Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Honokiol-Loaded Nanoemulsion
2.3. Optimization of the Honokiol-Loaded Nanoemulsion Preparation Process
2.4. Characterization of the Honokiol-Loaded Nanoemulsion
2.4.1. Lipid Droplet Size Determination
2.4.2. Lipid Droplet Zeta Potential Evaluation
2.4.3. pH Measurement
2.4.4. OSM Measurement
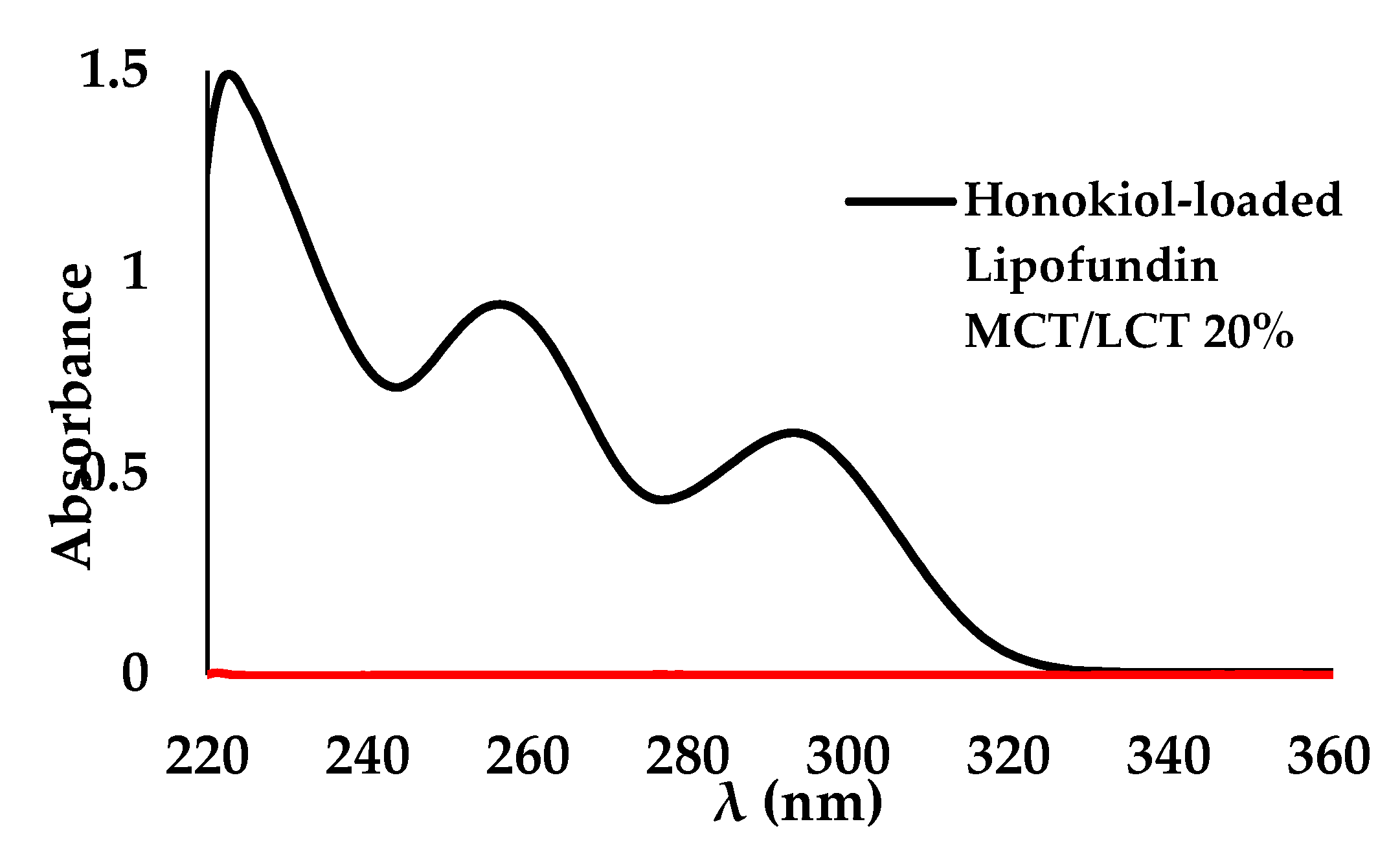
2.4.5. Determination of Honokiol Concentration in Nanoemulsion Using Spectrophotometry UV-VIS
2.4.6. Determination of Honokiol Concentration in Nanoemulsion Using HPLC-FLD Method
2.4.7. Loading Efficiency of Honokiol in Nanoemulsion
2.4.8. Short- and Long-Term Stability Studies
High-Temperature Effect
Oxidative Degradation
Photostability
Long-Term Stability
2.5. In Vitro Cytotoxicity Studies
3. Results
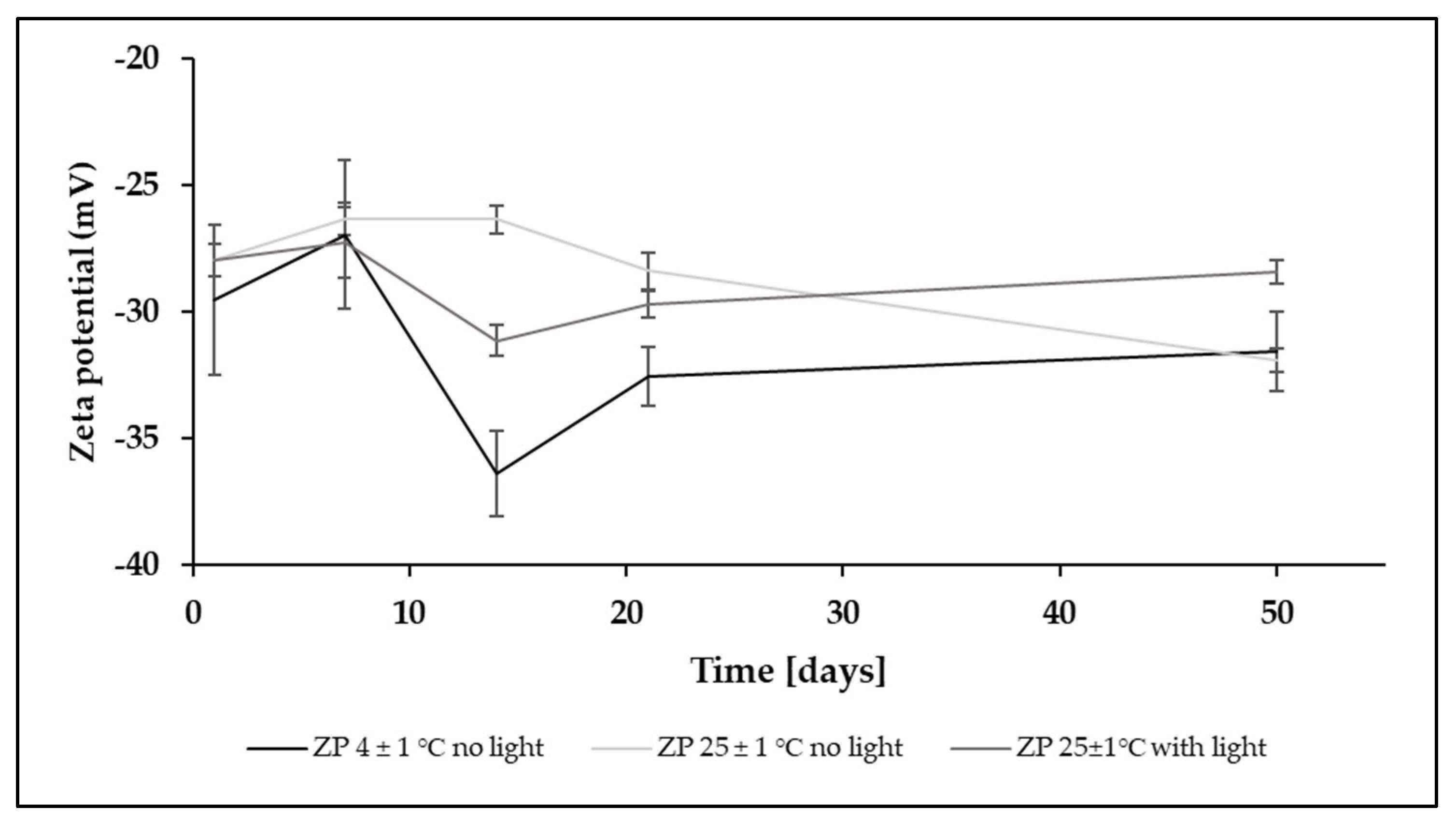
3.1. Short-Term and Long-Term Stability Studies
3.2. In Vitro Cytotoxicity Studies
4. Discussion
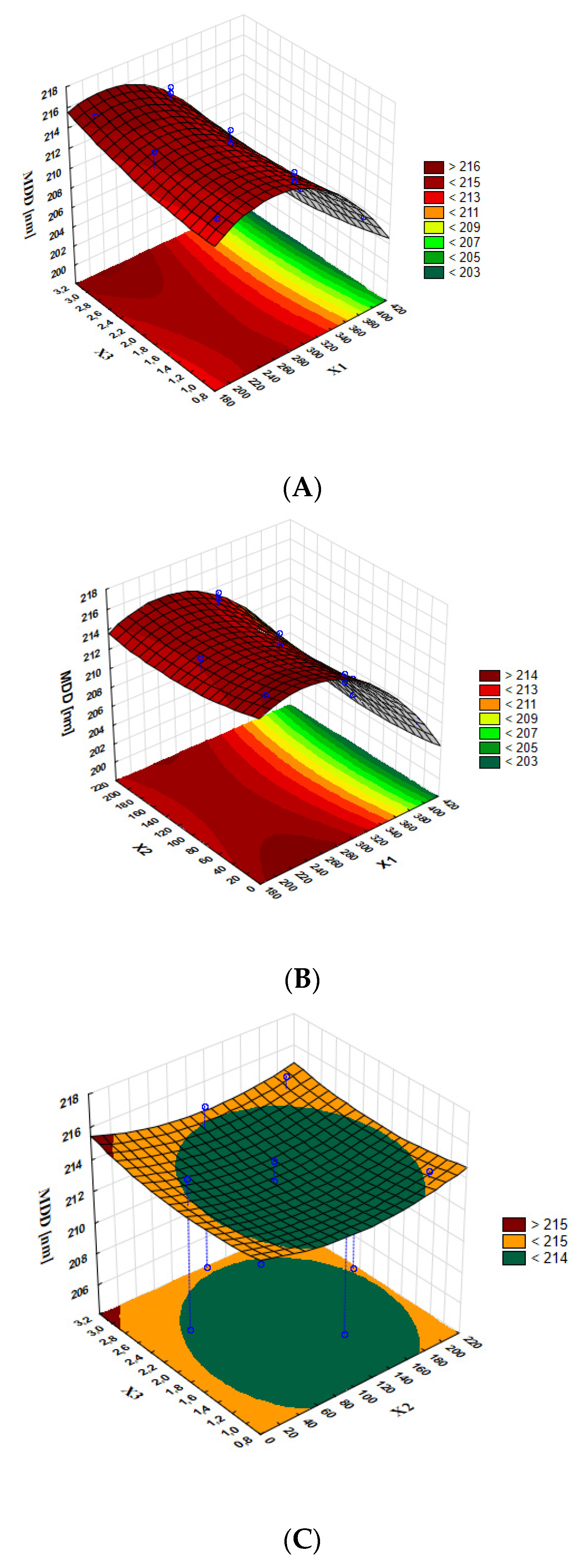
4.1. MDD, PDI, and ZP of Optimal Formulation
4.2. pH and OSM of Optimal Formulations
4.3. Stability Studies
4.4. MTT Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20 (Suppl. S4), iv1–iv86. [Google Scholar] [CrossRef]
- Canoll, P.; Goldman, J.E. The Interface between Glial Progenitors and Gliomas. Acta Neuropathol. 2008, 116, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA. Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xue, W.; Schachner, M.; Zhao, W. Honokiol Eliminates Glioma/Glioblastoma Stem Cell-Like Cells Via JAK-STAT3 Signaling and Inhibits Tumor Progression by Targeting Epidermal Growth Factor Receptor. Cancers 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-J.; Yang, S.-T.; Chen, R.-M. Major Contribution of Caspase-9 to Honokiol-Induced Apoptotic Insults to Human Drug-Resistant Glioblastoma Cells. Mol. Basel Switz. 2020, 25, 1450. [Google Scholar] [CrossRef]
- Chang, K.-H.; Yan, M.-D.; Yao, C.-J.; Lin, P.-C.; Lai, G.-M. Honokiol-Induced Apoptosis and Autophagy in Glioblastoma Multiforme Cells. Oncol. Lett. 2013, 6, 1435–1438. [Google Scholar] [CrossRef]
- Colin, M.; Delporte, C.; Janky, R.; Lechon, A.-S.; Renard, G.; Van Antwerpen, P.; Maltese, W.A.; Mathieu, V. Dysregulation of Macropinocytosis Processes in Glioblastomas May Be Exploited to Increase Intracellular Anti-Cancer Drug Levels: The Example of Temozolomide. Cancers 2019, 11, 411. [Google Scholar] [CrossRef]
- Jeong, J.J.; Lee, J.H.; Chang, K.C.; Kim, H.J. Honokiol Exerts an Anticancer Effect in T98G Human Glioblastoma Cells through the Induction of Apoptosis and the Regulation of Adhesion Molecules. Int. J. Oncol. 2012, 41, 1358–1364. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Chen, J.; Fan, Y.; Wang, C.; Du, Y.; Guo, C.; Chen, F.; Li, W. Liposomal Honokiol Inhibits Glioblastoma Growth through Regulating Macrophage Polarization. Ann. Transl. Med. 2021, 9, 1644. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, J.; Jiang, J.; He, Y.; Zhang, W.; Mo, X.; Kang, X.; Xu, Q.; Wang, B.; Huang, Y. Remodeling Tumor Immune Microenvironment (TIME) for Glioma Therapy Using Multi-Targeting Liposomal Codelivery. J. Immunother. Cancer 2020, 8, e000207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, X.; Shi, M.; Jiang, Z.; Wang, H.; Su, Q.; Liu, Q.; Li, G.; Jiang, G. Downregulation of STAT3 and Activation of MAPK Are Involved in the Induction of Apoptosis by HNK in Glioblastoma Cell Line U87. Oncol. Rep. 2014, 32, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Chang, Y.-A.; Lin, Y.-L.; Liu, S.H.; Chang, C.-K.; Chen, R.-M. Preclinical Effects of Honokiol on Treating Glioblastoma Multiforme via G1 Phase Arrest and Cell Apoptosis. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 517–527. [Google Scholar] [CrossRef]
- Wang, X.; Duan, X.; Yang, G.; Zhang, X.; Deng, L.; Zheng, H.; Deng, C.; Wen, J.; Wang, N.; Peng, C.; et al. Honokiol Crosses BBB and BCSFB, and Inhibits Brain Tumor Growth in Rat 9L Intracerebral Gliosarcoma Model and Human U251 Xenograft Glioma Model. PLoS ONE 2011, 6, e18490. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.; Chen, T.; Yang, Q.; Huang, T.; Fu, Y.; Gong, T.; Zhang, Z. Hyaluronic Acid-Modified Micelles Encapsulating Gem-C12 and HNK for Glioblastoma Multiforme Chemotherapy. Mol. Pharm. 2018, 15, 1203–1214. [Google Scholar] [CrossRef]
- Said Suliman, A.; Tom, R.; Palmer, K.; Tolaymat, I.; Younes, H.M.; Arafat, B.; Elhissi, A.M.A.; Najlah, M. Development, Characterization and Stability Evaluation of Ciprofloxacin-Loaded Parenteral Nutrition Nanoemulsions. Pharm. Dev. Technol. 2020, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Najlah, M.; Wan, K.-W.; Ahmed, W.; Crean, S.J.; Phoenix, D.A.; Taylor, K.M.G.; Elhissi, A.M.A. Stability of Parenteral Nanoemulsions Loaded with Paclitaxel: The Influence of Lipid Phase Composition, Drug Concentration and Storage Temperature. Pharm. Dev. Technol. 2014, 19, 999–1004. [Google Scholar] [CrossRef]
- Najlah, M.; Kadam, A.; Wan, K.-W.; Ahmed, W.; Taylor, K.M.G.; Elhissi, A.M.A. Novel Paclitaxel Formulations Solubilized by Parenteral Nutrition Nanoemulsions for Application against Glioma Cell Lines. Int. J. Pharm. 2016, 506, 102–109. [Google Scholar] [CrossRef]
- Nasr, M.; Nawaz, S.; Elhissi, A. Amphotericin B Lipid Nanoemulsion Aerosols for Targeting Peripheral Respiratory Airways via Nebulization. Int. J. Pharm. 2012, 436, 611–616. [Google Scholar] [CrossRef]
- Haidar, I.; Harding, I.H.; Bowater, I.C.; Eldridge, D.S.; Charman, W.N. The Role of Lecithin Degradation on the PH Dependent Stability of Halofantrine Encapsulated Fat Nano-Emulsions. Int. J. Pharm. 2017, 528, 524–535. [Google Scholar] [CrossRef]
- Müller, R.H.; Schmidt, S.; Buttle, I.; Akkar, A.; Schmitt, J.; Brömer, S. SolEmuls®—Novel Technology for the Formulation of i.v. Emulsions with Poorly Soluble Drugs. Int. J. Pharm. 2004, 269, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Akkar, A.; Müller, R.H. Formulation of Intravenous Carbamazepine Emulsions by SolEmuls Technology. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2003, 55, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Francke, N.M.; Bunjes, H. Influence of Drug Loading on the Physical Stability of Phospholipid-Stabilised Colloidal Lipid Emulsions. Int. J. Pharm. X 2020, 2, 100060. [Google Scholar] [CrossRef]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures. Pharmaceutics 2019, 12, 27. [Google Scholar] [CrossRef]
- Washington, C. Stability of Lipid Emulsions for Drug Delivery. Adv. Drug Deliv. Rev. 1996, 20, 131–145. [Google Scholar] [CrossRef]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. Clinical Nutrition of Critically Ill Patients in the Context of the Latest ESPEN Guidelines. Medicina 2019, 55, 770. [Google Scholar] [CrossRef]
- Tomczak, S.; Stawny, M.; Dettlaff, K.; Kieliszek, M.; Słomińska, D.; Jelińska, A. Physicochemical Compatibility and Stability of Linezolid with Parenteral Nutrition. Molecules 2019, 24, 1242. [Google Scholar] [CrossRef]
- Stawny, M.; Nadolna, M.; Jelińska, A. In Vitro Compatibility Studies of Vancomycin with Ready-to-Use Parenteral Nutrition Admixtures for Safer Clinical Practice. Clin. Nutr. 2020, 39, 2539–2546. [Google Scholar] [CrossRef]
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Physical Stability of an All-in-One Parenteral Nutrition Admixture for Preterm Infants upon Mixing with Micronutrients and Drugs. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, 36–42. [Google Scholar] [CrossRef]
- Stawny, M.; Olijarczyk, R.; Jaroszkiewicz, E.; Jelińska, A. Pharmaceutical Point of View on Parenteral Nutrition. Sci. World J. 2013, 2013, 415310. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Główka, E.; Ogrodowczyk, M. Effect of Lipid Emulsion on Stability of Ampicillin in Total Parenteral Nutrition. Nutrients 2019, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Nadolna, M.; Jelińska, A. Safe Practice of Y-Site Drug Administration: The Case of Colistin and Parenteral Nutrition. Pharmaceutics 2020, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. The United States Pharmacopeia and National Formulary, 38th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2015. [Google Scholar]
- Rosenblatt, K.M.; Bunjes, H. Evaluation of the Drug Loading Capacity of Different Lipid Nanoparticle Dispersions by Passive Drug Loading. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2017, 117, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.-E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics 2021, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F. Lipid Injectable Emulsions: Pharmacopeial and Safety Issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef]
- Peng, J.; Dong, W.-J.; Li, L.; Xu, J.-M.; Jin, D.-J.; Xia, X.-J.; Liu, Y.-L. Effect of High-Pressure Homogenization Preparation on Mean Globule Size and Large-Diameter Tail of Oil-in-Water Injectable Emulsions. J. Food Drug Anal. 2015, 23, 828–835. [Google Scholar] [CrossRef]
- Wang, J.-J.; Sung, K.C.; Hu, O.Y.-P.; Yeh, C.-H.; Fang, J.-Y. Submicron Lipid Emulsion as a Drug Delivery System for Nalbuphine and Its Prodrugs. J. Control. Release Off. J. Control. Release Soc. 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the Blood–Brain Tumor Barrier for Effective Glioblastoma Treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Ulmschneider, M.B.; Searson, P.C. Quantitative Analysis of the Enhanced Permeation and Retention (EPR) Effect. PLoS ONE 2015, 10, e0123461. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Roethlisberger, D.; Mahler, H.-C.; Altenburger, U.; Pappenberger, A. If Euhydric and Isotonic Do Not Work, What Are Acceptable PH and Osmolality for Parenteral Drug Dosage Forms? J. Pharm. Sci. 2017, 106, 446–456. [Google Scholar] [CrossRef]
- Stranz, M.; Kastango, E.S. A Review of PH and Osmolarity. Int. J. Pharm. Compd. 2002, 6, 216–220. [Google Scholar]
- Lee, Y.-C.; Zocharski, P.D.; Samas, B. An Intravenous Formulation Decision Tree for Discovery Compound Formulation Development. Int. J. Pharm. 2003, 253, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Porter, W.; Merdan, T.; Li, L.C. Recent Advances in Intravenous Delivery of Poorly Water-Soluble Compounds. Expert Opin. Drug Deliv. 2009, 6, 1261–1282. [Google Scholar] [CrossRef]
- Sweetana, S.; Akers, M.J. Solubility Principles and Practices for Parenteral Drug Dosage Form Development. PDA J. Pharm. Sci. Technol. 1996, 50, 330–342. [Google Scholar]
- Simamora, P.; Pinsuwan, S.; Surakitbanharn, Y.; Yalkowsky, S.H. Studies in Phlebitis VIII: Evaluations of PH Solubilized Intravenous Dexverapamil Formulations. PDA J. Pharm. Sci. Technol. 1996, 50, 123–128. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Jelińska, A.; Ogrodowczyk, M. Stability of High-Dose Thiamine in Parenteral Nutrition for Treatment of Patients with Wernicke’s Encephalopathy. Clin. Nutr. Edinb. Scotl. 2020, 39, 2929–2932. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Dettlaff, K.; Jelińska, A.; Ogrodowczyk, M. Stability Studies of Parenteral Nutrition with a High Dose of Vitamin C. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2020, 26, 1894–1902. [Google Scholar] [CrossRef]
- Gostyńska, A.; Starkowska, J.; Sobierajska, P.; Jelińska, A.; Stawny, M. All-in-One Pediatric Parenteral Nutrition Admixtures with an Extended Shelf Life-Insight in Correlations between Composition and Physicochemical Parameters. Pharmaceutics 2021, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Junwal, M.; Modhe, G.; Tiwari, H.; Kurmi, M.; Parashar, N.; Sidduri, P. Forced Degradation Studies to Assess the Stability of Drugs and Products. Trends Anal. Chem. 2013, 49, 71–88. [Google Scholar] [CrossRef]
- Bjerregaard, S.; Vermehren, C.; Söderberg, I.; Frokjaer, S. Accelerated Stability Testing of a Water-in-Oil Emulsion. J. Dispers. Sci. Technol. 2001, 22, 23–31. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Gehrcke, M.; Barbieri, A.V.; Zborowski, V.A.; Beck, R.C.R.; Nogueira, C.W.; Cruz, L. Pomegranate Seed Oil Nanoemulsions Improve the Photostability and in Vivo Antinociceptive Effect of a Non-Steroidal Anti-Inflammatory Drug. Colloids Surf. B Biointerfaces 2016, 144, 214–221. [Google Scholar] [CrossRef]
- Tiwari, N.; Ebenazer, A.; Franklyne, J.S.; Sivakumar, A.; Mukherjee, A.; Chandrasekaran, N. Drug Loaded Essential Oil Microemulsions Enhance Photostability and Evaluation of in Vitro Efficacy. Photodiagnosis Photodyn. Ther. 2020, 29, 101638. [Google Scholar] [CrossRef]
- Tazesh, S.; Tamizi, E.; Siahi Shadbad, M.; Mostaghimi, N.; Monajjemzadeh, F. Comparative Stability of Two Anti-Hyperpigmentation Agents: Kojic Acid as a Natural Metabolite and Its Di-Palmitate Ester, Under Oxidative Stress; Application to Pharmaceutical Formulation Design. Adv. Pharm. Bull. 2022, 12, 329–335. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.-Q. Comparison of Antioxidant Abilities of Magnolol and Honokiol to Scavenge Radicals and to Protect DNA. Biochimie 2011, 93, 1755–1760. [Google Scholar] [CrossRef]
- Khanum, R.; Thevanayagam, H. Lipid Peroxidation: Its Effects on the Formulation and Use of Pharmaceutical Emulsions. Asian J. Pharm. Sci. 2017, 12, 401–411. [Google Scholar] [CrossRef]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, PH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.M.C.; Vaz, M.M.O.L.L.; Fonseca, Y.M.; Bentley, M.V.L.B.; Fonseca, M.J.V. Characterization and Stability Study of a Water-in-Oil Microemulsion Incorporating Quercetin. Drug Dev. Ind. Pharm. 2011, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for Quality in Medicines and Healthcare (EDQM) European Pharmacopoeia 9.0; EDQM: Strasburg, France, 2017.
- Da Violante, G.; Zerrouk, N.; Richard, I.; Provot, G.; Chaumeil, J.C.; Arnaud, P. Evaluation of the Cytotoxicity Effect of Dimethyl Sulfoxide (DMSO) on Caco2/TC7 Colon Tumor Cell Cultures. Biol. Pharm. Bull. 2002, 25, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Scheim, D.E. Cytotoxicity of Unsaturated Fatty Acids in Fresh Human Tumor Explants: Concentration Thresholds and Implications for Clinical Efficacy. Lipids Health Dis. 2009, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Leaver, H.A.; Wharton, S.B.; Bell, H.S.; Leaver-Yap, I.M.M.; Whittle, I.R. Highly Unsaturated Fatty Acid Induced Tumour Regression in Glioma Pharmacodynamics and Bioavailability of Gamma Linolenic Acid in an Implantation Glioma Model: Effects on Tumour Biomass, Apoptosis and Neuronal Tissue Histology. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 283–292. [Google Scholar] [CrossRef]
- Miyake, J.A.; Benadiba, M.; Colquhoun, A. Gamma-Linolenic Acid Inhibits Both Tumour Cell Cycle Progression and Angiogenesis in the Orthotopic C6 Glioma Model through Changes in VEGF, Flt1, ERK1/2, MMP2, Cyclin D1, PRb, P53 and P27 Protein Expression. Lipids Health Dis. 2009, 8, 8. [Google Scholar] [CrossRef]
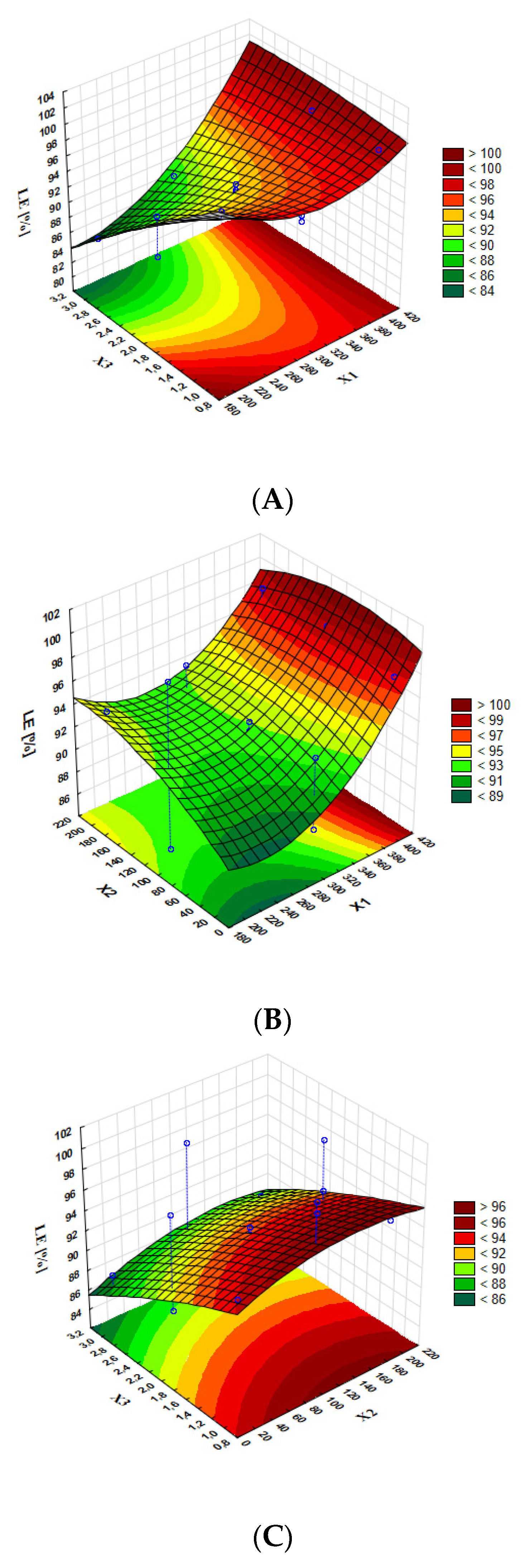
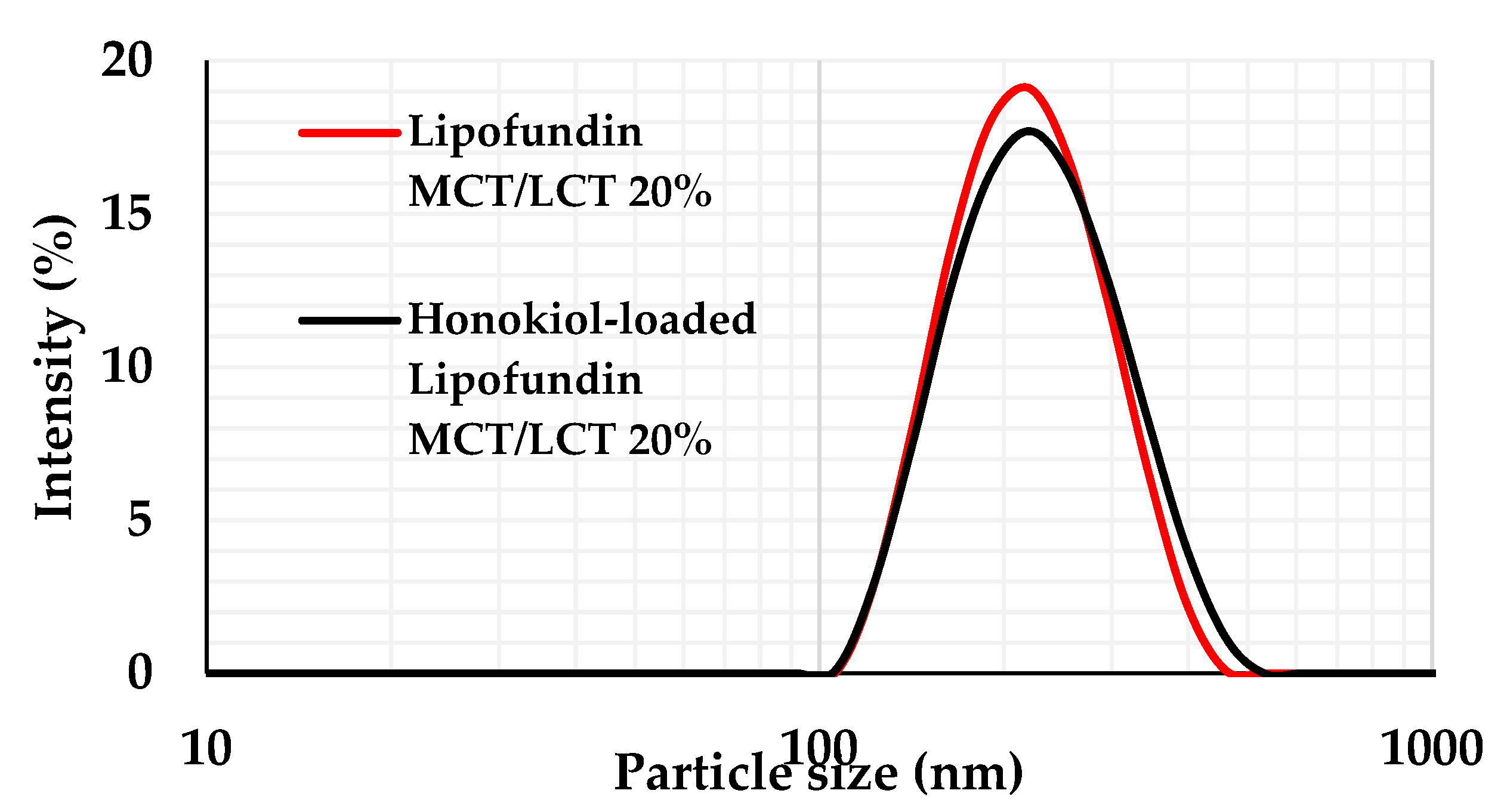


| Formulation Code | X1 Shaking Speed (rpm) | X2 Shaking Time (min) | X3 HON Concentration (mg/mL) |
|---|---|---|---|
| F1 | 200 | 15 | 2 |
| F2 | 400 | 15 | 2 |
| F3 | 200 | 195 | 2 |
| F4 | 400 | 195 | 2 |
| F5 | 200 | 105 | 1 |
| F6 | 400 | 105 | 1 |
| F7 | 200 | 105 | 3 |
| F8 | 400 | 105 | 3 |
| F9 | 300 | 15 | 1 |
| F10 | 300 | 195 | 1 |
| F11 | 300 | 15 | 3 |
| F12 | 300 | 195 | 3 |
| F13 | 300 | 105 | 2 |
| F14 | 300 | 105 | 2 |
| F15 | 300 | 105 | 2 |
| Formulation Code | LE% (SD) [%] | MDD (SD) [nm] | PDI (SD) | ZP (SD) [mV] |
|---|---|---|---|---|
| F1 | 88.52 (0.05) | 215.8 (1.5) | 0.07 ( 0.04) | −31.2 (0.6) |
| F2 | 97.85 (2.40) | 206.3 (1.5) | 0.07 (0.02) | −28.0 (0.2) |
| F3 | 93.78 (5.33) | 213.6 (0.8) | 0.07 (0.02) | −27.7 (0.8) |
| F4 | 98.75 (0.52) | 205.4 (2.0) | 0.09 (0.02) | −27.1 (0.3) |
| F5 | 99.77 (3.90) | 213.4 (2.1) | 0.09 (0.02) | −27.1 (0.1) |
| F6 | 98.71 (0.88) | 206.7 (1.1) | 0.08 (0.00) | −30.8 (0.5) |
| F7 | 85.41 (1.09) | 215.3 (1.0) | 0.08 (0.01) | −27.9 (0.3) |
| F8 | 97.74 (2.91) | 204.9 (1.1) | 0.10 (0.02) | −28.1 (0.3) |
| F9 | 94.02 (8.05) | 213.6 (2.1) | 0.04 (0.04) | −26.7 (0.3) |
| F10 | 94.81 (7.17) | 214.5 (0.8) | 0.08 (0.02) | −26.9 (0.1) |
| F11 | 87.78 (2.23) | 214.5 (1.6) | 0.09 (0.03) | −27.8 (0.2) |
| F12 | 89.35 (2.58) | 215.1 (1.9) | 0.07 (0.02) | −27.4 (0.9) |
| F13 | 92.62 (3.55) | 214.6 (0.5) | 0.09 (0.01) | −30.1 (0.5) |
| F14 | 92.40 (4.74) | 211.8 (2.2) | 0.07 (0.02) | −25.7 (0.2) |
| F15 | 93.38 (1.42) | 213.3 (0.8) | 0.09 (0.02) | −25.3 (0.4) |
| Source | dF | SS | MS | F-Values | p-Values | p-Values |
|---|---|---|---|---|---|---|
| Model | 9 | 210.879 | 23.431 | 16.83 | 0.003 | < 0.05 |
| Linear model | 3 | 152.351 | 50.784 | 36.47 | 0.001 | < 0.05 |
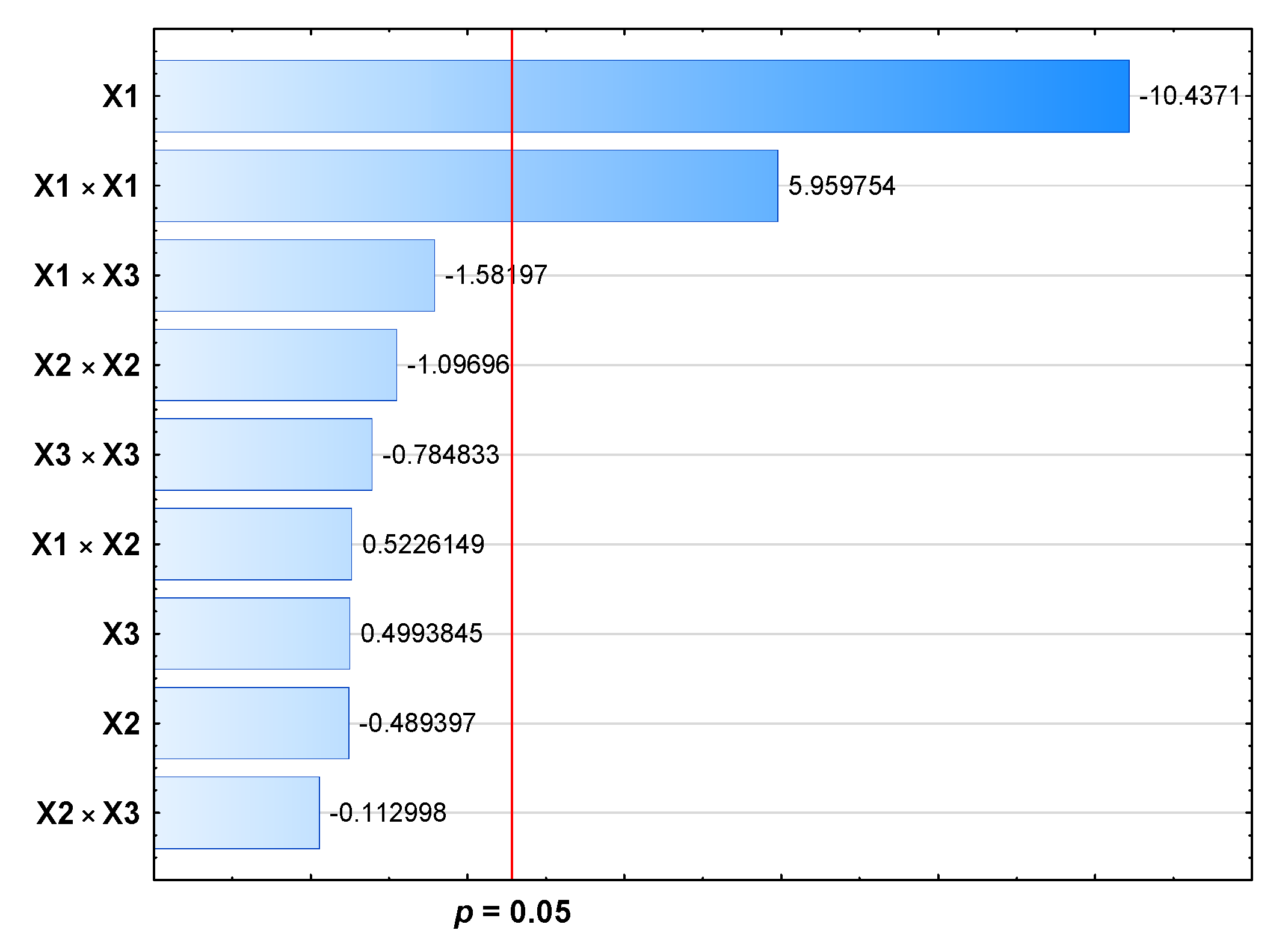
| X1 | 1 | 151.670 | 151.670 | 108.93 | 0.000 | < 0.05 |
| X2 | 1 | 0.333 | 0.333 | 0.24 | 0.645 | > 0.05 |
| X3 | 1 | 0.347 | 0.347 | 0.25 | 0.639 | > 0.05 |
| Quadratic model | 3 | 54.646 | 18.215 | 13.08 | 0.008 | < 0.05 |
| X1 × X1 | 1 | 49.453 | 49.453 | 35.52 | 0.002 | < 0.05 |
| X2 × X2 | 1 | 1.675 | 1.675 | 1.20 | 0.323 | > 0.05 |
| X3 × X3 | 1 | 0.858 | 0.858 | 0.62 | 0.468 | > 0.05 |
| Interactions | 3 | 3.882 | 1.294 | 0.93 | 0.491 | > 0.05 |
| X1 × X2 | 1 | 0.380 | 0.380 | 0.27 | 0.624 | > 0.05 |
| X1 × X3 | 1 | 3.484 | 3.484 | 2.50 | 0.175 | > 0.05 |
| X2 × X3 | 1 | 0.018 | 0.018 | 0.01 | 0.914 | > 0.05 |
| Error | 5 | 6.962 | 1.392 | |||
| Lack of fit | 3 | 3.030 | 1.010 | 0.51 | 0.713 | > 0.05 |
| Pure error | 2 | 3.932 | 1.966 | |||
| Total | 14 | 217.841 | ||||
| Regression equation | MDD [nm] = 191.36 + 0.1911 X1 − 0.0285 X2 + 0.116 X3 − 0.000366 X1 × X1 + 0.000083 X2 × X2 + 0.00482 X3 × X3 + 0.000034 X1×X2 − 0.000933 X1 × X3 − 0.000074 X2 × X3 | |||||
| Source | dF | SS | MS | F-values | p-Values | p-Values |
|---|---|---|---|---|---|---|
| Model | 9 | 269.446 | 29.9385 | 29.22 | 0.001 | <0.05 |
| Linear model | 3 | 182.006 | 60.6685 | 59.22 | 0.000 | <0.05 |
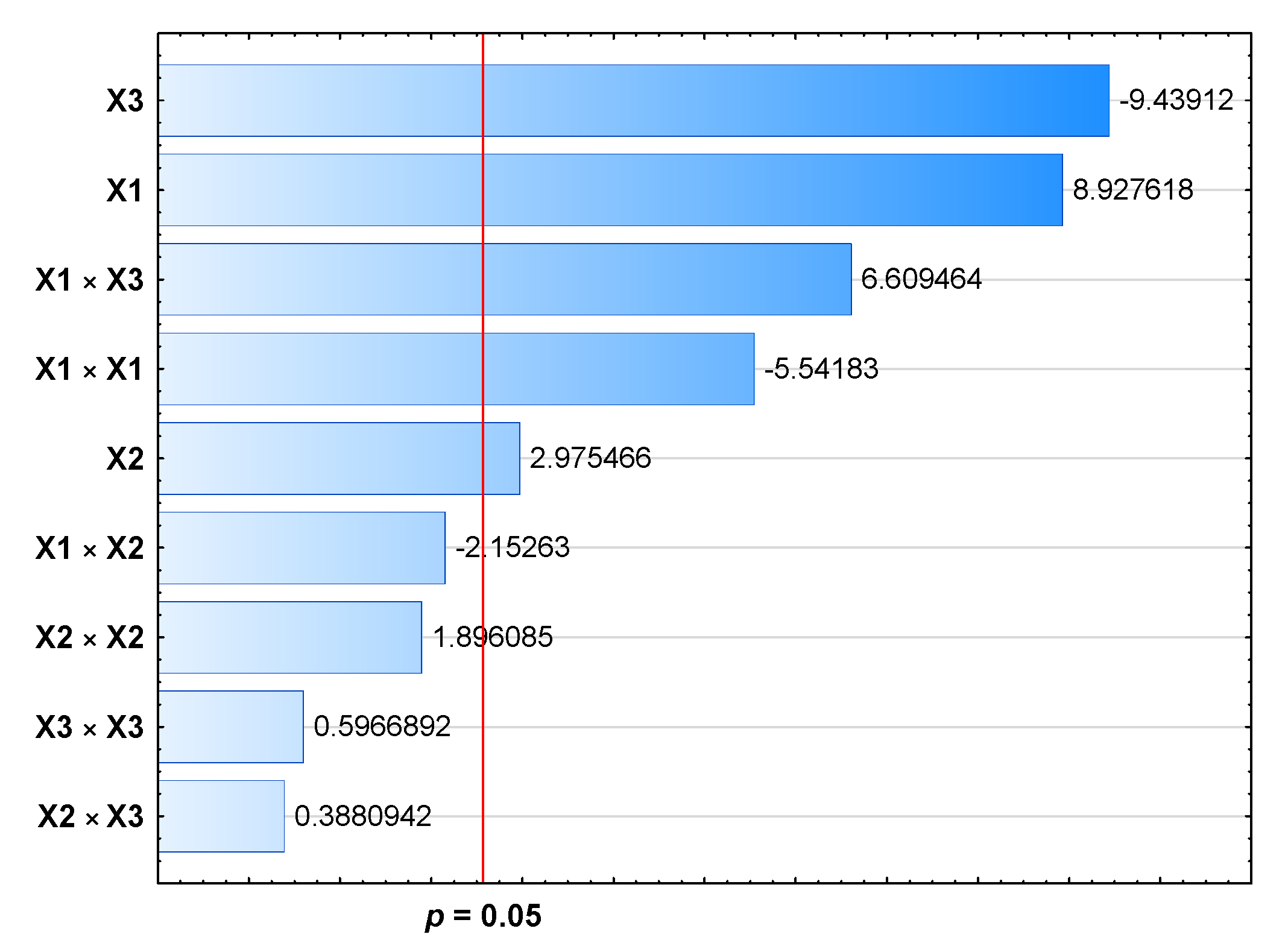
| X1 | 1 | 81.655 | 81.6552 | 79.70 | 0.000 | <0.05 |
| X2 | 1 | 9.070 | 9.0703 | 8.85 | 0.031 | <0.05 |
| X3 | 1 | 91.280 | 91.2800 | 89.10 | 0.000 | <0.05 |
| Quadratic model | 3 | 37.784 | 12.5946 | 12.29 | 0.010 | <0.05 |
| X1 × X1 | 1 | 31.464 | 31.4644 | 30.71 | 0.003 | <0.05 |
| X2 × X2 | 1 | 3.683 | 3.6832 | 3.60 | 0.116 | >0.05 |
| X3 × X3 | 1 | 0.365 | 0.3648 | 0.36 | 0.577 | >0.05 |
| Interactions | 3 | 49.657 | 16.5523 | 16.16 | 0.005 | <0.05 |
| X1 × X2 | 1 | 4.747 | 4.7473 | 4.63 | 0.084 | >0.05 |
| X1 × X3 | 1 | 44.755 | 44.7554 | 43.69 | 0.001 | <0.05 |
| X2 × X3 | 1 | 0.154 | 0.1543 | 0.15 | 0.714 | >0.05 |
| Error | 5 | 5.123 | 1.0245 | |||
| Lack of fit | 3 | 4.597 | 1.5322 | 5.83 | 0.150 | >0.05 |
| Pure error | 2 | 0.526 | 0.2629 | |||
| Total | 14 | 274.569 | ||||
| Regression equation | LE [%] = 129.10 − 0.1974 X1 + 0.0697 X2 1.238 X3 + 0.000292 X1 × X1 − 0.000123 X2 × X2 0.00314 X3 × X3 0.000121 X1 × X2 + 0.003345 X1 × X3 + 0.000218 X2 × X3 | |||||
| Time | No Stress Condition (4 ± 2 °C, No Light Exposure) | Oxidative Stress Condition (25 ± 2 °C, No Light Exposure) | High-Temperature Condition (60 ± 1 °C, No Light Exposure) | Accelerated-Light Condition (35 ± 2 °C) |
|---|---|---|---|---|
| t = 0 h | 100.00 | 100.00 | 100.00 | 100.00 |
| t = 24 h | 100.00 (0.47) | 99.92 (0.96) | 96.37 (3.62) | 91.72 (1.86) a |
| t = 48 h | 100.12 (3.05) | 99.76 (1.84) | 97.25 (2.08) | 99.05 (1.21) b |
| t = 72 h | 99.33 (1.29) | 99.15 (2.21) | 97.70 (1.34) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostyńska, A.; Czerniel, J.; Kuźmińska, J.; Brzozowski, J.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V.; Stawny, M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics 2023, 15, 448. https://doi.org/10.3390/pharmaceutics15020448
Gostyńska A, Czerniel J, Kuźmińska J, Brzozowski J, Majchrzak-Celińska A, Krajka-Kuźniak V, Stawny M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics. 2023; 15(2):448. https://doi.org/10.3390/pharmaceutics15020448
Chicago/Turabian StyleGostyńska, Aleksandra, Joanna Czerniel, Joanna Kuźmińska, Jakub Brzozowski, Aleksandra Majchrzak-Celińska, Violetta Krajka-Kuźniak, and Maciej Stawny. 2023. "Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay" Pharmaceutics 15, no. 2: 448. https://doi.org/10.3390/pharmaceutics15020448
APA StyleGostyńska, A., Czerniel, J., Kuźmińska, J., Brzozowski, J., Majchrzak-Celińska, A., Krajka-Kuźniak, V., & Stawny, M. (2023). Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics, 15(2), 448. https://doi.org/10.3390/pharmaceutics15020448










