Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques
Abstract
1. Introduction
2. Physicochemical Properties of Au at Nanoscale
- Their small size increases the number of surface atoms compared to the number of those in the volume. This happens because as the size decreases, there is a large increase in the surface/volume ratio (Figure 1). For this reason, any chemical reaction or phenomenon that occurs on the surface is enormously amplified [17]. Furthermore, the surface/volume ratio increase is responsible for the chemical and physical differences that nanomaterials possess that the bulk material does not.The high surface/volume ratio has some rather important implications for the behavior of the nanostructures themselves. If a bulk material is divided into smaller pieces, the volume remains constant, but the size of the surface undergoes a significant increase. Since each surface is associated with a specific surface energy, Esup, there will be an increase in the total Esup of the system. For this reason, nanostructured materials are metastable or thermodynamically unstable. This scenario explains why NPs have a strong tendency to agglomerate.To avoid agglomeration, some precautions can be adopted during the synthetic stage. These include the use of surfactants that work by adsorbing onto the AuNPs’ surface, increasing the repulsion forces among the NPs, thus preventing agglomeration.Controlling parameters such as temperature and synthetic times may possibly reduce the degree to which the AuNPs clump.Finally, an additional expedient is to use several AuNPs washes once they are synthesized. This allows them to be purified from organic and/or inorganic residues due to the presence of solvent materials used during the synthesis phase [19].

- In addition, Au is a noble metal material that has unique optical and electrical properties that are dependent on the LSPR phenomenon, which is typical of metal NPs. It refers to an oscillation of free electrons on the interface of the metal surface produced after the interaction with an incident electromagnetic wave. In particular, it happens if the frequency of the electromagnetic wave resonates with that of the electrons in the conduction band, generating a plasmon band (Figure 2).The result is an intense diffusion and strong absorption of light, combined by an enhancement of the electric field near the nanostructure surface [21,22].The LSPR phenomenon is more effective in Au, as well as more sensitive. Furthermore, its properties make it suitable for the biosensor’s development or as therapeutic tool, which exploit the phenomenon described above.

- The color of Au at the nanoscale is different from that of the material in the bulk form.At the macro scale, Au is yellow, with differences based on the brightness, depending on the surface roughness and on the purity of the material. When the size is reduced to the nanoscale, the color considerably changes due to the LSPR effect.

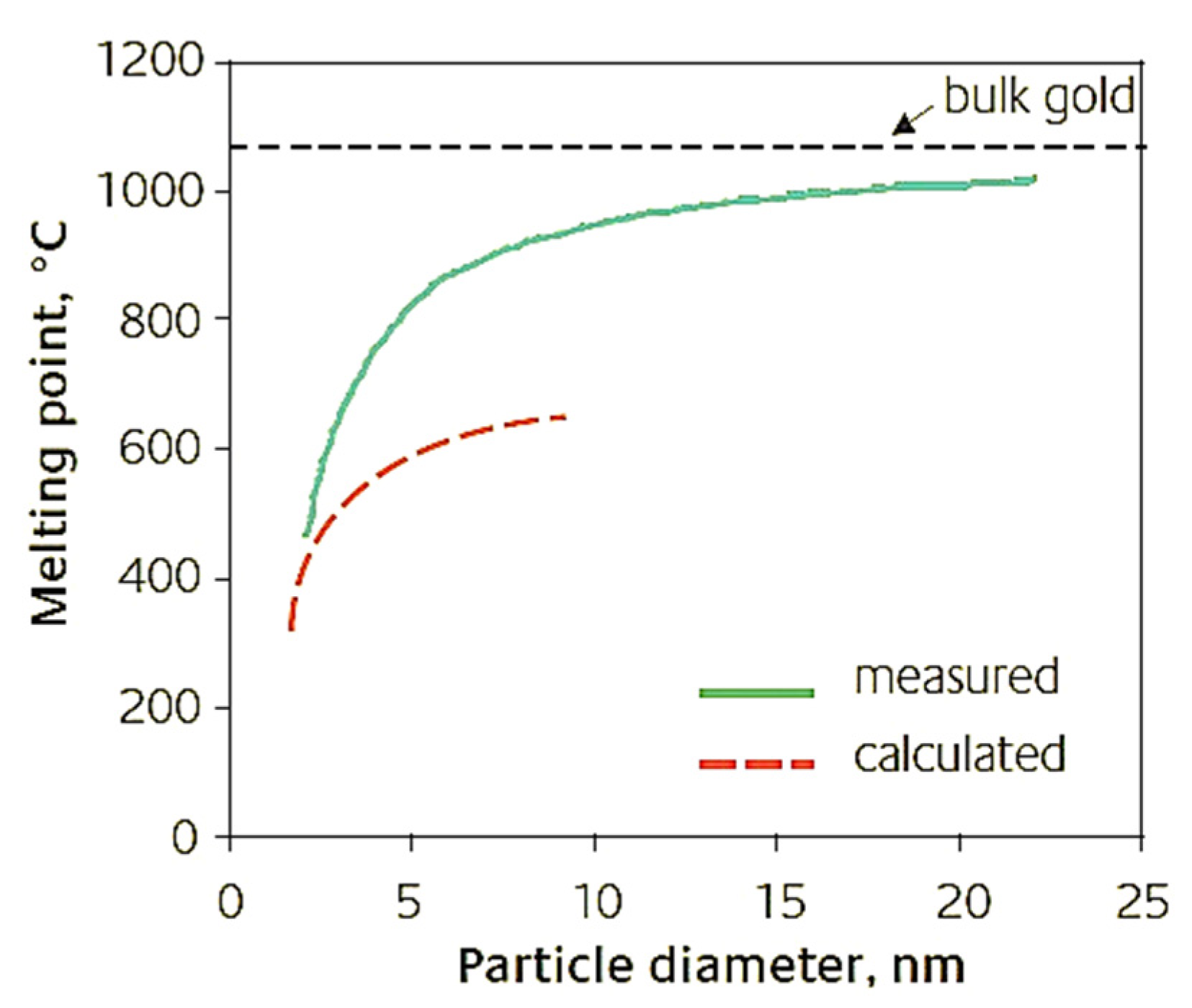
- Under standard conditions, the melting point of Au in the bulk form is ∼1064 °C. As the size of the AuNPs decreases to below 10 nm, the Tfus also decreases. This is due to the high surface/volume ratio.
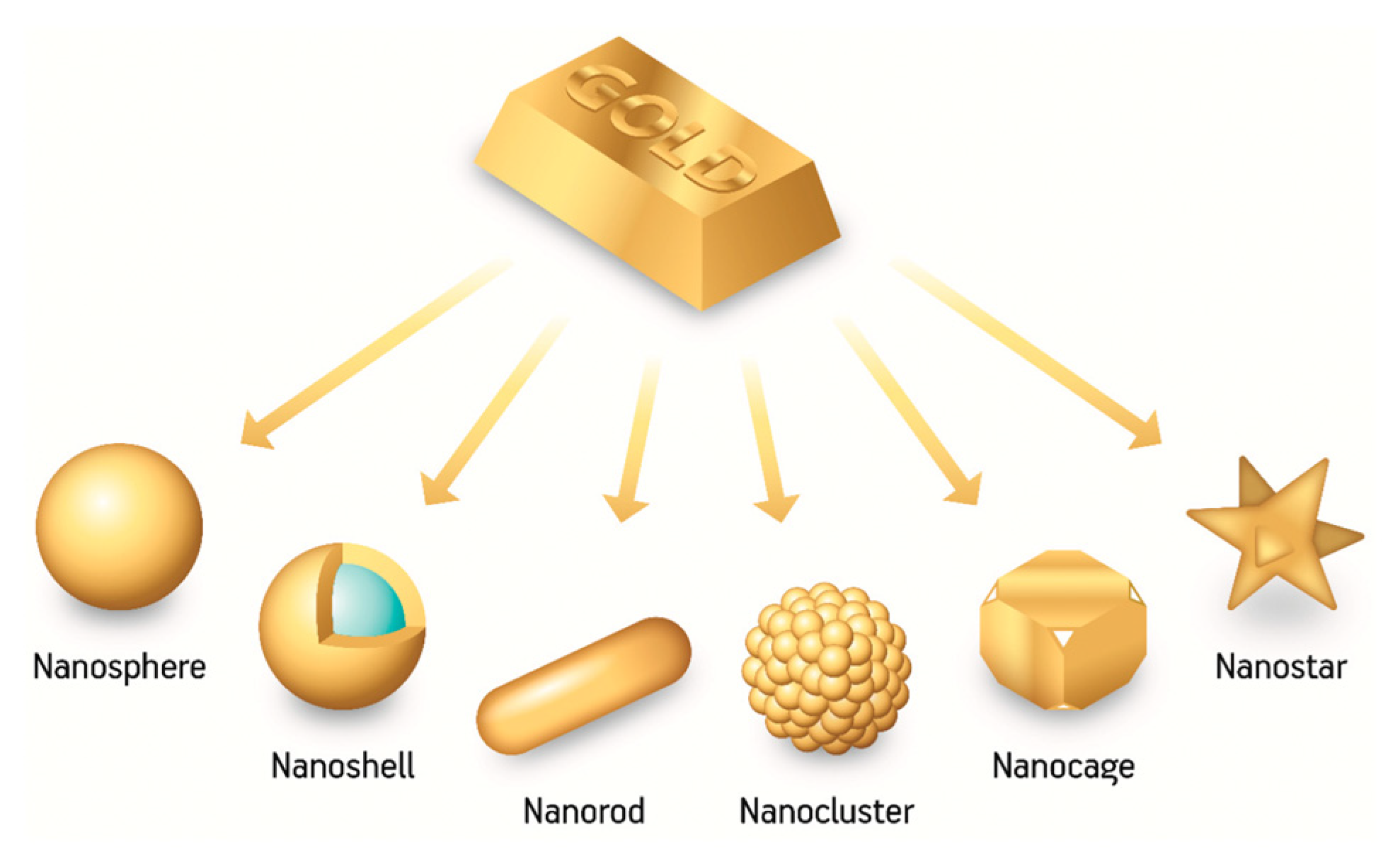
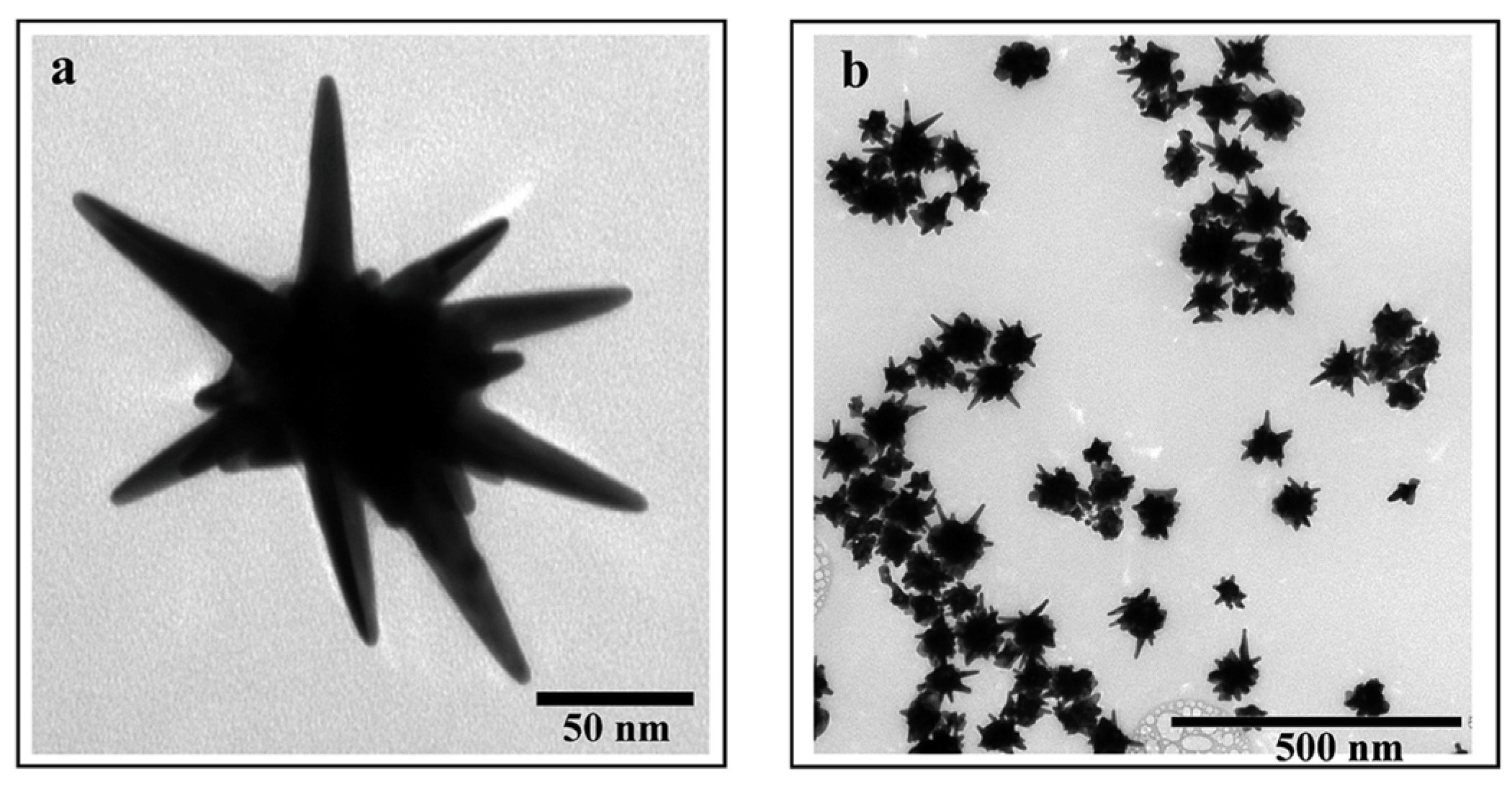
3. Why Au Star-Shaped NPs?
4. In Vitro and In Vivo Applications of AuNPs for Cancer Therapies
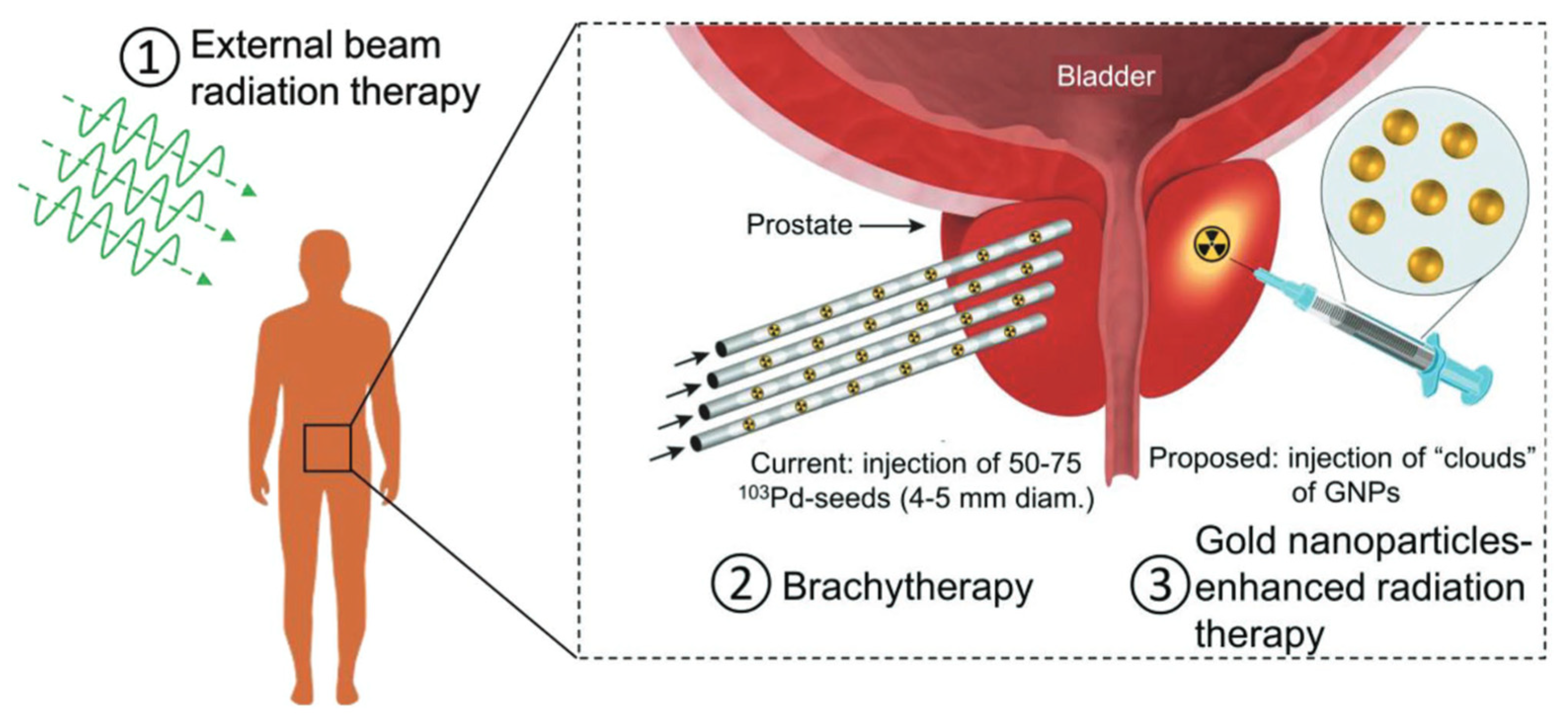
4.1. Radiotherapy
4.2. Photo-Thermal Therapy (PTT)
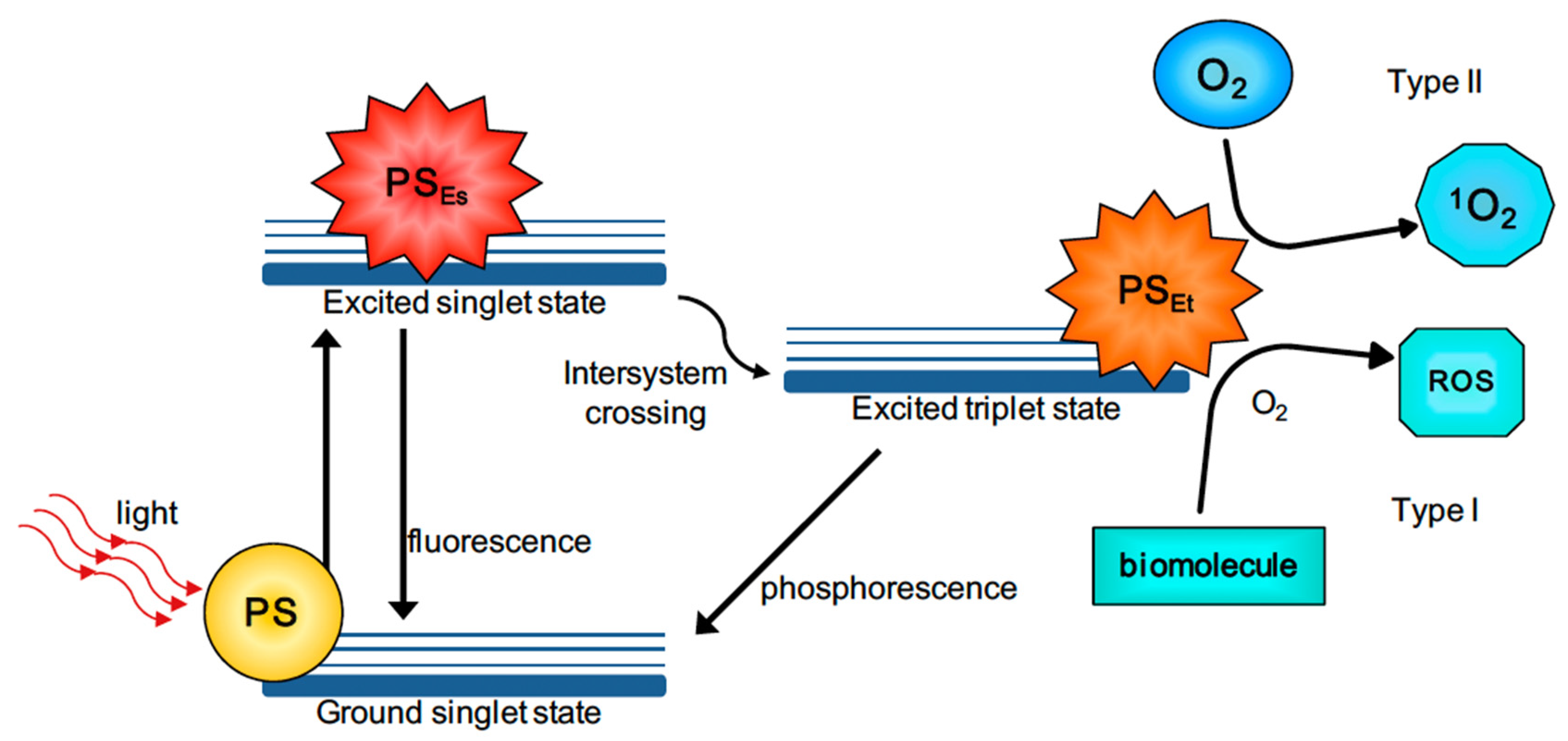
4.3. Photodynamic Therapy (PDT)
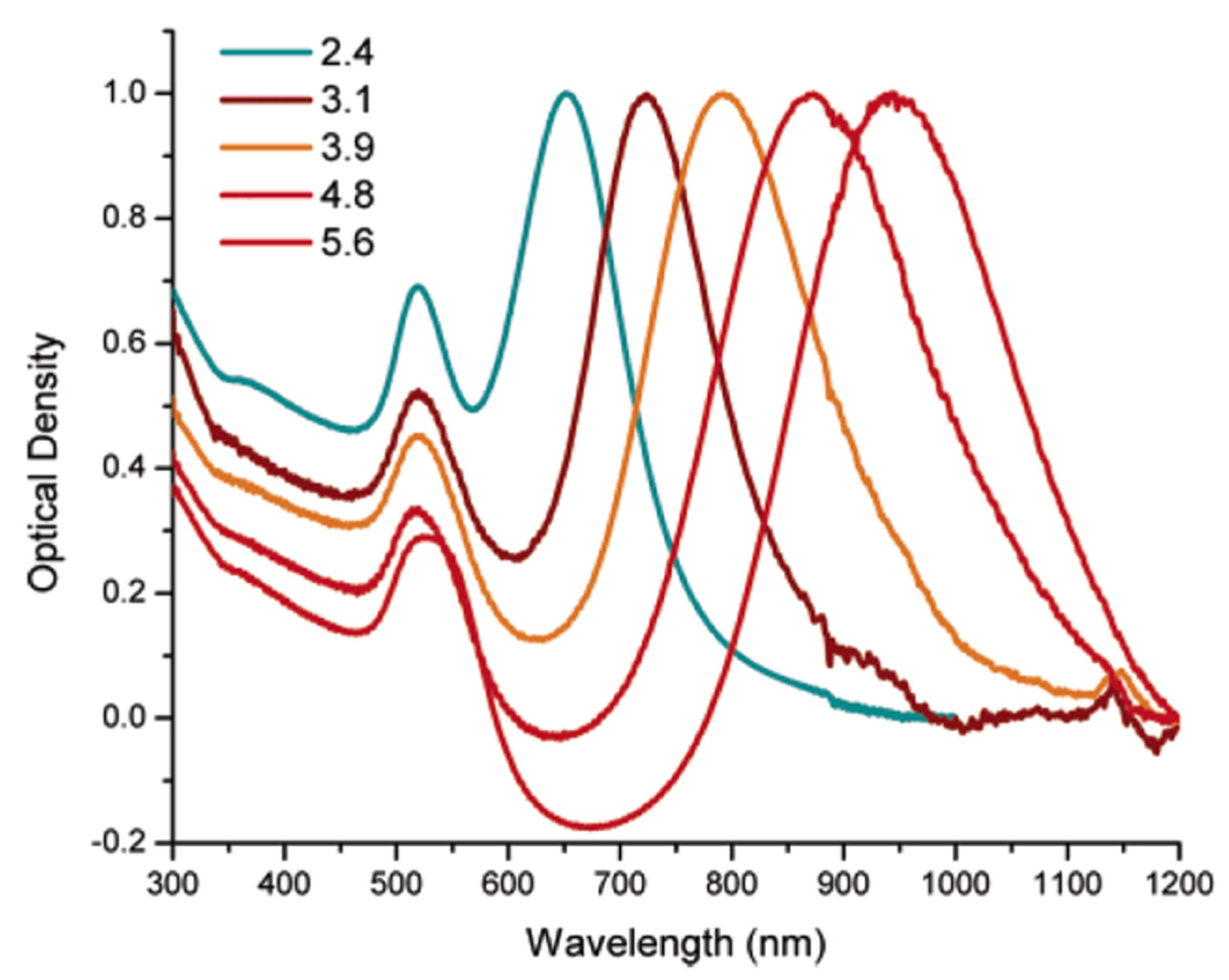
5. AuNPs for Applications in Cancer Imaging
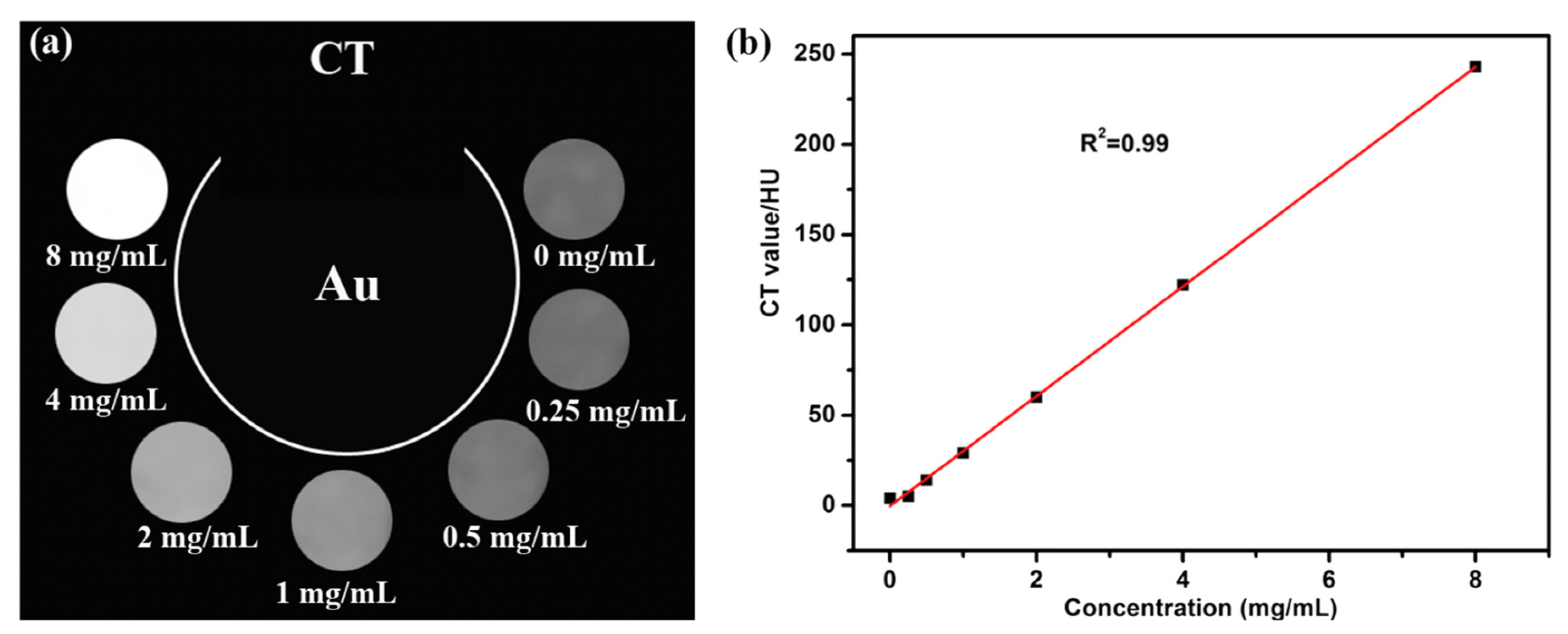
5.1. Computed Tomography (CT)
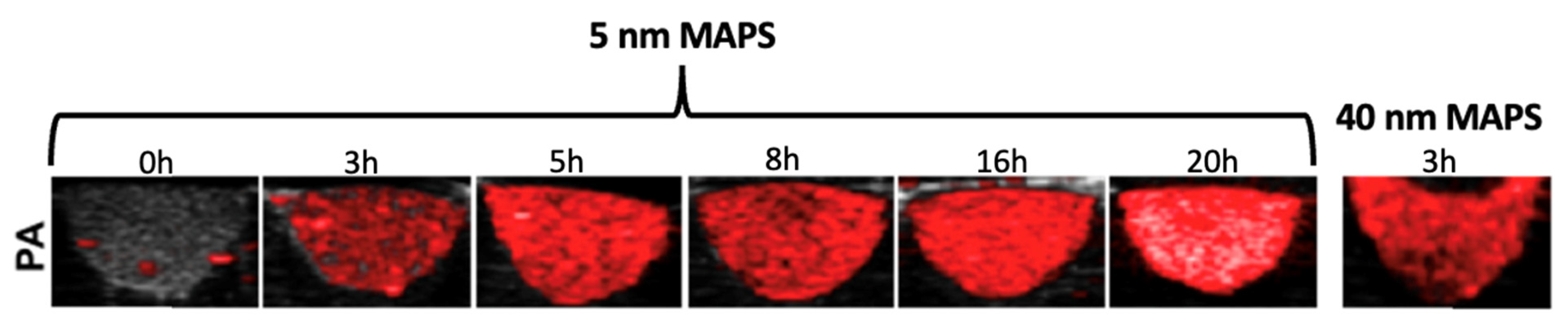
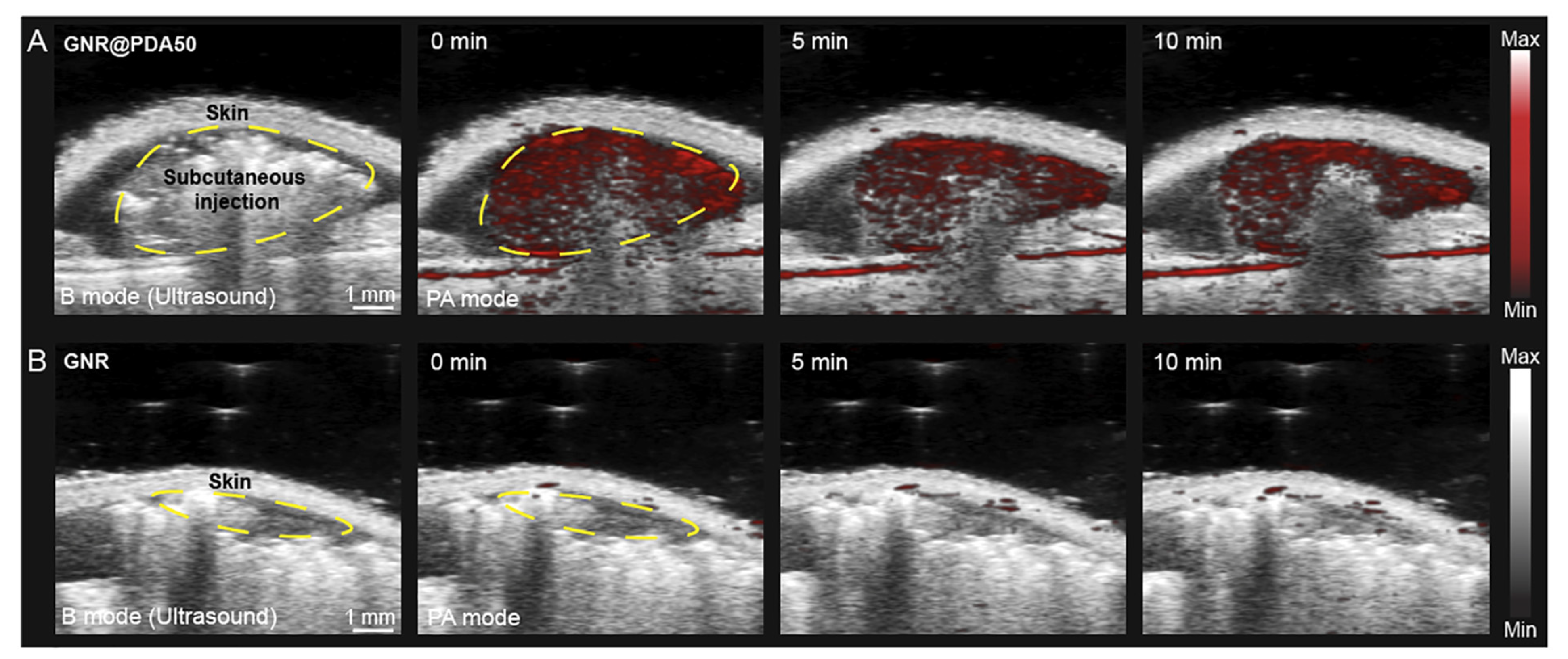
5.2. Photoacoustic Imaging (PAI)
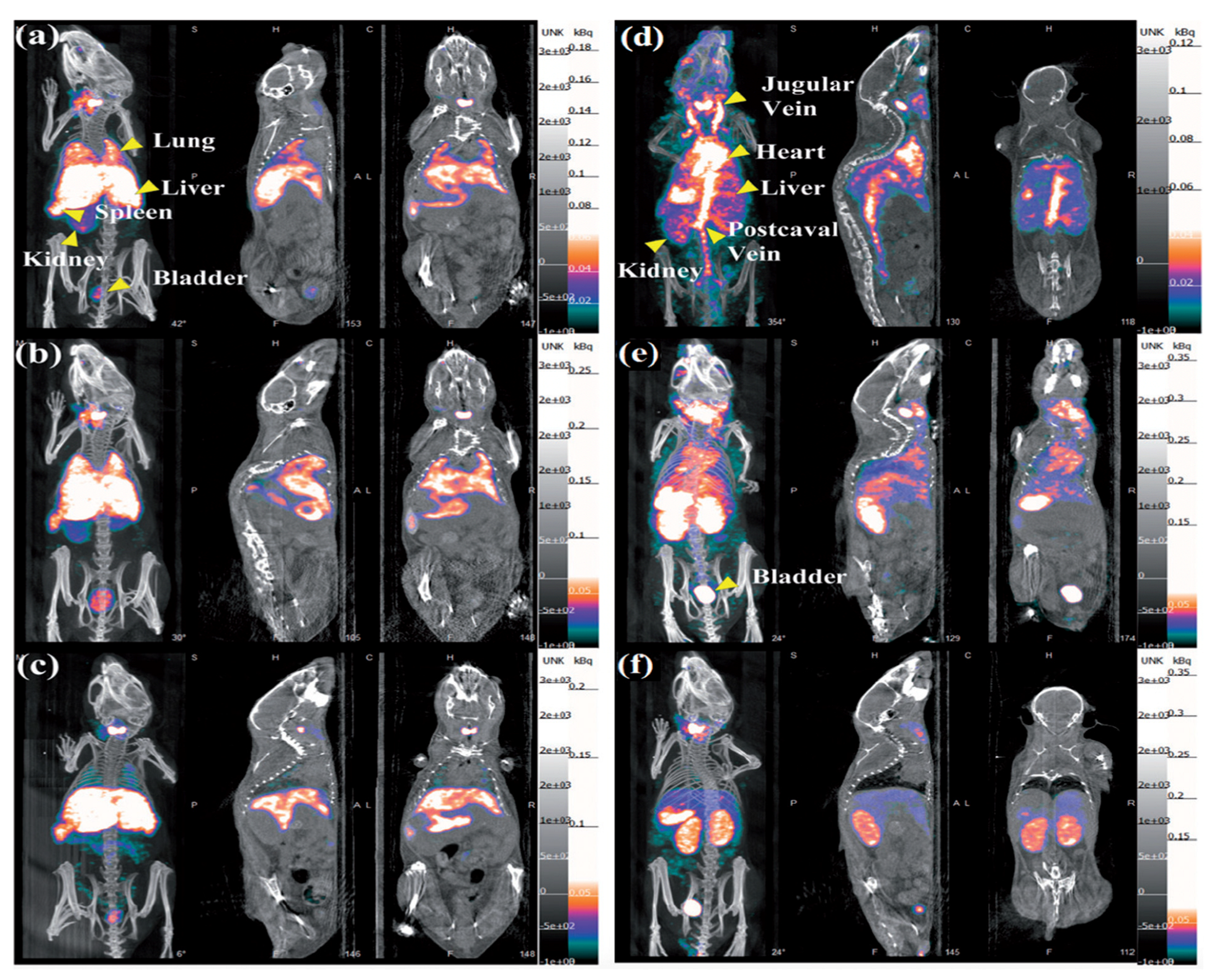
5.3. Single-Photon Emission Computed Tomography (SPECT)
6. Functionalized AuNPs for Cancer Therapy
7. AuNPs Toxicity
8. AuNPs Clinical Trials and Scientific Skepticism
9. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold nanoparticles in cancer theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2022, 33, 1–16. [Google Scholar] [CrossRef]
- Spyratou, E.; Makropoulou, M.; Efstathopoulos, E.P.; Georgakilas, A.G.; Sihver, L. Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers 2017, 9, 173. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Yang, C.; Liu, J. Recent advances of smart acid-responsive gold nanoparticles in tumor therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1619. [Google Scholar] [CrossRef]
- De Matteis, V.; Rizzello, L.; Cascione, M.; Liatsi-Douvitsa, E.; Apriceno, A.; Rinaldi, R. Green plasmonic nanoparticles and bio-inspired stimuli-responsive vesicles in cancer therapy application. Nanomaterials 2020, 10, 1083. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Rinaldi, R. Engineered gold nanoshells killing tumor cells: New perspectives. Curr. Pharm. Des. 2019, 25, 1477–1489. [Google Scholar] [CrossRef]
- Yang, C.; Bromma, K.; Chithrani, D. Peptide mediated in vivo tumor targeting of nanoparticles through optimization in single and multilayer in vitro cell models. Cancers 2018, 10, 84. [Google Scholar] [CrossRef]
- Cruz, E.; Kayser, V. Synthesis and enhanced cellular uptake in vitro of anti-HER2 multifunctional gold nanoparticles. Cancers 2019, 11, 870. [Google Scholar] [CrossRef]
- D’Acunto, M.; Cioni, P.; Gabellieri, E.; Presciuttini, G. Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology 2021, 32, 192001. [Google Scholar] [CrossRef]
- Dhanjal, D.S.; Mehta, M.; Chopra, C.; Singh, R.; Sharma, P.; Chellappan, D.K.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.; Gupta, G.; et al. Novel controlled release pulmonary drug delivery systems: Current updates and challenges. In Modeling and Control of Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Hassanen, E.I.; Korany, R.M.; Bakeer, A.M. Cisplatin-conjugated gold nanoparticles-based drug delivery system for targeting hepatic tumors. J. Biochem. Mol. Toxicol. 2021, 35, e22722. [Google Scholar] [CrossRef]
- Huai, Y.; Zhang, Y.; Xiong, X.; Das, S.; Bhattacharya, R.; Mukherjee, P. Gold Nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress 2019, 3, 267. [Google Scholar] [CrossRef]
- Tan, J.; Cho, T.J.; Tsai, D.-H.; Liu, J.; Pettibone, J.M.; You, R.; Hackley, V.A.; Zachariah, M.R. Surface modification of cisplatin-complexed gold nanoparticles and its influence on colloidal stability, drug loading, and drug release. Langmuir 2018, 34, 154–163. [Google Scholar] [CrossRef]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent advances of gold nanoparticles in biomedical applications: State of the art}. Cell Biochem. Biophys. 2019, 77, 123–137. [Google Scholar] [CrossRef]
- Taniguchi, N. On the basic concept of ‘nano-technology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Cortie, M. The weird world of nanoscale gold. Gold Bull. 2004, 37, 12–19. [Google Scholar] [CrossRef]
- Matteis, V.D.; Rinaldi, R. Toxicity assessment in the nanoparticle era. In Cellular and Molecular Toxicology of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–19. [Google Scholar]
- Das, A.; Chadha, R.; Maiti, N.; Kapoor, S. Role of surfactant in the formation of gold nanoparticles in aqueous medium. J. Nanopart. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Hammond, J.L.; Bhalla, N.; Rafiee, S.D.; Estrela, P. Localized surface plasmon resonance as a biosensing platform for developing countries. Biosensors 2014, 4, 172–188. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Zare, I.; Yaraki, M.T.; Speranza, G.; Najafabadi, A.H.; Shourangiz-Haghighi, A.; Nik, A.B.; Manshian, B.B.; Saraiva, C.; Soenen, S.J.; Kogan, M.J.; et al. Gold nanostructures: Synthesis, properties, and neurological applications. Chem. Soc. Rev. 2022, 51, 2601–2680. [Google Scholar] [CrossRef]
- Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H.K.; Modi, T.; Aguilar, Z.; Rodgers, H.; Hamilton, W.; Marutharaj, T.; Webb, C.; et al. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. (Landmark Ed.) 2014, 19, 1320–1344. [Google Scholar] [CrossRef]
- Njoki, P.N.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size correlation of optical and spectroscopic properties for gold nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.; Meisel, D. Size-dependent melting of silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef]
- Liu, H.; Ascencio, J.; Perez-Alvarez, M.; Yacaman, M. Melting behavior of nanometer sized gold isomers. Surf. Sci. 2001, 491, 88–98. [Google Scholar] [CrossRef]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold nanoparticles: Past, present, and future. Langmuir 2009, 25, 13840–13851. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.H.C.; dos Santos Batista, J.G.; Benévolo Lugão, A. An overview of the synthesis of gold nanoparticles using radiation technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Connor, E.; Mwamuka, J.; Gole, A.; Murphy, C.; Wyatt, M. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ricciardi, L.; Deitz, J.I.; Williams, R.E.; McComb, D.W.; Strangi, G. Manipulating acoustic and plasmonic modes in gold nanostars. Nanoscale Adv. 2019, 1, 2690–2698. [Google Scholar] [CrossRef]
- Pallares, M.R.; Stilson, T.; Choo, P.; Hu, J.; Odom, T.W. Using Good’s Buffers To Control the Anisotropic Structure and Optical Properties of Spiky Gold Nanoparticles for Refractive Index Sensing. ACS Appl. Nano Mater. 2019, 2, 5266–5271. [Google Scholar] [CrossRef]
- Becerril-Castro, I.B.; Calderon, I.; Pazos-Perez, N.; Guerrini, L.; Schulz, F.; Feliu, N.; Chakraborty, I.; Giannini, V.; Parak, W.J.; Alvarez-Puebla, R.A. Gold Nanostars: Synthesis, Optical and SERS Analytical Properties. Anal. Sens. 2022, 2, e202200005. [Google Scholar]
- Niidome, Y.; Haine, A.T.; Niidome, T. Anisotropic gold-based nanoparticles: Preparation, properties, and applications. Chem. Lett. 2016, 45, 488–498. [Google Scholar] [CrossRef]
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si-Au and Fe-Au core-shell on photothermal cancer treatment. Photodiagn. Photodyn. Ther. 2021, 33, 102144. [Google Scholar] [CrossRef]
- Trigari, S.; Rindi, A.; Margheri, G.; Sottini, S.; Dellepiane, G.; Giorgetti, E. Synthesis and modelling of gold nanostars with tunable morphology and extinction spectrum. J. Mater. Chem. 2011, 21, 6531–6540. [Google Scholar] [CrossRef]
- Sau, T.; Rogach, A.; Doeblinger, M.; Feldmann, J. One-Step High-Yield Aqueous Synthesis of Size-Tunable Multispiked Gold Nanoparticles. Small 2011, 7, 2188–2194. [Google Scholar] [CrossRef]
- Deveci, P. Synthesis, optical properties and photherapy applications of gold nanostars. J. Incl. Phenom. Macrocycl. Chem. 2021, 99, 23–31. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ringane, A.; Arya, A.; Das, G.; Dantham, V.; Laha, R.; Hussain, S. A high-yield, one-step synthesis of surfactant-free gold nanostars and numerical study for single-molecule SERS application. J. Nanopart. Res. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Nalbant Esenturk, E.; Hight Walker, A. Surface-enhanced Raman scattering spectroscopy via gold nanostars. J. Raman Spectrosc. 2009, 40, 86–91. [Google Scholar] [CrossRef]
- Huh, Y.S.; Chung, A.J.; Erickson, D. Surface enhanced Raman spectroscopy and its application to molecular and cellular analysis. Microfluid. Nanofluidics 2009, 6, 285–297. [Google Scholar] [CrossRef]
- Zheng, J.; He, L. Surface-enhanced Raman spectroscopy for the chemical analysis of food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 317–328. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Reagan, M. Causes of cancer: Genetic, epigenetic, viral, microenvironmental, and environmental contributions to cancer. In Cancer: Prevention, Early Detection, Treatment and Recovery; John Wiley & Sons: New York, NY, USA, 2019; pp. 53–74. [Google Scholar]
- Sánchez-Santos, M.E. Therapeutic Applications of Ionizing Radiations. In Biological and Medical Physics, Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2011; pp. 397–409. [Google Scholar]
- Haume, K.; Rosa, S.; Grellet, S.; Śmialek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 1–20. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Nie, X.-Y.; Ji, C.-C.; Lin, X.-X.; Liu, L.-J.; Chen, X.-M.; Yao, H.; Wu, S.-H. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J. Am. Heart Assoc. 2017, 6, e005633. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, X.; Wu, A.; Liu, H.; Wu, H.; Wu, Q.; Liu, Y.; Li, Y.; Cai, Y.; Liang, S. Pulmonary emphysema is a risk factor for radiation pneumonitis in NSCLC patients with squamous cell carcinoma after thoracic radiation therapy. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Mesbahi, A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep. Pract. Oncol. Radiother. 2010, 15, 176–180. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, J.-Q.; Ashby, C.R., Jr.; Zeng, L.; Fan, Y.-F.; Chen, Z.-S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Duke, S.; Jena, R.; Williams, M.V.; Burnet, N.G. Advances in radiotherapy. BMJ 2012, 345, e7765. [Google Scholar] [CrossRef]
- Laprise-Pelletier, M.; Simão, T.; Fortin, M.-A. Gold nanoparticles in radiotherapy and recent progress in nanobrachytherapy. Adv. Healthc. Mater. 2018, 7, 1701460. [Google Scholar] [CrossRef]
- Dendy, P.P.; Heaton, B. Physics for Diagnostic Radiology; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Qiao, L.; Chen, Y.; Liang, N.; Xie, J.; Deng, G.; Chen, F.; Wang, X.; Liu, F.; Li, Y.; Zhang, J. Targeting Epithelial-to-Mesenchymal Transition in Radioresistance: Crosslinked Mechanisms and Strategies. Front. Oncol. 2022, 12, 775238. [Google Scholar] [CrossRef]
- Cutter, D.J.; Darby, S.C.; Yusuf, S.W. Risks of heart disease after radiotherapy. Tex. Heart Inst. J. 2011, 38, 257. [Google Scholar]
- Travis, L.B.; Ng, A.K.; Allan, J.M.; Pui, C.-H.; Kennedy, A.R.; Xu, X.G.; Purdy, J.A.; Applegate, K.; Yahalom, J.; Constine, L.S.; et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J. Natl. Cancer Inst. 2012, 104, 357–370. [Google Scholar] [CrossRef]
- Schneider, U. Modeling the risk of secondary malignancies after radiotherapy. Genes 2011, 2, 1033–1049. [Google Scholar] [CrossRef]
- de Vathaire, F.; Hardiman, C.; Shamsaldin, A.; Campbell, S.; Grimaud, E.; Hawkins, M.; Raquin, M.; Oberlin, O.; Diallo, I.; Zucker, J.-M.; et al. Thyroid carcinomas after irradiation for a first cancer during childhood. Arch. Intern. Med. 1999, 159, 2713–2719. [Google Scholar] [CrossRef]
- Sklar, C.; Whitton, J.; Mertens, A.; Stovall, M.; Green, D.; Marina, N.; Greffe, B.; Wolden, S.; Robison, L. Abnormalities of the thyroid in survivors of Hodgkin’s disease: Data from the Childhood Cancer Survivor Study. J. Clin. Endocrinol. Metab. 2000, 85, 3227–3232. [Google Scholar] [CrossRef]
- Brenner, D.J.; Curtis, R.E.; Hall, E.J.; Ron, E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2000, 88, 398–406. [Google Scholar] [CrossRef]
- Rosen, E.M.; Day, R.; Singh, V.K. New approaches to radiation protection. Front. Oncol. 2015, 4, 381. [Google Scholar] [CrossRef]
- Choi, J.; Jung, K.O.; Graves, E.E.; Pratx, G. A gold nanoparticle system for the enhancement of radiotherapy and simultaneous monitoring of reactive-oxygen-species formation. Nanotechnology 2018, 29, 504001. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current trends in using polymer coated gold nanoparticles for cancer therapy. Int. J. Pharm. 2015, 484, 252–267. [Google Scholar] [CrossRef]
- Dobešová, L.; Gier, T.; Kopečná, O.; Pagáčová, E.; Vičar, T.; Bestvater, F.; Toufar, J.; Bačíková, A.; Kopel, P.; Fedr, R.; et al. Incorporation of low concentrations of gold nanoparticles: Complex effects on radiation response and fate of cancer cells. Pharmaceutics 2022, 14, 166. [Google Scholar] [CrossRef]
- Zhang, S.X.; Gao, J.; Buchholz, T.A.; Wang, Z.; Salehpour, M.R.; Drezek, R.A.; Yu, T.-K. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: A monte carlo simulation study. Biomed. Microdevices 2009, 11, 925–933. [Google Scholar] [CrossRef]
- Pratt, E.C.; Shaffer, T.M.; Zhang, Q.; Drain, C.M.; Grimm, J. Nanoparticles as multimodal photon transducers of ionizing radiation. Nat. Nanotechnol. 2018, 13, 418–426. [Google Scholar] [CrossRef]
- Porcel, E.; Li, S.; Usami, N.; Remita, Y.F.; Kobayashi, K.; Le Sech, C.; Lacombe, S. Nano-Sensitization under gamma rays and fast ion radiation. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2012; Volume 373, p. 012006. [Google Scholar]
- Sabbatucci, L.; Salvat, F. Theory and calculation of the atomic photoeffect. Radiat. Phys. Chem. 2016, 121, 122–140. [Google Scholar] [CrossRef]
- Schlathölter, T.; Eustache, P.; Porcel, E.; Salado, D.; Stefancikova, L.; Tillement, O.; Lux, F.; Mowat, P.; Biegun, A.K.; Van Goethem, M.-J.; et al. Improving proton therapy by metal-containing nanoparticles: Nanoscale insights. Int. J. Nanomed. 2016, 11, 1549. [Google Scholar] [CrossRef]
- Nagi, N.; Khair, Y.A.; Abdalla, A.M. Capacity of gold nanoparticles in cancer radiotherapy. Jpn. J. Radiol. 2017, 35, 555–561. [Google Scholar] [CrossRef]
- Shrestha, S.; Cooper, L.N.; Andreev, O.A.; Reshetnyak, Y.K.; Antosh, M.P. Gold nanoparticles for radiation enhancement in vivo. Jacobs J. Radiat. Oncol. 2016, 3, 26. [Google Scholar]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.-F.; Estève, F.; Ravanat, J.-L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Teraoka, S.; Kakei, Y.; Akashi, M.; Iwata, E.; Hasegawa, T.; Miyawaki, D.; Sasaki, R.; Komori, T. Gold nanoparticles enhance X-ray irradiation-induced apoptosis in head and neck squamous cell carcinoma in vitro. Biomed. Rep. 2018, 9, 415–420. [Google Scholar] [CrossRef]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A. A pH-controlled one-pot synthesis of gold nanostars by using a zwitterionic protein hydrolysate (gelatin): An enhanced radiosensitization of cancer cells. New J. Chem. 2021, 45, 13271–13279. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Brun, E.; Butterworth, K.T.; Coulter, J.A.; Douki, T.; Hirst, D.G.; Jain, S.; Kavanagh, A.P.; Krpetic, Z.; et al. Energy dependence of gold nanoparticle radiosensitization in plasmid DNA. J. Phys. Chem. C 2011, 115, 20160–20167. [Google Scholar] [CrossRef]
- Svaasand, L.O.; Gomer, C.J.; Morinelli, E. On the physical rationale of laser induced hyperthermia. Lasers Med. Sci. 1990, 5, 121–128. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2009, 23, 217–228. [Google Scholar] [CrossRef]
- Kayani, Z.; Islami, N.; Behzadpour, N.; Zahraie, N.; Imanlou, S.; Tamaddon, P.; Salehi, F.; Daneshvar, F.; Perota, G.; Sorati, E.; et al. Combating cancer by utilizing noble metallic nanostructures in combination with laser photothermal and X-ray radiotherapy. J. Drug Deliv. Sci. Technol. 2021, 65, 102689. [Google Scholar] [CrossRef]
- Doughty, A.C.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial applications in photothermal therapy for cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef]
- Ali, M.R.; Wu, Y.; El-Sayed, M.A. Gold-nanoparticle-assisted plasmonic photothermal therapy advances toward clinical application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef]
- Samali, A.; Holmberg, C.I.; Sistonen, L.; Orrenius, S. Thermotolerance and cell death are distinct cellular responses to stress: Dependence on heat shock proteins. FEBS Lett. 1999, 461, 306–310. [Google Scholar] [CrossRef]
- Li, J.-L.; Gu, M. Gold-nanoparticle-enhanced cancer photothermal therapy. IEEE J. Sel. Top. Quantum Electron. 2009, 16, 989–996. [Google Scholar]
- Dreifuss, T.; Barnoy, E.; Motiei, M.; Popovtzer, R. Theranostic gold nanoparticles for CT imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Berlin/Heidelberg, Germany, 2017; pp. 403–427. [Google Scholar]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Bi, C.; Chen, J.; Chen, Y.; Song, Y.; Li, A.; Li, S.; Mao, Z.; Gao, C.; Wang, D.; Möhwald, H.; et al. Realizing a record photothermal conversion efficiency of spiky gold nanoparticles in the second near-infrared window by structure-based rational design. Chem. Mater. 2018, 30, 2709–2718. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Banu, H.; Stanley, B.; Faheem, S.; Seenivasan, R.; Premkumar, K.; Vasanthakumar, G. Thermal chemosensitization of breast cancer cells to cyclophosphamide treatment using folate receptor targeted gold nanoparticles. Plasmonics 2014, 9, 1341–1349. [Google Scholar] [CrossRef]
- Du, B.; Ma, C.; Ding, G.; Han, X.; Li, D.; Wang, E.; Wang, J. Cooperative strategies for enhancing performance of photothermal therapy (PTT) agent: Optimizing its photothermal conversion and cell internalization ability. Small 2017, 13, 1603275. [Google Scholar] [CrossRef]
- Park, S.-E.; Lee, J.; Lee, T.; Bae, S.-B.; Kang, B.; Huh, Y.-M.; Lee, S.-W.; Haam, S. Comparative hyperthermia effects of silica--gold nanoshells with different surface coverage of gold clusters on epithelial tumor cells. Int. J. Nanomed. 2015, 10, 261. [Google Scholar]
- Yang, L.; Tseng, Y.-T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.-J.; Huang, C.-C.; Lin, C.-H. Photothermal therapeutic response of cancer cells to aptamer--gold nanoparticle-hybridized graphene oxide under NIR illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1609–1617. [Google Scholar] [CrossRef]
- Chatterjee, H.; Rahman, D.S.; Sengupta, M.; Ghosh, S.K. Gold nanostars in plasmonic photothermal therapy: The role of tip heads in the thermoplasmonic landscape. J. Phys. Chem. C 2018, 122, 13082–13094. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Ding, Y.; Sun, S. Understanding the photothermal effect of gold nanostars and nanorods for biomedical applications. RSC Adv. 2014, 4, 30375–30383. [Google Scholar] [CrossRef]
- Gao, J.; Sanchez-Purra, M.; Huang, H.; Wang, S.; Chen, Y.; Yu, X.; Luo, Q.; Hamad-Schifferli, K.; Liu, S. Synthesis of different-sized gold nanostars for Raman bioimaging and photothermal therapy in cancer nanotheranostics. Sci. China Chem. 2017, 60, 1219–1229. [Google Scholar] [CrossRef]
- Li, X.; Xing, L.; Zheng, K.; Wei, P.; Du, L.; Shen, M.; Shi, X. Formation of gold nanostar-coated hollow mesoporous silica for tumor multimodality imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 5817–5827. [Google Scholar] [CrossRef]
- Fiorentini, G.; Giovanis, P.; Rossi, S.; Dentico, P.; Paola, R.; Turrisi, G.; Bernardeschi, P. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. In Vivo 2006, 20, 721–724. [Google Scholar]
- Szasz, A. Current status of oncothermia therapy for lung cancer. Korean J. Thorac. Cardiovasc. Surg. 2014, 47, 77. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.-L.; Li, J.-J.; Chen, Y.-Y.; Wang, C.-Y.; Wang, Y.-S.; Chi, K.-H.; Wang, H.-E. Presence of Gold Nanoparticles in Cells Associated with the Cell-Killing Effect of Modulated Electro-Hyperthermia. ACS Appl. Bio Mater. 2019, 2, 3573–3581. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Du, H.; Wang, H. Intracellular gold nanoparticle aggregation and their potential applications in photodynamic therapy. Chem. Commun. 2014, 50, 7287–7290. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Qidwai, A.; Annu; Nabi, B.; Kotta, S.; Narang, J.K.; Baboota, S.; Ali, J. Role of nanocarriers in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 30, 101782. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I. Complement activation cascade and its regulation: Relevance for the response of solid tumors to photodynamic therapy. J. Photochem. Photobiol. B Biol. 2008, 93, 53–59. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic therapy: Past, present and future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Mokoena, D.R.; George, B.P.; Abrahamse, H. Enhancing breast cancer treatment using a combination of cannabidiol and gold nanoparticles for photodynamic therapy. Int. J. Mol. Sci. 2019, 20, 4771. [Google Scholar] [CrossRef]
- Pallavi, P.; Girigoswami, A.; Girigoswami, K.; Hansda, S.; Ghosh, R. Photodynamic therapy in cancer. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–24. [Google Scholar]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy—Current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Oh, J.; Yoon, H.; Park, J.-H. Nanoparticle platforms for combined photothermal and photodynamic therapy. Biomed. Eng. Lett. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H.; El-Boubbou, K.; Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; et al. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Qiao, G.; Zhuo, L.; Gao, Y.; Yu, L.; Li, N.; Tang, B. A tumor mRNA-dependent gold nanoparticle—Molecular beacon carrier for controlled drug release and intracellular imaging. Chem. Commun. 2001, 47, 7458–7460. [Google Scholar] [CrossRef]
- Cantatore, A.; Müller, P. Introduction to Computed Tomography; DTU Mechanical Engineering: Lyngby, Denmark, 2011. [Google Scholar]
- Hokamp, N.G.; Maintz, D.; Shapira, N.; Chang, D.-H.; Noel, P.B. Technical background of a novel detector-based approach to dual-energy computed tomography. Diagn. Interv. Radiol. 2020, 26, 68. [Google Scholar] [CrossRef]
- Mahan, M.M.; Doiron, A.L. Gold nanoparticles as X-ray, CT, and multimodal imaging contrast agents: Formulation, targeting, and methodology. J. Nanomater. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Xi, D.; Dong, S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. Rsc Adv. 2012, 2, 12515–12524. [Google Scholar] [CrossRef]
- Ahn, S.; Jung, S.Y.; Lee, S.J. Gold nanoparticle contrast agents in advanced X-ray imaging technologies. Molecules 2013, 18, 5858–5890. [Google Scholar] [CrossRef]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef]
- Hasebroock, K.M.; Serkova, N.J. Toxicity of MRI and CT contrast agents. Expert Opin. Drug Metab. Toxicol. 2009, 5, 403–416. [Google Scholar] [CrossRef]
- Iranpour, P.; Ajamian, M.; Safavi, A.; Iranpoor, N.; Abbaspour, A.; Javanmardi, S. Synthesis of highly stable and biocompatible gold nanoparticles for use as a new X-ray contrast agent. J. Mater. Sci. Mater. Med. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Rand, D.; Ortiz, V.; Liu, Y.; Derdak, Z.; Wands, J.R.; Tatíček, M.; Rose-Petruck, C. Nanomaterials for X-ray imaging: Gold nanoparticle enhancement of X-ray scatter imaging of hepatocellular carcinoma. Nano Lett. 2011, 11, 2678–2683. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Liu, Y.; Crawford, B.M.; Wang, H.-N.; Yuan, H.; Register, J.K.; Khoury, C.G. Shining Gold Nanostars: From Cancer Diagnostics to Photothermal Treatment and Immunotherapy. J. Immunol. Sci. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, H.; Kersey, F.R.; Register, J.K.; Parrott, M.C.; Vo-Dinh, T. Plasmonic gold nanostars for multi-modality sensing and diagnostics. Sensors 2015, 15, 3706–3720. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Monaco, I.; Franchini, M.C. Current concepts in nanostructured contrast media development for in vivo photoacoustic imaging. Biomater. Sci. 2019, 7, 1746–1775. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wen, Q.; Li, P.; Wang, Y.; Jiang, H.; Zhang, W. PEGylated gold nanorods with a broad absorption band in the first near-infrared window for in vivo multifunctional photoacoustic imaging. RSC Adv. 2020, 10, 4561–4567. [Google Scholar] [CrossRef]
- Du, J.; Yang, S.; Qiao, Y.; Lu, H.; Dong, H. Recent progress in near-infrared photoacoustic imaging. Biosens. Bioelectron. 2021, 191, 113478. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Sun, I.-C.; Ahn, C.-H.; Kim, K.; Emelianov, S. Photoacoustic imaging of cancer cells with glycol-chitosan-coated gold nanoparticles as contrast agents. J. Biomed. Opt. 2019, 24, 121903. [Google Scholar] [CrossRef]
- Han, S.; Bouchard, R.; Sokolov, K.V. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed. Opt. Express 2019, 10, 3472–3483. [Google Scholar] [CrossRef]
- Huang, X.; Shang, W.; Deng, H.; Zhou, Y.; Cao, F.; Fang, C.; Lai, P.; Tian, J. Clothing spiny nanoprobes against the mononuclear phagocyte system clearance in vivo: Photoacoustic diagnosis and photothermal treatment of early stage liver cancer with erythrocyte membrane-camouflaged gold nanostars. Appl. Mater. Today 2020, 18, 100484. [Google Scholar] [CrossRef]
- Neuschmelting, V.; Harmsen, S.; Beziere, N.; Lockau, H.; Hsu, H.-T.; Huang, R.; Razansky, D.; Ntziachristos, V.; Kircher, M.F. Dual-modality surface-enhanced resonance Raman scattering and multispectral optoacoustic tomography nanoparticle approach for brain tumor delineation. Small 2018, 14, 1800740. [Google Scholar] [CrossRef]
- Gao, D.; Hu, D.; Liu, X.; Sheng, Z.; Zheng, H. Recent advances in functional nanomaterials for photoacoustic imaging of glioma. Nanoscale Horiz. 2019, 4, 1037–1045. [Google Scholar] [CrossRef]
- Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled gold nanoparticles for imaging and therapy of cancer. Materials 2020, 14, 4. [Google Scholar] [CrossRef]
- Banstola, A.; Emami, F.; Jeong, J.-H.; Yook, S. Current applications of gold nanoparticles for medical imaging and as treatment agents for managing pancreatic cancer. Macromol. Res. 2018, 26, 955–964. [Google Scholar] [CrossRef]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold nanoparticles and nanorods in nuclear medicine: A mini review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef]
- Davis, K.M.; Ryan, J.L.; Aaron, V.D.; Sims, J.B. PET and SPECT imaging of the brain: History, technical considerations, applications, and radiotracers. In Seminars in Ultrasound, CT and MRI; Elsevier: Amsterdam, The Netherlands, 2020; Volume 41. [Google Scholar]
- Zhao, Y.; Pang, B.; Luehmann, H.; Detering, L.; Yang, X.; Sultan, D.; Harpstrite, S.; Sharma, V.; Cutler, C.S.; Xia, Y.; et al. Gold nanoparticles doped with 199Au atoms and their use for targeted cancer imaging by SPECT. Adv. Healthc. Mater. 2016, 5, 928–935. [Google Scholar] [CrossRef]
- Daems, N.; Michiels, C.; Lucas, S.; Baatout, S.; Aerts, A. Gold nanoparticles meet medical radionuclides. Nucl. Med. Biol. 2021, 100, 61–90. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, S.; Zhu, M.; Li, D.; Xing, Y.; Shen, M.; Shi, X.; Zhao, J. 99mTc-labelled multifunctional polyethylenimine-entrapped gold nanoparticles for dual mode SPECT and CT imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, 488–498. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, L.; Zhao, P.; Yang, J.; Shi, J.; Zhao, J. Charge-conversional polyethylenimine-entrapped gold nanoparticles with 131 I-labeling for enhanced dual mode SPECT/CT imaging and radiotherapy of tumors. Biomater. Sci. 2020, 8, 3956–3965. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, L.; Zhu, J.; Li, Y.; Song, N.; Xing, Y.; Qiao, W.; Huang, H.; Zhao, J. 131I-labeled polyethylenimine-entrapped gold nanoparticles for targeted tumor SPECT/CT imaging and radionuclide therapy. Int. J. Nanomed. 2019, 14, 4367. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.-M.; Liu, P.-X.; Liang, X.-J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef]
- Majidi, F.S.; Mohammadi, E.; Mehravi, B.; Nouri, S.; Ashtari, K.; Neshasteh-Riz, A. Investigating the effect of near infrared photo thermal therapy folic acid conjugated gold nano shell on melanoma cancer cell line A375. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2161–2170. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Gao, L.; Hu, P.; Jiang, L.; Ren, T.; Fu, R.; Yang, D.; Jiang, X. Aptamer-conjugated gold nanostars for targeted cancer photothermal therapy. J. Mater. Sci. 2018, 53, 14138–14148. [Google Scholar] [CrossRef]
- Dixit, S.; Miller, K.; Zhu, Y.; McKinnon, E.; Novak, T.; Kenney, M.E.; Broome, A.-M. Dual receptor-targeted theranostic nanoparticles for localized delivery and activation of photodynamic therapy drug in glioblastomas. Mol. Pharm. 2015, 12, 3250–3260. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Inorganic nanoparticles applied for active targeted photodynamic therapy of breast cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, G.; You, M.; Song, E.; Shukoor, M.I.; Zhang, K.; Altman, M.B.; Chen, Y.; Zhu, Z.; Huang, C.Z.; et al. Assembly of aptamer switch probes and photosensitizer on gold nanorods for targeted photothermal and photodynamic cancer therapy. ACS Nano 2012, 6, 5070–5077. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chang, C.-N.; Chang, Y.-T.; Yang, M.-H.; Chien, Y.-H.; Chen, S.-J.; Yeh, C.-S. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew. Chem. 2010, 122, 2771–2775. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef]
- Chen, J.S.; Chen, J.; Bhattacharjee, S.; Cao, Z.; Wang, H.; Swanson, S.D.; Zong, H.; Baker, J.R.; Wang, S.H. Functionalized nanoparticles with targeted antibody to enhance imaging of breast cancer in vivo. J. Nanobiotechnol. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Yuan, H.; Wilson, C.M.; Xia, J.; Doyle, S.L.; Li, S.; Fales, A.M.; Liu, Y.; Ozaki, E.; Mulfaul, K.; Hanna, G.; et al. Plasmonics-enhanced and optically modulated delivery of gold nanostars into brain tumor. Nanoscale 2014, 6, 4078–4082. [Google Scholar] [CrossRef]
- Sun, I.-C.; Na, J.H.; Jeong, S.Y.; Kim, D.-E.; Kwon, I.C.; Choi, K.; Ahn, C.-H.; Kim, K. Biocompatible glycol chitosan-coated gold nanoparticles for tumor-targeting CT imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Khademi, S.; Sarkar, S.; Shakeri-Zadeh, A.; Attaran, N.; Kharrazi, S.; Ay, M.R.; Azimian, H.; Ghadiri, H. Targeted gold nanoparticles enable molecular CT imaging of head and neck cancer: An in vivo study. Int. J. Biochem. Cell Biol. 2019, 114, 105554. [Google Scholar] [CrossRef]
- Hu, P.; Hou, X.; Yu, X.; Wei, X.; Li, Y.; Yang, D.; Jiang, X. Folic acid-conjugated gold nanostars for computed tomography imaging and photothermal/radiation combined therapy. ACS Appl. Bio Mater. 2021, 4, 4862–4871. [Google Scholar] [CrossRef]
- Yim, W.; Zhou, J.; Mantri, Y.; Creyer, M.N.; Moore, C.A.; Jokerst, J.V. Gold nanorod--melanin hybrids for enhanced and prolonged photoacoustic imaging in the near-infrared-II window. ACS Appl. Mater. Interfaces 2021, 13, 14974–14984. [Google Scholar] [CrossRef]
- Umehara, Y.; Kageyama, T.; Son, A.; Kimura, Y.; Kondo, T.; Tanabe, K. Biological reduction of nitroimidazole-functionalized gold nanorods for photoacoustic imaging of tumor hypoxia. RSC Adv. 2019, 9, 16863–16868. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Du, Z.; Pu, X.; Zhu, B. 131I labeled pH-responsive gold nanoparticles for bimodal tumor diagnosis. Mater. Lett. 2023, 330, 133202. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, X.; Wu, A.; Li, J.; Tian, J.; Chong, Y.; Chai, Z.; Zhao, Y.; Chen, C.G.; Ge, C. Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Appl. Mater. Interfaces 2015, 7, 20568–20575. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, H.-Y.; Wu, D.; Wang, Y.-Y.; Chang, J.-H.; Zhai, Z.-B.; Meng, A.-M.; Liu, P.-X.; Zhang, L.-A.; Fan, F.-Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Ma, J.; Wang, X.; Zhang, Y.; Wang, W.; Yuan, Z. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf. B: Biointerfaces 2018, 167, 260–266. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Tanaka, A.; Fuzimori, C.; Echigoya, M.; Taira, Y.; Taira, I.; Shimizu, Y.; Akimoto, Y.; Kawakami, H.; Ishida, I. Toxicity of gold nanoparticles in mice due to nanoparticle/drug interaction induces acute kidney damage. Nanoscale Res. Lett. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz--Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 1–15. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Yang, N.; Zhao, B.; Zhang, H.; Sun, Y.-M.; Zhang, L.-A.; Fan, F.-Y. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int. J. Nanomed. 2011, 6, 2071. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Das, S.; Debnath, N.; Mitra, S.; Datta, A.; Goswami, A. Comparative analysis of stability and toxicity profile of three differently capped gold nanoparticles for biomedical usage. Biometals 2012, 25, 1009–1022. [Google Scholar] [CrossRef]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2022, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Brandão, A.; Soares, M.E.; Morais, T.; Duarte, J.A.; Pereira, L.; Soares, L.; Neves, C.; Pereira, E.; de Lourdes Bastos, M.; et al. Short-and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1757–1766. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and cytotoxicity of gold nanoparticles: Recent advances in methodologies and regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Bloise, N.; Strada, S.; Dacarro, G.; Visai, L. Gold Nanoparticles Contact with Cancer Cell: A Brief Update. Int. J. Mol. Sci. 2022, 23, 7683. [Google Scholar] [CrossRef]
- Luan, X.; Yuan, H.; Song, Y.; Hu, H.; Wen, B.; He, M.; Zhang, H.; Li, Y.; Feng, L.; Shu, P.; et al. Reappraisal of anticancer nanomedicine design criteria in three types of preclinical cancer models for better clinical translation. Biomaterials 2021, 275, 120910. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, S.; Gao, W. What went wrong with anticancer nanomedicine design and how to make it right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarantino, S.; Caricato, A.P.; Rinaldi, R.; Capomolla, C.; De Matteis, V. Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques. Pharmaceutics 2023, 15, 500. https://doi.org/10.3390/pharmaceutics15020500
Tarantino S, Caricato AP, Rinaldi R, Capomolla C, De Matteis V. Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques. Pharmaceutics. 2023; 15(2):500. https://doi.org/10.3390/pharmaceutics15020500
Chicago/Turabian StyleTarantino, Simona, Anna Paola Caricato, Rosaria Rinaldi, Caterina Capomolla, and Valeria De Matteis. 2023. "Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques" Pharmaceutics 15, no. 2: 500. https://doi.org/10.3390/pharmaceutics15020500
APA StyleTarantino, S., Caricato, A. P., Rinaldi, R., Capomolla, C., & De Matteis, V. (2023). Cancer Treatment Using Different Shapes of Gold-Based Nanomaterials in Combination with Conventional Physical Techniques. Pharmaceutics, 15(2), 500. https://doi.org/10.3390/pharmaceutics15020500









