Peptide Vaccines in Melanoma: Chemical Approaches towards Improved Immunotherapeutic Efficacy
Abstract
1. Introduction
2. The Role of the Immune System in Cancers/Melanoma
3. Immunotherapeutic Approaches for the Treatment of Melanoma
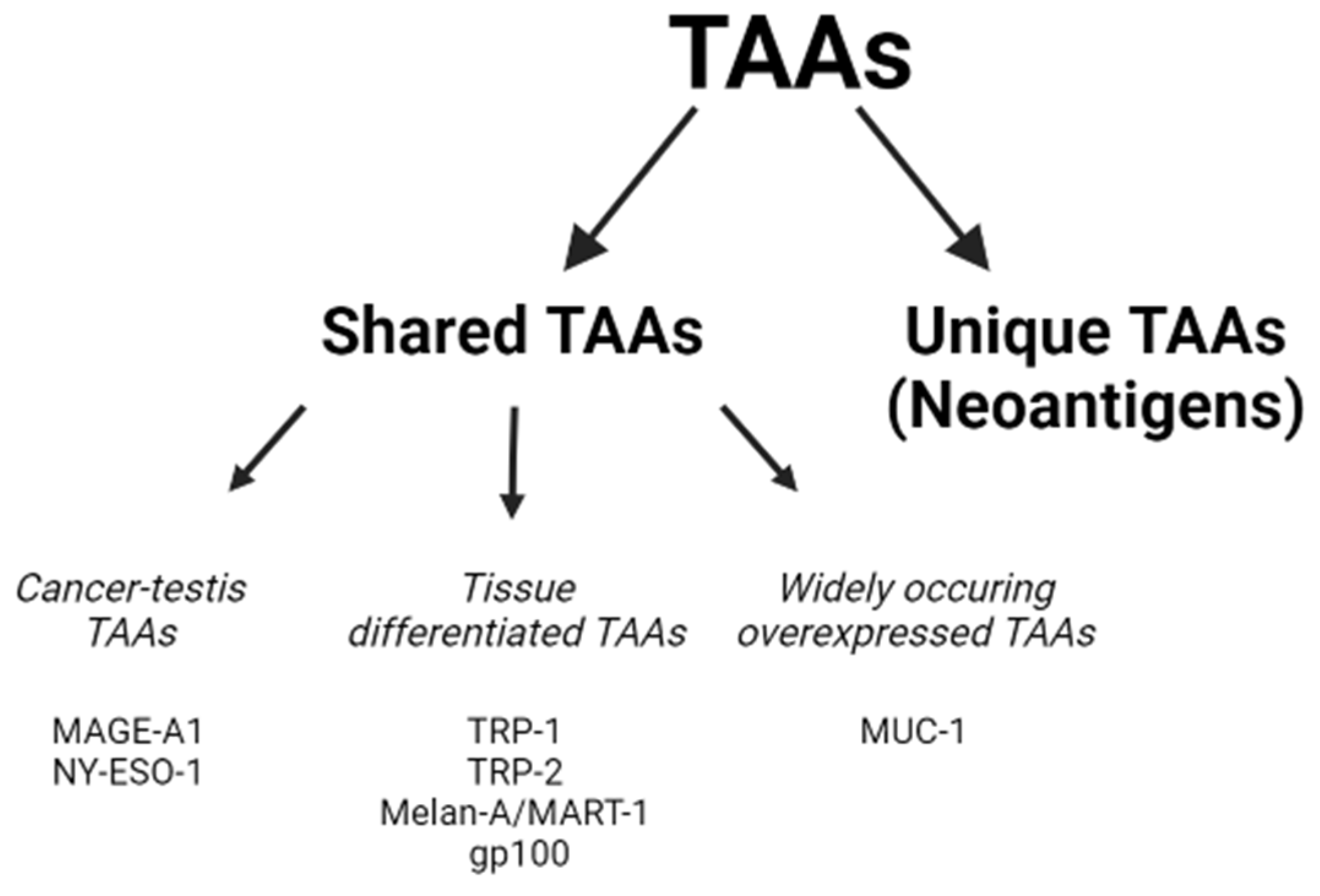
4. Types of Antigens: Overexpressed Antigens, Cancer Testis Antigens, Mutated Oncogenes and Patient-Specific Mutated Neoantigens
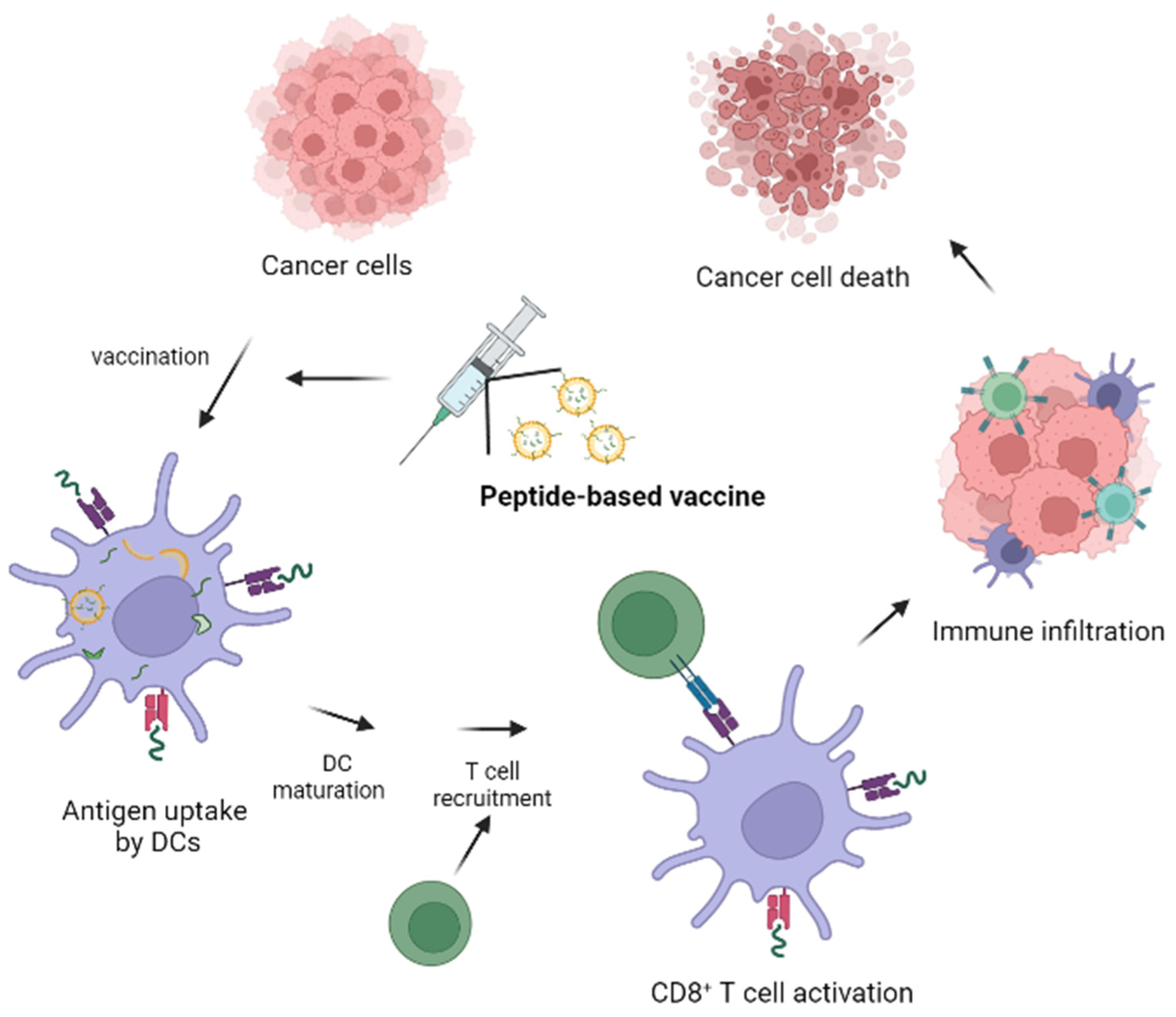
5. Peptide Vaccines: Benefits, Drawbacks, Types and Composition
6. How to Improve the Peptide?
6.1. Enhancing the Binding Affinity
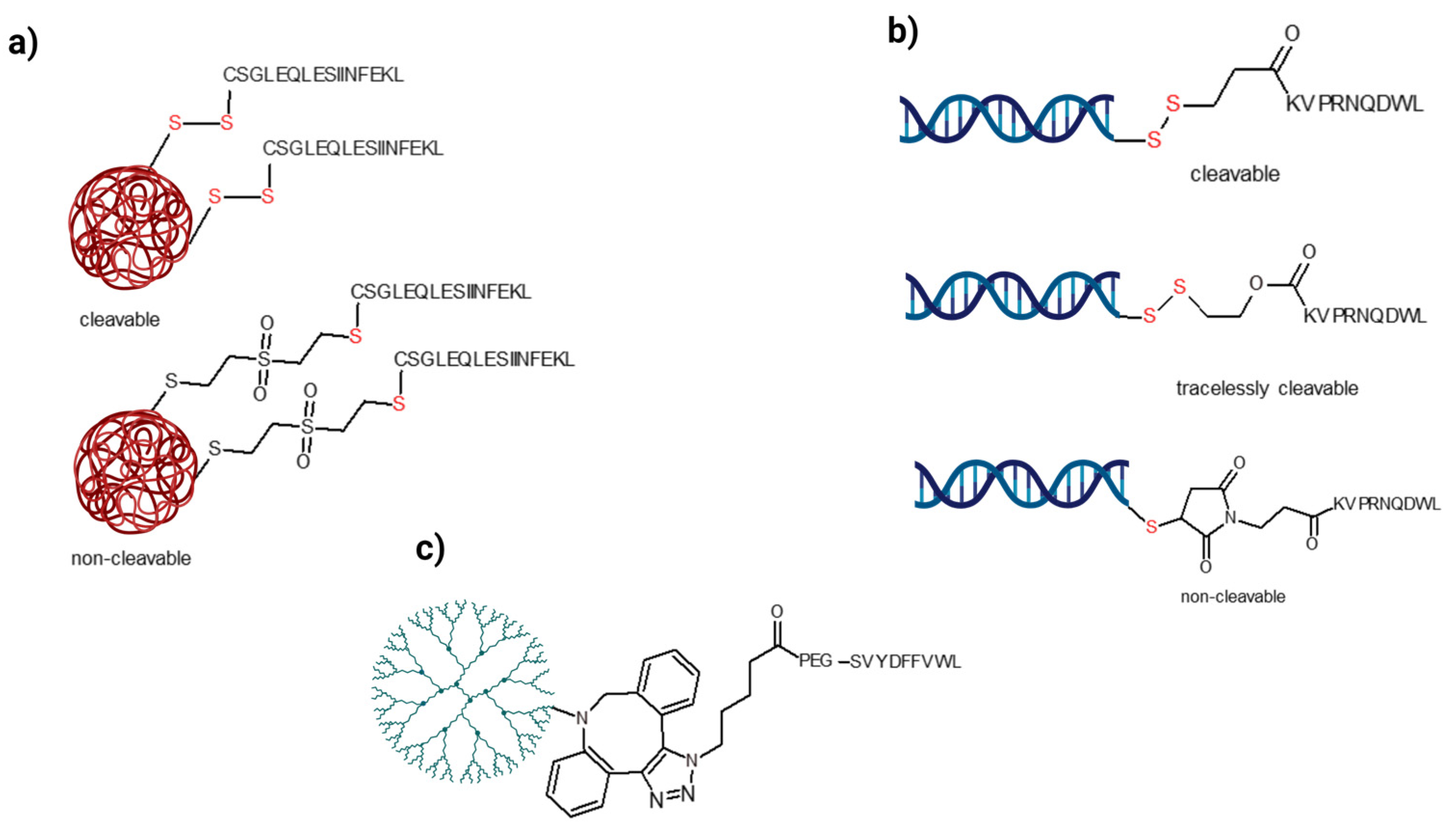
6.2. Usage of a Covalent Linkage between the Antigen and the Nanocarrier
6.3. Self-Assembling Moieties for Improved Efficacy
6.4. Incorporation of Lymph Node Targeting Moieties
6.5. Usage of Cell-Penetrating Peptides
7. Adjuvants and Formulation
7.1. Adjuvants in Melanoma Vaccines
7.1.1. Alum
7.1.2. Emulsions
7.1.3. Freund’s Adjuvants
7.1.4. Poly-ICLC (aka Hiltonol®)
7.1.5. Immunostimulatory Proteins
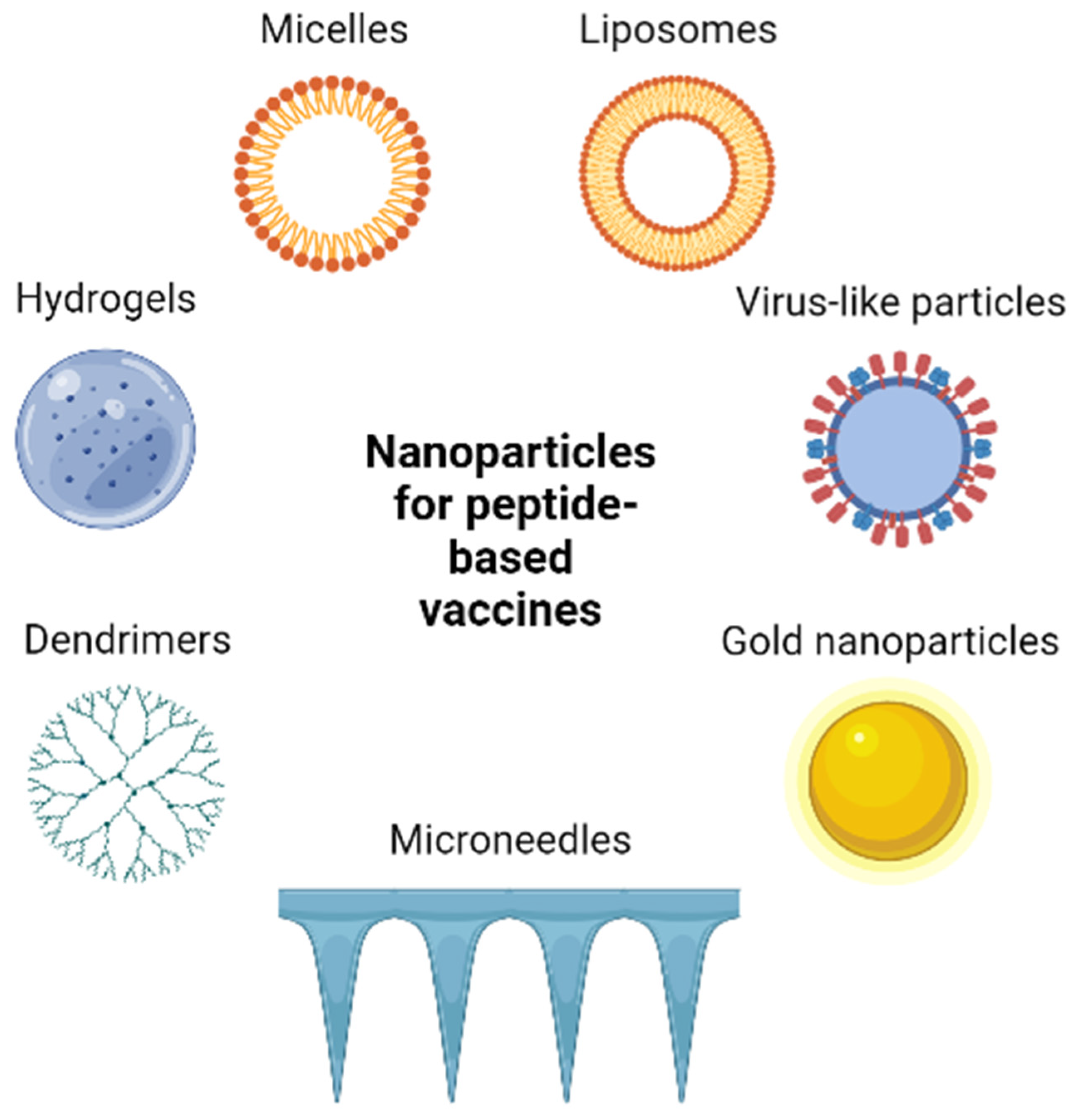
7.2. Vaccine Delivery Systems in Melanoma
7.2.1. Liposomes
7.2.2. Hydrogels
7.2.3. Micelles
7.2.4. Inorganic Nanoparticles
7.2.5. Virus-like Particles
8. Ongoing Clinical Trials
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Fisher, D.E.; Rizos, H. Biology of Melanocytes and Primary Melanoma. In Cutaneous Melanoma; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–38. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Survival Rates for Melanoma Skin Cancer. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html (accessed on 3 December 2022).
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Ehrlich, P. Ueber Den Jetzigen Stand Der Karzinomforschung. Ned. Tijdschr. Voor Geneeskd. 1908, 5, 273–290. [Google Scholar]
- Thomas, L. Discussion. In Cellular and Humoral Aspects of the Hypersensitive States; Lawrence, H.S., Ed.; Hoeber-Harper: New York, NY, USA, 1959; pp. 529–532. [Google Scholar]
- Burnet, M. Cancer—A Biological Approach: III. Viruses Associated with Neoplastic Conditions. IV. Practical Applications. BMJ 1957, 1, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Phoon, Y.P.; Tannenbaum, C.; Diaz-Montero, C.M. Immunobiology of Melanoma. Clin. Plast. Surg. 2021, 48, 561–576. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the cells of origin of squamous cell carcinomas. Nat. Rev. Cancer 2018, 18, 549–561. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef]
- Bhatia, A.; Kumar, Y. Cancer-Immune Equilibrium: Questions Unanswered. Cancer Microenviron. 2011, 4, 209–217. [Google Scholar] [CrossRef]
- Bernardone, I.S. Role of NK cells and adaptive immunity in “immunoediting”: Recent developments. Inmunología 2008, 27, 141–146. [Google Scholar] [CrossRef]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; Plevak, M.F.; Melton, L.J., 3rd. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2002, 346, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Mahnke, Y.D.; Schwendemann, J.; Beckhove, P.; Schirrmacher, V. Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells. Immunology 2005, 115, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. Cell transfer immunotherapy for metastatic solid cancer—What clinicians need to know. Nat. Rev. Clin. Oncol. 2011, 8, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.D.; Katz, K.H.; Windsor, J.; Thrush, G.; Scheuermann, R.H.; Uhr, J.W.; Street, N.E. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-gamma in establishing and maintaining the tumor-dormant state. J. Immunol. 1999, 162, 2842–2849. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef]
- Felsher, D.W. Tumor Dormancy: Death and Resurrection of Cancer As Seen through Transgenic Mouse Models. Cell Cycle 2006, 5, 1808–1811. [Google Scholar] [CrossRef]
- Holmgren, L.; O’Reilly, M.S.; Folkman, J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1995, 1, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lee, Y.; Liu, W.; Krausz, T.; Chong, A.; Schreiber, H.; Fu, Y.-X. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 2005, 201, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, L. The capacity of the immune system to control cancer. Eur. J. Cancer 2009, 45, 2068–2070. [Google Scholar] [CrossRef]
- Vence, L.; Palucka, A.K.; Fay, J.W.; Ito, T.; Liu, Y.-J.; Banchereau, J.; Ueno, H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 2007, 104, 20884–20889. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Merino, L.; Grande-Pulido, E.; Albero-Tamarit, A.; de Villena, M.E.C.-M. Cancer and Immune Response: Old and New Evidence for Future Challenges. Oncologist 2008, 13, 1246–1254. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Yang, J.; Zhang, M.; Jin, H.; Ma, X.; Shi, H. Combination of Immunotherapy with Targeted Therapy: Theory and Practice in Metastatic Melanoma. Front. Immunol. 2019, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Candido, S.; Falzone, L.; Spandidos, D.A.; Libra, M. Cutaneous melanoma and the immunotherapy revolution. Int. J. Oncol. 2020, 57, 609–618. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S. Cytotoxic T-Lymphocyte–Associated Antigen-4. Clin. Cancer Res. 2007, 13, 5238–5242. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Bentebibel, S.-E.; Diab, A. Cytokines in the Treatment of Melanoma. Curr. Oncol. Rep. 2021, 23, 83. [Google Scholar] [CrossRef]
- Borgers, J.S.; Haanen, J.B. Cellular Therapy and Cytokine Treatments for Melanoma. Hematol. Clin. N. Am. 2020, 35, 129–144. [Google Scholar] [CrossRef]
- Foley, K.C.; Nishimura, M.I.; Moore, T.V. Combination immunotherapies implementing adoptive T-cell transfer for advanced-stage melanoma. Melanoma Res. 2018, 28, 171–184. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Z.; Cohen, C.J.; Gattinoni, L.; Palmer, D.; Restifo, N.P.; Rosenberg, S.A.; Morgan, R.A. High-Efficiency Transfection of Primary Human and Mouse T Lymphocytes Using RNA Electroporation. Mol. Ther. 2006, 13, 151–159. [Google Scholar] [CrossRef]
- Clay, T.M.; Custer, M.C.; Sachs, J.; Hwu, P.; Rosenberg, S.A.; Nishimura, M.I. Efficient Transfer of a Tumor Antigen-Reactive TCR to Human Peripheral Blood Lymphocytes Confers Anti-Tumor Reactivity. J. Immunol. 1999, 163, 507–513. [Google Scholar] [CrossRef]
- Tsuji, T.; Yasukawa, M.; Matsuzaki, J.; Ohkuri, T.; Chamoto, K.; Wakita, D.; Azuma, T.; Niiya, H.; Miyoshi, H.; Kuzushima, K.; et al. Generation of tumor-specific, HLA class I-restricted human Th1 and Tc1 cells by cell engineering with tumor peptide-specific T-cell receptor genes. Blood 2005, 106, 470–476. [Google Scholar] [CrossRef]
- Peng, P.D.; Cohen, C.J.; Yang, S.; Hsu, C.; Jones, S.; Zhao, Y.; Zheng, Z.; Rosenberg, S.A.; Morgan, R.A. Efficient nonviral Sleeping Beauty transposon-based TCR gene transfer to peripheral blood lymphocytes confers antigen-specific antitumor reactivity. Gene Ther. 2009, 16, 1042–1049. [Google Scholar] [CrossRef]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; van der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Met, O.; Jensen, K.M.; Chamberlain, C.A.; Donia, M.; Svane, I.M. Principles of adoptive T cell therapy in cancer. Semin. Immunopathol. 2018, 41, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Antigen-specific vaccines for cancer treatment. Hum. Vaccines Immunother. 2014, 10, 3332–3346. [Google Scholar] [CrossRef] [PubMed]
- Chaux, P.; Luiten, R.; Demotte, N.; Vantomme, V.; Stroobant, V.; Traversari, C.; Russo, V.; Schultz, E.; Cornelis, G.R.; Boon, T.; et al. Identification of Five MAGE-A1 Epitopes Recognized by Cytolytic T Lymphocytes Obtained by In Vitro Stimulation with Dendritic Cells Transduced with MAGE-A1. J. Immunol. 1999, 163, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Traversari, C.; Van Der Bruggen, P.; Luescher, I.F.; Lurquin, C.; Chomez, P.; Van Pel, A.; De Plaen, E.; Amar-Costesec, A.; Boon, T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 1992, 176, 1453–1457. [Google Scholar] [CrossRef]
- Jäger, E.; Chen, Y.-T.; Drijfhout, J.W.; Karbach, J.; Ringhoffer, M.; Jäger, D.; Arand, M.; Wada, H.; Noguchi, Y.; Stockert, E.; et al. Simultaneous Humoral and Cellular Immune Response against Cancer–Testis Antigen NY-ESO-1: Definition of Human Histocompatibility Leukocyte Antigen (HLA)-A2–binding Peptide Epitopes. J. Exp. Med. 1998, 187, 265–270. [Google Scholar] [CrossRef]
- Van Der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van Den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; Van Der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Wang, R.-F.; Appella, E.; Kawakami, Y.; Kang, X.; Rosenberg, S.A. Identification of TRP-2 as a Human Tumor Antigen Recognized by Cytotoxic T Lymphocytes. J. Exp. Med. 1996, 184, 2207–2216. [Google Scholar] [CrossRef]
- Kawakami, Y.; Eliyahu, S.; Sakaguchi, K.; Robbins, P.F.; Rivoltini, L.; Yannelli, J.R.; Appella, E.; Rosenberg, S.A. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 1994, 180, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Schreurs, M.W.; De Boer, A.J.; Kawakami, Y.; Rosenberg, S.A.; Adema, G.J.; Figdor, C. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 1994, 179, 1005–1009. [Google Scholar] [CrossRef]
- Kawakami, Y.; Robbins, P.F.; Wang, X.; Tupesis, J.P.; Parkhurst, M.R.; Kang, X.; Sakaguchi, K.; Appella, E.; Rosenberg, S.A. Identification of New Melanoma Epitopes on Melanosomal Proteins Recognized by Tumor Infiltrating T Lymphocytes Re-stricted by HLA-A1, -A2, and -A3 Alleles. J. Immunol. 1998, 161, 6985–6992. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Eliyahu, S.; Jennings, C.; Sakaguchi, K.; Kang, X.; Southwood, S.; Robbins, P.F.; Sette, A.; Appella, E.; Rosenberg, S.A. Recognition of Multiple Epitopes in the Human Melanoma Antigen Gp100 by Tumor-Infiltrating T Lymphocytes Asso-ciated with in Vivo Tumor Regression. J. Immunol. 1995, 154, 3961–3968. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Morgan, R.A.; Dudley, M.E.; Cassard, L.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Royal, R.E.; Sherry, R.M.; Wunderlich, J.R.; et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009, 114, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer Neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Yamamoto, T.N.; Kishton, R.J.; Restifo, N.P. Developing neoantigen-targeted T cell–based treatments for solid tumors. Nat. Med. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Davis, L.; Tarduno, A.; Lu, Y.-C. Neoantigen-Reactive T Cells: The Driving Force behind Successful Melanoma Immunotherapy. Cancers 2021, 13, 6061. [Google Scholar] [CrossRef]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Löwer, M.; Van de Roemer, N.; De Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C.; et al. Exploiting the Mutanome for Tumor Vaccination. Cancer Res 2012, 72, 1081–1091. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2018, 565, 234–239. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines. Cancers 2020, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef] [PubMed]
- Truex, N.L.; Holden, R.L.; Wang, B.-Y.; Chen, P.-G.; Hanna, S.; Hu, Z.; Shetty, K.; Olive, O.; Neuberg, D.; Hacohen, N.; et al. Automated Flow Synthesis of Tumor Neoantigen Peptides for Personalized Immunotherapy. Sci. Rep. 2020, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Horváti, K.; Gyulai, G.; Csámpai, A.; Rohonczy, J.; Kiss, E.; Bosze, S. Surface Layer Modification of Poly(d,l-lactic-co-glycolic acid) Nanoparticles with Targeting Peptide: A Convenient Synthetic Route for Pluronic F127–Tuftsin Conjugate. Bioconjug. Chem. 2018, 29, 1495–1499. [Google Scholar] [CrossRef]
- Horváti, K.; Bacsa, B.; Kiss, E.; Gyulai, G.; Fodor, K.; Balka, G.; Rusvai, M.; Szabó, E.; Hudecz, F.; Bősze, S. Nanoparticle Encapsulated Lipopeptide Conjugate of Antitubercular Drug Isoniazid: In Vitro Intracellular Activity and in Vivo Efficacy in a Guinea Pig Model of Tuberculosis. Bioconjug. Chem. 2014, 25, 2260–2268. [Google Scholar] [CrossRef]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2015, 7, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Mahdevar, E.; Safavi, A.; Abiri, A.; Kefayat, A.; Hejazi, S.H.; Miresmaeili, S.M.; Mobarakeh, V.I. Exploring the cancer-testis antigen BORIS to design a novel multi-epitope vaccine against breast cancer based on immunoinformatics approaches. J. Biomol. Struct. Dyn. 2021, 40, 6363–6380. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, R.; Barrett, T.; Beck, J.; Benson, D.A.; Bollin, C.; Bolton, E.; Bourexis, D.; Brister, J.R.; Bryant, S.H.; Canese, K.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Bui, H.-H.; Sidney, J.; Li, W.; Fusseder, N.; Sette, A. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC Bioinform. 2007, 8, 361. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. Identifying candidate subunit vaccines using an alignment-independent method based on principal amino acid properties. Vaccine 2007, 25, 856–866. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Nielsen, M.; Lundegaard, C.; Lund, O.; Keşmir, C. The role of the proteasome in generating cytotoxic T-cell epitopes: Insights obtained from improved predictions of proteasomal cleavage. Immunogenetics 2005, 57, 33–41. [Google Scholar] [CrossRef]
- Diez-Rivero, C.M.; Chenlo, B.; Zuluaga, P.; Reche, P.A. Quantitative modeling of peptide binding to TAP using support vector machine. Proteins Struct. Funct. Bioinform. 2009, 78, 63–72. [Google Scholar] [CrossRef]
- Castiglione, F.; Deb, D.; Srivastava, A.P.; Liò, P.; Liso, A. From Infection to Immunity: Understanding the Response to SARS-CoV2 Through In-Silico Modeling. Front. Immunol. 2021, 12, 3433. [Google Scholar] [CrossRef]
- Zhang, Z.; Tongchusak, S.; Mizukami, Y.; Kang, Y.J.; Ioji, T.; Touma, M.; Reinhold, B.; Keskin, D.B.; Reinherz, E.L.; Sasada, T. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials 2011, 32, 3666–3678. [Google Scholar] [CrossRef]
- Valmori, D.; Fonteneau, J.F.; Lizana, C.M.; Gervois, N.; Liénard, D.; Rimoldi, D.; Jongeneel, V.; Jotereau, F.; Cerottini, J.C.; Romero, P. Enhanced Generation of Specific Tumor-Reactive CTL in Vitro by Selected Melan-A/MART-1 Immunodominant Peptide Analogues. J. Immunol. 1998, 160, 1750–1758. [Google Scholar] [CrossRef]
- Falk, K.; Rötzschke, O.; Stevanovié, S.; Jung, G.; Rammensee, H.-G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991, 351, 290–296. [Google Scholar] [CrossRef]
- Blanchet, J.-S.; Valmori, D.; Dufau, I.; Ayyoub, M.; Nguyen, C.; Guillaume, P.; Monsarrat, B.; Cerottini, J.-C.; Romero, P.; Gairin, J.E. A New Generation of Melan-A/MART-1 Peptides That Fulfill Both Increased Immunogenicity and High Resistance to Biodegradation: Implication for Molecular Anti-Melanoma Immunotherapy. J. Immunol. 2001, 167, 5852–5861. [Google Scholar] [CrossRef] [PubMed]
- Douat-Casassus, C.; Marchand-Geneste, N.; Diez, E.; Gervois, N.; Jotereau, F.; Quideau, S. Synthetic Anticancer Vaccine Candidates: Rational Design of Antigenic Peptide Mimetics That Activate Tumor-Specific T-Cells. J. Med. Chem. 2007, 50, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Tarbe, M.; Azcune, I.; Balentová, E.; Miles, J.J.; Edwards, E.E.; Miles, K.M.; Do, P.; Baker, B.M.; Sewell, A.K.; Aizpurua, J.M.; et al. Design, synthesis and evaluation of β-lactam antigenic peptide hybrids; unusual opening of the β-lactam ring in acidic media. Org. Biomol. Chem. 2010, 8, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Tarbe, M.; Miles, J.J.; Edwards, E.S.J.; Miles, K.M.; Sewell, A.K.; Baker, B.M.; Quideau, S. Synthesis and Biological Evaluation of Hapten-Clicked Analogues of The Antigenic Peptide Melan-A/MART-1 26(27L)-35. ChemMedChem 2020, 15, 799–807. [Google Scholar] [CrossRef]
- Slansky, J.E.; Nakayama, M. Peptide mimotopes alter T cell function in cancer and autoimmunity. Semin. Immunol. 2020, 47, 101395. [Google Scholar] [CrossRef]
- Duan, F.; Duitama, J.; Al Seesi, S.; Ayres, C.M.; Corcelli, S.A.; Pawashe, A.P.; Blanchard, T.; McMahon, D.; Sidney, J.; Sette, A.; et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J. Exp. Med. 2014, 211, 2231–2248. [Google Scholar] [CrossRef]
- Smith, A.R.; Alonso, J.A.; Ayres, C.M.; Singh, N.K.; Hellman, L.M.; Baker, B.M. Structurally silent peptide anchor modifications allosterically modulate T cell recognition in a receptor-dependent manner. Proc. Natl. Acad. Sci. USA 2021, 118, e2018125118. [Google Scholar] [CrossRef]
- Hirosue, S.; Kourtis, I.C.; Van der Vlies, A.J.; Hubbell, J.A.; Swartz, M.A. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine 2010, 28, 7897–7906. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Song, Q.; Wu, T.; Zhuang, X.; Bao, Y.; Kong, M.; Qi, Y.; Tan, S.; Zhang, Z. Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma. ACS Nano 2015, 9, 6918–6933. [Google Scholar] [CrossRef]
- Skakuj, K.; Wang, S.; Qin, L.; Lee, A.; Zhang, B.; Mirkin, C.A. Conjugation Chemistry-Dependent T-Cell Activation with Spherical Nucleic Acids. J. Am. Chem. Soc. 2018, 140, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Van Dinther, D.; Venegas, M.L.; Veninga, H.; Olesek, K.; Hoogterp, L.; Revet, M.; Ambrosini, M.; Kalay, H.; Stöckl, J.; Van Kooyk, Y.; et al. Activation of CD8+ T Cell Responses after Melanoma Antigen Targeting to CD169+ Antigen Presenting Cells in Mice and Humans. Cancers 2019, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Kakwere, H.; Ingham, E.S.; Allen, R.; Mahakian, L.M.; Tam, S.M.; Zhang, H.; Silvestrini, M.T.; Lewis, J.S.; Ferrara, K.W. Toward Personalized Peptide-Based Cancer Nanovaccines: A Facile and Versatile Synthetic Approach. Bioconjug. Chem. 2017, 28, 2756–2771. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wang, S.; Xu, K.; Tang, Q.; Li, W.; Zheng, W.; Shi, H.; Su, K.; Liu, Y.; Hong, Z. A Potent Micron Neoantigen Tumor Vaccine GP-Neoantigen Induces Robust Antitumor Activity in Multiple Tumor Models. Adv. Sci. 2022, 9, 2201496. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, G.; Fan, J.; Zheng, R.; Zhao, L.; Yuan, P.; Zhao, X.; Yu, X.; Li, S. A Self-Delivery Chimeric Peptide for Photodynamic Therapy Amplified Immunotherapy. Macromol. Biosci. 2018, 19, e1800410. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, F.; Jiang, X.; Lv, Q.; Luo, N.; Gong, C.; Wang, C.; Yang, L.; He, G. Discovery of a self-assembling and self-adjuvant lipopeptide as a saccharide-free peptide vaccine targeting EGFRvIII positive cutaneous melanoma. Biomater. Sci. 2018, 6, 1120–1128. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Feng, Z.; Wang, C.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Synthetic, Supramolecular, and Self-Adjuvanting CD8 + T-Cell Epitope Vaccine Increases the Therapeutic Antitumor Immunity. Adv. Ther. 2019, 2, 1900010. [Google Scholar] [CrossRef]
- Shi, X.; Song, H.; Wang, C.; Zhang, C.; Huang, P.; Kong, D.; Zhang, J.; Wang, W. Co-assembled and self-delivered epitope/CpG nanocomplex vaccine augments peptide immunogenicity for cancer immunotherapy. Chem. Eng. J. 2020, 399, 125854. [Google Scholar] [CrossRef]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef]
- Moynihan, K.D.; Holden, R.L.; Mehta, N.K.; Wang, C.; Karver, M.R.; Dinter, J.; Liang, S.; Abraham, W.; Melo, M.B.; Zhang, A.Q.; et al. Enhancement of Peptide Vaccine Immunogenicity by Increasing Lymphatic Drainage and Boosting Serum Stability. Cancer Immunol. Res. 2018, 6, 1025–1038. [Google Scholar] [CrossRef]
- Zhu, G.; Lynn, G.M.; Jacobson, O.; Chen, K.; Liu, Y.; Zhang, H.; Ma, Y.; Zhang, F.; Tian, R.; Ni, Q.; et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017, 8, 1954. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.M.; Holden, R.L.; Moynihan, K.D.; Garafola, D.; Farquhar, C.; Mehta, N.K.; Maiorino, L.; Pham, S.; Iorgulescu, J.B.; Reardon, D.A.; et al. Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity. Proc. Natl. Acad. Sci. USA 2022, 119, e2204078119. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Becker, K.W.; Knight, F.C.; Baljon, J.J.; Sevimli, S.; Shae, D.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Poly(propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials 2018, 182, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Di Berardino-Besson, W.; Gaertner, H.; Carboni, S.; Dunand-Sauthier, I.; Cerini, F.; Suso-Inderberg, E.-M.; Wälchli, S.; König, S.; Salazar, A.M.; et al. Enhancing Antitumor Immune Responses by Optimized Combinations of Cell-penetrating Peptide-based Vaccines and Adjuvants. Mol. Ther. 2016, 24, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef]
- Luthra, R.; Datta, S.; Roy, A. Role of Different Peptides for Cancer Immunotherapy. Int. J. Pept. Res. Ther. 2021, 27, 2777–2793. [Google Scholar] [CrossRef]
- He, X.; Abrams, S.I.; Lovell, J.F. Peptide Delivery Systems for Cancer Vaccines. Adv. Ther. 2018, 1, 1800060. [Google Scholar] [CrossRef]
- Park, W.H.; Schroder, M.C. Diphtheria Toxin-Antitoxin and Toxoid. Am. J. Public Health Nations Health 1932, 22, 7–16. [Google Scholar] [CrossRef]
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines—A way to modulate the immune response. Semin. Cell Dev. Biol. 2021, 115, 3–9. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Mitkus, R.J.; King, D.B.; Hess, M.A.; Forshee, R.A.; Walderhaug, M.O. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine 2011, 29, 9538–9543. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, E.B.; Schønberg, N.E. Aluminum Adjuvants: Preparation, Application, Dosage, and Formulation with Antigen. In Vaccine Adjuvants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 41–58. [Google Scholar] [CrossRef]
- Hamid, O.; Solomon, J.C.; Scotland, R.; Garcia, M.; Sian, S.; Ye, W.; Groshen, S.L.; Weber, J.S. Alum with Interleukin-12 Augments Immunity to a Melanoma Peptide Vaccine: Correlation with Time to Relapse in Patients with Resected High-Risk Disease. Clin. Cancer Res. 2007, 13, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Aluminum Vaccine Adjuvants: Are they Safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2018, 9, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef]
- Speiser, D.; Liénard, D.; Pittet, M.J.; Batard, P.; Rimoldi, D.; Guillaume, P.; Cerottini, J.-C.; Romero, P. In vivo activation of melanoma-specific CD8+ T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur. J. Immunol. 2002, 32, 731–741. [Google Scholar] [CrossRef]
- Moresco, E.M.Y.; LaVine, D.; Beutler, B. Toll-like receptors. Curr. Biol. 2011, 21, R488–R493. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef]
- Tambunlertchai, S.; Geary, S.M.; Salem, A.K. Topically Applied Resiquimod versus Imiquimod as a Potential Adjuvant in Melanoma Treatment. Pharmaceutics 2022, 14, 2076. [Google Scholar] [CrossRef]
- Dockrell, D.H.; Kinghorn, G.R. Imiquimod and resiquimod as novel immunomodulators. J. Antimicrob. Chemother. 2001, 48, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Beebe, M.; Qin, M.; Moi, M.; Wu, S.; Heiati, H.; Walker, L.; Newman, M.; Fikes, J.; Ishioka, G.Y. Formulation and characterization of a ten-peptide single-vial vaccine EP-2101, designed to induce cytotoxic T-lymphocyte responses for cancer immunotherapy. Hum. Vaccines 2008, 4, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.L.; Bullard, D.C.; Weaver, C.; Jenkins, M.K. Preferential Accumulation of Antigen-specific Effector CD4 T Cells at an Antigen Injection Site Involves CD62E-dependent Migration but Not Local Proliferation. J. Exp. Med. 2003, 197, 751–762. [Google Scholar] [CrossRef]
- Redmond, W.L.; Sherman, L.A. Peripheral Tolerance of CD8 T Lymphocytes. Immunity 2005, 22, 275–284. [Google Scholar] [CrossRef]
- Khong, H.; Overwijk, W.W. Adjuvants for peptide-based cancer vaccines. J. Immunother. Cancer 2016, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Aucouturier, J.; Dupuis, L.; Deville, S.; Ascarateil, S.; Ganne, V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines 2002, 1, 111–118. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.B.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Qian, L.; Ke, Y.; Feng, X.; Chen, X.; Liu, F.; Yu, L.; Zhang, L.; Tao, Y.; Xu, R.; et al. Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. J. Nanobiotechnol. 2022, 20, 190. [Google Scholar] [CrossRef]
- Meneveau, M.O.; Petroni, G.R.; Salerno, E.P.; Lynch, K.T.; Smolkin, M.; Woodson, E.; Chianese-Bullock, K.A.; Olson, W.C.; Deacon, D.; Patterson, J.W.; et al. Immunogenicity in humans of a transdermal multipeptide melanoma vaccine administered with or without a TLR7 agonist. J. Immunother. Cancer 2021, 9, e002214. [Google Scholar] [CrossRef]
- Sultan, H.; Salazar, A.M.; Celis, E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin. Immunol. 2020, 49, 101414. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Burke, A.; Pavlick, A.; Blazquez, A.; Gimenez, G.; Meseck, M.; Donovan, M.; Rodriguez, D.; Castillo-Martin, M.; Thin, T.H.; et al. Abstract CT108: Immunogenicity of Poly-ICLC matured dendritic cells as an adjuvant for NY-ESO-1 and Melan-A-MART-1 peptide vaccination compared to Montanide® ISA-51 VG, in study subjects with melanoma in complete clinical remission but at high risk of disease recurrence. Cancer Res. 2022, 82, CT108. [Google Scholar] [CrossRef]
- Rivera-Patron, M.; Moreno, M.; Baz, M.; Roehe, P.M.; Cibulski, S.P.; Silveira, F. ISCOM-like Nanoparticles Formulated with Quillaja brasiliensis Saponins Are Promising Adjuvants for Seasonal Influenza Vaccines. Vaccines 2021, 9, 1350. [Google Scholar] [CrossRef]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2013, 66, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Mooney, D.J. Biomaterials for enhancing anti-cancer immunity. Curr. Opin. Biotechnol. 2016, 40, 1–8. [Google Scholar] [CrossRef]
- Nakamura, T.; Ono, K.; Suzuki, Y.; Moriguchi, R.; Kogure, K.; Harashima, H. Octaarginine-Modified Liposomes Enhance Cross-Presentation by Promoting the C-Terminal Trimming of Antigen Peptide. Mol. Pharm. 2014, 11, 2787–2795. [Google Scholar] [CrossRef]
- Muraoka, D.; Harada, N.; Hayashi, T.; Tahara, Y.; Momose, F.; Sawada, S.-I.; Mukai, S.-A.; Akiyoshi, K.; Shiku, H. Nanogel-Based Immunologically Stealth Vaccine Targets Macrophages in the Medulla of Lymph Node and Induces Potent Antitumor Immunity. ACS Nano 2014, 8, 9209–9218. [Google Scholar] [CrossRef]
- Fujita, Y.; Taguchi, H. Current status of multiple antigen-presenting peptide vaccine systems: Application of organic and inorganic nanoparticles. Chem. Cent. J. 2011, 5, 48. [Google Scholar] [CrossRef]
- García-García, P.; Briffault, E.; Landin, M.; Evora, C.; Diaz-Rodriguez, P.; Delgado, A. Tailor-made oligonucleotide-loaded lipid-polymer nanosystems designed for bone gene therapy. Drug Deliv. Transl. Res. 2021, 11, 598–607. [Google Scholar] [CrossRef]
- Bellini, C.; Horváti, K. Recent Advances in the Development of Protein- and Peptide-Based Subunit Vaccines against Tuberculosis. Cells 2020, 9, 2673. [Google Scholar] [CrossRef]
- Kruit, W.H.; Suciu, S.; Dreno, B.; Mortier, L.; Robert, C.; Chiarion-Sileni, V.; Maio, M.; Testori, A.; Dorval, T.; Grob, J.-J.; et al. Selection of Immunostimulant AS15 for Active Immunization with MAGE-A3 Protein: Results of a Randomized Phase II Study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 2013, 31, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Homsi, J.; Torres-Cabala, C.A.; Bassett, R.; Popuri, R.M.; James, M.L.; Vence, L.M.; Hwu, W.-J. A phase II trial of recombinant MAGE-A3 protein with immunostimulant AS15 in combination with high-dose Interleukin-2 (HDIL2) induction therapy in metastatic melanoma. BMC Cancer 2018, 18, 1274. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Davis, I.D.; McArthur, G.; Chen, L.; Haydon, A.; Parente, P.; Dimopoulos, N.; Jackson, H.; Xiao, K.; Maraskovsky, E.; et al. Low-dose cyclophosphamide enhances antigen-specific CD4+ T cell responses to NY-ESO-1/ISCOMATRIX™ vaccine in patients with advanced melanoma. Cancer Immunol. Immunother. 2015, 64, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kündig, T.M.; et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.; Schwarz, K.; Baumgaertner, P.; Manolova, V.; Devevre, E.; Sterry, W.; Walden, P.; Zippelius, A.; Conzett, K.B.; Senti, G.; et al. Memory and Effector CD8 T-cell Responses After Nanoparticle Vaccination of Melanoma Patients. J. Immunother. 2010, 33, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Z.; Zhang, M.; Ling, G.; Zhang, P. Research progress of microneedles in the treatment of melanoma. J. Control. Release 2022, 348, 631–647. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Miura, N.; Akita, H.; Tateshita, N.; Nakamura, T.; Harashima, H. Modifying Antigen-Encapsulating Liposomes with KALA Facilitates MHC Class I Antigen Presentation and Enhances Anti-tumor Effects. Mol. Ther. 2017, 25, 1003–1013. [Google Scholar] [CrossRef]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Jois, S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control. Release 2020, 329, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Duan, S.; Ye, F.; Hou, X.; Li, X.; Zhao, J.; Yu, X.; Hu, Z.; Tang, Z.; Mo, F.; et al. The enhanced antitumor-specific immune response with mannose- and CpG-ODN-coated liposomes delivering TRP2 peptide. Theranostics 2018, 8, 1723–1739. [Google Scholar] [CrossRef]
- Warner, N.; Núñez, G. MyD88: A Critical Adaptor Protein in Innate Immunity Signal Transduction. J. Immunol. 2013, 190, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Hatamipour, M.; Alani, B.; Nikzad, H.; Roshan, N.M.; Verdi, J.; Jaafari, M.R.; Noureddini, M.; Badiee, A. Liposomal gp100 vaccine combined with CpG ODN sensitizes established B16F10 melanoma tumors to anti PD-1 therapy. Ir. J. Basic Med. Sci. 2020, 23, 1065–1077. [Google Scholar] [CrossRef]
- Su, Q.; Wang, C.; Song, H.; Zhang, C.; Liu, J.; Huang, P.; Zhang, Y.; Zhang, J.; Wang, W. Co-delivery of anionic epitope/CpG vaccine and IDO inhibitor by self-assembled cationic liposomes for combination melanoma immunotherapy. J. Mater. Chem. B 2021, 9, 3892–3899. [Google Scholar] [CrossRef]
- Seo, H.S.; Wang, C.-P.J.; Park, W.; Park, C.G. Short Review on Advances in Hydrogel-Based Drug Delivery Strategies for Cancer Immunotherapy. Tissue Eng. Regen. Med. 2021, 19, 263–280. [Google Scholar] [CrossRef]
- Hori, Y.; Winans, A.M.; Huang, C.C.; Horrigan, E.M.; Irvine, D.J. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials 2008, 29, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Yahia, L. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 13. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J. In situ forming polymeric drug delivery systems. Indian J. Pharm. Sci. 2009, 71, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Wang, B.; Shivji, G.M.; Toto, P.; Amerio, P.; Sauder, D.N.; Tomai, M.A.; Miller, R.L. Imiquimod, a Topical Immune Response Modifier, Induces Migration of Langerhans Cells. J. Investig. Dermatol. 2000, 114, 135–141. [Google Scholar] [CrossRef]
- Schön, M.P. TLR7 and TLR8 as targets in cancer therapy. Oncogene 2008, 27, 190–199. [Google Scholar] [CrossRef]
- Wakabayashi, R.; Sakuragi, M.; Kozaka, S.; Tahara, Y.; Kamiya, N.; Goto, M. Solid-in-Oil Peptide Nanocarriers for Transcutaneous Cancer Vaccine Delivery against Melanoma. Mol. Pharm. 2018, 15, 955–961. [Google Scholar] [CrossRef]
- Dai, X.; Meng, J.; Deng, S.; Zhang, L.; Wan, C.; Lu, L.; Huang, J.; Hu, Y.; Zhang, Z.; Li, Y.; et al. Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics 2020, 10, 3049–3063. [Google Scholar] [CrossRef]
- Tam, J.P. Synthetic peptide vaccine design: Synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA 1988, 85, 5409–5413. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials 2021, 280, 121303. [Google Scholar] [CrossRef]
- Rawding, P.A.; Bu, J.; Wang, J.; Kim, D.W.; Drelich, A.J.; Kim, Y.; Hong, S. Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response. WIREs Nanomed. Nanobiotechnol. 2021, 14, e1752. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Xu, L.; Chao, Y.; Wang, C.; Han, X.; Dong, Z.; Chang, H.; Peng, R.; Cheng, Y.; et al. Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials 2019, 207, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Wang, X.-Y.; Yang, H. “Double-punch” strategy for delivery of viral immunotherapy with prolonged tumor retention and enhanced transfection efficacy. J. Control. Release 2020, 329, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticle drug delivery systems: An excellent carrier for tumor peptide vaccines. Drug Deliv. 2018, 25, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jia, H.; Zheng, M.; Wang, H.; Yang, W.; Gao, L.; Zhang, Z.; Xue, J.; Xu, B.; Yang, W.; et al. Trp2 Peptide-Assembled Nanoparticles with Intrinsically Self-Chelating 64Cu Properties for PET Imaging Tracking and Dendritic Cell-Based Immunotherapy against Melanoma. ACS Appl. Bio Mater. 2021, 4, 5707–5716. [Google Scholar] [CrossRef]
- Huo, M.; Zhao, Y.; Satterlee, A.B.; Wang, Y.; Xu, Y.; Huang, L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J. Control. Release 2017, 245, 81–94. [Google Scholar] [CrossRef]
- Chang, R.; Zou, Q.; Xing, R.; Yan, X. Peptide-Based Supramolecular Nanodrugs as a New Generation of Therapeutic Toolboxes against Cancer. Adv. Ther. 2019, 2, 1900048. [Google Scholar] [CrossRef]
- Liang, R.; Xie, J.; Li, J.; Wang, K.; Liu, L.; Gao, Y.; Hussain, M.; Shen, G.; Zhu, J.; Tao, J. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Xu, K.; Wen, Y.; Zhang, X.; Liu, Y.; Qiu, D.; Li, B.; Zheng, L.; Wu, Y.; Xing, M.; Li, J. Injectable host-guest gel nanovaccine for cancer immunotherapy against melanoma. Mater. Today Adv. 2022, 15, 100236. [Google Scholar] [CrossRef]
- Zeng, L.; Liao, Z.; Li, W.; Yuan, Q.; Wu, P.; Gu, Z.; Liu, Z.; Liao, G. Non-covalent glycosylated gold nanoparticles/peptides nanovaccine as potential cancer vaccines. Chin. Chem. Lett. 2019, 31, 1162–1164. [Google Scholar] [CrossRef]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Q.; Li, L.; Zeng, Q.; Li, H.; Gong, T.; Zhang, Z.; Sun, X. Turning the Old Adjuvant from Gel to Nanoparticles to Amplify CD8+ T Cell Responses. Adv. Sci. 2017, 5, 1700426. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jiang, H.; Song, Y.; Zhu, Y.; Qin, M.; He, C.; Du, G.; Sun, X. Aluminum nanoparticles deliver a dual-epitope peptide for enhanced anti-tumor immunotherapy. J. Control. Release 2022, 344, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Du, T.; Li, Y.; Qi, Y.; Min, H.; Wang, Y.; Zhang, Q.; Wang, C.; Zhou, Y.; Li, L.; et al. Dual-Antigen-Loaded Hepatitis B Virus Core Antigen Virus-like Particles Stimulate Efficient Immunotherapy Against Melanoma. ACS Appl. Mater. Interfaces 2020, 12, 53682–53690. [Google Scholar] [CrossRef]
- Kramer, K.; Al-Barwani, F.; Baird, M.A.; Young, V.L.; Larsen, D.S.; Ward, V.K.; Young, S.L. Functionalisation of Virus-Like Particles Enhances Antitumour Immune Responses. J. Immunol. Res. 2019, 2019, 5364632. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Heath, M.; Cabral-Miranda, G.; Lipp, C.; Zeltins, A.; Sande, M.; Stein, J.V.; Riether, C.; Roesti, E.S.; Zha, L.; et al. Correction to: Vaccination with nanoparticles combined with micro-adjuvants protects against cancer. J. Immunother. Cancer 2019, 7, 137. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Vogel, M.; Riether, C.; Muller, J.; Salatino, S.; Ternette, N.; Gomes, A.C.; Cabral-Miranda, G.; El-Turabi, A.; Ruedl, C.; et al. Targeting Mutated Plus Germline Epitopes Confers Pre-clinical Efficacy of an Instantly Formulated Cancer Nano-Vaccine. Front. Immunol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Clinical Trials. Studies Found for: Melanoma. Available online: https://clinicaltrials.gov/ct2/results?cond=melanoma&term=&cntry=&state=&city=&dist=&Search=Search (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Immunotherapy. Available online: https://clinicaltrials.gov/ct2/results?cond=melanoma&term=immunotherapy&cntry=&state=&city=&dist=&Search=Search (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine. Available online: https://clinicaltrials.gov/ct2/results?cond=melanoma&term=vaccine&cntry=&state=&city=&dist=&Search=Search (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Immunotherapy + Peptide. Available online: https://clinicaltrials.gov/ct2/results?cond=melanoma&term=immunotherapy+peptide&cntry=&state=&city=&dist=&Search=Search (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide. Available online: https://clinicaltrials.gov/ct2/results?cond=melanoma&term=vaccine+peptide&cntry=&state=&city=&dist=&Search=Search (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide, Completed Studies | Melanoma. Available online: https://clinicaltrials.gov/ct2/results?term=vaccine+peptide&cond=melanoma&Search=Apply&recrs=e&age_v=&gndr=&type=&rslt= (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide, Completed Studies, Studies with Results. Available online: https://clinicaltrials.gov/ct2/results?term=vaccine+peptide&cond=melanoma&recrs=e&age_v=&gndr=&type=&rslt=With&Search=Apply (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide + Stage IV Melanoma. Available online: https://clinicaltrials.gov/ct2/results?term=vaccine+peptide+AND+stage+IV+melanoma&cond=melanoma (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide + Metastatic Melanoma. Available online: https://clinicaltrials.gov/ct2/results?term=vaccine+peptide+AND+Metastatic+Melanoma&cond=melanoma (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide + Phase 1. Available online: https://clinicaltrials.gov/ct2/results?term=vaccine+peptide&cond=melanoma&flds=aby&age_v=&gndr=&type=&rslt=&phase=0&Search=Apply (accessed on 11 December 2022).
- Clinical Trials. Studies Found for: Melanoma + Vaccine + Peptide + Phase 2. Available online: https://clinicaltrials.gov/ct2/results?term=vaccine+peptide&cond=melanoma&flds=aby&age_v=&gndr=&type=&rslt=&phase=1&Search=Apply (accessed on 11 December 2022).
- Clinical Trial NCT00254397. Melanoma Vaccine with Peptides and Leuprolide. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00254397 (accessed on 11 December 2022).
- Clinical Trial NCT00118274. Vaccine Therapy with or without Cyclophosphamide in Treating Patients Who Have Undergone Surgery for Stage II, Stage III, or Stage IV Melanoma. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00118274 (accessed on 11 December 2022).
- Clinical Trial NCT01876212. Dendritic Cell Vaccines + Dasatinib for Metastatic Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01876212 (accessed on 11 December 2022).
- Clinical Trial NCT00003895. Vaccine Therapy in Treating HLA-A2 Positive Patients with Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00003895 (accessed on 11 December 2022).
- Clinical Trial NCT00960752. Tumor and Vaccine Site with a Toll Like Receptor (TLR) Agonist. Available online: https://clinicaltrials.gov/ct2/show/NCT00960752 (accessed on 11 December 2022).
- Clinical Trial NCT01961115. Epacadostat and Vaccine Therapy in Treating Patients with Stage III–IV Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01961115 (accessed on 11 December 2022).
- Clinical Trial NCT00084656. Monoclonal Antibody Therapy and Vaccine Therapy in Treating Patients with Resected Stage III or Stage IV Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00084656 (accessed on 11 December 2022).
- Clinical Trial NCT01989572. Sargramostim, Vaccine Therapy, or Sargramostim and Vaccine Therapy in Preventing Disease Recurrence in Patients with Melanoma That Has Been Removed By Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT01989572 (accessed on 11 December 2022).
- Clinical Trial NCT00019682. Aldesleukin with or Without Vaccine Therapy in Treating Patients with Locally Advanced or Metastatic Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00019682 (accessed on 11 December 2022).
- Clinical Trial NCT00112242. Immunotherapy of Stage III/IV Melanoma Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT00112242 (accessed on 11 December 2022).
- Clinical Trial NCT00518206. Study of NY-ESO-1 ISCOMATRIX® in Patients with Measurable Stage III or IV Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00518206 (accessed on 11 December 2022).
- Clinical Trial NCT00142454. NY-ESO-1 Protein Vaccine with Imiquimod in Melanoma (Adjuvant Setting). Available online: https://clinicaltrials.gov/ct2/show/NCT00142454 (accessed on 11 December 2022).
- Nelde, A.; Rammensee, H.-G.; Walz, J.S. The Peptide Vaccine of the Future. Mol. Cell. Proteom. 2021, 20, 100022. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- Brossart, P. The Role of Antigen Spreading in the Efficacy of Immunotherapies. Clin. Cancer Res. 2020, 26, 4442–4447. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Drawbacks |
|---|---|
| Simple, reproducible, cost-effective production | Poorly immunogenic |
| Chemically and physically fully and thoroughly characterizable | Require the use of adjuvants and delivery systems |
| Non-natural amino acids and peptidomimetics can be introduced | Susceptibility for enzymatic degradation |
| Lipids, carbohydrate and phosphate groups can be incorporated | Unrelated immune responses during degradation |
| Does not require expression: pure and easily detectable product | Often not uniformly recognized by all patients |
| Large-scale production can be economically carried out | |
| Usage of nanoparticles: water solubility and stability can be reached | |
| Safe storage after lyophilization | |
| Biodegradability | |
| Possibility of multiepitope peptides |
| NCT Number | Stage of Melanoma | Vaccine Composition | Groups | Completed Clinical Trial | Ref |
|---|---|---|---|---|---|
| NCT00254397 | not labelled | drug: Leuprolide (D) antigen(s): gp100(209–217) (Gp209), MAGE-3 peptide (M3) adjuvant(s): - | Group 1: D+Gp209 Group 2: Gp209 Group 3: Gp209+M3+D Group 4: Gp209+M3 | Phase II | [204] |
| NCT00118274 | II III IV | drug: Cyclophosphamide (D) antigen(s): melanoma helper peptide (MHP), multiepitope melanoma peptide (MeMP), vaccine tetanus toxoid helper peptide (TTHP) adjuvant(s): incomplete Freund’s adjuvant (IFA), Montanide ISA-51 (ISA) | Group 1: MeMP+TTHP+IFA Group 2: D+MeMP+TTHP+IFA Group 3: MHP+MeMP+ISA Group 4: D+MHP+MeMP+ISA | Phase I Phase II | [205] |
| NCT01876212 | not labelled | drug: Dasatinib (D) antigen(s): αDC1 1 incorporation with TBVA 2-derived peptides (DC+TBVA) adjuvant(s): - | Group 1: D+(DC+TBVA) (cycle 1+cycle2) Group 2: D+(DC+TBVA) (cycle 1+cycle1) | Phase II | [206] |
| NCT00003895 | IA, IB, IIA, IIB, IIC, IIIA, IIIB, IIIC | drug: - antigen(s): HPV16E7(12–20) 3 (HPV12), gp100(209–217) (Gp209) adjuvant(s): Montanide ISA-51 (ISA) | Group 1: p209+HPV12+ISA in every 2 weeks for 6 months Group 2: Gp209+HPV12+ISA in every 3 weeks for 6 months | Phase II | [207] |
| NCT00960752 | not labelled | drug: - antigen(s): gp100(209–217) (Gp209), MAGE-3 (M3) adjuvant(s): Resiquimod (R848) | Group 1: Gp209+M3+R848 Group 2: Gp209+M3 Group 3 (Metastatic melanoma patients): Gp209+M3+R848 | Phase II | [208] |
| NCT01961115 | IIIA, IIIB, IIIC IV | drug: Epacadostat (D) antigen(s): multiepitope peptide antigens (MELITAC 12.1) adjuvant(s): - | Group 1: D+MELITAC 12.1 | Phase II | [209] |
| NCT00084656 | III IV | drug: ipilimumab (D) antigen(s): multiepitope melanoma peptide(MeMP) 4 adjuvant(s): Montanide ISA-51 (ISA) | Group 1: D+MeMP+ISA | Phase II | [210] |
| NCT01989572 | IIA, IIB, IIC, IIIA, IIIB, IIIC, IV | drug: - antigen(s): multiepitope melanoma peptide(MeMP) 4 adjuvant(s): GM-CSF, Montanide ISA-51 (ISA) | Group 1: MeMP+GM-CSF+ISA Group 2: MeMP+placebo Group 3: GM-CSF+placebo Group 4: placebo+placebo | Phase III | [211] |
| NCT00019682 | IIIA, IIIB, IIIC, IV | drug: - antigen(s): gp100(209–217) (Gp209) Cytokine: IL2 adjuvant(s): Montanide ISA-51 (ISA) | Group 1: IL2 Group 2: Gp209+ISA+IL2 | Phase III | [212] |
| NCT00112242 | not labelled | drug: - antigen(s): Melan-A analogs (ELA or EAA), NY-ESO-1 analogs (b and LP), MAGE-A10, gp100(209–217) (Gp209) Cytokine: IL2 adjuvant(s): Montanide ISA-51 (ISA), CpG | Group 1: ELA + ISA Group 2: ELA+ b+MAGE-A10+ISA Group 3: EAA+ELA+ Mage-A10+b+ISA+CpG Group 4: ELA+Mage-A10+LP+ISA+CpGGroup 5: EAA+ELA+ Mage-A10+LP+ISA+CpG+IL2 | [213] | |
| NCT00518206 | not labelled | drug: Cyclophosphamide (D) antigen(s): NY-ESO-1 analogs Cytokine: IL2 adjuvant(s): ISOMATRIX® | Group 1: NY-ESO-1+ISOMATRIX® Group 2: D+ NY-ESO-1+ISOMATRIX® | Phase II | [214] |
| NCT00142454 | not labelled | drug: Imiquimod (D) antigen(s): NY-ESO-1 analogs adjuvant(s): - | Group 1: D+NY-ESO-1 | Phase I | [215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biri-Kovács, B.; Bánóczi, Z.; Tummalapally, A.; Szabó, I. Peptide Vaccines in Melanoma: Chemical Approaches towards Improved Immunotherapeutic Efficacy. Pharmaceutics 2023, 15, 452. https://doi.org/10.3390/pharmaceutics15020452
Biri-Kovács B, Bánóczi Z, Tummalapally A, Szabó I. Peptide Vaccines in Melanoma: Chemical Approaches towards Improved Immunotherapeutic Efficacy. Pharmaceutics. 2023; 15(2):452. https://doi.org/10.3390/pharmaceutics15020452
Chicago/Turabian StyleBiri-Kovács, Beáta, Zoltán Bánóczi, Anitha Tummalapally, and Ildikó Szabó. 2023. "Peptide Vaccines in Melanoma: Chemical Approaches towards Improved Immunotherapeutic Efficacy" Pharmaceutics 15, no. 2: 452. https://doi.org/10.3390/pharmaceutics15020452
APA StyleBiri-Kovács, B., Bánóczi, Z., Tummalapally, A., & Szabó, I. (2023). Peptide Vaccines in Melanoma: Chemical Approaches towards Improved Immunotherapeutic Efficacy. Pharmaceutics, 15(2), 452. https://doi.org/10.3390/pharmaceutics15020452






