit Polymer
3. Characterization of Eudragit
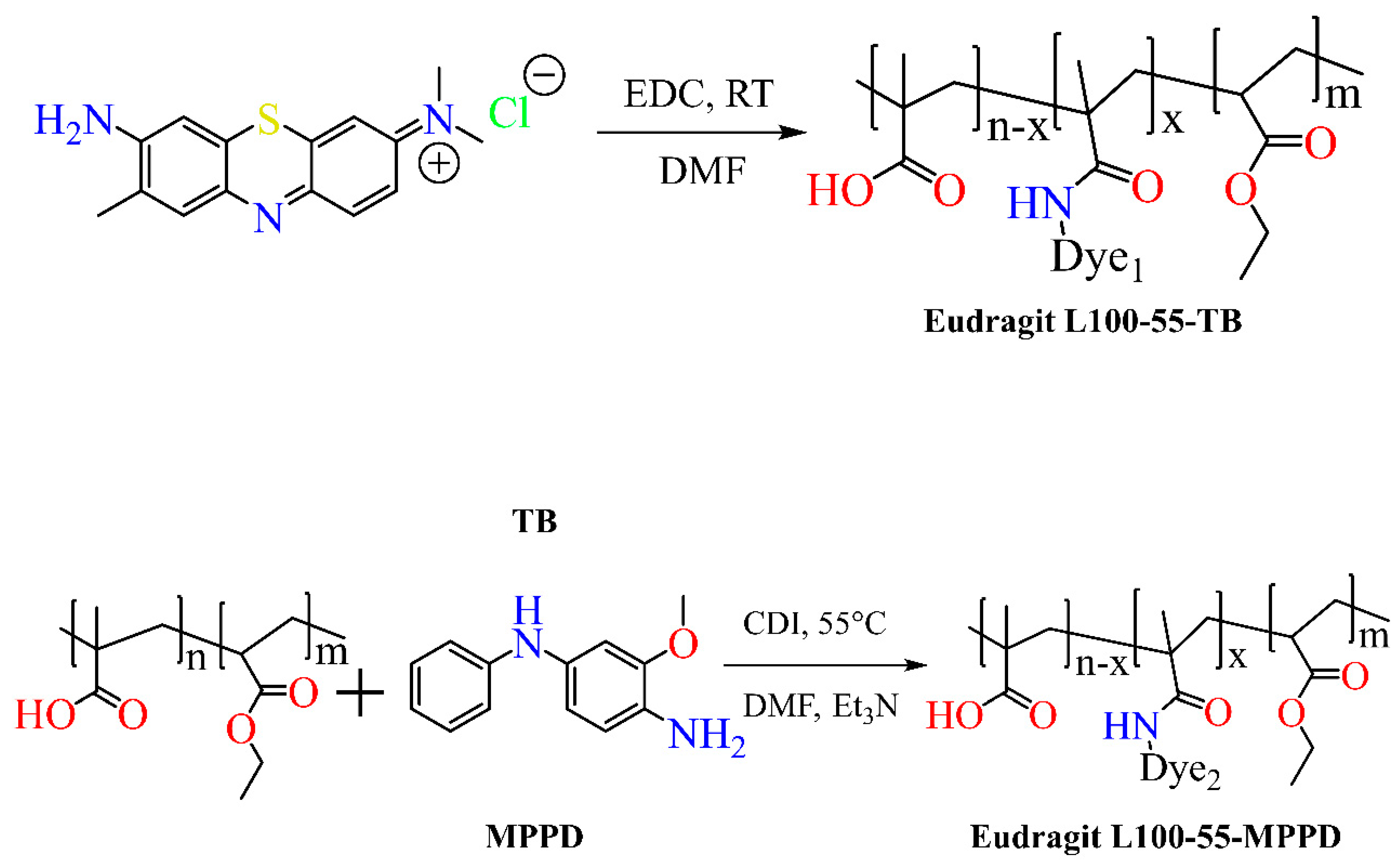
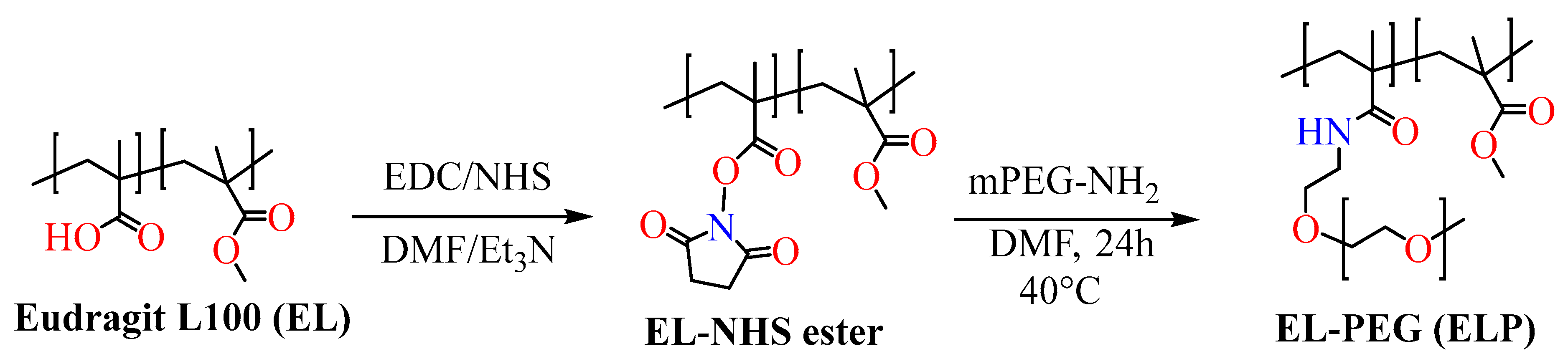
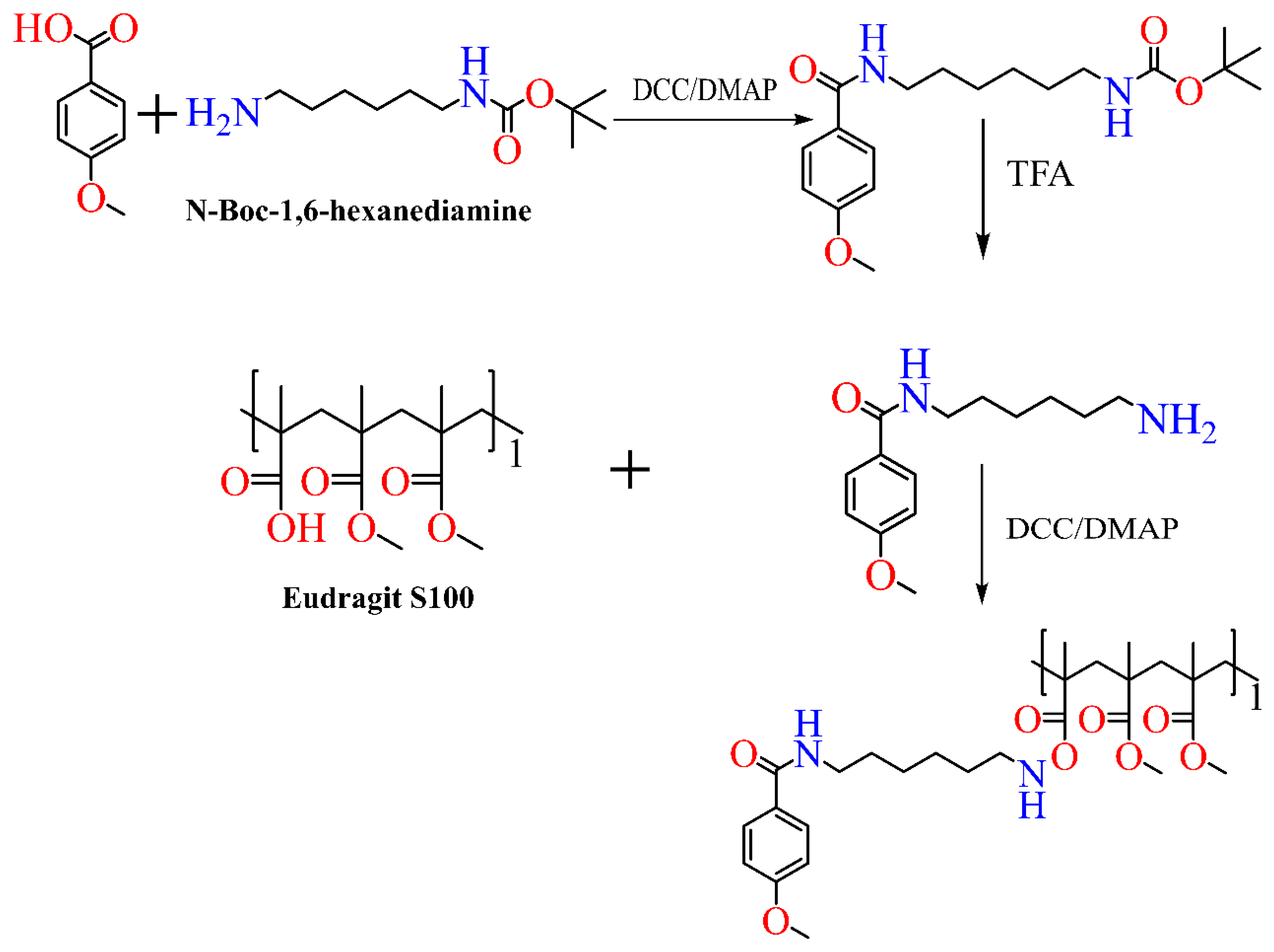
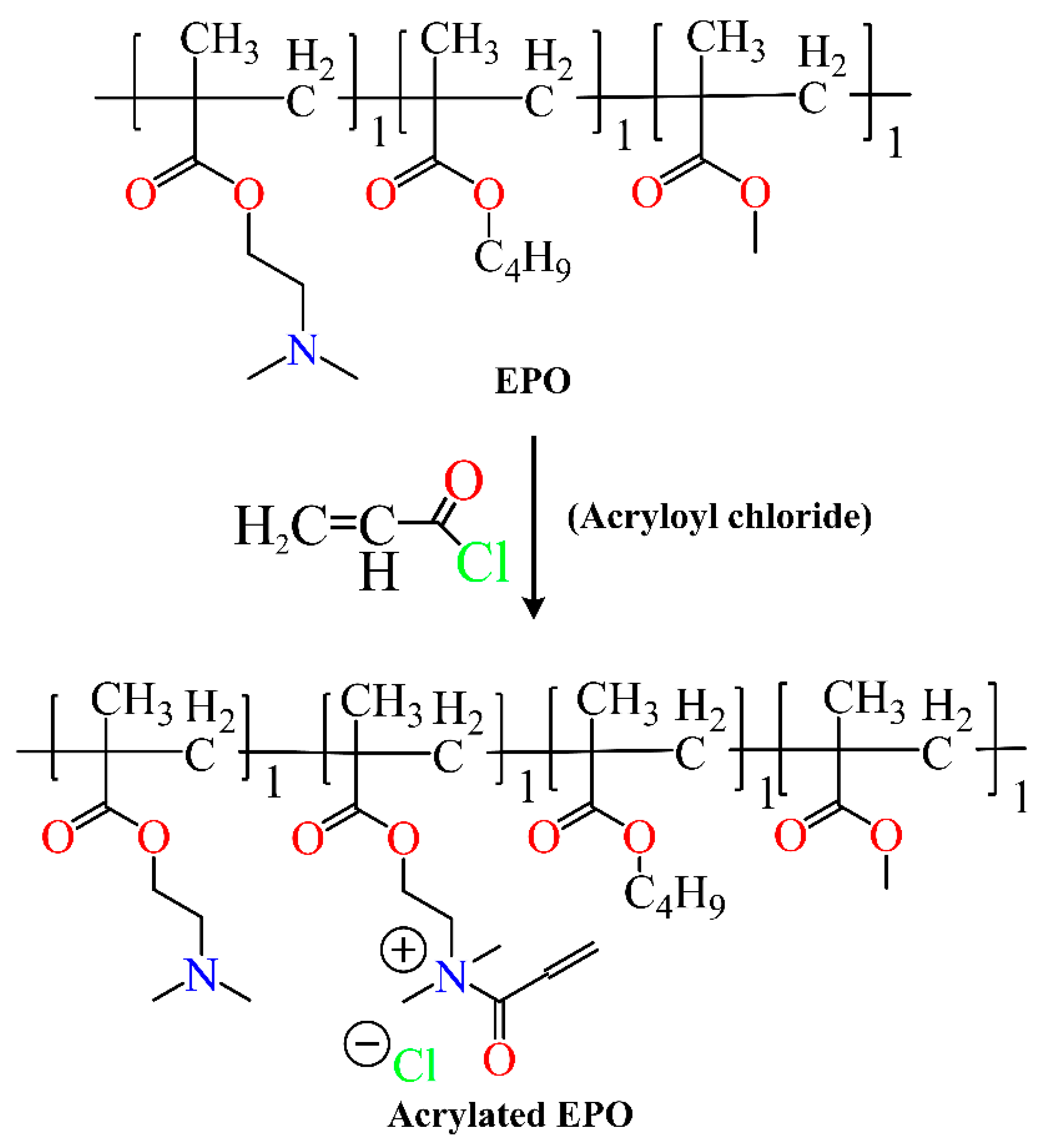
4. Synthesis of Eudragit Polymer
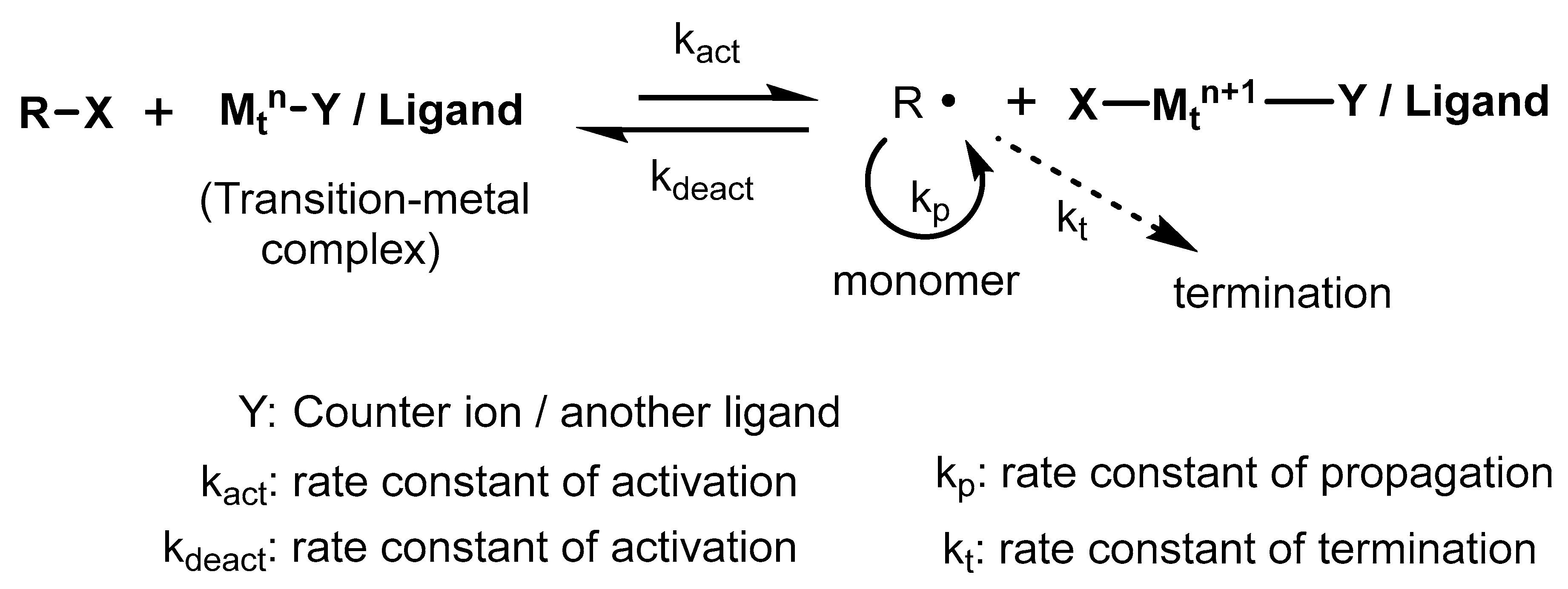
4.1. Atom Transfer Radical Polymerization
4.2. Reversible Addition–Fragmentation
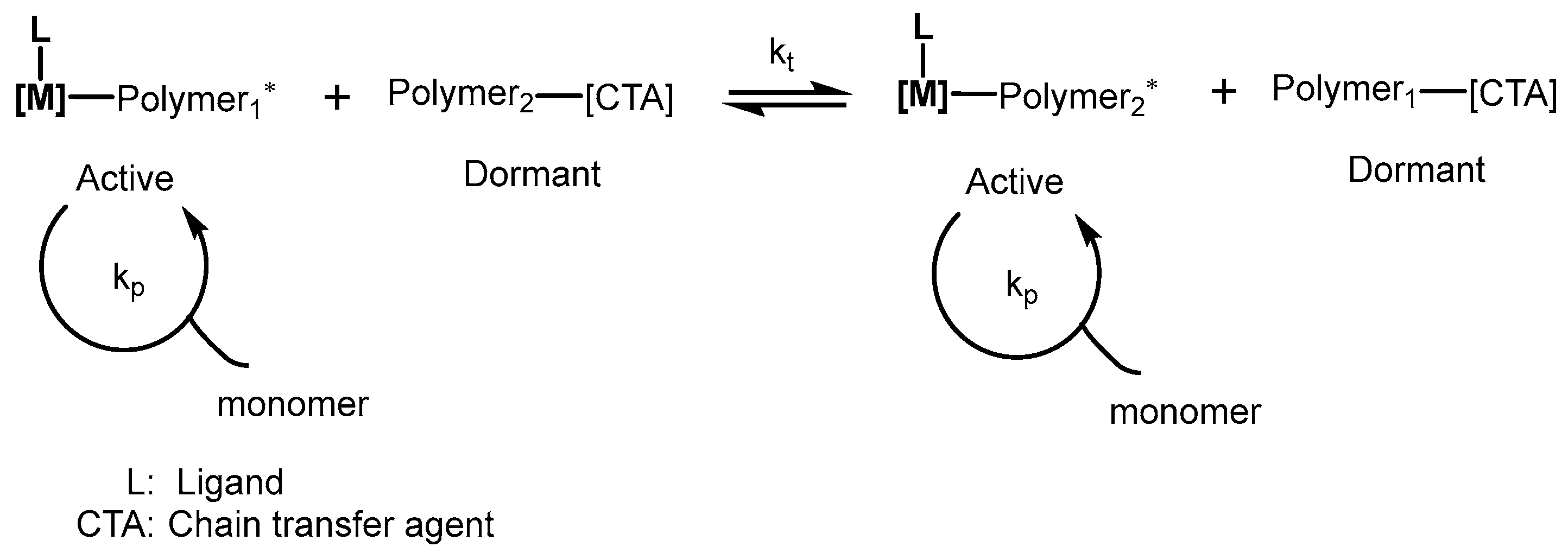
4.3. Chain Transfer Polymerization
5. Functionalized Eudragit-Based Nanomedicine for Targeted Drug Delivery
5.1. Eudragit-Based Hydrogel Drug Delivery
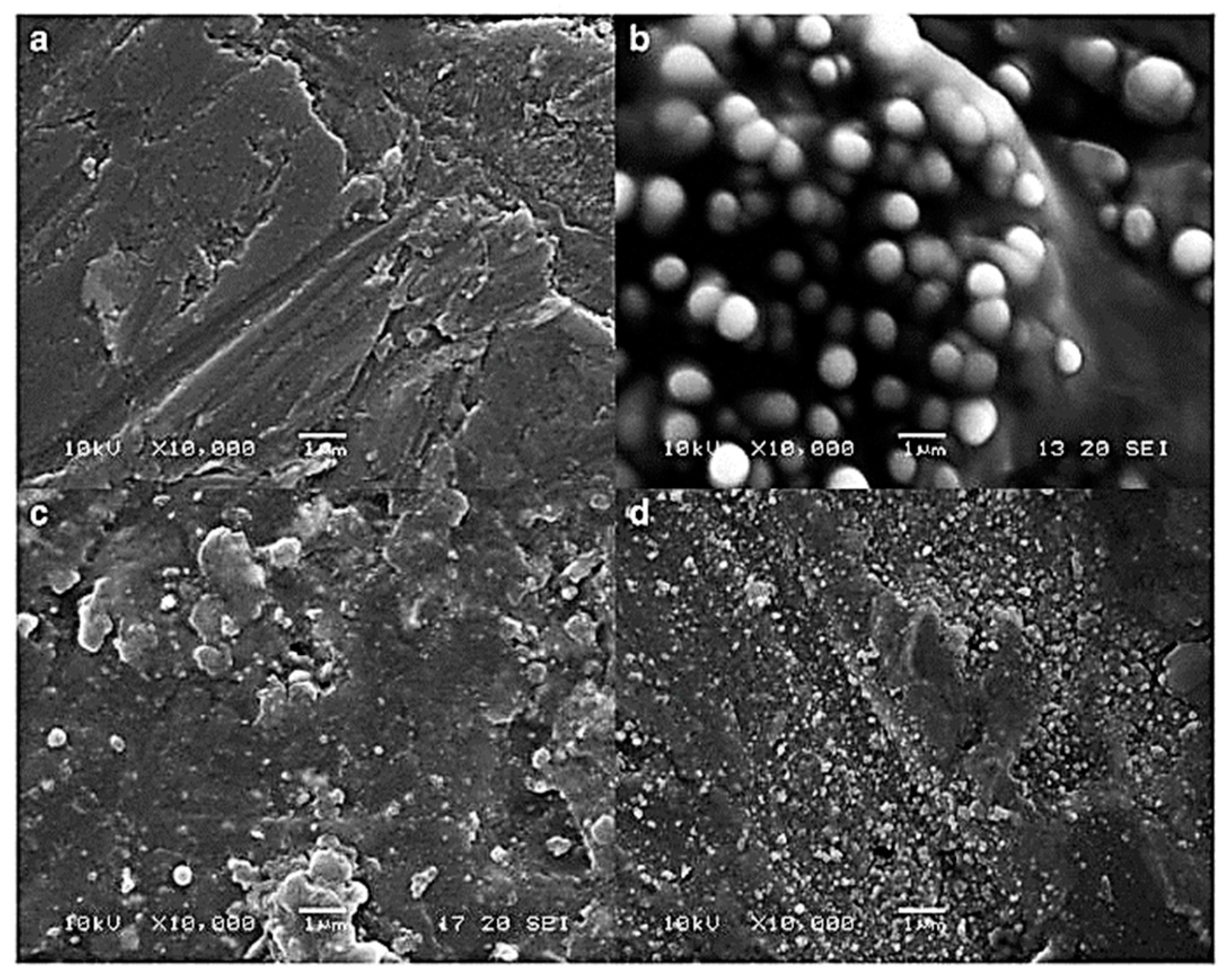
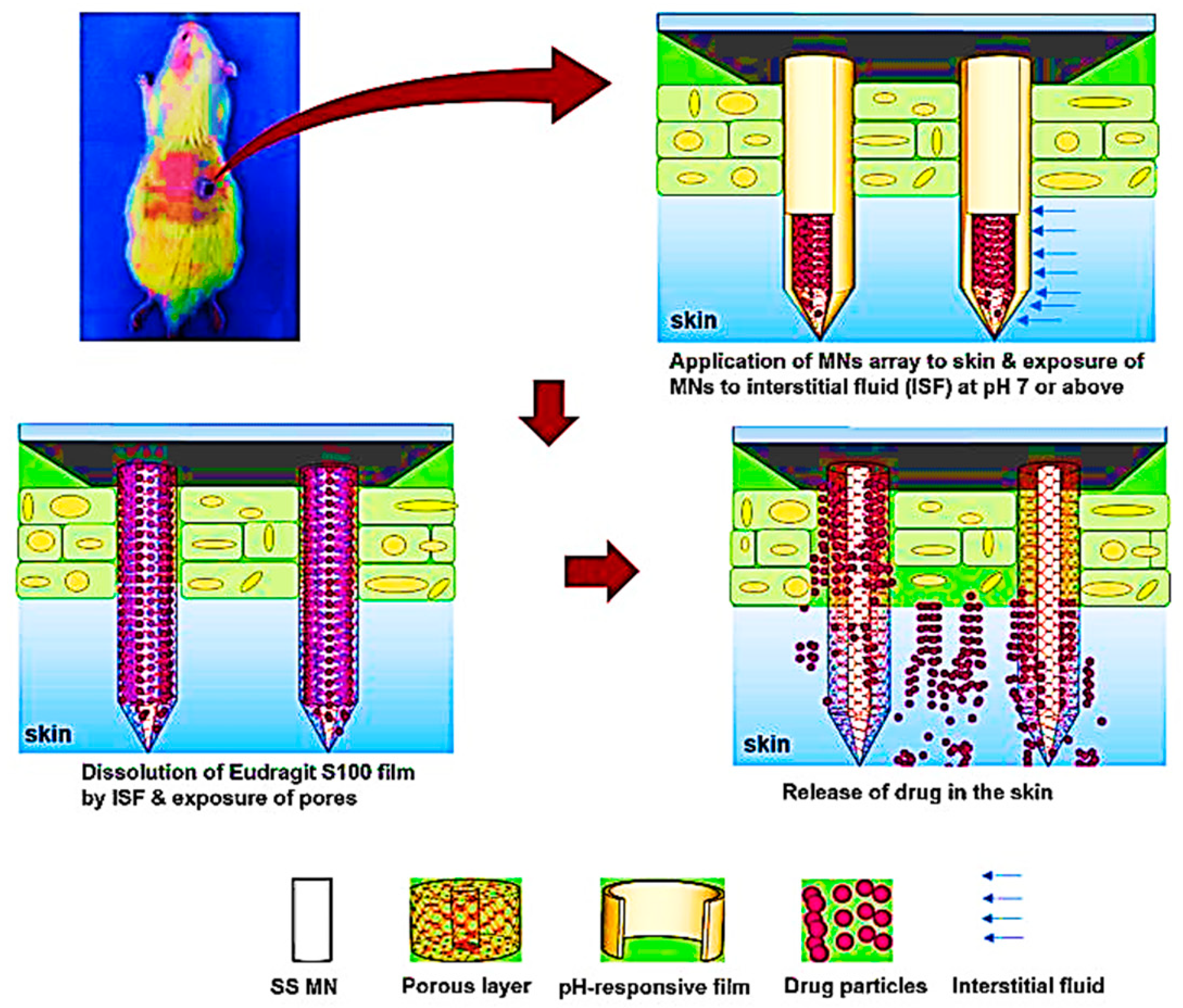
5.2. Eudragit-Based Microneedle Drug Delivery
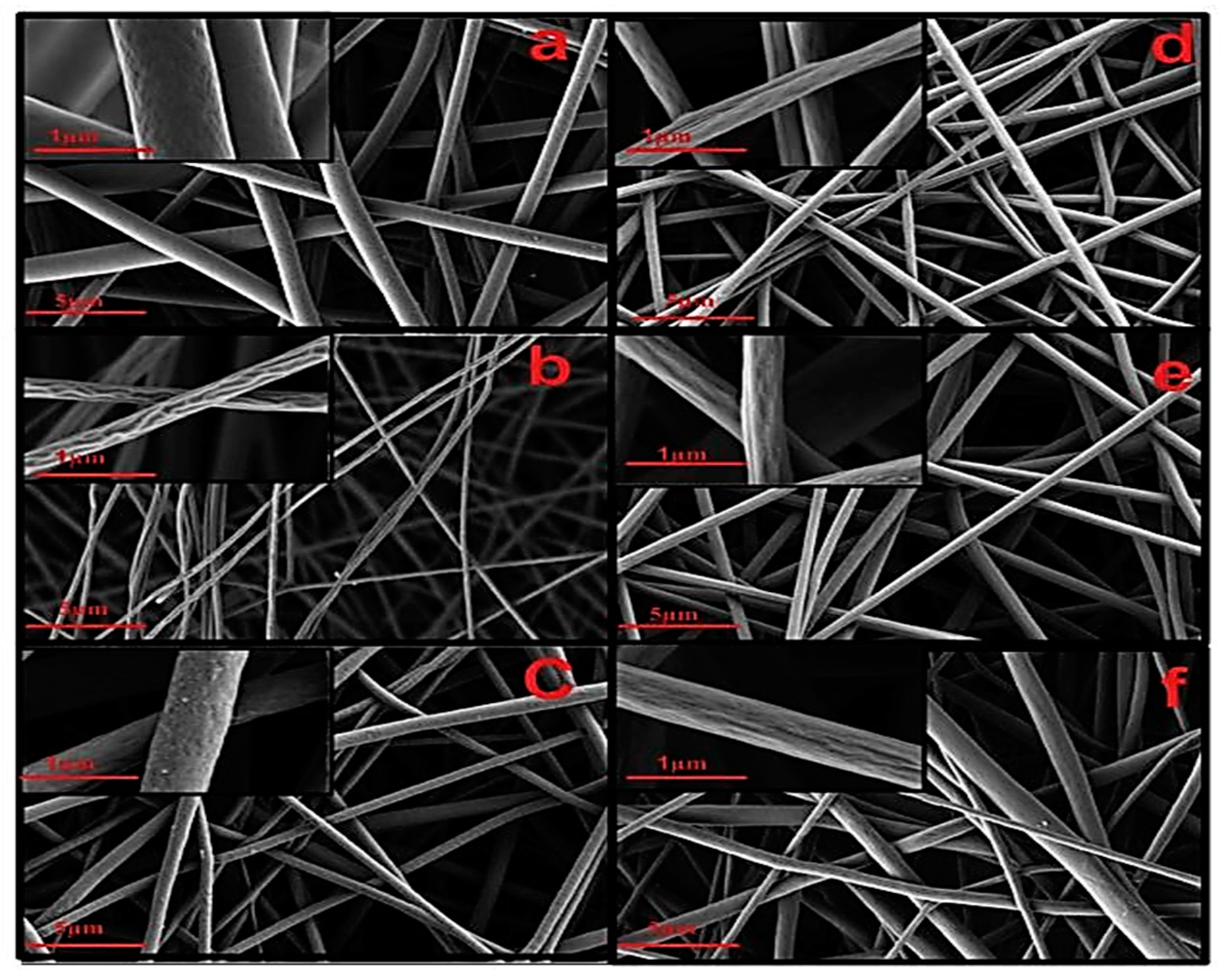
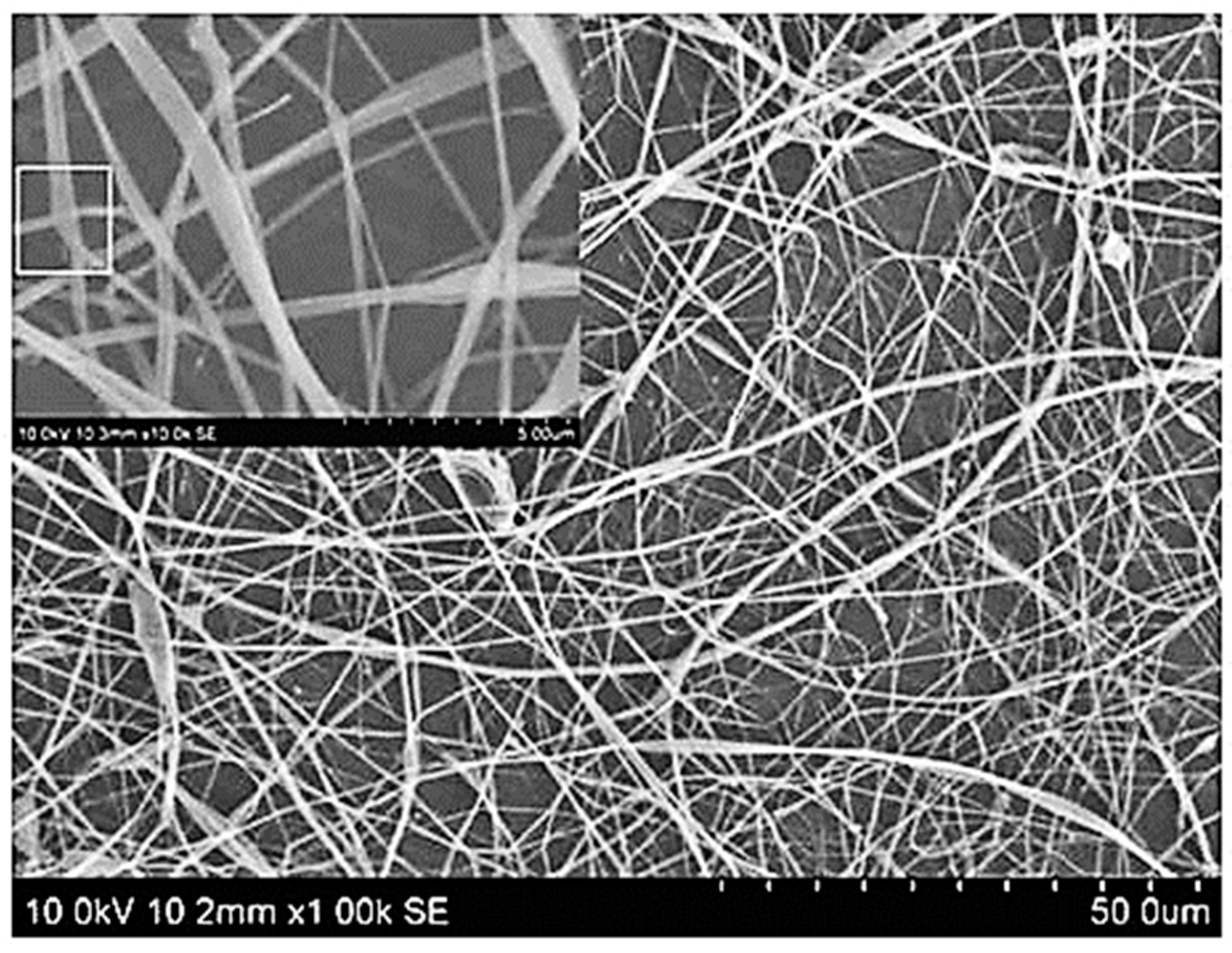
5.3. Eudragit-Based Nanofiber Drug Delivery
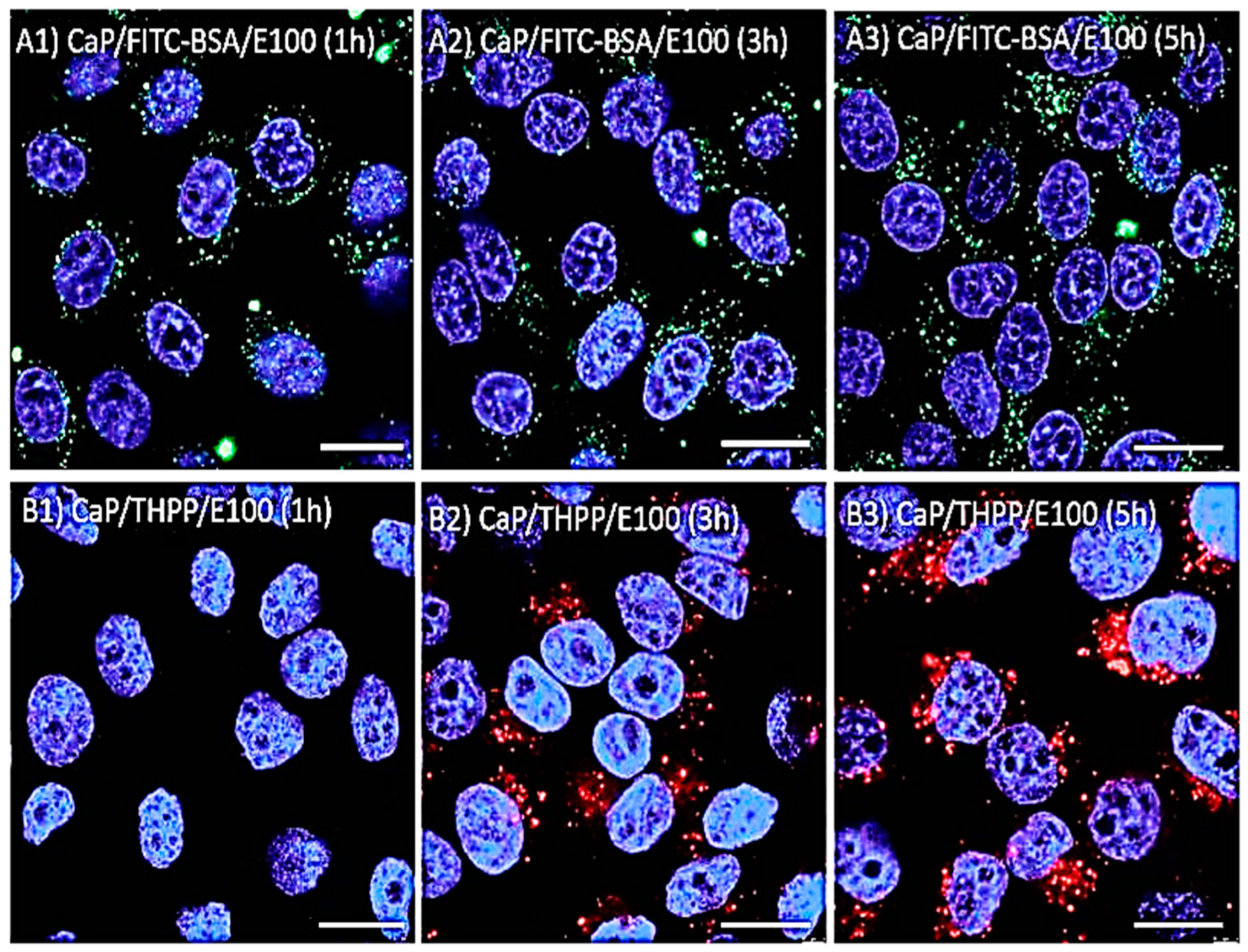
5.4. Eudragit-Based Nanoparticles Drug Delivery
6. Gene-Based Drug Delivery
Eudragit-Based Drug Delivery against DNA/RNA
7. Cancer-Based Drug Delivery
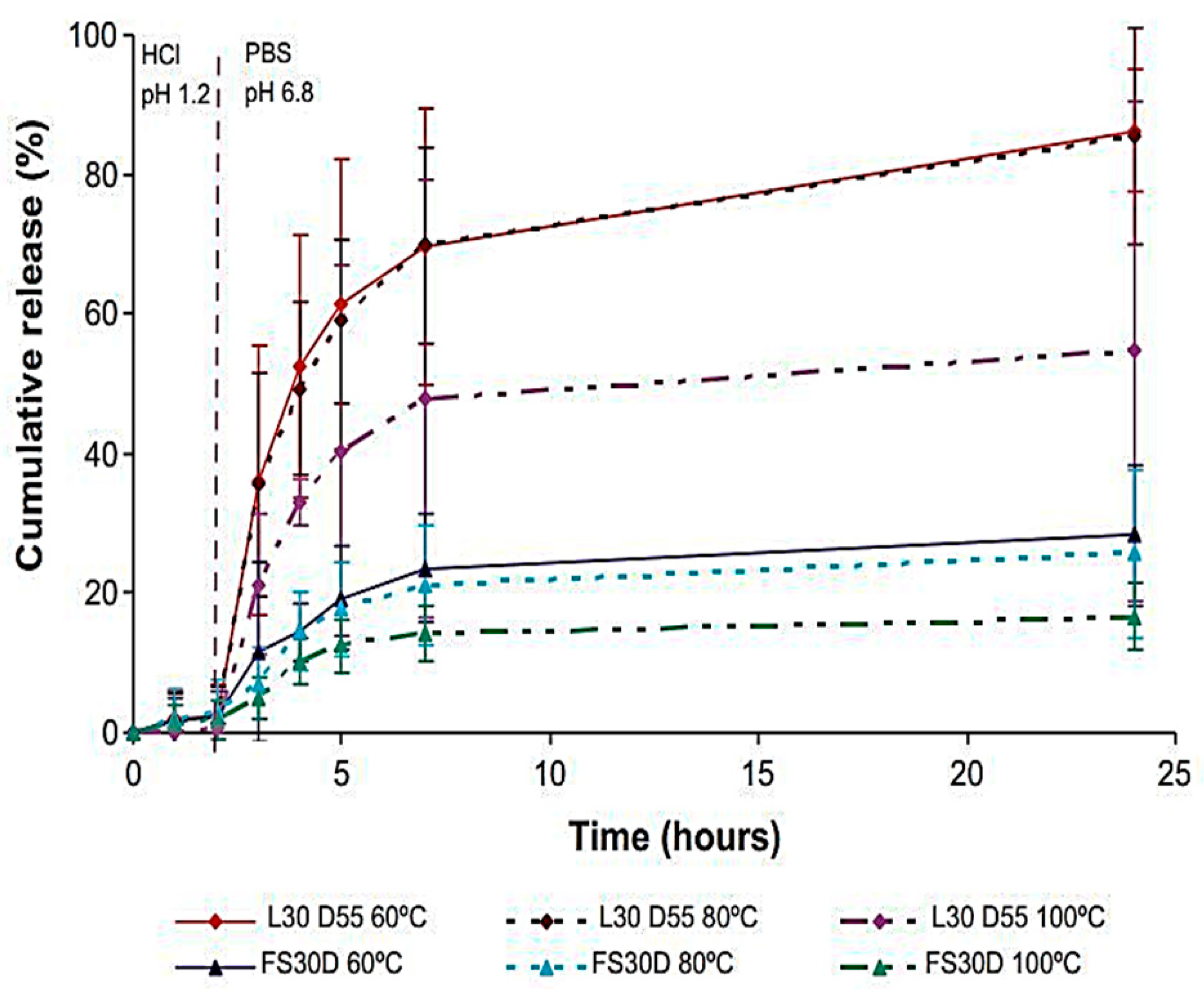
7.1. Eudragit-Based Drug Delivery against Colon Cancer
7.2. Eudragit-Based Drug Delivery against Oral and Buccal Cancer
8. Applications of Eudragit in Biosensor
9. Patent on Eudragit-Based Pharmaceutical Formulation for Drug Delivery
10. Future Prospective
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RAFT | Reversible addition–fragmentation chain transfer |
| ATRP | Atom transfer radical polymerization |
| GIT | Gastro-intestine tract |
| DSC | Differential scanning calorimetry |
| FT-IR | Fourier-transform infrared spectroscopy |
| TGA | Thermal gravimetric analysis |
| DMSO | Dimethyl sulfoxide |
| TB | Toluidine blue |
| MPPD | 2-methoxy-N−4-phenyl-1,4-phenylenediamine |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| CDI | 1,1′-Carbonyldiimidazole |
| DMF | Dimethyl formamide |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| SEM | Scanning electron microscopy |
| ROS | Reactive oxygen species |
| DMNs | Dissolving microneedles |
| PVP-K90 | Polyvinylpyrrolidone K90 |
| MNs | Microneedles |
| PLGA | poly(lactic acid-co-glycolic acid) |
| FESEM | Field emission scanning electron microscopy |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| 5-FU | 5-fluourouacil |
| VLPVPR | Val-LeuPro-Val-Pro-Arg |
| FITC-BSA | Fluorescein isothiocyanate labeled bovine serum albumin |
| MHC | Major histocompatibility complex |
| PBS | Phosphate-buffered saline |
| BSA | Bovine serum albumin |
| TBSS-IER | Tract-based spatial statistics-ion exchange resins |
| TNBS | 2: 4, 6-triniteobenzenesulfonic acid |
| E-CPNs | Eudragit S100-loaded Citrus-Pectin Nanoparticles |
| WGMR | Whispering Gallery Mode Resonator |
| GO | glucose oxidase |
References
- Shah, H.; Jain, A.; Laghate, G.; Prabhudesai, D. Pharmaceutical Excipients. In Remington: The Science and Practice of Pharmacy; Academic Press: Cambridge, MA, USA, 2020; pp. 633–643. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical Significance of Eudragit: A Review. Futur. J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A Technology Evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, K.S.; Run-Chu, L. Organic Esters of Plasticizers Affecting the Water Absorption, Adhesive Property, Glass Transition Temperature and Plasticizer Permanence of Eudragit Acrylic Films. J. Control. Release 2000, 68, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, V.; Panda, A.K.; Majumdar, D.K. Development of Enteric Submicron Particles Formulation of α-Amylase for Oral Delivery. Pharm. Dev. Technol. 2013, 18, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, M. Physicomechanical Properties of Naproxen-Loaded Microparticles Prepared from Eudragit L100. AAPS PharmSciTech 2009, 10, 120–128. [Google Scholar] [CrossRef]
- Lin, S.Y.; Yu, H.L. Thermal Stability of Methacrylic Acid Copolymers of Eudragits L, S, and L30D and the Acrylic Acid Polymer of Carbopol. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2061–2067. [Google Scholar] [CrossRef]
- Lin, S.Y.; Liao, C.M.; Liang, R.C. Use of Microscopic FT-IR/DSC Combined System for the Study of Glass Transition Temperatures of Polymers. Polym. J. 1995, 27, 201–204. [Google Scholar] [CrossRef]
- Parsons, R.L. Drug Absorption in Gastrointestinal Disease with Particular Reference to Malabsorption Syndromes. Clin. Pharm. 1977, 2, 45–60. [Google Scholar] [CrossRef]
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Impact of Gastrointestinal Disease States on Oral Drug Absorption—Implications for Formulation Design—A PEARRL Review. J. Pharm. Pharmacol. 2019, 71, 674–698. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The Use of Mucoadhesive Polymers in Buccal Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef]
- Gilhotra, R.M.M.; Ikram, S.; Srivastava, N.; Gilhotra, N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014, 28, 81. [Google Scholar] [CrossRef]
- Li, H.; Singh, B.; Park, T.; Hong, Z.; Kang, S.; Cho, C.; Choi, Y. European Journal of Pharmaceutical Sciences Mannan-Decorated Thiolated Eudragit Microspheres for Targeting Antigen Presenting Cells via Nasal Vaccination. PHASCI 2015, 80, 16–25. [Google Scholar] [CrossRef]
- Majdanski, T.C.; Schubert, S.; Windhab, N.; Schubert, U.S. Synthesis and Characterization of Colored EUDRAGIT V as Enteric Coating Material. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2386–2393. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.H.; Nguyen, D.; Lee, H.S.; Kang, N.; Baek, M.; An, J.; Yoo, S.; Mun, Y.; Lee, W.; et al. Preparation and Evaluation of Eudragit L100-PEG Proliponiosomes for Enhanced Oral Delivery of Celecoxib. Pharmaceutics 2020, 12, 718. [Google Scholar] [CrossRef]
- Porfiryeva, N.N.; Nasibullin, S.F.; Abdullina, S.G.; Tukhbatullina, I.K.; Moustafine, R.I.; Khutoryanskiy, V.V. Acrylated Eudragit® E PO as a Novel Polymeric Excipient with Enhanced Mucoadhesive Properties for Application in Nasal Drug Delivery. Int. J. Pharm. 2019, 562, 241–248. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Kemenova, V.A.; Rombaut, P.; Van Den Mooter, G. Eudragit e PO as a Complementary Material for Designing Oral Drug Delivery Systems with Controlled Release Properties: Comparative Evaluation of New Interpolyelectrolyte Complexes with Countercharged Eudragit L100 Copolymers. Mol. Pharm. 2013, 10, 2630–2641. [Google Scholar] [CrossRef]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine Towards Cancer. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Sitenkov, A.Y.; Bukhovets, A.V.; Nasibullin, S.F.; Appeltans, B.; Kabanova, T.V.; Khutoryanskiy, V.V.; Van den Mooter, G. Indomethacin-Containing Interpolyelectrolyte Complexes Based on Eudragit® E PO/S 100 Copolymers as a Novel Drug Delivery System. Int. J. Pharm. 2017, 524, 121–133. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Keddie, D.J. A Guide to the Synthesis of Block Copolymers Using Reversible-Addition Fragmentation Chain Transfer (RAFT) Polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Ourique, A.F.; Guterres, S.S.; Pohlmann, A.R. Spray-Dried Polymeric Nanoparticles for Pharmaceutics: A Review of Patents. Recent Pat. Drug Deliv. Formul. 2012, 6, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Dos, P.; Chaves, S.; Frank, L.A.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S.; Carlos, R.; Beck, R. Mucoadhesive Properties of Eudragit ® RS100, Eudragit ® S100, and Poly ( ε -Caprolactone ) Nanocapsules: In Fl Uence of the Vehicle and the Mucosal Surface. AAPS PharmSciTech. 2018, 19, 1637–1646. [Google Scholar] [CrossRef]
- Gupta, V.K.; Assmus, M.W.; Beckert, T.E.; Price, J.C. A Novel PH- and Time-Based Multi-Unit Potential Colonic Drug Delivery System. II. Optimization of Multiple Response Variables. Int. J. Pharm. 2001, 213, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Buabeid, M.A.; Ullah, K.; Murtaza, G.; Mannan, A. Synthesis, Characterization and Safety Profiling of Eudragit-Based PH Responsive Hydrogels: A Promising Platform for Colonic Delivery of Losartan Synthesis, Characterization and Safety Profiling of Eudragit-Based PH- Responsive Hydrogels: A Promising Platform for colonic drug delivery of losartan potassium. Curr. Drug Deliv. 2020, 16, 548–564. [Google Scholar] [CrossRef]
- Cazorla-luna, R.; Mart, A.; Notario-p, F.; Bedoya, L.M. Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for PH Responsive Release of Tenofovir. Mar. Drugs 2020, 18, 44. [Google Scholar] [CrossRef]
- Hironaka, K.; Inokuchi, Y.; Fujisawa, T.; Shimazaki, H.; Akane, M.; Tozuka, Y. European Journal of Pharmaceutics and Biopharmaceutics Edaravone-Loaded Liposomes for Retinal Protection against Oxidative Stress-Induced Retinal Damage. Eur. J. Pharm. Biopharm. 2011, 79, 119–125. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs. 2020, 18, 64. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Ngawhirunpat, T.; Tekko, I.A.; Vora, L.; McCarthy, H.O.; Donnelly, R.F. Dissolving Polymeric Microneedle Arrays for Enhanced Site-Specific Acyclovir Delivery. Eur. J. Pharm. Sci. 2018, 121, 200–209. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The Immunology of the Porcine Skin and Its Value as a Model for Human Skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, Characterization and Comparison of α-Arbutin Loaded Dissolving and Hydrogel Forming Microneedles. Int. J. Pharm. 2020, 586, 119508. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A Proposed Model Membrane and Test Method for Microneedle Insertion Studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirumpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. Enhancement of transdermal delivery of resveratrol using Eudragit and polyvinyl pyrrolidone-based dissolving microneedle patches. J. Drug Deliv. Sci. Technol. 2020, 102284. [Google Scholar] [CrossRef]
- Ullah, A.; Jang, M.; Khan, H.; Jin, H.; An, S.; Kim, D.; Kim, Y.; Kim, U.; Man, G. Sensors and Actuators: B. Chemical Microneedle Array with a PH-Responsive Polymer Coating and Its Application in Smart Drug Delivery for Wound Healing. Sens. Actuators B. Chem. 2021, 345, 130441. [Google Scholar] [CrossRef]
- Yang, G.; He, M.; Zhang, S.; Wu, M.; Gao, Y. An Acryl Resin-Based Swellable Microneedles for Controlled Release Intradermal Delivery of Granisetron. Drug Dev. Ind. Pharm. 2017, 44, 1–9. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Edition. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Mansfield, K.; Sanders, E.; Kenawy, E.; Cooper, J.; Simpson, G.; Sanders, E.H.; Wnek, G.E. Release of Tetracycline Hydrochloride from Electrospun Poly ( Ethylene-Co- Vinylacetate ), Poly ( Lactic Acid ), And a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar]
- Sill, T.J.; Recum, H.A. Von Electrospinning: Applications in Drug Delivery and Tissue Engineering Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Erik, L.; Rajan, A.; Amarjargal, A.; Prasad, A.; Tshool, S.; Hee, C.; Sang, C. Electrospun Polyurethane / Eudragit 1 L100-55 Composite Mats for the PH Dependent Release of Paclitaxel on Duodenal Stent Cover Application. Int. J. Pharm. 2015, 478, 1–8. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Guhathakarta, S.; Rajaram, R.; Sai, P. Electrospun Zein / Eudragit Nanofibers Based Dual Drug Delivery System for the Simultaneous Delivery of Aceclofenac and Pantoprazole. Int. J. Pharm. 2012, 438, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B: Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.; Hanes, J. Mucus-Penetrating Nanoparticles for Drug and Gene Delivery to Mucosal Tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic Property in Pharmaceutical Formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Esfahani, M. Preparation of Microparticles of Naproxen with Eudragit RS and Talc by Spherical Crystallization Technique. Pharm. Dev. Technol. 2009, 14, 442–450. [Google Scholar] [CrossRef]
- Nandy, B.C.; Mazumder, B. Formulation and Characterizations of Delayed Release Multi-Particulates System of Indomethacin: Optimization by Response Surface Methodology. Curr. Drug Deliv. 2014, 11, 72–86. [Google Scholar] [CrossRef]
- Khachane, P.; Date, A.A.; Nagarsenker, M.S. Eudragit EPO Nanoparticles: Application in Improving Therapeutic Efficacy and Reducing Ulcerogenicity of Meloxicam on Oral Administration Eudragit EPO Nanoparticles: Application in Improving Therapeutic Efficacy and Reducing Ulcerogenicity of Meloxicam on Oral Administration. J. Biomed. Nanotechnol. 2011, 7, 590–597. [Google Scholar] [CrossRef]
- Li, P.; Yang, Z.; Wang, Y.; Peng, Z.; Li, S.; Kong, L.; Wang, Q. Microencapsulation of Coupled Folate and Chitosan Nanoparticles for Targeted Delivery of Combination Drugs to Colon. J. Microencapsul. Micro Nano Carr. 2014, 2048, 1–6. [Google Scholar] [CrossRef]
- Singh, G.; Pai, R.S. Atazanavir-Loaded Eudragit RL 100 Nanoparticles to Improve Oral Bioavailability: Optimization and in Vitro / in Vivo Appraisal Atazanavir-Loaded Eudragit RL 100 Nanoparticles to Improve Oral Bioavailability: Optimization and in Vitro / in Vivo Appraisal. Drug Deliv. 2016, 23, 7544. [Google Scholar] [CrossRef]
- Yoo, J.; Giri, N.; Lee, C.H. PH-Sensitive Eudragit Nanoparticles for Mucosal Drug Delivery PH-Sensitive Eudragit Nanoparticles for Mucosal Drug Delivery. Int. J. Pharm. 2010, 403, 262–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Zhang, Y.; Li, G.; Cai, C.; Xu, J. Thiolated Eudragit-Based Nanoparticles for Oral Insulin Delivery: Preparation, Characterization, and Evaluation Using Intestinal Epithelial Cells In Vitro. Macromol. Biosci. 2014, 14, 842–852. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Zhu, L.; Wang, B.; Guo, T. Colloids and Surfaces B: Biointerfaces Preparation of Eudragit L 100-55 Enteric Nanoparticles by a Novel Emulsion Diffusion Method. Colloids Surf. B Biointerfaces 2013, 108, 127–133. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Zakeri-milani, P.; Siahi-shadbad, M.R.; Loveymi, B.D.; Nokhodchi, A.; Azari, Z.; Valizadeh, H. Development of PH-Sensitive Insulin Nanoparticles Using Eudragit L100-55 and Chitosan with Different Molecular Weights. AAPS PharmSciTech 2010, 11, 1237–1242. [Google Scholar] [CrossRef]
- Haiyan, S.; Dong, L.; Xuwei, T.; Yanli, C. Preparation and in Vitro / in Vivo Characterization of Enteric-Coated Nanoparticles Loaded with the Antihypertensive Peptide VLPVPR. Int. J. Nanomed. 2014, 1709, 1716. [Google Scholar] [CrossRef]
- Pignatello, R.; Ricupero, N.; Bucolo, C.; Maugeri, F.; Maltese, A.; Puglisi, G.; Farmaceutiche, S.; Universitaria, C.; Doria, V.A. Preparation and Characterization of Eudragit Retard Nanosuspensions for the Ocular Delivery of Cloricromene. AAPS PharmSciTech 2006, 7, 1–7. [Google Scholar] [CrossRef]
- Tayel, S.A.; El-nabarawi, M.A.; Tadros, M.I.; Abd-elsalam, W.H. Positively Charged Polymeric Nanoparticle Reservoirs of Terbinafine Hydrochloride: Preclinical Implications for Controlled Drug Delivery in the Aqueous Humor of Rabbits. AAPS PharmSciTech 2013, 14, 782–793. [Google Scholar] [CrossRef]
- Doerdelmann, G.; Kozlova, D.; Epple, M. A PH-Sensitive Poly(Methyl Methacrylate) Copolymer for Efficient Drug and Gene Delivery across the Cell Membrane. J. Mater. Chem. B 2014, 2, 7123–7131. [Google Scholar] [CrossRef]
- Esposito, E.; Sebben, S.; Cortesi, R.; Menegatti, E.; Nastruzzi, C. Preparation and Characterization of Cationic Microspheres for Gene Delivery. Int. J. Pharm. 1999, 189, 29–41. [Google Scholar] [CrossRef]
- Voltan, R.; Castaldello, A.; Brocca-Cofano, E.; Altavilla, G.; Caputo, A.; Laus, M.; Sparnacci, K.; Ensoli, B.; Spaccasassi, S.; Ballestri, M.; et al. Preparation and Characterization of Innovative Protein-Coated Poly(Methylmethacrylate) Core-Shell Nanoparticles for Vaccine Purposes. Pharm. Res. 2007, 24, 1870–1882. [Google Scholar] [CrossRef]
- Basarkar, A.; Singh, J. Poly (Lactide-Co-Glycolide)-Polymethacrylate Nanoparticles for Intramuscular Delivery of Plasmid Encoding Interleukin-10 to Prevent Autoimmune Diabetes in Mice. Pharm. Res. 2009, 26, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Año, G.; Esquisabel, A.; Pastor, M.; Talavera, A.; Cedré, B.; Fernández, S.; Sifontes, S.; Aranguren, Y.; Falero, G.; García, L.; et al. A New Oral Vaccine Candidate Based on the Microencapsulation by Spray-Drying of Inactivated Vibrio Cholerae. Vaccine 2011, 29, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Haining, W.N.; Anderson, D.G.; Little, S.R.; von Berwelt-Baildon, M.S.; Cardoso, A.A.; Alves, P.; Kosmatopoulos, K.; Nadler, L.M.; Langer, R.; Kohane, D.S. PH-Triggered Microparticles for Peptide Vaccination. J. Immunol. 2004, 173, 2578–2585. [Google Scholar] [CrossRef]
- Lale, S.V.; Gill, H.S. Pollen Grains as a Novel Microcarrier for Oral Delivery of Proteins. Int. J. Pharm. 2018, 552, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, D.A.; Manzo, R.H.; Allemandi, D.A. Design of a Colonic Delivery System Based on Cationic Polymethacrylate (Eudragit E100)-Mesalamine Complexes. Drug Deliv. 2010, 17, 208–213. [Google Scholar] [CrossRef]
- Dong, K.; Zeng, A.; Wang, M.; Dong, Y.; Wang, K.; Guo, C.; Yan, Y.; Zhang, L.; Shi, X.; Xing, J. In Vitro and In Vivo Study of a Colon-Targeting Resin Microcapsule Loading a Novel Prodrug, 3,4,5-Tributyryl Shikimic Acid. RSC Adv. 2016, 6, 16882–16890. [Google Scholar] [CrossRef]
- Tsai, S.W.; Yu, D.S.; Tsao, S.W.; Hsu, F.Y. Hyaluronan—Cisplatin Conjugate Nanoparticles Embedded in Eudragit S100-Coated Pectin/Alginate Microbeads for Colon Drug Delivery. Int. J. Nanomed. 2013, 8, 2399–2407. [Google Scholar] [CrossRef]
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N.P. Electrospun Diclofenac Sodium Loaded Eudragit® L 100-55 Nanofibers for Colon-Targeted Drug Delivery. Int. J. Pharm. 2011, 408, 200–207. [Google Scholar] [CrossRef]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- Loveymi, B.D.; Jelvehgari, M.; Zakeri-Milani, P.; Valizadeh, H. Statistical Optimization of Oral Vancomycin-Eudragit RS Nanoparticles Using Response Surface Methodology. Iran. J. Pharm. Res. 2012, 11, 1001–1012. [Google Scholar]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit Nanoparticles Containing Genistein: Formulation, Development, and Bioavailability Assessment. Int. J. Nanomed. 2011, 6, 2429–2435. [Google Scholar]
- Momoh, M.A.; Kenechukwu, F.C.; Adedokun, M.O.; Odo, C.E.; Attama, A.A. Pharmacodynamics of Diclofenac from Novel Eudragit Entrapped Microspheres. Drug Deliv. 2014, 21, 193–203. [Google Scholar] [CrossRef]
- Cetin, M.; Atila, A.; Kadioglu, Y. Formulation and in Vitro Characterization of Eudragit® L100 and Eudragit® L100-PLGA Nanoparticles Containing Diclofenac Sodium. AAPS PharmSciTech 2010, 11, 1250–1256. [Google Scholar] [CrossRef]
- Jain, D.; Panda, A.K.; Majumdar, D.K. Eudragit S100 Entrapped Insulin Microspheres for Oral Delivery. AAPS PharmSciTech 2005, 6, 100–107. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. A Review on Impedimetric Biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef]
- Xiuyun, W.; Shunichi, U. Polymers for Biosensors Construction. State Art Biosens. Gen. Asp. 2013, 3, 67–85. [Google Scholar]
- Giannetti, A.; Berneschi, S.; Baldini, F.; Cosi, F.; Conti, G.N.; Soria, S. Performance of Eudragit Coated Whispering Gallery Mode Resonator-Based Immunosensors. Sensors 2012, 12, 14604–14611. [Google Scholar] [CrossRef]
- Tzianni, E.I.; Hrbac, J.; Christodoulou, D.K.; Prodromidis, M.I. A Portable Medical Diagnostic Device Utilizing Free-Standing Responsive Polymer Film-Based Biosensors and Low-Cost Transducer for Point-of-Care Applications. Sens. Actuators B Chem. 2020, 304, 127356. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Campuzano, S.; Pingarrón, J.M.; Esteban Fernández de Ávila, B.; Wang, J. Direct Electrochemical Biosensing in Gastrointestinal Fluids. Anal. Bioanal. Chem. 2019, 411, 4597–4604. [Google Scholar] [CrossRef]
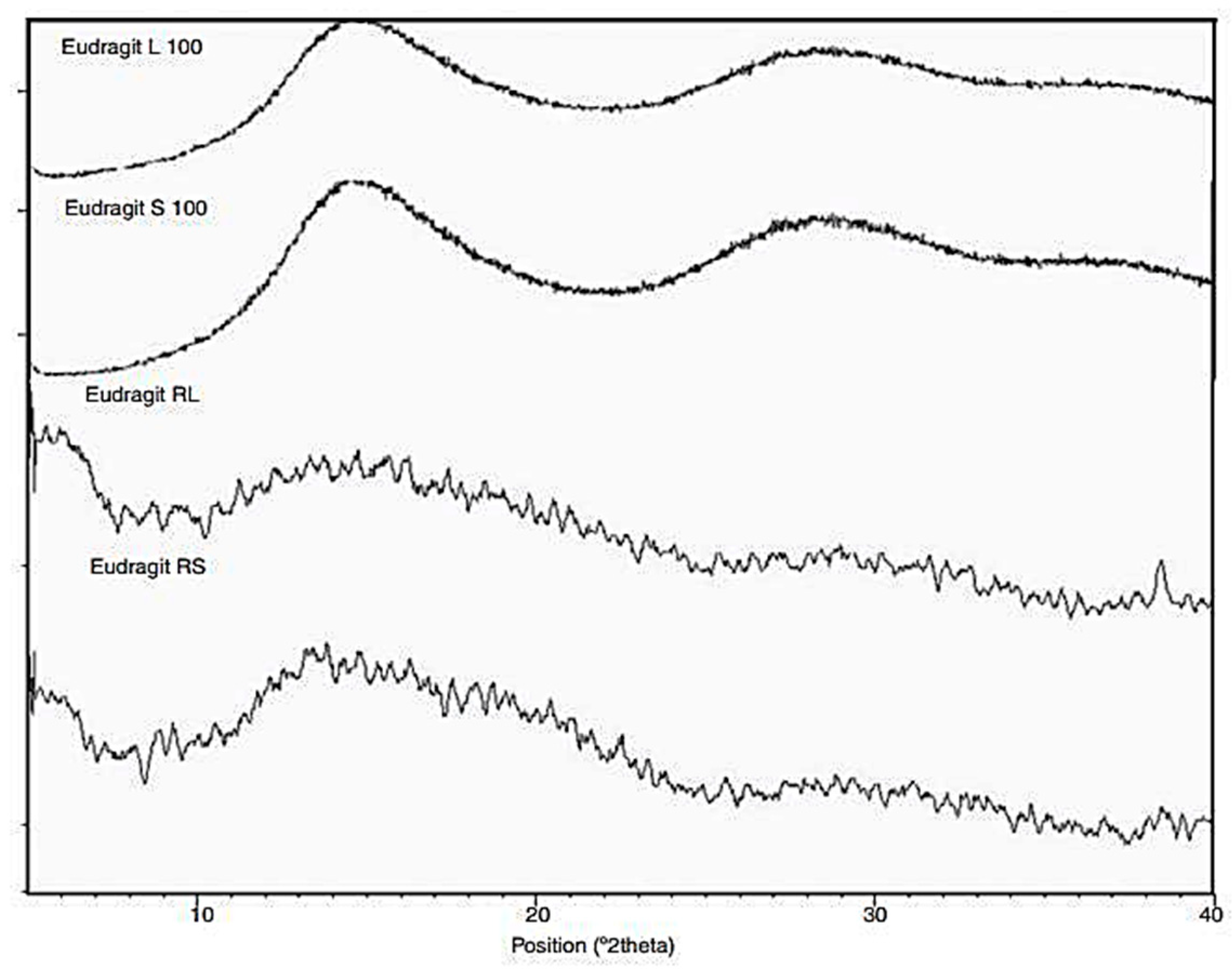
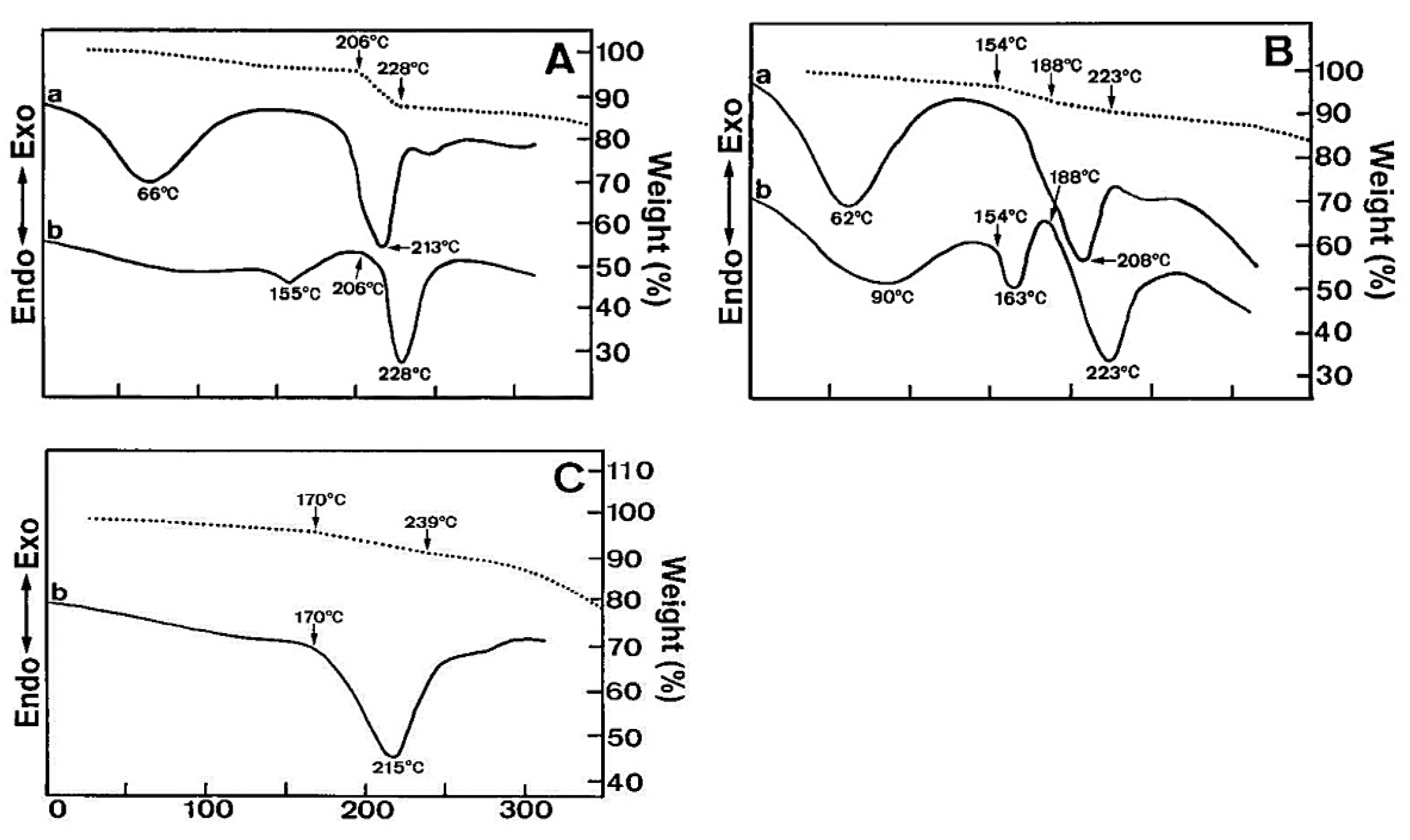


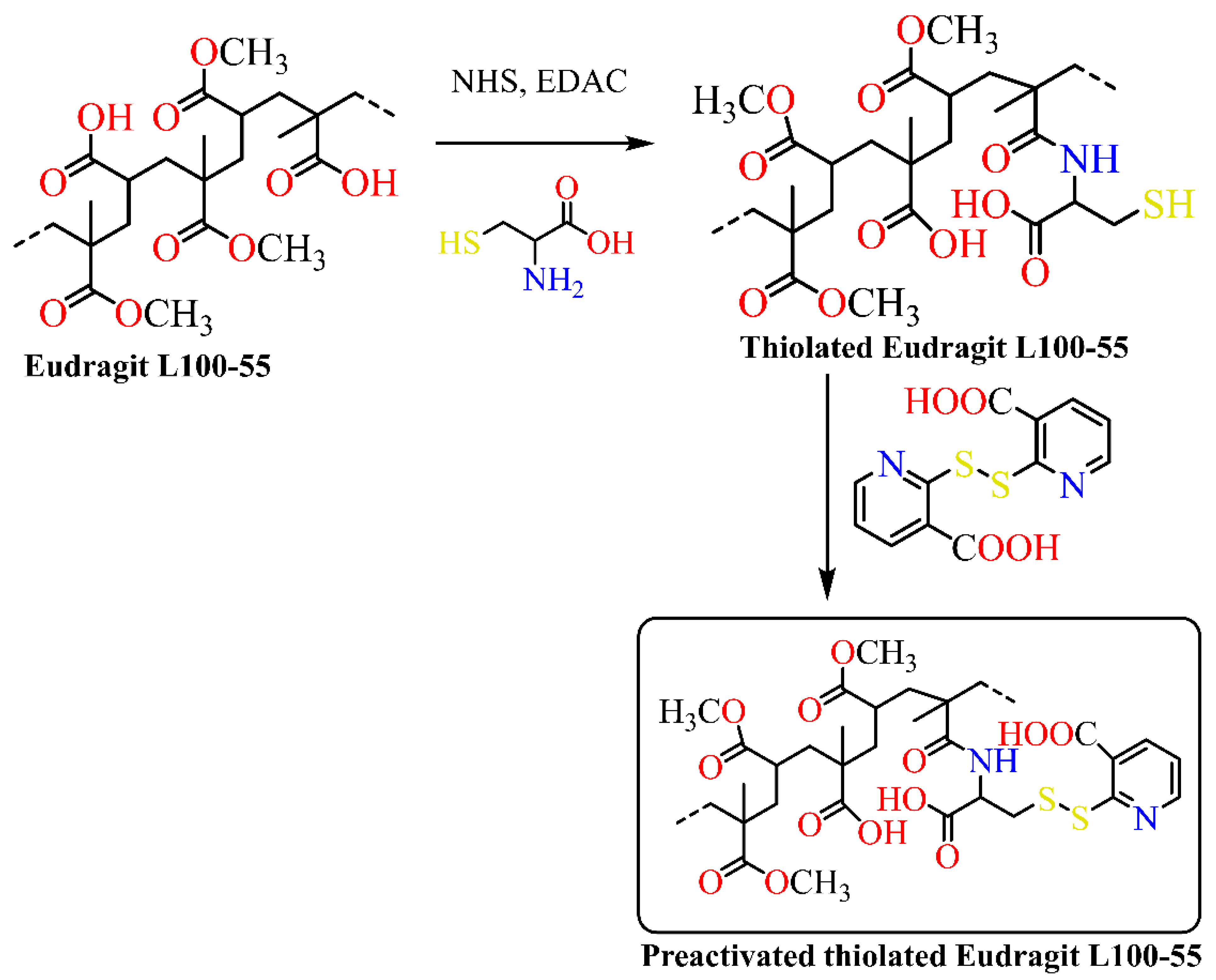
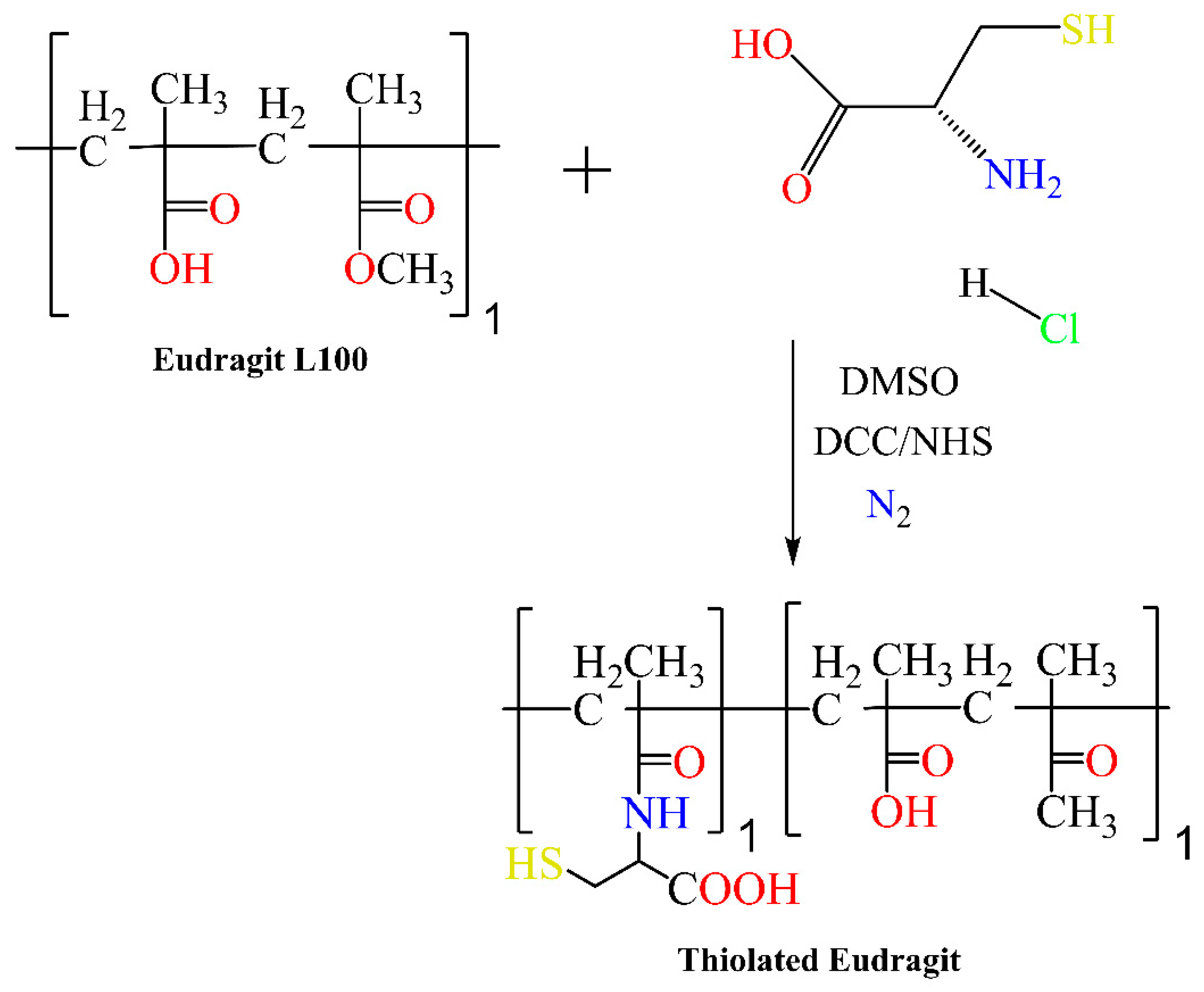




| Eudragit Grade | Applications | Chemical Composition | Solubility |
|---|---|---|---|
| Cationic (Aminoalkylmethacrylate copolymers) Eudragit E 12.5 Eudragit E 100 | Increased geriatric and pediatric patient compliance. Increased bioavailability and dissolution profile. Increased anti-inflammatory action. High oral bioavailability | Poly(butyl methacrylate, (2-dimethyl aminoethyl) methacrylate, methyl methacrylate) 1:2:1 Poly(butyl methacrylate, (2-dimethyl aminoethyl) methacrylate, methyl methacrylate) 1:2:1 | Both are soluble in gastric fluid to pH 5 |
| Anionic (Methacrylic acid copolymers) Eudragit L 100 Eudragit L 100-55 Eudragit L 12.5 Eudragit L 12.5 P Eudragit S 12.5 Eudragit S 12.5 P Eudragit L 30 D-55 Eudragit S 100 Eudragit FS 30 D | pH-dependent and high release. Increased oral absorption. Increased taste masking. Controlled release. Colonic-specific drug delivery. Targeting drug delivery. Delay release profile. High oral bioavailability | Poly(methacrylic acid, methyl methacrylate) 1:1 Poly(methacrylic acid, ethyl acrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:2 Poly(methacrylic acid, methyl methacrylate) 1:2 Poly(methacrylic acid, ethyl acrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:2 Methyl acrylate, methyl methacrylate, and methacrylic acid | Soluble in intestinal fluid around pH 6 Soluble in intestinal fluid around pH 5.5 Soluble in intestinal fluid around pH 6 Soluble in intestinal fluid around pH 6 Soluble in intestinal fluid around pH 7 Soluble in intestinal fluid around pH 7 Soluble in intestinal fluid from pH 5.5 Soluble in intestinal fluid around pH 7 Soluble above pH 6.8 |
| Neutral (Methacrylic acid copolymers) 1. Eudragit RL PO 2. Eudragit RL 30 D 3. Eudragit RL 100 (Type A) 4. Eudragit RS PO 5. Eudragit RS 30 D 6. Eudragit RS 100 (Type B) | Increased release time and ocular bioavailability. Increased shelf life for ophthalmic dosage form. Sustainable drug release for more than 6 h. Sustained release with significance for vaginal drug delivery. Improved permeation and increased bioavailability as well as shelf life. | Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.2 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.2 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.2 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.1 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.1 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.1 | Permeability is High Permeability is High Permeability is High Permeability is Low Permeability is Low Permeability is Low |
| Neutral (Methacrylic acid copolymers) 1. Eudragit NM 30 D 2. Eudragit NE 30 D 3. Eudragit NE 40 D | Poly(ethyl acrylate, methyl methacrylate) with 0.7% (PEG stearyl ether) 2:1 Poly(ethyl acrylate, methyl methacrylate) with 1.5% (nonoxynol) 2:1 Poly(ethyl acrylate, methyl methacrylate) with 1.5% (nonoxynol) 2:1 | Permeable, swellable Permeable, swellable Permeable, swellable |
| Grades of Eudragit Polymer | Glass Transition Temperature (°C) |
|---|---|
| Eudragit E 100/E PO | 48 |
| Eudragit FS 30 D | 48 |
| Eudragit NE 30 D | 9 |
| Eudragit ME 30 D | 11 |
| Eudragit L 100-55 | 110 |
| Eudragit RL 100 | 70 |
| Eudragit RS 100 | 65 |
| Eudragit Grade | Drug Name | Dosage form/Delivery System | Method of Preparation | Application | References |
|---|---|---|---|---|---|
| Eudragit S100 | 5-fluourouacil (5-FU) and leucovorin | Nanoparticles microencapsulated with enteric polymers | Ionic gelation followed by a solvent evaporation method | Chemotherapy for colon cancer that targets specific drugs for delivery to the colon. | [51] |
| Eudragit RL100 | Atazanavir | Nanoparticles | Nanoprecipitation method | To improve bioavailability in prolonged drug release | [52] |
| Eudragit L100 | Insulin | Thiolated Eudragit-based nanoparticles with reduced glutathione | Nanotechnology | Facilitate insulin permeation through the intestinal epithelium | [54] |
| Eudragit L100-55 | Omeprazole | Nanoparticles | Ultrasonic dispersion and diffusion solidification | Nanoparticles showed a strong pH-sensitive release in vitro | [55] |
| Eudragit L100-55 | Insulin | Enteric nanoparticles | Complex coacervation method | Complex coacervation process using chitosan and Eudragit L100-55 polymers may provide a useful approach for entrapment of hydrophilic polypeptides without affecting their conformation | [56] |
| Eudragit S100 | Peptide Val-LeuPro-Val-Pro-Arg (VLPVPR) | Enteric-coated nanoparticles | Double emulsion method followed by freeze-drying | Nanoparticles almost completely released at pH 7.4 after 8 h reduced blood pressure for more than 30 h | [57] |
| Eudragit RS100 and RL100 | Cloricromene | Nanoparticle suspensions | Quasi-emulsion solvent diffusion technique | Improves the shelf life and bioavailability of this drug after ophthalmic application | [58] |
| Eudragit RS100 | Terbinafine hydrochloride | Positively charged controlled-release polymeric Nanoparticles as an eye drop | Nanoprecipitation method | Increased drug means residence time and improved ocular bioavailability four-fold | [59] |
| Sr. No | Title of the Patent | Essence of the Invention | Patent Number | Inventors | Date |
|---|---|---|---|---|---|
| 1 | Sustained release pharmaceutical composition | Controlled dissolution of the active principle independently of the pH, which consists of microparticles containing the active principle, coated with a mixture of ethyl cellulose and Eudragit RS | EP0322277 | H. Stevens, M. Chariot, F. Arnold, G. Lewis | 22 January 1992 |
| 2 | Ketoprofen micro granules, the method for preparing same and pharmaceutical compositions | Ketoprofen micro granules of Eudragit RL and RS exhibited prolonged release | WO/2000/064432 | L. C. Marechal, D.S. Pascal | 2 November 2000 |
| 3 | Improved stabilization of misoprostol | Misoprostol was complexed with various grades of Eudragit RS series, Eudragit RL series, Eudragit S, and Eudragit L. The solid dispersions were stable and showed sustain release | EP0896823 | C. David Tsay, R. Jen Lin Hue In Lu Shu-bin | 25 September 2002 |
| 4 | Formulation stabilizer for proton pump inhibitors | The polymeric base is cholestyramine-OH, Eudragit EPO, chitosan, or a mixture thereof. The composition stabilizes the benzimidazole derivative proton pump inhibitor in a humid environment | US 20060013880 | F. Robert, R. Narayan, Z. Joseph H. Ping | 19 January 2006 |
| 5 | Modified release tablet formulations with enhanced mechanical properties | Eudragit L100-55 for a said pharmaceutical formulation achieves the desired hardness for tablets made from the formulation | US 20070104782 | S. H. Amir C.E. Melissa | 8 February 2007 |
| 6 | Colonic delivery using zn/pectin beads with a Eudragit coating | The systems include pectin beads cross-linked with zinc or any divalent cation of interest, which beads are then coated with Eudragit®-type polymers | US 20080124279 | A. Andremont H. Huguet | 29 May 2008 |
| 7 | Colonic delivery of metal-dependent enzymes | Pectin beads are crosslinked with zinc ions, and the pectin beads are coated with a Eudragit® polymer. | US 20080199528 | A. Andremont, H. Huguet | 21 August 2008 |
| 8 | Coated senna extract granules | Senna extract with 20% sennosides is granulated with Eudragit L 100 and then coated with Eudragit L 30 D 55 | WO/2011/014976 | P. H. Jorge | 2 October 2011 |
| 9 | Ursodeoxycholic acid-synthetic hydrotalcite-Eudragit hybrid, a pharmaceutical composition containing the same method for preparing the same | The ursodeoxycholic acid synthetic hydrotalcite-Eudragit hybrid was used for bitter taste-blocking effect and improved body absorption rate with high solubility | US 20120156263 | J.H. Choy, G.E. Choi, M. C. Park, H. C. Chang | 21 June 2012 |
| 10 | Curcuminoid complexes with enhanced stability, solubility, and/or bioavailability | Curcuminoid–Eudragit complex, which enhances the bioavailability of the curcumin component | US20140271530 | H. Tummala, S. Kumar | 18 September 2014 |
| 11 | Oral drug delivery formulations | One active substance and at least one coat comprising Eudragit E. The formulation may be used for releasing up to about 55% of a total dose as a loading dose to manage pain | US 20150250733 | O. Isa | 10 September 2015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. https://doi.org/10.3390/pharmaceutics15020587
Nikam A, Sahoo PR, Musale S, Pagar RR, Paiva-Santos AC, Giram PS. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics. 2023; 15(2):587. https://doi.org/10.3390/pharmaceutics15020587
Chicago/Turabian StyleNikam, Aniket, Priya Ranjan Sahoo, Shubham Musale, Roshani R. Pagar, Ana Cláudia Paiva-Santos, and Prabhanjan Shridhar Giram. 2023. "A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare" Pharmaceutics 15, no. 2: 587. https://doi.org/10.3390/pharmaceutics15020587
APA StyleNikam, A., Sahoo, P. R., Musale, S., Pagar, R. R., Paiva-Santos, A. C., & Giram, P. S. (2023). A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics, 15(2), 587. https://doi.org/10.3390/pharmaceutics15020587











