Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
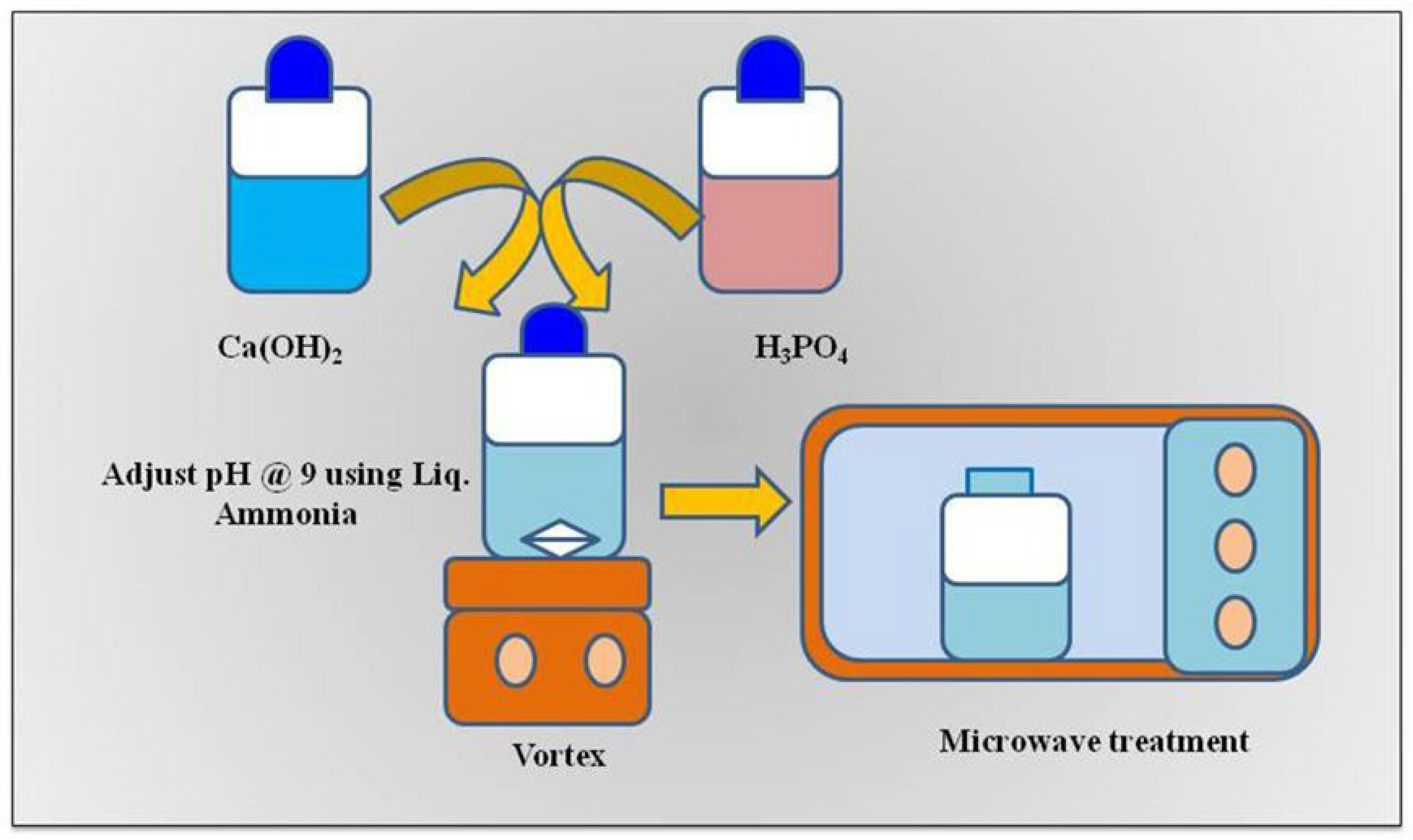
2.2. Synthesis and Characterization of HANPs
2.3. Bacterial Strain and Culture Conditions
2.4. Antibiofilm Property of HANPs
2.4.1. Static Biofilm Assay
2.4.2. In Situ Analysis of Biofilm Formation
3. Results and Discussion
3.1. Synthesis and Characterization of HANPs
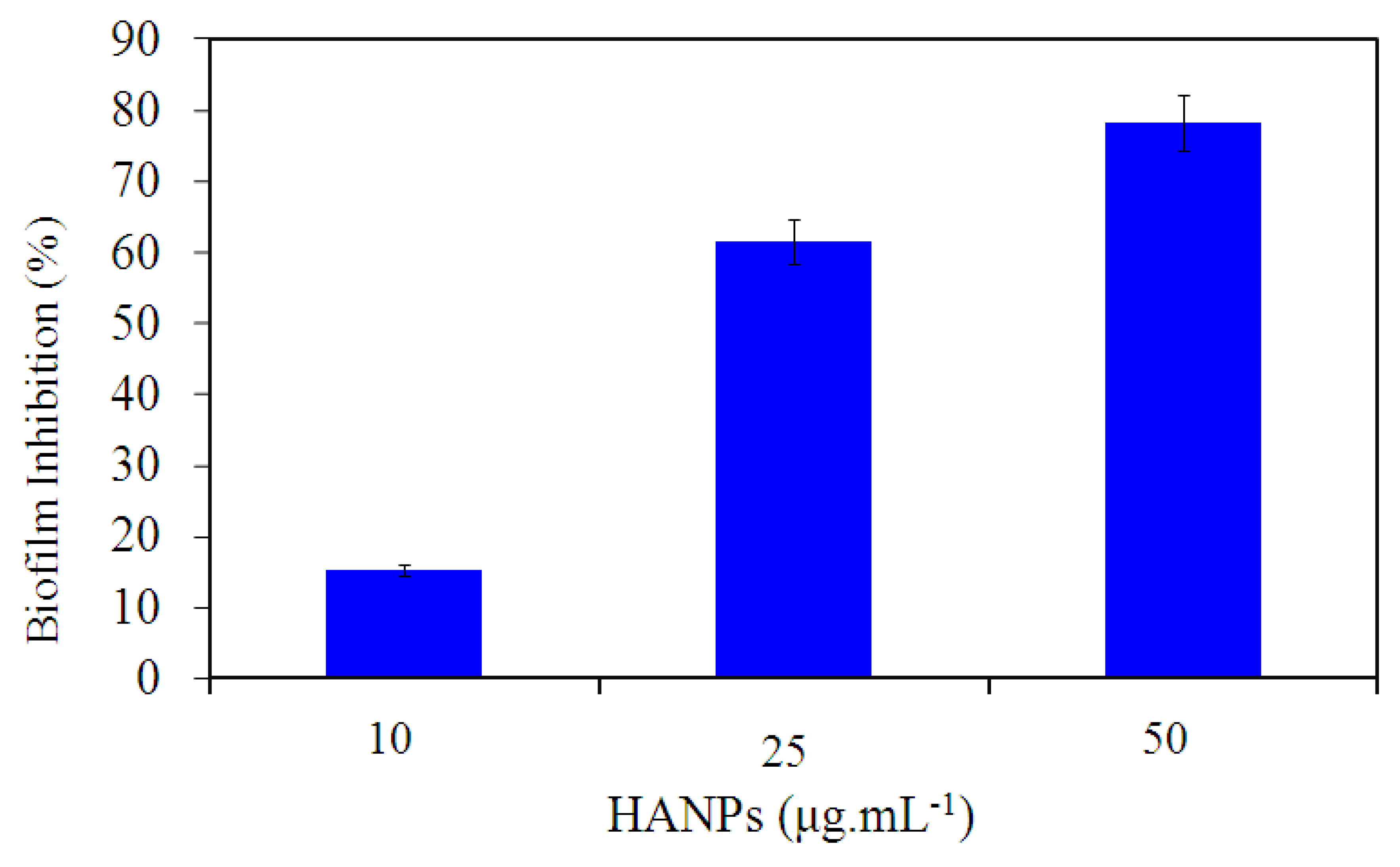
3.2. Quantitative Analysis of P. aeruginosa ATCC 10145 Treated with HANPs
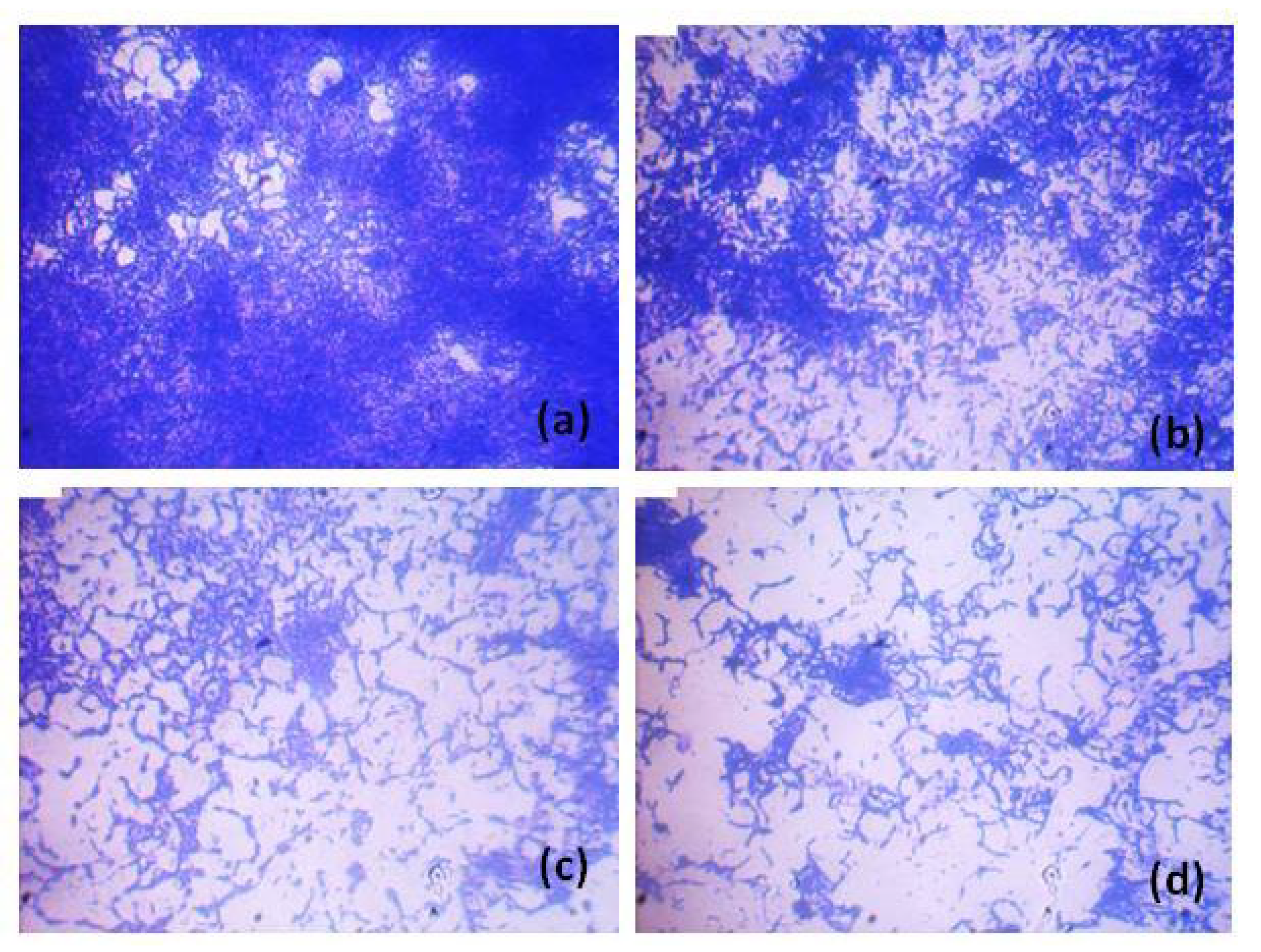
3.3. Qualitative Analysis of P. aeruginosa ATCC 10145 Treated with HANPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Sang-Yul, L.; Seong-Cheol, K.; Jung-Wan, K. One-step synthesis and characterization of broad spectrum antimicrobial efficacy of cellulose/silver nanobiocomposites using solution plasma process. RSC Adv. 2015, 5, 35052–35060. [Google Scholar]
- MubarakAli, D.; Arunkumar, J.; Pooja, P.; Subramanian, G.; Thajuddin, N.; Alharbi, N.S. Synthesis and characterization of biocompatibility of tenorite nanoparticles and potential property against biofilm formation. Saudi Pharm. J. 2015, 23, 421–428. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Park, B.-R.; Rhee, W.-J.; Lee, S.-Y.; Kim, J.-W. Antioxidant potentials of nanoceria synthesized by solution plasma process and its biocompatibility study. Arch. Biochem. Biophys. 2018, 645, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.; Tredwin, C.; Handy, R. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Davoodbasha, M. Imaging bacteria and biofilm by field emission scanning electron microscopy. In Analytical Methodologies for Biofilm Research; Springer: Berlin, Germany, 2021; pp. 205–222. [Google Scholar]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Venkatkumar, G.; Saravanan, M.; Mubarak Ali, D. Tribulus terrestris leaf mediated biosynthesis of stable antibacterial silver nanoparticles. Pharm. Nanotechnol. 2015, 3, 26–34. [Google Scholar] [CrossRef]
- Koley, S.; Mukherjee, M. Genetic basis of biofilm formation and spread of nosocomial infections. In Analytical Methodologies for Biofilm Research; Nag, M., Lahiri, D., Eds.; Springer: New York, NY, USA, 2021; pp. 269–298. [Google Scholar]
- Chari, N.; Felix, L.; Davoodbasha, M.; Ali, A.S.; Nooruddin, T. In vitro and in vivo antibiofilm effect of copper nanoparticles against aquaculture pathogens. Biocatal Agric. Biotechnol. 2017, 10, 336–341. [Google Scholar] [CrossRef]
- LewisOscar, F.; MubarakAli, D.; Nithya, C.; Priyanka, R.; Gopinath, V.; Alharbi, N.S.; Thajuddin, N. One pot synthesis and potential antibiofilm property of copper nanoparticles against clinically isolated strains of Pseudomonas aeruginosa. Biofouling 2015, 31, 379–391. [Google Scholar] [CrossRef]
- Srinivasan, R.; Vigneshwari, L.; Rajavel, T.; Durgadevi, R.; Kannappan, A.; Balamurugan, K.; Pandima Devi, K.; Veera Ravi, A. Biogenic synthesis of silver nanoparticles using Piper betle aqueous extract and evaluation of its anti-quorum sensing and antibiofilm potential against uropathogens with cytotoxic effects: An in vitro and in vivo approach. Environ. Sci. Pollu. Res. 2018, 25, 10538–10554. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointer. 2012, 96, 69–74. [Google Scholar] [CrossRef]
- MubarakAli, D.; Arunkumar, J.; Nag, K.H.; SheikSyedIshack, K.A.; Baldev, E.; Pandiaraj, D.; Thajuddin, N. Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms—Comparative studies on synthesis and its application on biolabelling. Colloids Surf. B Biointer. 2013, 103, 166–173. [Google Scholar] [CrossRef]
- Høiby, N.; Koch, C. Cystic fibrosis. 1. Pseudomonas aeruginosa infection in cystic fibrosis and its management. Thorax 1990, 45, 881. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Kristiansen, S.; Phipps, R.; Nielsen, A.K.; Jensen, P.Ø.; Høiby, N.; Givskov, M. Silver against Pseudomonas aeruginosa biofilms. APMIS 2007, 115, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- We, M.; Ruys, A.; Swain, M.; Milthorpe, B.; Sorrell, C. Hydroxyapatite-coated metals: Interfacial reactions during sintering. J. Mater. Sci. Mater. Med. 2005, 16, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Rameshbabu, N.; Bose, A.C.; Muthupandi, V.; Subramanian, S.; MubarakAli, D.; Thajuddin, N. Fabrication of corrosion resistant, bioactive and antibacterial silver substituted hydroxyapatite/titania composite coating on Cp Ti. Ceram. Int. 2012, 38, 731–740. [Google Scholar] [CrossRef]
- MubarakAli, D. Microwave irradiation mediated synthesis of needle-shaped hydroxyapatite nanoparticles as a flocculant for Chlorella vulgaris. Biocatal Agric. Biotechnol. 2019, 17, 203–206. [Google Scholar] [CrossRef]
- Ravindran, D.; Ramanathan, S.; Arunachalam, K.; Jeyaraj, G.; Shunmugiah, K.; Arumugam, V. Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: An in vitro study. J Appl. Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, A.; Gowrishankar, S.; Srinivasan, R.; Pandian, S.K.; Ravi, A.V. Antibiofilm activity of Vetiveria zizanioides root extract against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2017, 110, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, A.; Sivaranjani, M.; Srinivasan, R.; Rathna, J.; Pandian, S.K.; Ravi, A.V. Inhibitory efficacy of geraniol on biofilm formation and development of adaptive resistance in Staphylococcus epidermidis RP62A. J. Med. Microbiol. 2017, 66, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, A.; Balasubramaniam, B.; Ranjitha, R.; Srinivasan, R.; Packiavathy, I.A.S.V.; Balamurugan, K.; Pandian, S.K.; Ravi, A.V. In vitro and in vivo biofilm inhibitory efficacy of geraniol-cefotaxime combination against Staphylococcus spp. Food Chem Toxicol. 2019, 125, 322–332. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori. Sci. Rep. 2019, 9, 5787. [Google Scholar] [CrossRef]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Beyene, Z.; Ghosh, R. Effect of zinc oxide addition on antimicrobial and antibiofilm activity of hydroxyapatite: A potential nanocomposite for biomedical applications. Mater. Today Commun. 2019, 21, 100612. [Google Scholar] [CrossRef]
- Ahmed, H.Y.; Safwat, N.; Shehata, R.; Althubaiti, E.H.; Kareem, S.; Atef, A.; Qari, S.H.; Aljahani, A.H.; Al-Meshal, A.S.; Youssef, M.; et al. Synthesis of Natural Nano-Hydroxyapatite from Snail Shells and Its Biological Activity: Antimicrobial, Antibiofilm, and Biocompatibility. Membranes 2022, 12, 408. [Google Scholar] [CrossRef]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalińska, A.; Chodara, A.; Szałaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun membrane surface modification by sonocoating with ha and ZnO:Ag nanoparticles-characterization and evaluation of osteoblasts and bacterial cell behavior in vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef]
- Nica, I.C.; Popa, M.; Marutescu, L.; Dinischiotu, A.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, D. Biocompatibility and antibiofilm properties of samarium doped hydroxyapatite coatings: An in vitro study. Coatings 2021, 11, 1185. [Google Scholar] [CrossRef]
- Swathirajan, C.R.; Rameshkumar, M.R.; Solomon, S.S.; Vignesh, R.; Balakrishnan, P. Changing drug resistance profile in Pseudomonas aeruginosa infection among HIV patients from 2010–2017: A retrospective study. J. Glob. Antimicrob. Resist. 2019, 16, 274–277. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—How P. aeruginosa can escape antibiotics. Front. Microbioly 2019, 10, 913. [Google Scholar] [CrossRef]
- Nam, S.; MubarakAli, D.; Kim, J. Characterization of alginate/silver nanobiocomposites synthesized by solution plasma process and their antimicrobial properties. J. Nanomater. 2016, 2016, 4712813. [Google Scholar] [CrossRef]
- Costescu, A.; Pasuk, I.; Ungureanu, F.; Dinischiotu, A.; Costache, M.; Huneau, F.; Galaup, S.; Le Coustumer, P.; Predoi, D. Physico-chemical properties of nano-sized hexagonal hydroxyapatite powder synthesized by sol-gel. Dig. J. Nanomater. Bios (DJNB) 2010, 5, 989–1000. [Google Scholar]
- Ganeshbabu, M.; Kannan, N.; Venkatesh, P.S.; Paulraj, G.; Jeganathan, K.; MubarakAli, D. Synthesis and characterization of BiVO(4) nanoparticles for environmental applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Barik, R.C.; Natarajan, D.; Kiran, M.; Pattanayak, D.K. Synthesis, phase stability of hydroxyapatite–silver composite with antimicrobial activity and cytocompatability. Ceram. Int. 2014, 40, 10831–10838. [Google Scholar] [CrossRef]
- Akhavan, A.; Sheikh, N.; Khoylou, F.; Naimian, F.; Ataeivarjovi, E. Synthesis of antimicrobial silver/hydroxyapatite nanocomposite by gamma irradiation. Radiat. Phys. Chem. 2014, 98, 46–50. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. Biomed. Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Basche, S.; Burghardt, T.; Al-Ahmad, A.; Hannig, M. Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin. Oral Investig. 2013, 17, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollu. Res. Int. 2018, 25, 10362–10370. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MubarakAli, D.; Arunachalam, K.; Lakshmanan, M.; Badar, B.; Kim, J.-W.; Lee, S.-Y. Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy. Pharmaceutics 2023, 15, 463. https://doi.org/10.3390/pharmaceutics15020463
MubarakAli D, Arunachalam K, Lakshmanan M, Badar B, Kim J-W, Lee S-Y. Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy. Pharmaceutics. 2023; 15(2):463. https://doi.org/10.3390/pharmaceutics15020463
Chicago/Turabian StyleMubarakAli, Davoodbasha, Kannappan Arunachalam, Murugan Lakshmanan, Bazigha Badar, Jung-Wan Kim, and Sang-Yul Lee. 2023. "Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy" Pharmaceutics 15, no. 2: 463. https://doi.org/10.3390/pharmaceutics15020463
APA StyleMubarakAli, D., Arunachalam, K., Lakshmanan, M., Badar, B., Kim, J.-W., & Lee, S.-Y. (2023). Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy. Pharmaceutics, 15(2), 463. https://doi.org/10.3390/pharmaceutics15020463







