Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
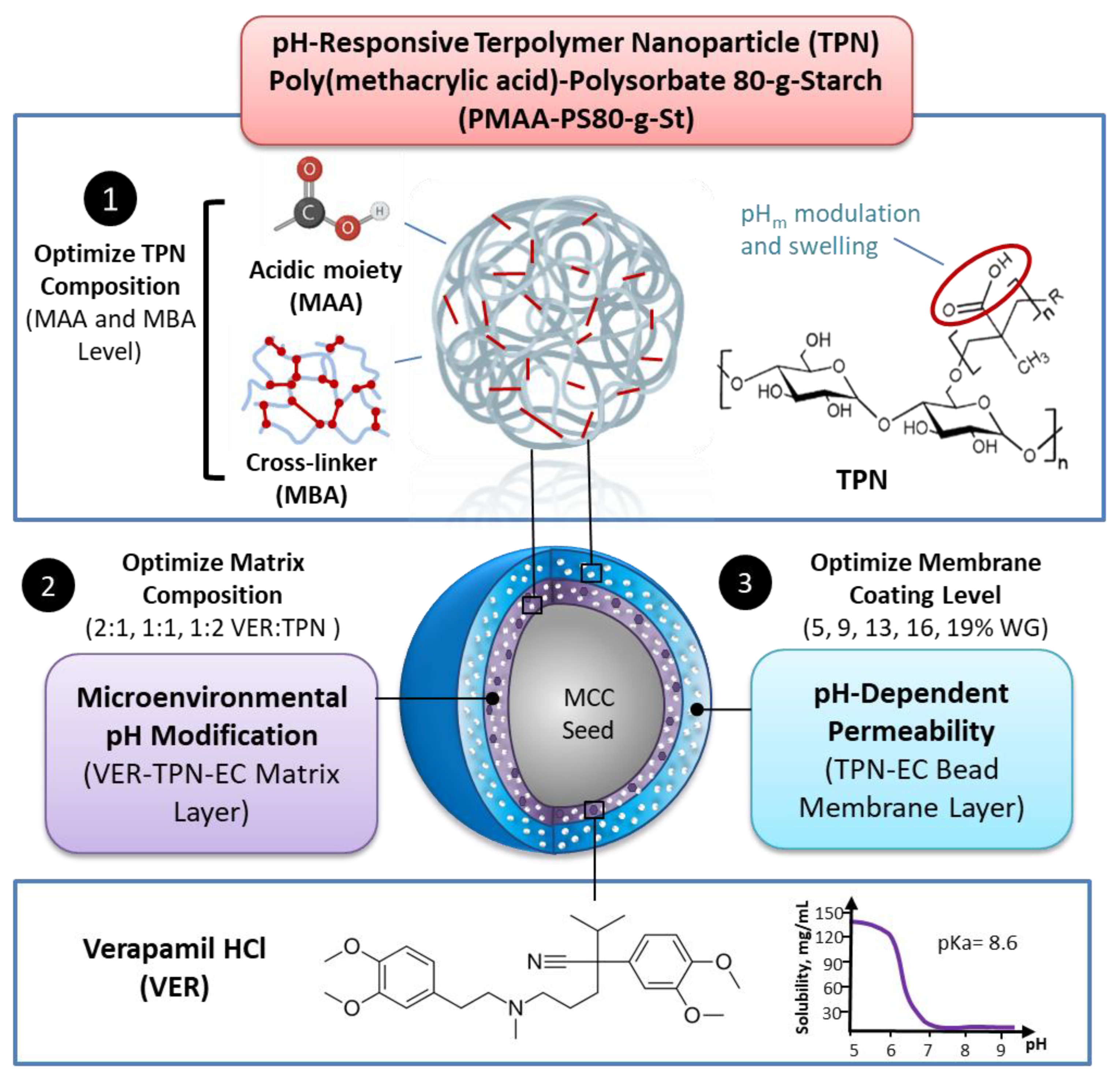
2.2. Synthesis and Characterization of TPN
2.3. Preparation and Evaluation of Matrix Free-Films of VER-TPN-EC
2.3.1. Film-Casting and Structural Characterization of Composite Matrix Formulations
2.3.2. Determination of Drug Release from VER-TPN-EC Composite Matrix Films
2.3.3. pH-Dependence of Medium Uptake of TPN-EC Composite Matrix Films
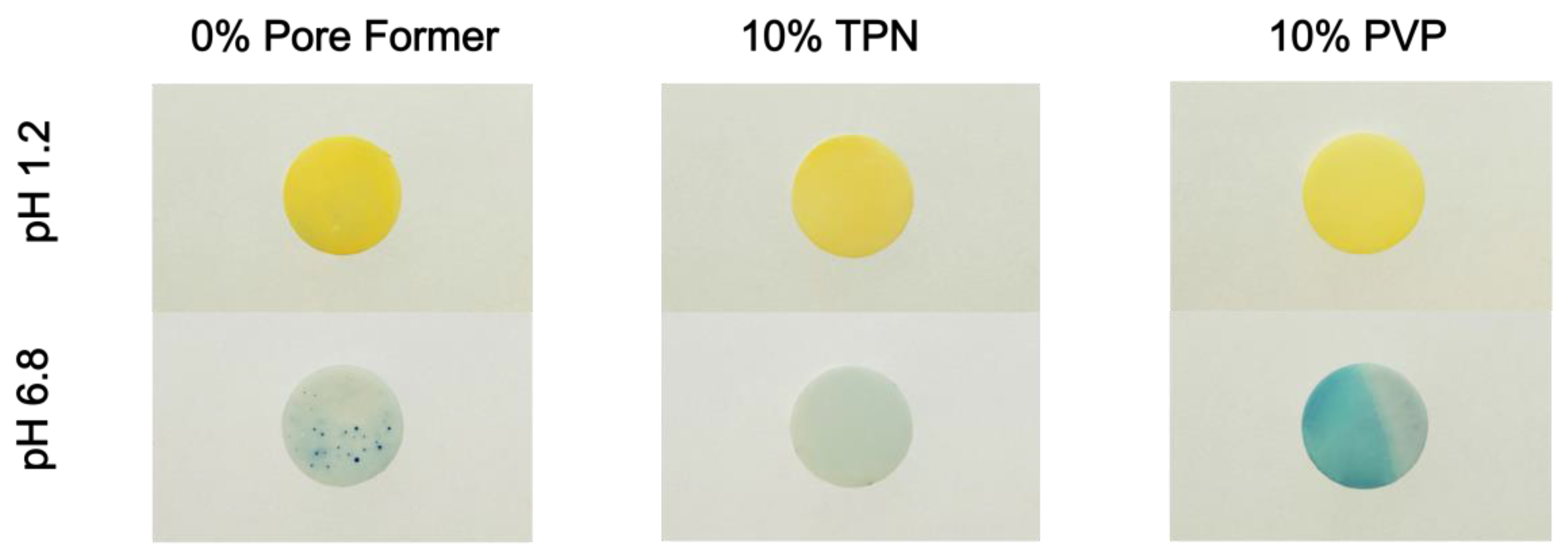
2.3.4. Preparation and Photography of pH Indicator Dye-Incorporated Matrix Films
2.3.5. Preparation and Measurement of pHm of Fluorescent Dye-Labeled Matrix Films
2.4. Preparation of TPN-Containing Bilayer-Coated Beads
2.4.1. Application of Drug-Loaded Matrix Coating Layer (VER-TPN-EC)
2.4.2. Application of TPN-EC Enteric Coating Membrane Layer
2.5. Determination of Drug Release from Bilayer-Coated Beads
2.6. Statistical Analysis
3. Results
3.1. Characterization of Synthesized TPN and TPN Films
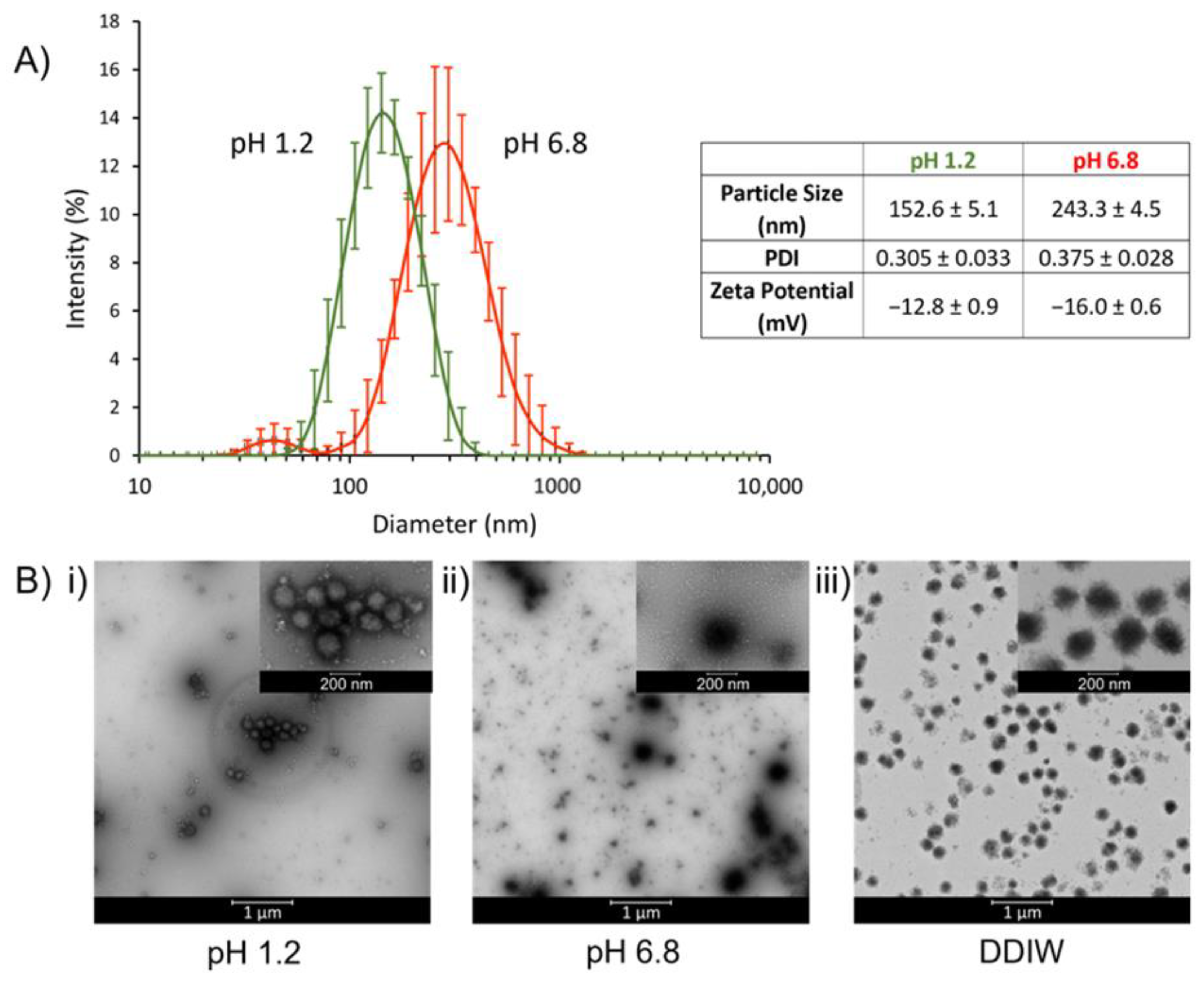
3.1.1. Particle Size, Zeta Potential, and Morphology of TPN
3.1.2. Investigation of Component Interactions within TPN-EC and VER-TPN-EC Films
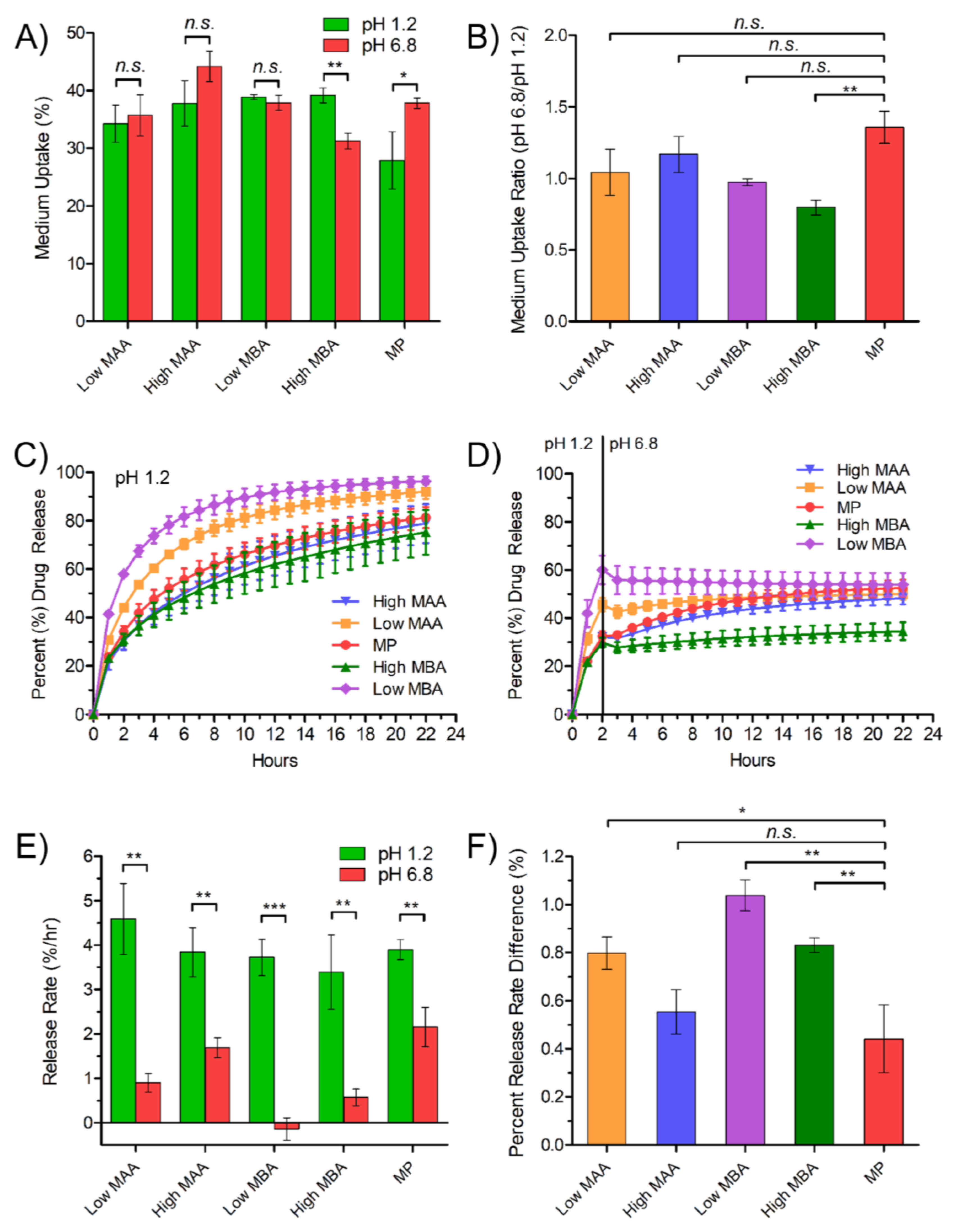
3.2. Investigating the Effect of TPN Composition on Medium Uptake and Drug Release Using Free-Film Matrices
3.3. Investigation of Microenvironmental pH within the Free-Film Matrices
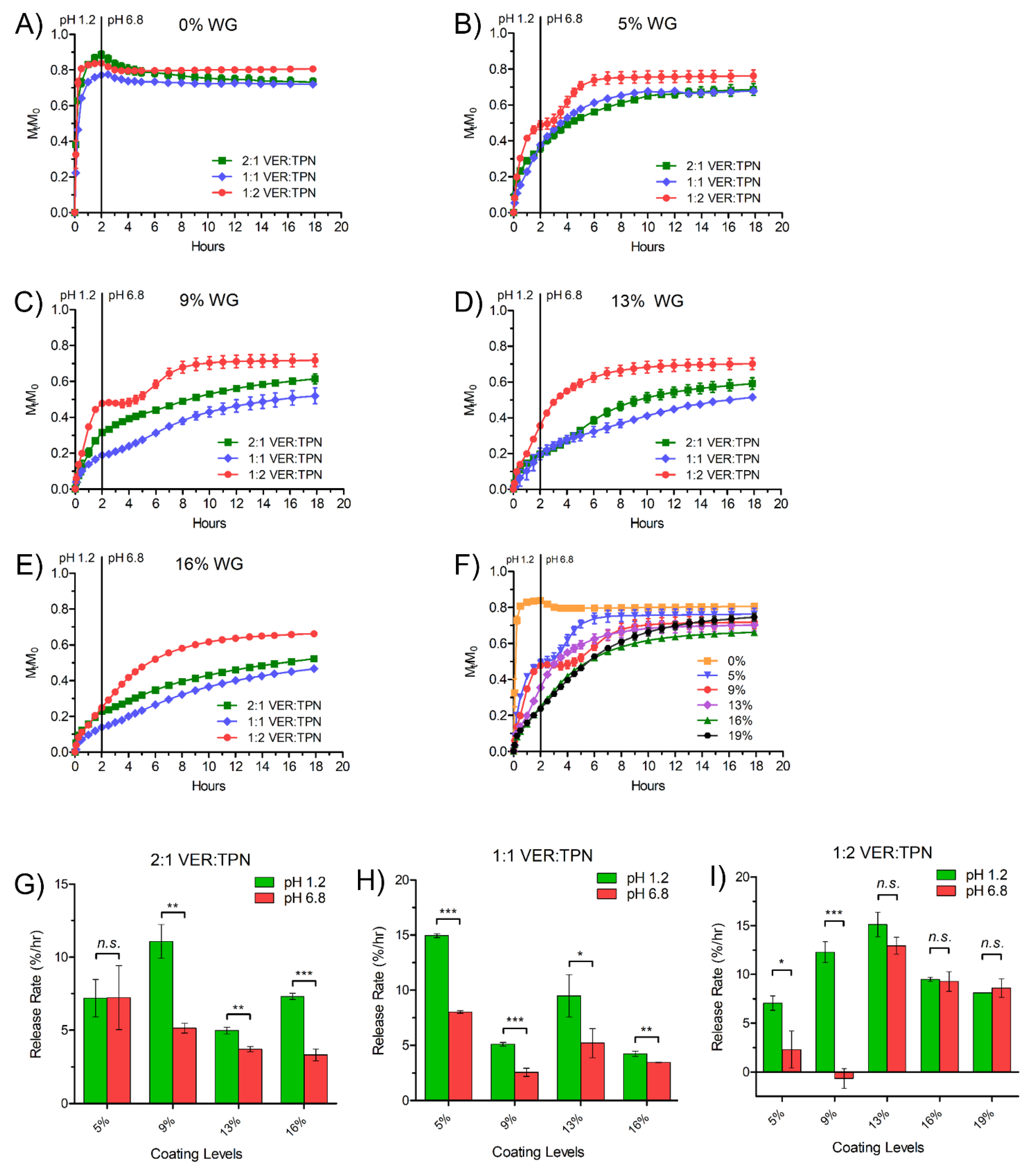
3.4. Effect of VER:TPN Ratio in TPN-EC-Matrix Layer and TPN-EC Membrane Coating Level on Drug Release from TPN Bilayer-Coated Beads
3.5. Achieving pH Transition-Independent Controlled Drug Release from Optimized TPN Bilayer-Coated Beads
4. Discussion
4.1. pH-Dependent Permeability Counteracts VER Solubility
4.2. Microenvironmental pH Modification by TPN
4.3. pH-Dependent Complexation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvořáčková, K.; Doležel, P.; Mašková, E.; Muselík, J.; Kejdušová, M.; Vetchý, D. The effect of acid pH modifiers on the release characteristics of weakly basic drug from hydrophlilic-lipophilic matrices. AAPS Pharmscitech 2013, 14, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. pH modulation: A mechanism to obtain pH-independent drug release. Expert Opin. Drug Deliv. 2010, 7, 845–857. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Measurement of intraluminal pH changes in the gastrointestinal tract of mice with gastrointestinal diseases. Biochem. Biophys. Res. Commun. 2022, 620, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Thoma, K.; Ziegler, I. The pH-independent release of fenoldopam from pellets with insoluble film coats. Eur. J. Pharm. Biopharm. 1998, 46, 105–113. [Google Scholar] [CrossRef]
- Bolourchian, N.; Dadashzadeh, S. PH-independent release of propranolol hydrochloride from HPMC-based matrices using organic acids. Daru 2008, 16, 136–142. [Google Scholar]
- Nie, S.; Pan, W.; Li, X.; Wu, X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev. Ind. Pharm. 2004, 30, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, R.; Hong, E.; Villafuerte, L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int. J. Pharm. 2000, 201, 165–173. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Dashevsky, A.; Bodmeier, R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J. Control. Release 2000, 67, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, A. Modulation of the micro-environmental pH and its influence on the gel layer behavior and release of theophylline from hydrophilic matrices. Int. J. Pharma. Bio Sci. 2013, 4, P794–P802. [Google Scholar]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Assessment of tailor-made HPMC-based matrix minitablets comprising a weakly basic drug compound. Drug Dev. Ind. Pharm. 2008, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Siepe, S.; Lueckel, B.; Kramer, A.; Ries, A.; Gurny, R. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int. J. Pharm. 2006, 316, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Yoshioka, M.; Horibe, H.; Hirai, S.; Kitamori, N.; Toguchi, H. pH-independent controlled-release microspheres using polyglycerol esters of fatty acids. J. Pharm. Sci. 1994, 83, 1600–1607. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Blends of enteric and GIT-insoluble polymers used for film coating: Physicochemical characterization and drug release patterns. J. Control. Release 2003, 89, 457–471. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C.; White, E.F.T. Polymer/polymer interaction in blends of ethyl cellulose with both cellulose derivatives and polyethylene glycol 6000. Int. J. Pharm. 1986, 34, 93–103. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C. Phase separation and morphology in ethylcellulose/cellulose acetate phthalate blends. J. Appl. Polym. Sci. 1991, 43, 845–855. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. pH-Sensitive polymer blends used as coating materials to control drug release from spherical beads: Elucidation of the underlying mass transport mechanisms. Pharm. Res. 2005, 22, 1129–1141. [Google Scholar] [CrossRef]
- Khan, M.Z.; Prebeg, Z.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- John, R.; Howard, P.T. Controlled Release Formulation. U.S. Patent US4792452A, 20 December 1988. [Google Scholar]
- Amighi, K.; Timmermans, J.; Puigdevall, J.; Baltes, E.; Moës, A.J. Peroral sustained-release film-coated pellets as a means to overcome physicochemical and biological drug-related problems. I. In vitro development and evaluation. Drug Dev. Ind. Pharm. 1998, 24, 509–515. [Google Scholar] [CrossRef]
- Gruber, P.; Brickl, R.; Bozler, G.; Stricker, H. Dipyricamole Sustained Release Forms Comprising Lacquer-Coated Particles and the Preparation Thereof. U.S. Patent US4367217A, 4 January 1983. [Google Scholar]
- Cole, E.T.; Scott, R.A.; Connor, A.L.; Wilding, I.R.; Petereit, H.U.; Schminke, C.; Beckert, T.; Cadé, D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002, 231, 83–95. [Google Scholar] [CrossRef]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug. Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Frohoff-Hülsmann, M.A.; Lippold, B.C.; McGinity, J.W. Aqueous ethyl cellulose dispersion containing plasticizers of different water solubility and hydroxypropyl methyl-cellulose as coating material for diffusion pellets II: Properties of sprayed films. Eur. J. Pharm. Biopharm. 1999, 48, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Luliński, P. Molecularly imprinted polymers based drug delivery devices: A way to application in modern pharmacotherapy. A review. Mater. Sci. Eng. C 2017, 76, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.; Mohammadi, A.; Manshadi, M.K.D.; Sanati-Nezhad, A. Mathematical and computational modeling of nano-engineered drug delivery systems. J. Control. Release 2019, 307, 150–165. [Google Scholar] [CrossRef]
- Al Ragib, A.; Chakma, R.; Dewan, K.; Islam, T.; Kormoker, T.; Idris, A.M. Current advanced drug delivery systems: Challenges and potentialities. J. Drug Deliv. Sci. Technol. 2022, 76, 103727. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheung, R.; Wu, X.Y.; Ballinger, J.R.; Bendayan, R.; Rauth, A.M. A study of doxorubicin loading onto and release from sulfopropyl dextran ion-exchange microspheres. J. Control. Release 2001, 77, 213–224. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J. Pharm. Sci. 2004, 93, 1993–2008. [Google Scholar] [CrossRef]
- Shalviri, A.; Raval, G.; Prasad, P.; Chan, C.; Liu, Q.; Heerklotz, H.; Rauth, A.M.; Wu, X.Y. pH-Dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur. J. Pharm. Biopharm. 2012, 82, 587–597. [Google Scholar] [CrossRef]
- Shalviri, A.; Chan, H.K.; Raval, G.; Abdekhodaie, M.J.; Liu, Q.; Heerklotz, H.; Wu, X.Y. Design of pH-responsive nanoparticles of terpolymer of poly(methacrylic acid), polysorbate 80 and starch for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2013, 101, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chang, H.H.R.; Shalviri, A.; Li, J.; Lugtu-Pe, J.A.; Kane, A.; Wu, X.Y. Investigation of a new pH-responsive nanoparticulate pore former for controlled release enteric coating with improved processability and stability. Eur. J. Pharm. Biopharm. 2017, 120, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.I.; Gomes, E.C.L.; Soares, C.D.V.; Cunha, A.F.; Oliveira, M.A. Thermal Analysis Applied to Verapamil Hydrochloride Characterization in Pharmaceutical Formulations. Molecules 2010, 15, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Shaw, J.; Yip, C.; Wu, X.Y. Microdomain pH gradient and kinetics inside composite polymeric membranes of pH and glucose sensitivity. Pharm. Res. 2008, 25, 1150–1157. [Google Scholar] [CrossRef]
- Li, Y.; Wong, H.L.; Shuhendler, A.J.; Rauth, A.M.; Wu, X.Y. Molecular interactions, internal structure and drug release kinetics of rationally developed polymer-lipid hybrid nanoparticles. J. Control. Release 2008, 128, 60–70. [Google Scholar] [CrossRef]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional albumin-MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- SNARF™-4F 5-(and-6)-Carboxylic Acid. Available online: https://www.thermofisher.com/order/catalog/product/S23920 (accessed on 25 January 2021).
- Munday, D.L. Film Coated Pellets Containing Verapamil Hydrochloride: Enhanced Dissolution into Neutral Medium. Drug Dev. Ind. Pharm. 2003, 29, 575–583. [Google Scholar] [CrossRef]
- Fahier, J.; Vukosavljevic, B.; De Kinder, L.; Florin, H.; Goossens, J.-F.; Windbergs, M.; Siepmann, F.; Siepmann, J.; Muschert, S. Towards a Better Understanding of Verapamil Release from Kollicoat SR:IR Coated Pellets Using Non-Invasive Analytical Tools. Pharmaceutics 2021, 13, 1723. [Google Scholar] [CrossRef]
- Yasuda, H.; Peterlin, A.; Colton, C.K.; Smith, K.A.; Merrill, E.W. Permeability of solutes through hydrated polymer membranes. Part III. Theoretical background for the selectivity of dialysis membranes. Die Makromol. Chem. 1969, 126, 177–186. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J. Appl. Polym. Sci. 1988, 36, 735–747. [Google Scholar] [CrossRef]
- Abdekhodaie, M.J.; Wu, X.Y. Modeling of a glucose sensitive composite membrane for closed-loop insulin delivery. J. Membr. Sci. 2009, 335, 21–31. [Google Scholar] [CrossRef]
- Shalviri, A.; Liu, Q.; Abdekhodaie, M.J.; Wu, X.Y. Novel modified starch–xanthan gum hydrogels for controlled drug delivery: Synthesis and characterization. Carbohydr. Polym. 2010, 79, 898–907. [Google Scholar] [CrossRef]
- Siegel, R.A. pH-sensitive gels: Swelling equilibria, kinetics, and applications for drug delivery. In Pulsed and Self-Regulated Drug Delivery, 1st ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 129–155. [Google Scholar]
- Chiu, H.-C.; Lin, Y.-F.; Hung, S.-H. Equilibrium Swelling of Copolymerized Acrylic Acid−Methacrylated Dextran Networks: Effects of pH and Neutral Salt. Macromolecules 2002, 35, 5235–5242. [Google Scholar] [CrossRef]
- Hasa, J.; Ilavský, M.; Dušek, K. Deformational, swelling, and potentiometric behavior of ionized poly(methacrylic acid) gels. I. Theory. J. Polym. Sci. 1975, 13, 253–262. [Google Scholar] [CrossRef]
- Brøndsted, H.; Kopeček, J.I. Hydrogels for site-specific oral drug delivery: Synthesis and characterization. Biomaterials 1991, 12, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, K.; Winnicka, K. Ethylcellulose-A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [Green Version]



| TPN Composition | Variables (mmol) | MAA:MBA:PS 80:Starch Molar Ratio | ||||
|---|---|---|---|---|---|---|
| MAA (x1) | MBA (x2) | MAA | MBA | PS80 | Starch | |
| Low MAA | 13.47 | 3.24 | 2.75 | 0.66 | 0.12 | 1.00 |
| High MAA | 33.01 | 3.24 | 6.74 | 0.66 | 0.12 | 1.00 |
| Mid-point (MP) | 23.24 | 3.24 | 4.74 | 0.66 | 0.12 | 1.00 |
| Low MBA | 23.24 | 0.52 | 4.74 | 0.11 | 0.12 | 1.00 |
| High MBA | 23.24 | 5.97 | 4.74 | 1.22 | 0.12 | 1.00 |
| Materials | VER:TPN (%w/w of Dispersion) | ||
|---|---|---|---|
| 2:1 | 1:1 | 1:2 | |
| VER | 2 | 2 | 2 |
| TPN | 1 | 2 | 4 |
| Surelease® | 40 | 36 | 28 |
| DDI water | 57 | 60 | 66 |
| Materials | %w/w of Dispersion | %w/w of Solid Content |
|---|---|---|
| TPN | 1 | 10 |
| Surelease® | 36 | 90 |
| DDI water | 63 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.H.R.; Chen, K.; Lugtu-Pe, J.A.; AL-Mousawi, N.; Zhang, X.; Bar-Shalom, D.; Kane, A.; Wu, X.Y. Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery. Pharmaceutics 2023, 15, 547. https://doi.org/10.3390/pharmaceutics15020547
Chang HHR, Chen K, Lugtu-Pe JA, AL-Mousawi N, Zhang X, Bar-Shalom D, Kane A, Wu XY. Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery. Pharmaceutics. 2023; 15(2):547. https://doi.org/10.3390/pharmaceutics15020547
Chicago/Turabian StyleChang, Hao Han R., Kuan Chen, Jamie Anne Lugtu-Pe, Nour AL-Mousawi, Xuning Zhang, Daniel Bar-Shalom, Anil Kane, and Xiao Yu Wu. 2023. "Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery" Pharmaceutics 15, no. 2: 547. https://doi.org/10.3390/pharmaceutics15020547
APA StyleChang, H. H. R., Chen, K., Lugtu-Pe, J. A., AL-Mousawi, N., Zhang, X., Bar-Shalom, D., Kane, A., & Wu, X. Y. (2023). Design and Optimization of a Nanoparticulate Pore Former as a Multifunctional Coating Excipient for pH Transition-Independent Controlled Release of Weakly Basic Drugs for Oral Drug Delivery. Pharmaceutics, 15(2), 547. https://doi.org/10.3390/pharmaceutics15020547







