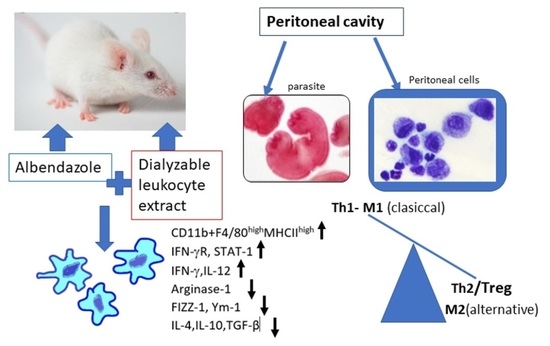
Co-Treatment with Human Leukocyte Extract and Albendazole Stimulates Drug’s Efficacy and Th1 Biased Immune Response in Mesocestoides vogae (Cestoda) Infection via Modulation of Transcription Factors, Macrophage Polarization, and Cytokine Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Biochemicals
2.2. Mice, Infection, and Experiment Design
2.3. Isolation of Exudates and Cell Sample Preparation
2.4. In Vitro Experiments on Adherent PECs from Infected Mice
2.5. RNA Isolation and Real-Time PCR
2.6. Staining of Adherent PECs
2.7. Flow Cytometric Analysis
2.8. Determination of Nitrite Production by Peritoneal Cells Ex Vivo
2.9. Cytokine Detection in Mouse Peritoneal Exudates
2.10. Preparation and Analysis of Standard ABZ and Its Metabolites via LC-MS
2.11. Chromatographic Conditions
2.12. Metacestode Burden in the Peritoneal Cavity
2.13. Statistical Analysis
3. Results
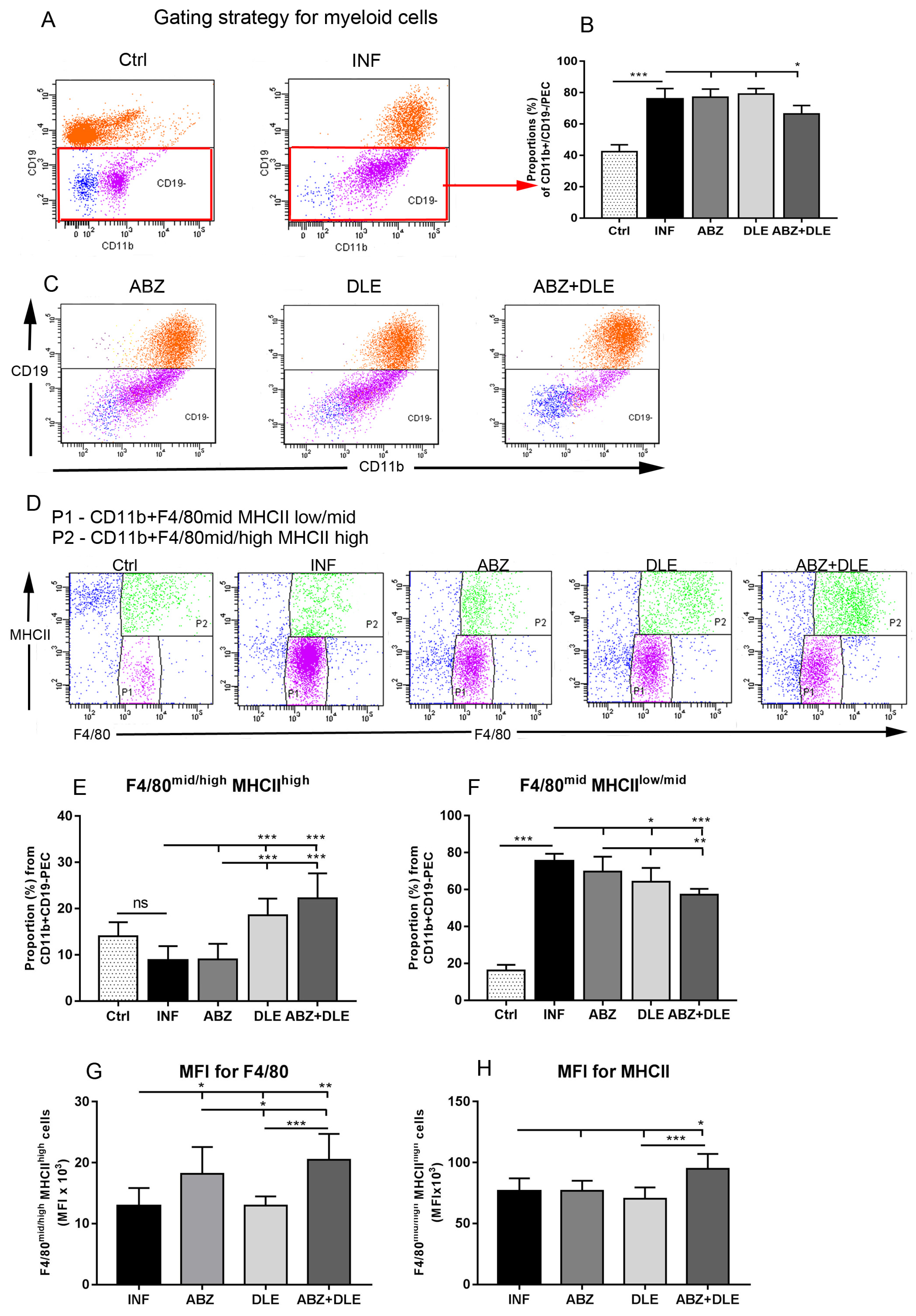
3.1. Proportions of Myeloid Peritoneal Exudate Cells
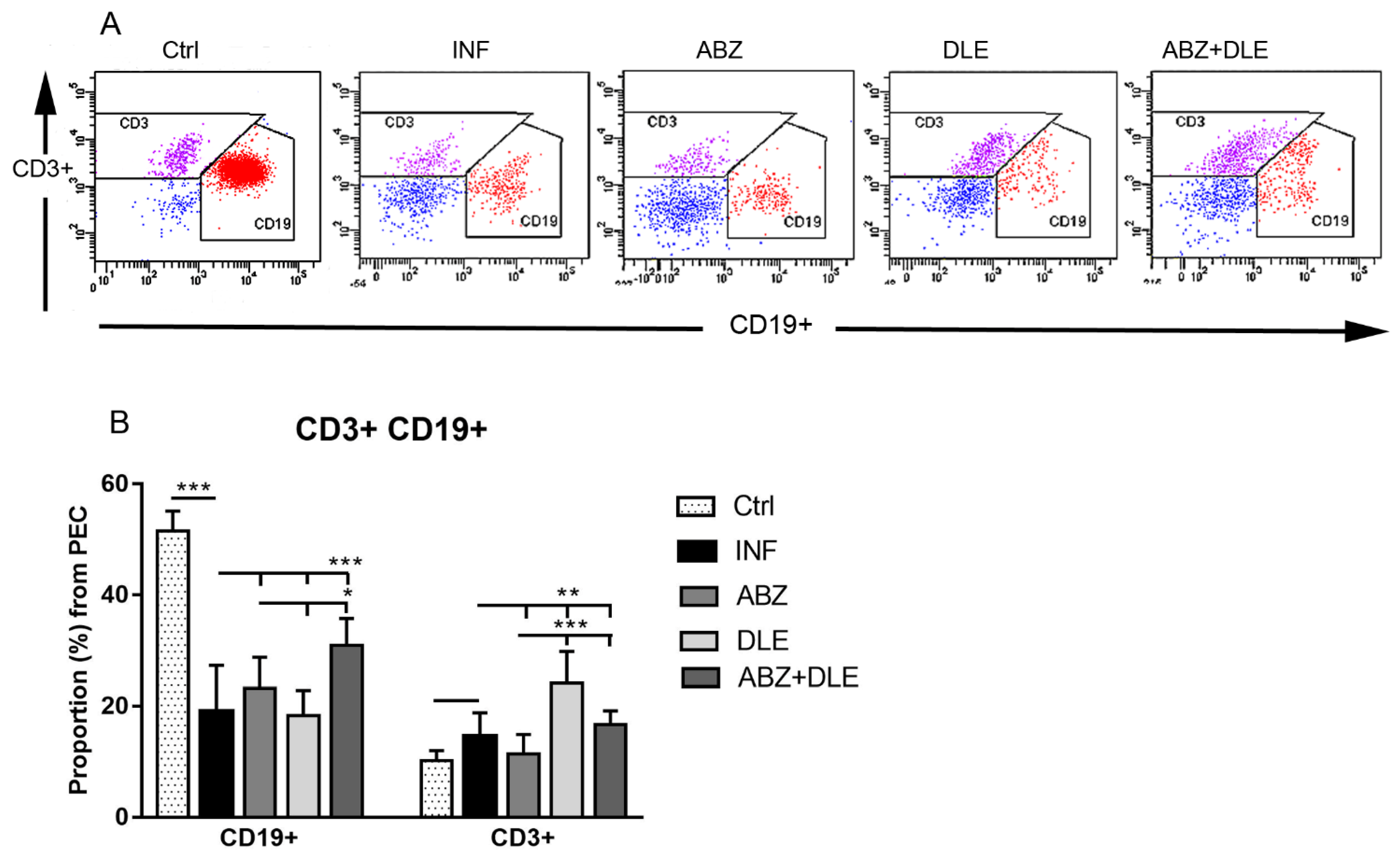
3.2. Proportions of Lymphoid Peritoneal Exudate Cells
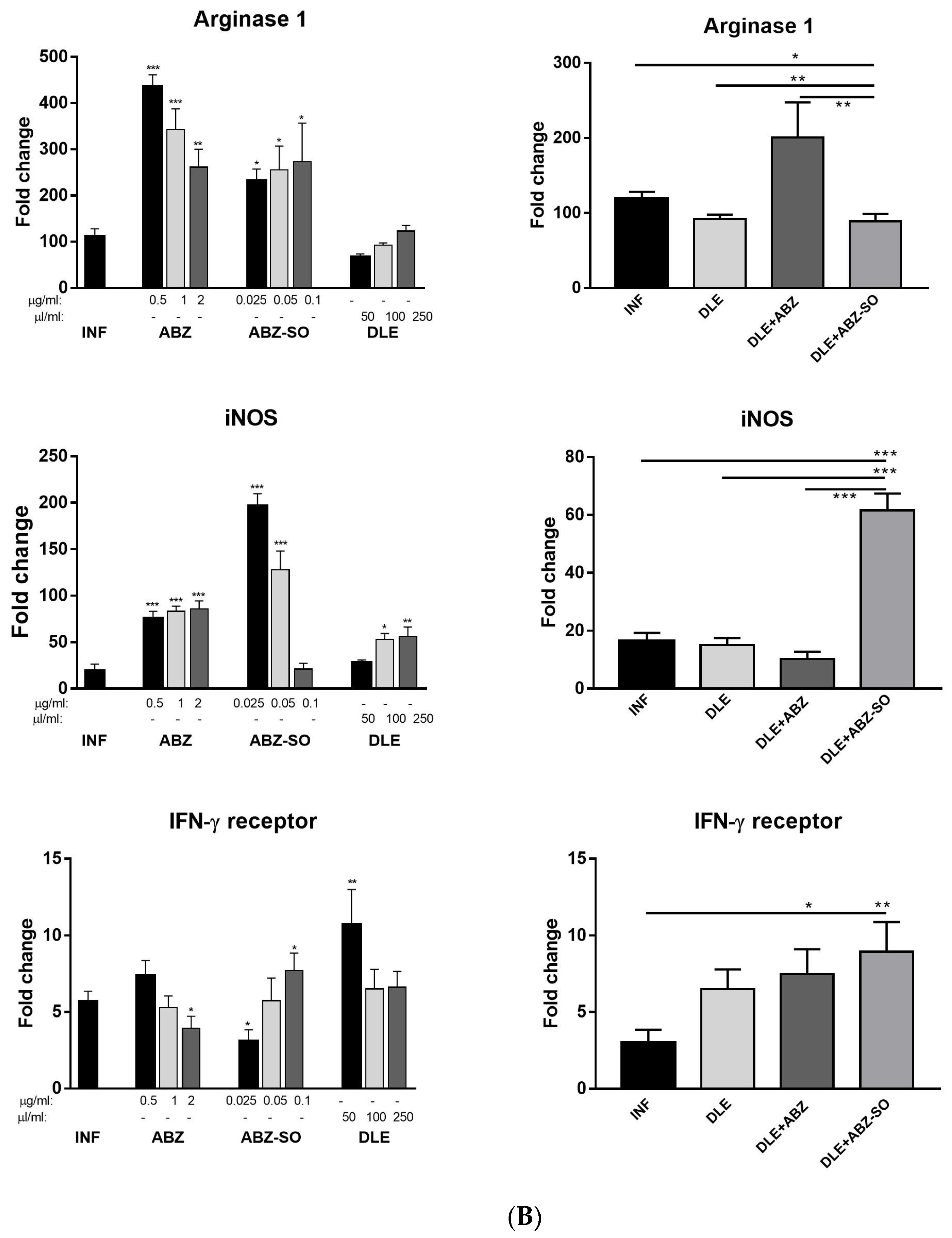
3.3. Gene Expression of Myeloid Cell Markers in Peritoneal Exudate Cells
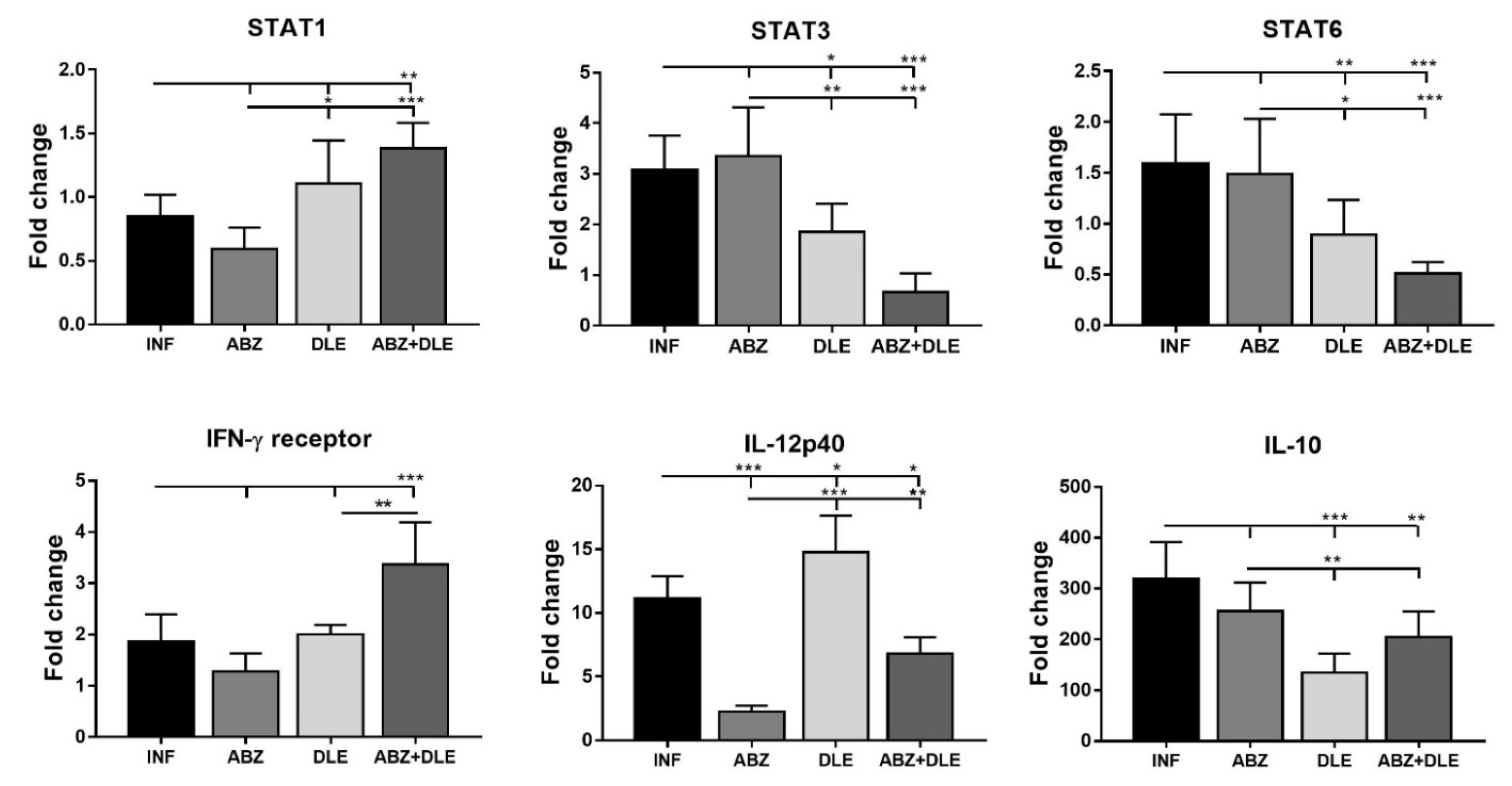
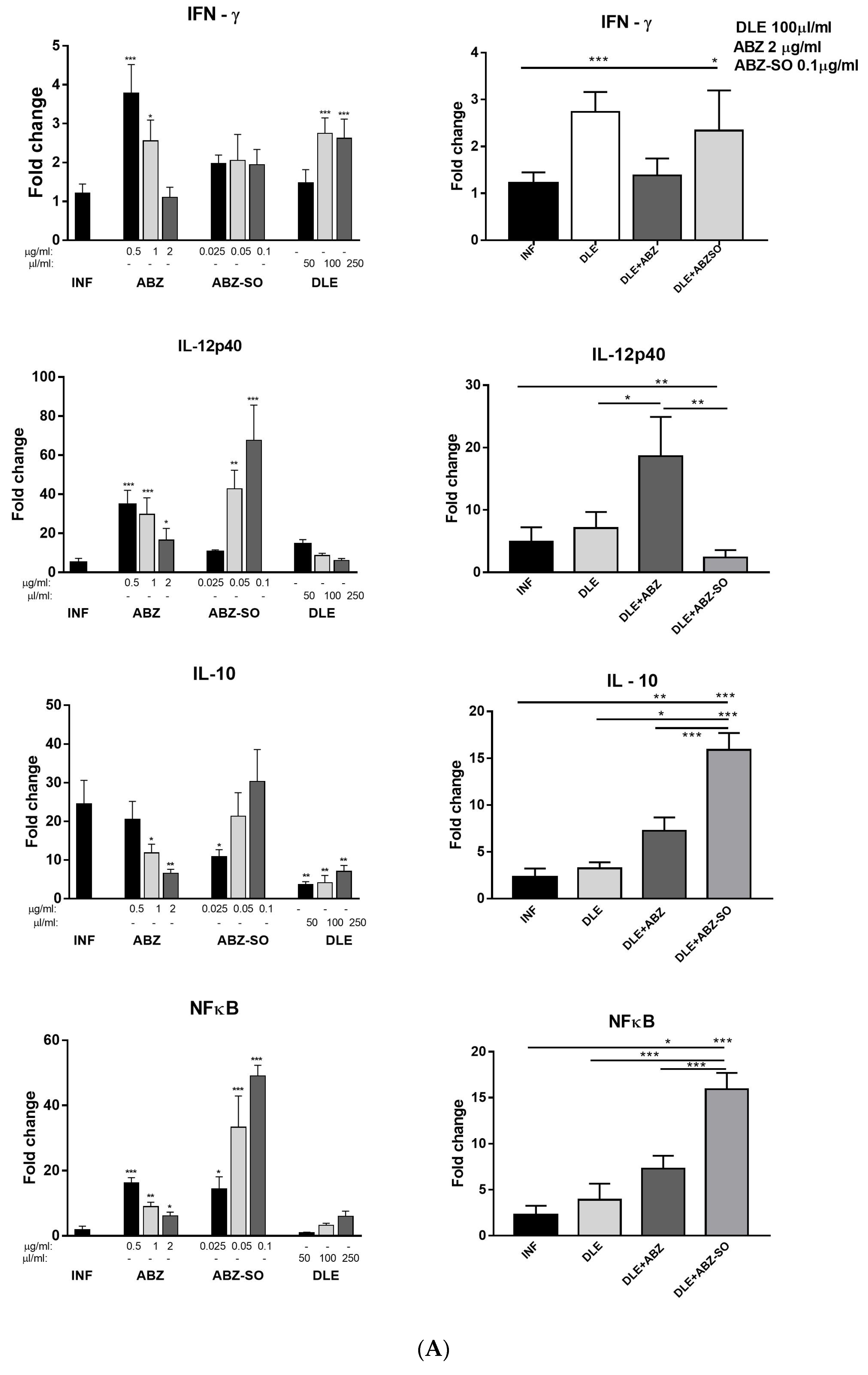
3.4. m-RNA Transcription Profiles of Cytokines and NFκB in Peritoneal Exudate Cells
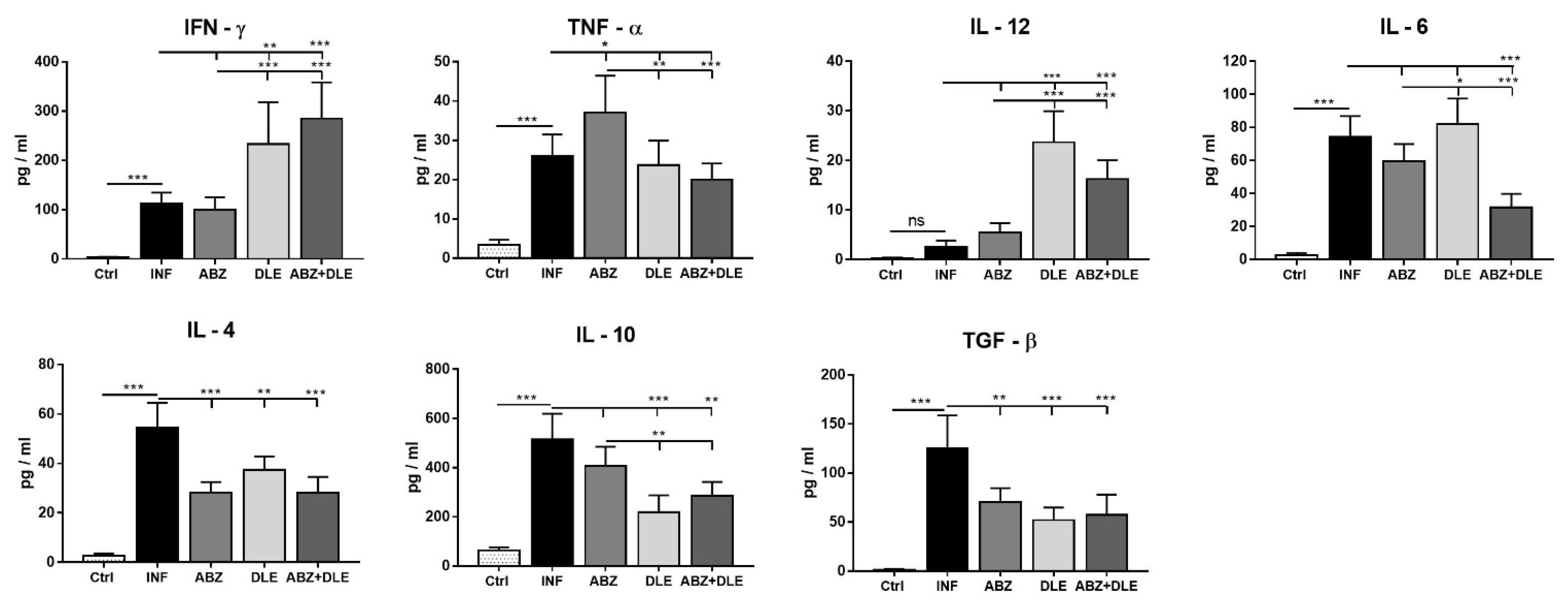
3.5. Cytokine Concentrations in the Peritoneal Exudates
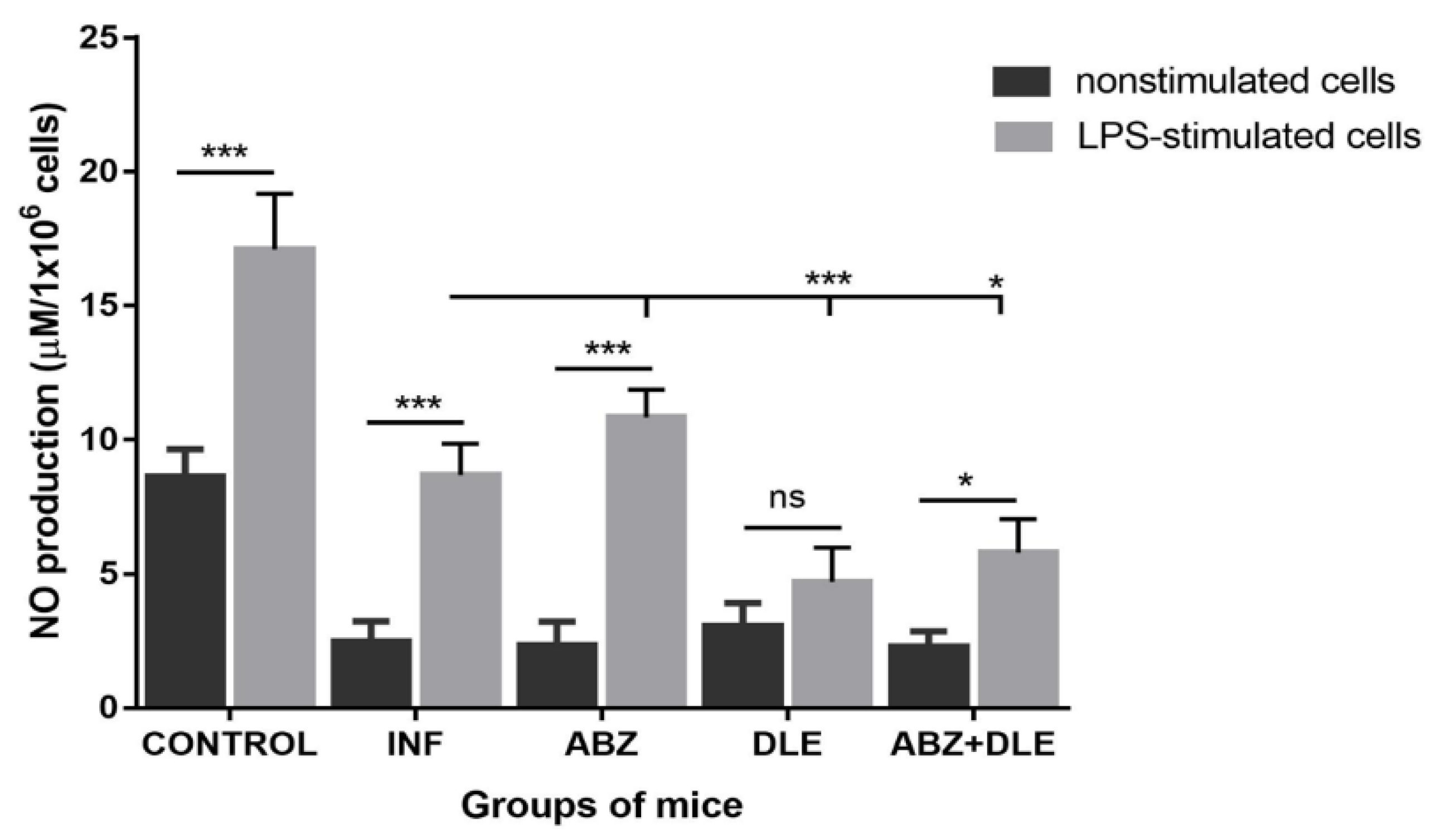
3.6. Production of NO by Peritoneal Cells Ex Vivo
3.7. Morphological and Phenotypic Analysis of Adherent Peritoneal Cells
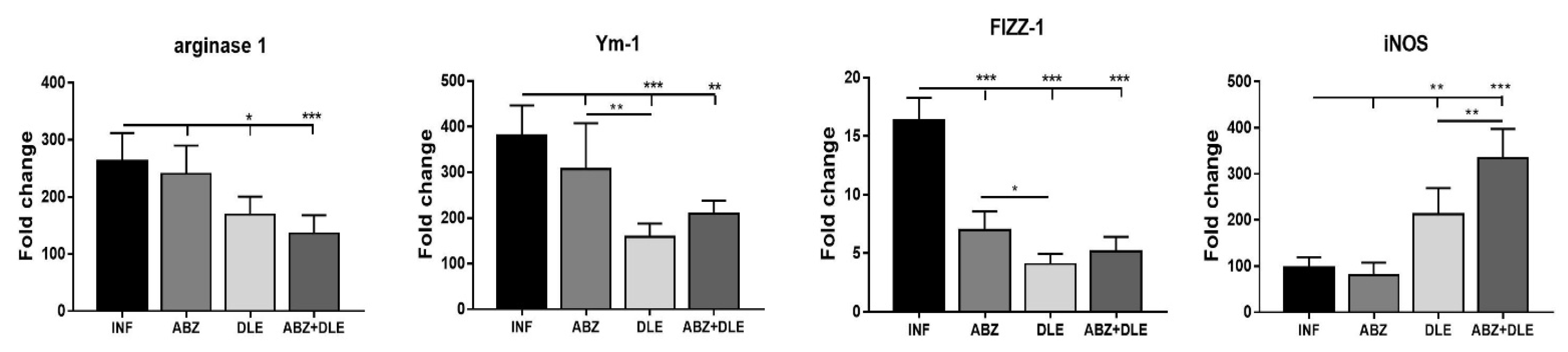
3.8. Gene Expression of Selected Markers in Adherent Macrophages
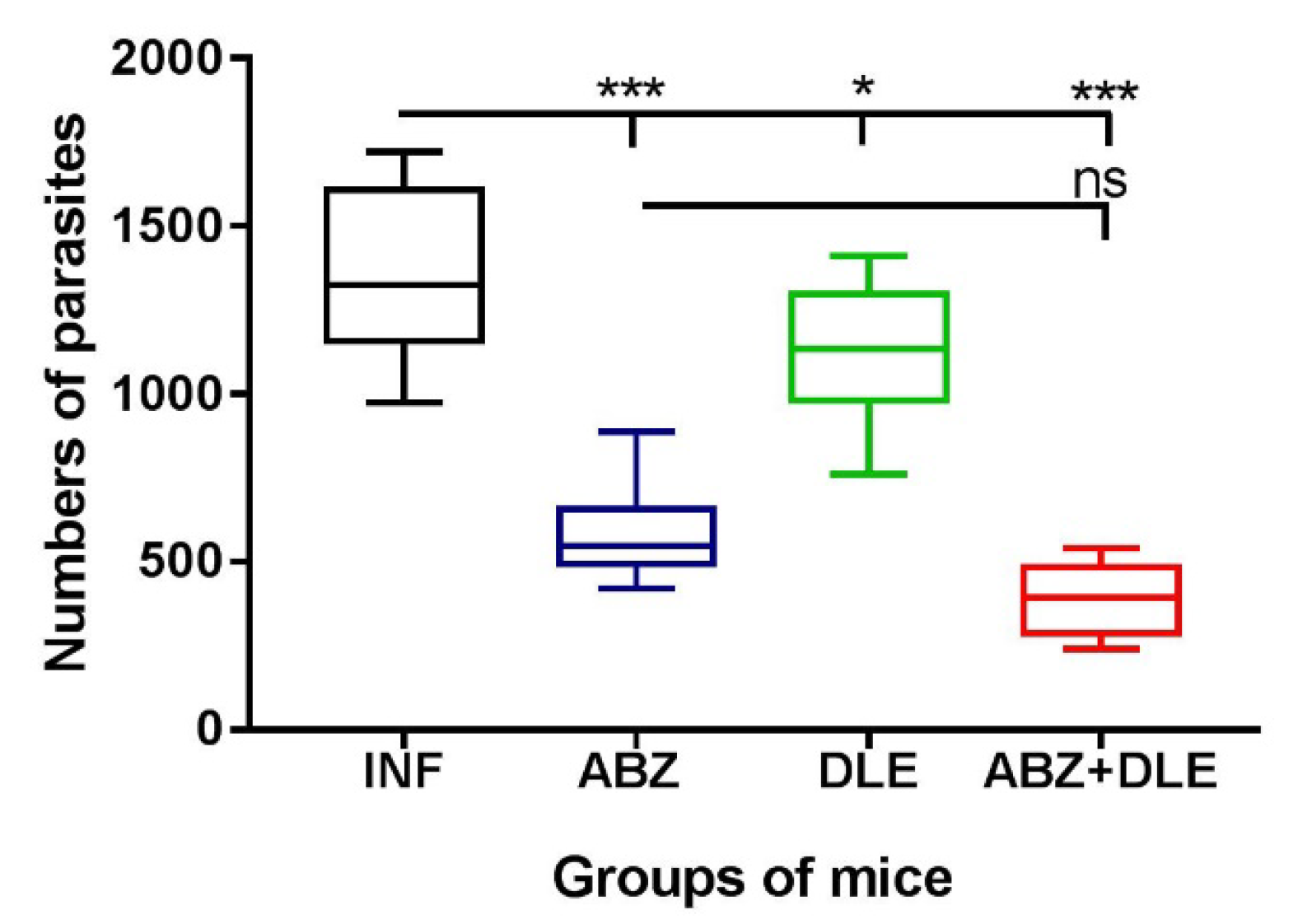
3.9. Parasite Numbers in the Peritoneal Cavities
3.10. In Vitro Experiments on Adherent Peritoneal Macrophages/Monocytes from Infected Mice
3.11. Pharmacokinetic Analysis of ABZ Metabolites in Plasma of Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Echinococcosis; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (accessed on 31 August 2021).
- Vuitton, D.A. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: What is the consensus? Expert Rev. Anti Infect. Ther. 2009, 7, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Qavi, A.; Garg, R.K.; Malhotra, H.S.; Jain, A.; Kumar, N.; Malhotra, K.P.; Srivastava, P.K.; Verma, R.; Sharma, P.K. Disseminated cysticercosis: Clinical spectrum, Toll-like receptor-4 gene polymorphisms and role of albendazole: A prospective follow-up of 60 cases with a review of 56 published cases. Medicine 2016, 95, e4882. [Google Scholar] [CrossRef] [PubMed]
- Rasib, Q.; Khan, A.; Ahmed, H.; Nizamuddin, S.; Asif, F.; Afzal, M.S.; Simsek, S.; Khurshid, F.; Irum, S.; Hussain, N.; et al. Demographics of cystic echinococcosis patients treated surgically in Lahore, Pakistan: A single centre study from 2007–2018. Helminthologia 2021, 58, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. Albendazole for the treatment of echinococcosis. Fund. Clin. Pharmacol. 2003, 17, 205–212. [Google Scholar]
- Hemphill, A.; Müller, J. Alveolar and cystic echinococcosis: Towards novel chemotherapeutical treatment options. J. Helminthol. 2009, 83, 99–111. [Google Scholar] [CrossRef]
- Lacey, E. Mode of action of benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- Lopez, L.M.; Pensel, P.E.; Fabbri, J.; Albani, C.M.; Elissondo, N.; Gambino, G.; Elissondo, M.C. The combination of carvacrol and albendazole enhanced the efficacy of monotherapy in experimental alveolar echinococcosis. Acta Tropica 2022, 225, 106198. [Google Scholar] [CrossRef]
- Hamprecht, K.H.; Vötsch, W.; Anderer, F.A. A dialysable acid factor from human leukocyte extracts activates tumor cell lysis mediated by human monocytes and natural killer cells. Onkologie 1989, 12, 120–127. [Google Scholar] [CrossRef]
- Doelker, I.; Anderer, F.A. The CySF-L2 factor from dialysable human leucocyte extract activates natural killer cytotoxicity by induction of interferon gamma. Cancer Immunol. Immunother. 1992, 34, 299–305. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H. Transfer factors: Identification of conserved sequences in transfer factor molecules. Mol. Med. 2000, 6, 332–341. [Google Scholar] [CrossRef]
- Arnaudov, A.; Kostova, Z. Dialysable leukocyte extracts in immunotherapy. Biotechnol. Biotechnol. Equip. 2015, 29, 1017–1023. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H. Structural nature and functions of transfer factors. Ann. N. Y. Acad. 1993, 685, 362–368. [Google Scholar] [CrossRef]
- Zuniga-Navarrete, F.; Zavala-Meneses, S.G.; Zelnik, V.; Kopacek, J.; Skultety, L. Initial proteomic characterization of IMMODIN, commercially available dialysable leukocytes extract. Chem. Pap. 2021, 75, 1959–1968. [Google Scholar] [CrossRef]
- Demečkovám, V.; Solár, P.; Hrčková, G.; Mudroňová, D.; Bojková, B.; Kassayová, M.; Gancarčiková, S. Immodin and its immune system supportive role in paclitaxel therapy of 4T1 mouse breast cancer. Biomed. Pharmacother. 2017, 89, 245–256. [Google Scholar] [CrossRef]
- Solár, P.; Sačková, V.; Hrčková, G.; Demečková, V.; Kassayová, M.; Bojková, B.; Mudroňová, D.; Gancarčíková, S.; Jendželovský, R.; Fedoročko, P. Antitumor effect of the combination of manumycin A and Immodin is associated with antiplatelet activity and increased granulocyte tumor infiltration in a 4T1 breast tumor model. Oncol. Rep. 2017, 37, 368–378. [Google Scholar] [CrossRef]
- Hernández-Esquivel, M.A.; Pérez-Torres, A.; Romero-Romero, L.; Reyes-Matute, A.; Loaiza, B.; Mellado-Sánchez, G.; Pavón, L.; Medina-Rivero, E.; Pestell, R.G.; Pérez-Tapia, S.M.; et al. The dialyzable leukocyte extract Transferon TM inhibits tumor growth and brain metastasis in a murine model of prostate cancer. Biomed. Pharmacother. 2018, 101, 938–944. [Google Scholar] [CrossRef]
- Cherenko, S.O.; Reva, O.A.; Rekalova, O.M.; Kibizova, N.I.; Yasir, S.G.; Matvienko, Y.O. Immunotherapy with leukocyte immunomodulator dialysate in patients with multidrug-resistant tuberculosis. Asthma Allergy 2013, 3, 13–20. [Google Scholar]
- Byston, J.; Cech, K.; Pekarek, J.; Jilkova, J. Effect of anti-herpes specific transfer factor. Biotherapy 1996, 9, 73–75. [Google Scholar] [CrossRef]
- Vacek, A.; Hofer, M.; Barnet, K.; Cech, K.; Pekárek, J.; Schneiderová, H. Positive effects of dialyzable leukocyte extract (DLE) on recovery of mouse haemopoiesis suppressed by ionizing radiation and on proliferation of haemopoietic progenitor cells in vitro. Int. J. Immunopharmacol. 2000, 22, 623–634. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Allen, J.E.; Maizels, R. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kreider, T.; Anthony, R.M.; Urban, J.F., Jr.; Gause, W.C. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 2007, 19, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Horsnell, W.G.; Brombacher, F. Genes associated with alternatively activated macrophages discretely regulate helminth infection and pathogenesis in experimental mouse models. Immunobiology 2010, 215, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Hrčková, G.; Velebný, S.; Solár, P. Dynamics of hepatic stellate cells, collagen types I and III synthesis and gene expression of selected cytokines during hepatic fibrogenesis following Mesocestoides vogae (Cestoda) infection in mice. Int. J. Parasitol. 2010, 40, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Vendelova, E.; Lutz, M.B.; Hrčková, G. Immunity and immune modulation elicited by the larval cestode Mesocestoides vogae and its products. Parasite Immunol. 2015, 37, 493–504. [Google Scholar] [CrossRef]
- Cardoso, F.M.; Tomkova, M.; Petrovajova, D.; Bubanova, M.; Ragac, O.; Hornakova, T. New and cost effective cell-based assay for Dialyzed Leukocyte Extract (DLE)-induced Jurkat cells proliferation under azathioprine treatment. J. Pharm. Biomed. Anal. 2017, 138, 100–108. [Google Scholar] [CrossRef]
- Mačák Kubašková, T.; Mudroňová, D.; Vargová, M.; Reiterová, K.; Hrčková, G. Cellular and humoral peritoneal immunity to Mesocestoides vogae metacestode infection in mice. Parasites Vectors 2021, 14, 54. [Google Scholar] [CrossRef]
- Mingjie, W.; Shuhua, X.; Junjie, C.; Bin, L.; Cheng, F.; Weixia, S.; Hotez, P. Albendazole-soybean oil emulsion for the treatment of human cystic echinococcosis: Evaluation of bioavailability and bioequivalence. Acta Trop. 2002, 83, 177–181. [Google Scholar] [CrossRef]
- O’connell, A.E.; Kerepesi, L.A.; Vandergrift, G.L.; Herbert, D.R.; Van Winkle, T.J.; Hooper, D.C.; Pearce, E.J.; Abraham, D. IL-4(-/-) mice with lethal Mesocestoides corti infections--reduced Th2 cytokines and alternatively activated macrophages. Parasite Immunol. 2009, 31, 741–749. [Google Scholar] [CrossRef]
- Raes, G.; Noël, W.; Beschin, A.; Brys, L.; De Baetselier, P.; Hassanzadeh, G.H.G. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev. Immunol. 2002, 9, 151–159. [Google Scholar] [CrossRef]
- Nair, M.G.; Gallagher, I.J.; Taylor, M.D.; Loke Pn Coulson, P.S.; Wilson, R.A.; Maizels, R.M.; Allen, J.E. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 2005, 73, 385–394. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Mačák Kubašková, T.; Mudroňová, D.; Velebný, S.; Hrčková, G. The utilisation of human dialyzable leukocyte extract (IMMODIN) as adjuvant in albendazole therapy on mouse model of larval cestode infection: Immunomodulatory and hepatoprotective effects. Int. Immunopharmacol. 2018, 65, 148–158. [Google Scholar] [CrossRef]
- Dvorožňáková, E.; Porubcová, J.; Ševcíková, Z. Immune response of mice with alveolar echinococcosis to therapy with transfer factor, alone and in combination with albendazole. Parasitol. Res. 2009, 105, 1067–1076. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hardy, R.R.; Herzenberg, L.A. Peritoneal Ly-1 B cells: Genetic control, autoantibody production, increased lambda light chain expression. Eur. J. Immunol. 1986, 16, 450–456. [Google Scholar] [CrossRef]
- Gordon, S.; Lawson, L.; Rabinowitz, S.; Crocker, P.R.; Morris, L.; Perry, V.H. Antigen markers of macrophage differentiation in murine tissues. Curr. Top Microbiol. Immunol. 1992, 181, 1–37. [Google Scholar]
- Jiménez-Uribe, A.P.; Valencia-Martínez, H.; Carballo-Uicab, G.; Vallejo-Castillo, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; Velasco-Velázquez, M.A.; Mellado-Sánchez, G.; Estrada-Parra, S.; et al. CD80 expression correlates with IL-6 production in THP-1-like macrophages costimulated with LPS and dialyzable leukocyte extract (Transferon®). J. Immunol. Res. 2019, 2019, 2198508. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Locati, M. New vistas on macrophage differentiation and activation. Eur. J. Immunol. 2007, 37, 14–16. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; Harris, N.L. Interactions between macrophages and helminths. Parasite Immunol. 2020, 42, e12717. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: Granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Gong, W.; Zhang, X.; Xu, M.; Wang, Y.; Xu, Y.; Cao, J.; Shen, Y.; Chen, J. Arginase promotes immune evasion of Echinococcus granulosus in mice. Parasit. Vectors 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Makepeace, B.L.; Martin, C.; Turner, J.D.; Specht, S. Granulocytes in helminth infection–who is calling the shots? Curr. Med. Chem. 2012, 19, 1567–1586. [Google Scholar] [CrossRef]
- Arulanandam, R.; Batenchuk, C.; Varette, O.; Zakaria, C.; Garcia, V.; Forbes, N.E.; Davis, C.; Krishnan, R.; Karmacharya, R.; Cox, J.; et al. Microtubule disruption synergizes with oncolytic virotherapy by inhibiting interferon translation and potentiating bystander killing. Nat. Commun. 2015, 6, 6410. [Google Scholar] [CrossRef]
- Riganò, R.; Profumo, E.; Buttari, B.; Teggi, A.; Siracusano, A. Cytokine gene expression in peripheral blood mononuclear cells (PBMC) from patients with pharmacologically treated cystic echinococcosis. Clin. Exp. Immunol. 1999, 118, 95–101. [Google Scholar] [CrossRef]
- Godot, V.; Harraga, S.; Beurton, I.; Deschaseaux, M.; Sarciron, E.; Gottstein, B.; Vuitton, D.A. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. I. Comparison of patients with progressive and abortive lesions. Clin. Exp. Immunol. 2000, 121, 484–490. [Google Scholar]
- Fabre, R.A.; Pérez, T.M.; Aguilar, L.D.; Rangel, M.J.; Estrada-Garcìa, I.; Hernández-Pando, R.; Estrada Parra, S. Transfer factors as immunotherapy and supplement of chemotherapy in experimental pulmonary tuberculosis. Clin. Exp. Immunol. 2004, 136, 215–223. [Google Scholar] [CrossRef]
- Murray, P.J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006, 6, 379–386. [Google Scholar] [CrossRef]
- Cooper, A.M.; Khader, S.A. IL-12p40: An inherently agonistic cytokine. Trends Immunol. 2007, 28, 33–38. [Google Scholar] [CrossRef]
- Rawat, J.; Dixon, J.B.; Macintyre, A.R.; Mcgarry, H.F.; Taylor, M.J. IL-4 dependent resistance to the tapeworm Mesocestoides corti (Cestoda) in mice. Parasite Immunol. 2003, 25, 553–557. [Google Scholar] [CrossRef]
- Jenkins, P.; Spiers, S.; Dixon, J.B.; Carter, S.D.; May, S. The effects of tumour necrosis factor on host-parasite relations in murine Mesocestoides corti (Cestoda) infection. Parasitology 1992, 105 Pt 3, 453–459. [Google Scholar] [CrossRef]
- Amiot, F.; Vuong, P.; Defontaines, M.; Pater, C.; Dautry, F.; Liance, M. Secondary alveolar echinococcosis in lymphotoxin-alpha and tumour necrosis factor-alpha deficient mice: Exacerbation of Echinococcus multilocularis larval growth is associated with cellular changes in the periparasitic granuloma. Parasite Immunol. 1999, 21, 475–483. [Google Scholar] [CrossRef]
- Baska, P.; Norbury, L.J. The Role of Nuclear Factor Kappa B (NF-kappa B) in the Immune Response against Parasites. Pathogens 2022, 11, 310. [Google Scholar] [CrossRef]
- Stark, G.R. How cells respond to interferons revisited: From early history to current complexity. Cytokine Growth Factor Rev. 2007, 18, 419–423. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Cassado Ados, A.; D’império Lima, M.R.; Bortoluci, K.R. Revisiting mouse peritoneal macrophages: Heterogeneity, development, and function. Front. Immunol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity 1996, 4, 313–319. [Google Scholar] [CrossRef]
- Ramirez, T.; Benitez-Bribiesca, L.; Ostrosky-Wegman, P.; Herrera, L.A. In vitro effects of albendazole and its metabolites on the cell proliferation kinetics and micronuclei frequency of stimulated human lymphocytes. Arch. Med. Res. 2001, 32, 119–122. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, I.; Ruiz-Olmedo, M.I.; Cardenas, G.; Jung-Cook, H. A simple LC-MS/MS method to determine plasma and cerebrospinal fluid levels of albendazole metabolites (albendazole sulfoxide and albendazole sulfone) in patients with neurocysticercosis. Biomed. Chromatogr. 2012, 26, 267–272. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrčková, G.; Mačak Kubašková, T.; Mudroňová, D.; Jurčacková, Z.; Ciglanová, D. Co-Treatment with Human Leukocyte Extract and Albendazole Stimulates Drug’s Efficacy and Th1 Biased Immune Response in Mesocestoides vogae (Cestoda) Infection via Modulation of Transcription Factors, Macrophage Polarization, and Cytokine Profiles. Pharmaceutics 2023, 15, 541. https://doi.org/10.3390/pharmaceutics15020541
Hrčková G, Mačak Kubašková T, Mudroňová D, Jurčacková Z, Ciglanová D. Co-Treatment with Human Leukocyte Extract and Albendazole Stimulates Drug’s Efficacy and Th1 Biased Immune Response in Mesocestoides vogae (Cestoda) Infection via Modulation of Transcription Factors, Macrophage Polarization, and Cytokine Profiles. Pharmaceutics. 2023; 15(2):541. https://doi.org/10.3390/pharmaceutics15020541
Chicago/Turabian StyleHrčková, Gabriela, Terézia Mačak Kubašková, Dagmar Mudroňová, Zuzana Jurčacková, and Denisa Ciglanová. 2023. "Co-Treatment with Human Leukocyte Extract and Albendazole Stimulates Drug’s Efficacy and Th1 Biased Immune Response in Mesocestoides vogae (Cestoda) Infection via Modulation of Transcription Factors, Macrophage Polarization, and Cytokine Profiles" Pharmaceutics 15, no. 2: 541. https://doi.org/10.3390/pharmaceutics15020541
APA StyleHrčková, G., Mačak Kubašková, T., Mudroňová, D., Jurčacková, Z., & Ciglanová, D. (2023). Co-Treatment with Human Leukocyte Extract and Albendazole Stimulates Drug’s Efficacy and Th1 Biased Immune Response in Mesocestoides vogae (Cestoda) Infection via Modulation of Transcription Factors, Macrophage Polarization, and Cytokine Profiles. Pharmaceutics, 15(2), 541. https://doi.org/10.3390/pharmaceutics15020541