Antibiotic-Loaded Gold Nanoparticles: A Nano-Arsenal against ESBL Producer-Resistant Pathogens
Abstract
1. Introduction
- Antibiotic-loaded AuNPs as magic bullets to overcome resistance
- Plausible antibacterial mechanism of AuNPs
- Clinical translation status and Toxicity aspects of AuNP-based drug delivery system
- Future prospects of antibiotic-loaded AuNPs
- Challenges associated with antibiotic-loaded AuNPs
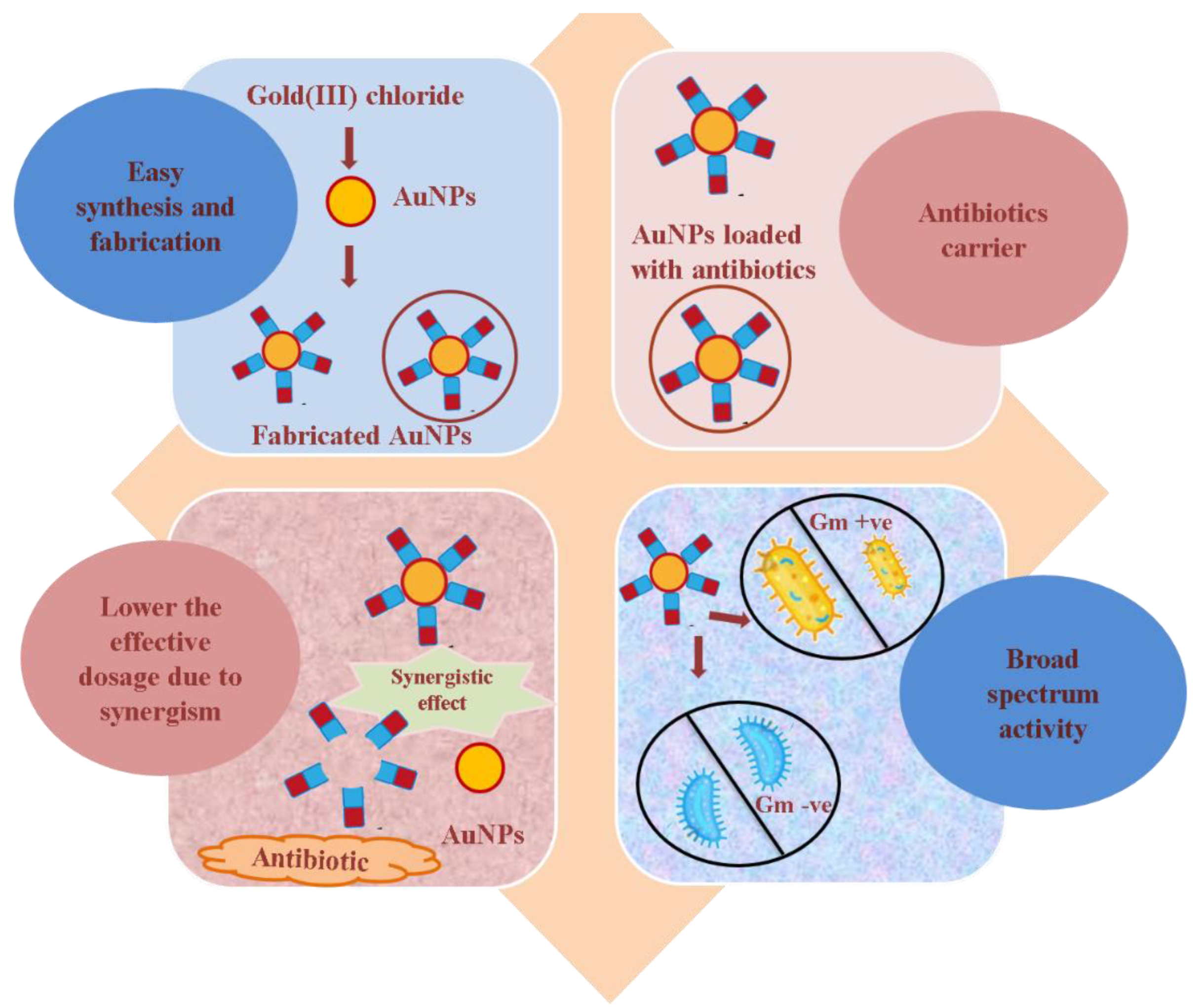
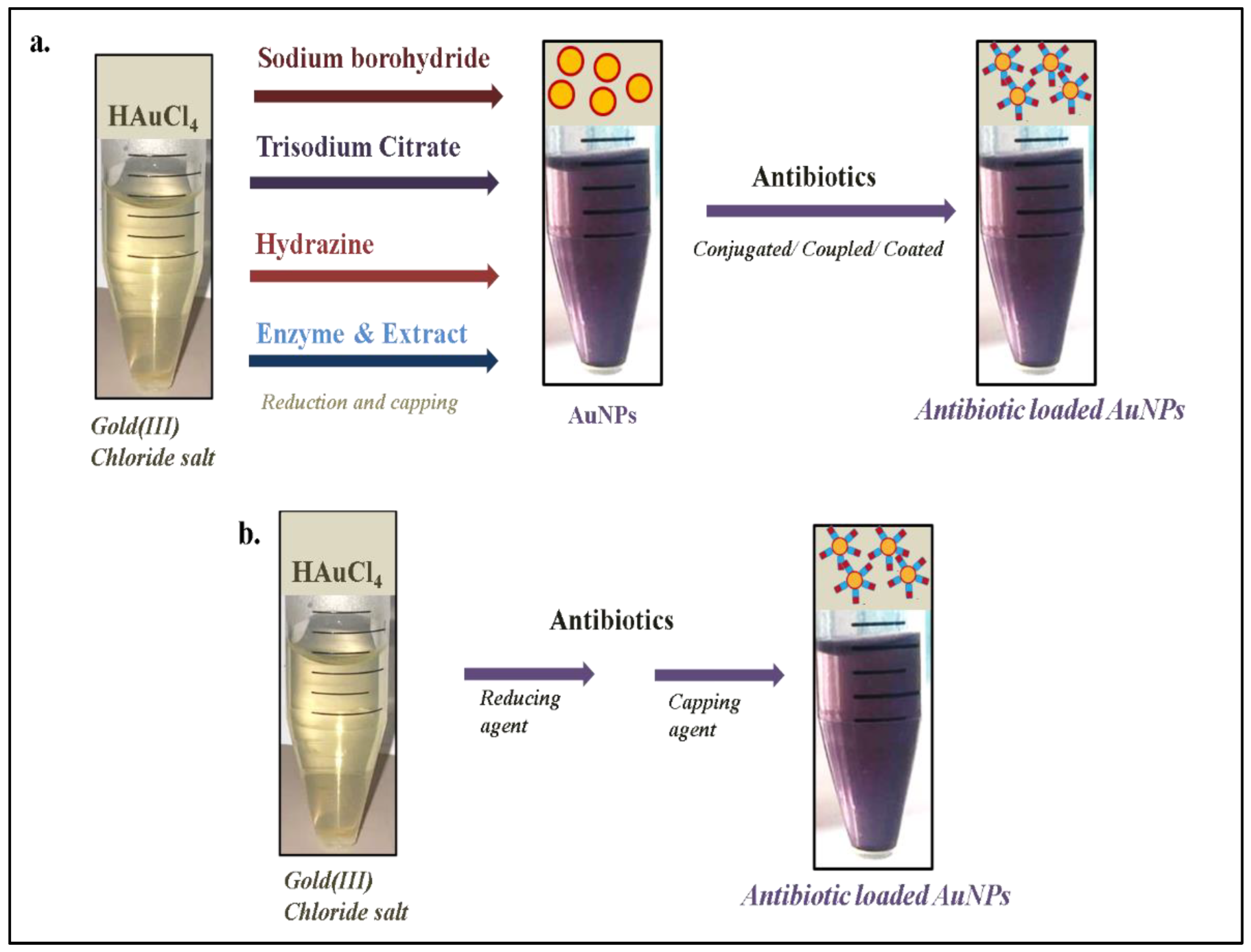
2. Antibiotic-Loaded AuNPs as Magic Bullets to Overcome Resistance
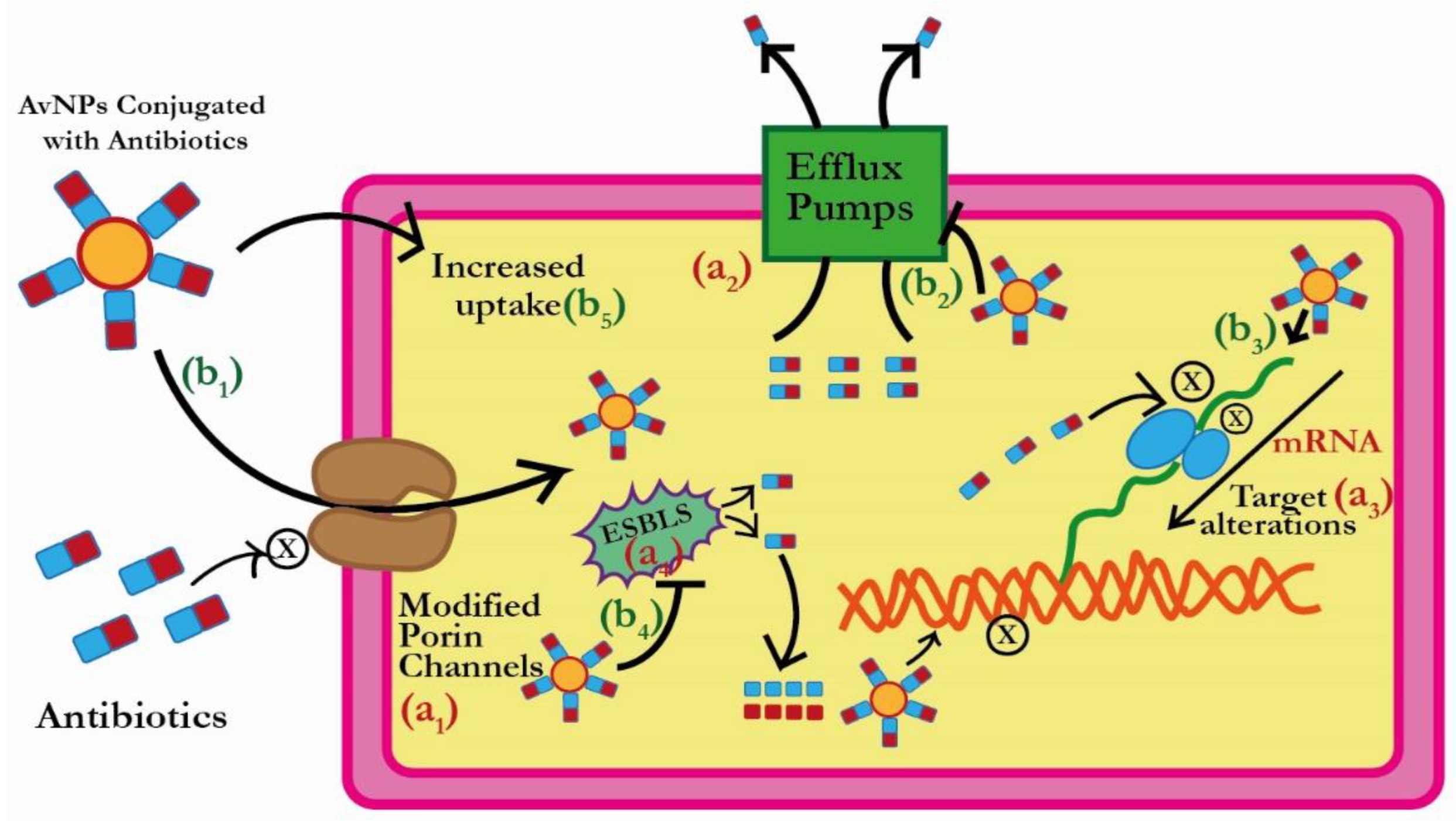
3. Plausible Antibacterial Mechanism of AuNPs
3.1. Reduction in the Antibiotic Uptake by ESBLs Producing Bacterial Pathogens
3.2. Efflux of Antibiotics by ESBLs Producing Bacterial Pathogens
3.3. Inactivation of Antibiotics by ESBLs Producing Bacterial Pathogens
4. Clinical Translation Status and Toxicity Aspects of AuNP-Based Drug Delivery System
4.1. Clinical Translation Status
4.2. Toxicity Aspects
5. Future Prospects of Antibiotic-Loaded or -Conjugated Gold Nanoparticles
6. Challenges Associated with Antibiotic-Loaded or -Conjugated Gold Nanoparticles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.A.; Hasan, F.; Ahmed, S.; Hameed, A. Characteristics, epidemiology and clinical importance of emerging strains of Gram-negative bacilli producing extended-spectrum beta-lactamases. Res. Microbiol. 2004, 155, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.H.; Clark, J. ESBLs: A Clear and Present Danger? Crit. Care Res. Pract. 2012, 2012, 625170. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Pascual, A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev. Anti Infect. Ther. 2008, 6, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 2: Management strategies and new agents. Pharm. Ther. 2015, 40, 344–352. [Google Scholar]
- Chaudhuri, A.; Martinez-Martin, P.; Kennedy, P.G.; Andrew Seaton, R.; Portegies, P.; Bojar, M.; Steiner, I. EFNS guideline on the management of community-acquired bacterial meningitis: Report of an EFNS Task Force on acute bacterial meningitis in older children and adults. Eur. J. Neurol. 2008, 15, 649–659. [Google Scholar] [CrossRef]
- Teklu, D.S.; Negeri, A.A.; Legese, M.H.; Bedada, T.L.; Woldemariam, H.K.; Tullu, K.D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control 2019, 8, 39. [Google Scholar] [CrossRef]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Hussain, T.; Ahmed, A.B.F.; Alshammari, T.M.; Moin, A.; Ahmed, M.Q.; Barreto, G.E.; Kamal, M.A.; Ashraf, G.M. Gold nanoparticles: A plausible tool to combat neurological bacterial infections in humans. Biomed. Pharmacother. 2018, 107, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rizvi, S.M.D.; Avaish, M.; Arshad, M.; Bagga, P.; Khan, M.S. A novel process for size controlled biosynthesis of gold nanoparticles using bromelain. Mater. Lett. 2015, 159, 373–376. [Google Scholar] [CrossRef]
- Camas, M.; Sazak Camas, A.; Kyeremeh, K. Extracellular Synthesis and Characterization of Gold Nanoparticles Using Mycobacterium sp. BRS2A-AR2 Isolated from the Aerial Roots of the Ghanaian Mangrove Plant, Rhizophora racemosa. Indian J. Microbiol. 2018, 58, 214–221. [Google Scholar] [CrossRef]
- Khandelwal, P.; Singh, D.K.; Poddar, P. Advances in the Experimental and Theoretical Understandings of Antibiotic Conjugated Gold Nanoparticles for Antibacterial Applications. ChemistrySelect 2019, 4, 6719–6738. [Google Scholar] [CrossRef]
- Wang, S.G.; Chen, Y.C. Antibacterial gold nanoparticle-based photothermal killing of vancomycin-resistant bacteria. Nanomedicine 2018, 13, 1405–1416. [Google Scholar] [CrossRef]
- Chavan, C.; Kamble, S.; Murthy, A.V.R.; Kale, S.N. Ampicillin-mediated functionalized gold nanoparticles against ampicillin-resistant bacteria: Strategy, preparation and interaction studies. Nanotechnology 2020, 31, 215604. [Google Scholar] [CrossRef]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef]
- Fuller, M.; Whiley, H.; Köper, I. Antibiotic delivery using gold nanoparticles. SN Appl. Sci. 2020, 2, 1022. [Google Scholar] [CrossRef]
- Nishanthi, R.; Malathi, S.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar]
- Pradeepa; Vidya, S.M.; Mutalik, S.; Udaya Bhat, K.; Huilgol, P.; Avadhani, K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016, 153, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Mahalunkar, S.; Shriram, V.; Gosavi, S.; Kumar, V. Embelin-loaded chitosan gold nanoparticles interact synergistically with ciprofloxacin by inhibiting efflux pumps in multidrug-resistant Pseudomonas aeruginosa and Escherichia coli. Environ. Res. 2021, 199, 111321. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Rizvi, S.M.D.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.S.; Alshammari, F.; Khafagy, E.S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, F.; Alshammari, B.; Moin, A.; Alamri, A.; Al Hagbani, T.; Alobaida, A.; Baker, A.; Khan, S.; Rizvi, S.M.D. Ceftriaxone Mediated Synthesized Gold Nanoparticles: A Nano-Therapeutic Tool to Target Bacterial Resistance. Pharmaceutics 2021, 13, 1896. [Google Scholar] [CrossRef] [PubMed]
- Alafnan, A.; Rizvi, S.M.D.; Alshammari, A.S.; Faiyaz, S.S.M.; Lila, A.S.A.; Katamesh, A.A.; Khafagy, E.S.; Alotaibi, H.F.; Ahmed, A.B.F. Gold Nanoparticle-Based Resuscitation of Cefoxitin against Clinical Pathogens: A Nano-Antibiotic Strategy to Overcome Resistance. Nanomaterials 2022, 12, 3643. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Huwaimel, B.; Alobaida, A.; Hussain, T.; Rafi, Z.; Mehmood, K.; Abdallah, M.H.; Hagbani, T.A.; Rizvi, S.M.D.; Moin, A.; et al. Delafloxacin-Capped Gold Nanoparticles (DFX-AuNPs): An Effective Antibacterial Nano-Formulation of Fluoroquinolone Antibiotic. Materials 2022, 15, 5709. [Google Scholar] [CrossRef]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Hussain, T.; Alshammari, T.M.; Ahmad, W.; Tabrez, S.; Al-Qahtani, M.H.; Abuzenadah, A.M. Synthesis and Characterization of Cefotaxime Conjugated Gold Nanoparticles and Their Use to Target Drug-Resistant CTX-M-Producing Bacterial Pathogens. J. Cell Biochem. 2017, 118, 2802–2808. [Google Scholar] [CrossRef]
- Shaker, M.A.; Shaaban, M.I. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: In vitro antibacterial study. Int. J. Pharm. 2017, 525, 71–84. [Google Scholar] [CrossRef]
- Kalita, S.; Kandimalla, R.; Sharma, K.K.; Kataki, A.C.; Deka, M.; Kotoky, J. Amoxicillin functionalized gold nanoparticles reverts MRSA resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 720–727. [Google Scholar] [CrossRef]
- Hur, Y.E.; Park, Y. Vancomycin-Functionalized Gold and Silver Nanoparticles as an Antibacterial Nanoplatform Against Methicillin-Resistant Staphylococcus aureus. J. Nanosci. Nanotechnol. 2016, 16, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Liu, K.; He, J.; Zhang, Y.; Chen, S.; Ma, G.; Cui, Y.; Wang, L.; Gao, D. Synthesis of gold nanoflowers stabilized with amphiphilic daptomycin for enhanced photothermal antitumor and antibacterial effects. Int. J. Pharm. 2020, 580, 119231. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Samanta, S.; Agarwal, S.; Nair, K.K.; Harris, R.A.; Swart, H. Biomolecular assisted synthesis and mechanism of silver and gold nanoparticles. Mater. Res. Express 2019, 6, 82009. [Google Scholar] [CrossRef]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis—A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining Rules for the Shape Evolution of Gold Nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, 1801451. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Jagannathan, R.; Khandelwal, P.; Abraham, P.M.; Poddar, P. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: An experimental and density functional theory investigation. Nanoscale 2013, 5, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.M.; Zhang, L. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef]
- Rastogi, L.; Kora, A.J.; Arunachalam, J. Highly stable, protein capped gold nanoparticles as effective drug delivery vehicles for amino-glycosidic antibiotics. Mater. Sci. Eng. C 2012, 32, 1571–1577. [Google Scholar] [CrossRef]
- Tom, R.T.; Suryanarayanan, V.; Reddy, P.G.; Baskaran, S.; Pradeep, T. Ciprofloxacin-protected gold nanoparticles. Langmuir 2004, 20, 1909–1914. [Google Scholar] [CrossRef]
- Ahangari, A.; Salouti, M.; Saghatchi, F. Gentamicin-gold nanoparticles conjugate: A contrast agent for X-ray imaging of infectious foci due to Staphylococcus aureus. IET Nanobiotechnol. 2016, 10, 190–194. [Google Scholar] [CrossRef]
- Grace, A.N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P. Continuous release of gentamicin from gold nanocarriers. RSC Adv. 2014, 4, 51904–51910. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shen, Y.; Yu, Y.; Bai, X. Synthesis of antibacterial gold nanoparticles with different particle sizes using chlorogenic acid. R Soc. Open Sci. 2020, 7, 191141. [Google Scholar] [CrossRef] [PubMed]
- Pourali, P.; Benada, O.; Pátek, M.; Neuhöferová, E.; Dzmitruk, V.; Benson, V. Response of Biological Gold Nanoparticles to Different pH Values: Is It Possible to Prepare Both Negatively and Positively Charged Nanoparticles? Appl. Sci. 2021, 11, 11559. [Google Scholar] [CrossRef]
- Khan, S.; Rizvi, S.M.; Saeed, M.; Srivastava, A.K.; Khan, M.A. Novel Approach for the synthesis of gold nanoparticles using Trypsin. Adv. Sci. Lett. 2014, 20, 1061–1065. [Google Scholar] [CrossRef]
- Marcelo, G.A.; Duarte, M.P.; Oliveira, E. Gold@mesoporous silica nanocarriers for the effective delivery of antibiotics and by-passing of β-lactam resistance. SN Appl. Sci. 2020, 2, 1354. [Google Scholar] [CrossRef]
- Haddada, M.B.; Jeannot, K.; Spadavecchia, J. Novel Synthesis and Characterization of Doxycycline-Loaded Gold Nanoparticles: The Golden Doxycycline for Antibacterial Applications. Part. Part. Syst. Charact. 2019, 36, 1800395. [Google Scholar] [CrossRef]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.; Santra, C.; Dasgupta, A.K.; Karmakar, P. In Vitro Structural and Functional Evaluation of Gold Nanoparticles Conjugated Antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Sobhana, S.S.; Jacob, J.P.; Kumar, M.G.; Devi, M.P.; Sastry, T.P.; Mandal, A.B. Preparation, characterization and evaluation of a biopolymeric gold nanocomposite with antimicrobial activity. Biotechnol. Appl. Biochem. 2010, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hagbani, T.A.; Yadav, H.; Moin, A.; Lila, A.S.A.; Mehmood, K.; Alshammari, F.; Khan, S.; Khafagy, E.S.; Hussain, T.; Rizvi, S.M.D.; et al. Enhancement of Vancomycin Potential against Pathogenic Bacterial Strains via Gold Nano-Formulations: A Nano-Antibiotic Approach. Materials 2022, 15, 1108. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Fayaz, A.; Girilal, M.; Mahdy, S.A.; Somsundar, S.S.; Venkatesan, R.; Kalaichelvan, P.T. Vancomycin bound biogenic gold nanoparticles: A different perspective for development of anti VRSA agents. Process Biochem. 2011, 46, 636–641. [Google Scholar] [CrossRef]
- Esmaeillou, M.; Zarrini, G.; Ahangarzadeh Rezaee, M.; Shahbazi Mojarrad, J.; Bahadori, A. Vancomycin Capped with Silver Nanoparticles as an Antibacterial Agent against Multi-Drug Resistance Bacteria. Adv. Pharm. Bull. 2017, 7, 479–483. [Google Scholar] [CrossRef]
- Song, Y.-Z.; Zhu, A.-F.; Song, Y.; Cheng, Z.-P.; Xu, J.; Zhou, J.-F. Experimental and theoretical study on the synthesis of gold nanoparticles using ceftriaxone as a stabilizing reagent for and its catalysis for dopamine. Gold Bull. 2012, 45, 153–160. [Google Scholar] [CrossRef]
- Nishanthi, R.; Palani, P. Green synthesis of gold nanoparticles from the rind extract of Garcinia mangostana and its synergistic effect with antibiotics against human pathogenic bacteria. In Proceedings of the 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), Sendai, Japan, 22–25 August 2016; pp. 431–434. [Google Scholar]
- Al-Khafaji, M.H.; Hashim, M.H. The synergistic effect of biosynthesized gold nanoparticles with antibiotic against clinical isolates. J. Biotechnol. Res. Cent. 2019, 13, 58–62. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Maurya, N.; Jangra, M.; Tambat, R.; Nandanwar, H. Alliance of Efflux Pumps with β-Lactamases in Multidrug-Resistant Klebsiella pneumoniae Isolates. Microb. Drug Resist. 2019, 25, 1155–1163. [Google Scholar] [CrossRef]
- Yasufuku, T.; Shigemura, K.; Shirakawa, T.; Matsumoto, M.; Nakano, Y.; Tanaka, K.; Arakawa, S.; Kinoshita, S.; Kawabata, M.; Fujisawa, M. Correlation of overexpression of efflux pump genes with antibiotic resistance in Escherichia coli Strains clinically isolated from urinary tract infection patients. J. Clin. Microbiol. 2011, 49, 189–194. [Google Scholar] [CrossRef]
- Yedekci, S.; Erac, B.; Limoncu, M.H. Detection of the efflux pump-mediated quinolone resistance in ESBL positive Escherichia coli and Klebsiella pneumoniae isolates by phe-Arg-beta naphthylamide. Turk. J. Pharm. Sci. 2012, 9, 67–74. [Google Scholar]
- Tsai, Y.K.; Fung, C.P.; Lin, J.C.; Chen, J.H.; Chang, F.Y.; Chen, T.L.; Siu, L.K. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2011, 55, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Mahendran, K.R.; Molitor, A.; Bolla, J.M.; Bessonov, A.N.; Winterhalter, M.; Pagès, J.M. How beta-lactam antibiotics enter bacteria: A dialogue with the porins. PLoS ONE 2009, 4, e5453. [Google Scholar] [CrossRef]
- Nitzan, Y.; Deutsch, E.B.; Pechatnikov, I. Diffusion of beta-lactam antibiotics through oligomeric or monomeric porin channels of some gram-negative bacteria. Curr. Microbiol. 2002, 45, 446–455. [Google Scholar] [CrossRef]
- Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. How to Enter a Bacterium: Bacterial Porins and the Permeation of Antibiotics. Chem. Rev. 2021, 121, 5158–5192. [Google Scholar] [CrossRef]
- Dé, E.; Baslé, A.; Jaquinod, M.; Saint, N.; Malléa, M.; Molle, G.; Pagès, J.M. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 2001, 41, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.M.; Abd El-Aziz, A.M.; Hassan, R.; Abdelmegeed, E.S. β-lactam resistance associated with β-lactamase production and porin alteration in clinical isolates of E. coli and K. pneumoniae. PLoS ONE 2021, 16, e0251594. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, S.; Subha, A. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and Escherichia coli. Indian J. Med. Microbiol. 2005, 23, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, L. Extended-spectrum beta-lactamases and the permeability barrier. Clin. Microbiol. Infect. 2008, 14 (Suppl. S1), 82–89. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef]
- Chen, J.; Hessler, J.A.; Putchakayala, K.; Panama, B.K.; Khan, D.P.; Hong, S.; Mullen, D.G.; Dimaggio, S.C.; Som, A.; Tew, G.N.; et al. Cationic nanoparticles induce nanoscale disruption in living cell plasma membranes. J. Phys. Chem. B 2009, 113, 11179–11185. [Google Scholar] [CrossRef] [PubMed]
- Białas, N.; Sokolova, V.; van der Meer, S.B.; Knuschke, T.; Ruks, T.; Klein, K.; Westendorf, A.M.; Epple, M. Bacteria (E. coli) take up ultrasmall gold nanoparticles (2 nm) as shown by different optical microscopic techniques (CLSM, SIM, STORM). Nano Select 2022, 3, 1407–1420. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Le Guével, X.; Fleury, J.B.; Hanssen, E.; Nguyen, T.H.; Juodkazis, S.; Bryant, G.; Crawford, R.J.; Stoodley, P.; et al. Antibacterial action of nanoparticles by lethal stretching of bacterial cell membranes. Adv. Mater. 2020, 32, 2005679. [Google Scholar] [CrossRef] [PubMed]
- Giri, K.; Yepes, L.R.; Duncan, B.; Parameswaran, P.K.; Yan, B.; Jiang, Y.; Bilska, M.; Moyano, D.F.; Thompson, M.; Rotello, V.M.; et al. Targeting bacterial biofilms via surface engineering of gold nanoparticles. RSC Adv. 2015, 5, 105551–105559. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target Gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Adhikari, M.D.; Goswami, S.; Panda, B.R.; Chattopadhyay, A.; Ramesh, A. Membrane-directed high bactericidal activity of (gold nanoparticle)-polythiophene composite for niche applications against pathogenic bacteria. Adv. Healthc. Mater. 2013, 2, 599–606. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhang, X.; Wu, F.-G.; Chen, Y. Colorimetric and test stripe-based assay of bacteria by using vancomycin-modified gold nanoparticles. Sens. Actuators B Chem. 2019, 281, 408–414. [Google Scholar] [CrossRef]
- Mobasseri, G.; Lin, T.K.; Teh, C.S.J. Association of Efflux Pump and OMPs with Antibiotic Susceptibility Among ESBL-Producing Klebsiella Pneumoniae Clinical Strains in Malaysia. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Dorri, K.; Modaresi, F.; Shakibaie, M.R.; Moazamian, E. Effect of gold nanoparticles on the expression of efflux pump mexA and mexB genes of Pseudomonas aeruginosa strains by Quantitative real-time PCR. Pharmacia 2022, 69, 125–133. [Google Scholar] [CrossRef]
- Miller, L.M.; Silver, C.D.; Herman, R.; Duhme-Klair, A.-K.; Thomas, G.H.; Krauss, T.F.; Johnson, S.D. Surface-Bound Antibiotic for the Detection of β-Lactamases. ACS Appl. Mater. Interfaces 2019, 11, 32599–32604. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, B.; Yan, B. Regulation of enzyme activity through interactions with nanoparticles. Int. J. Mol. Sci. 2009, 10, 4198–4209. [Google Scholar] [CrossRef]
- Chatterjee, T.; Das, G.; Ghosh, S.; Chakrabarti, P. Effect of gold nanoparticles on the structure and neuroprotective function of protein L-isoaspartyl methyltransferase (PIMT). Sci. Rep. 2021, 11, 1–3. [Google Scholar] [CrossRef]
- Ramalingam, V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci. 2019, 271, 101989. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Tian, E.K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold nanoparticle: Recent progress on its antibacterial applications and mechanisms. J. Nanomater. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.G. Gold nanoparticles induce a reactive oxygen species-independent apoptotic pathway in Escherichia coli. Colloids Surf. B Biointerfaces 2018, 167, 1–7. [Google Scholar] [CrossRef]
- Ahmed, F.; Faisal, S.M.; Ahmed, A.; Husain, Q. Beta galactosidase mediated bio-enzymatically synthesized nano-gold with aggrandized cytotoxic potential against pathogenic bacteria and cancer cells. J. Photochem. Photobiol. B 2020, 209, 111923. [Google Scholar] [CrossRef] [PubMed]
- Global Newswire. Available online: https://www.globenewswire.com/news-release/2022/10/13/2534150/0/en/Global-Gold-Nanoparticles-.Market-to-Reach-7-6-Billion-by-2027.html (accessed on 23 November 2022).
- IMARC. Available online: https://www.imarcgroup.com/gold-nanoparticles-market (accessed on 23 November 2022).
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/gold-nanoparticles-market-A08997 (accessed on 23 November 2022).
- FDA. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf7/k070804.pdf (accessed on 23 November 2022).
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2022, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Tsoli, M.; Kuhn, H.; Brandau, W.; Esche, H.; Schmid, G. Cellular uptake and toxicity of Au55 clusters. Small 2005, 1, 841–844. [Google Scholar] [CrossRef]
- Mironava, T.; Hadjiargyrou, M.; Simon, M.; Jurukovski, V.; Rafailovich, M.H. Gold nanoparticles cellular toxicity and recovery: Effect of size, concentration and exposure time. Nanotoxicology 2010, 4, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjugate Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Hauck, T.S.; Ghazani, A.A.; Chan, W.C. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 2008, 4, 153–159. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hung, Y.C.; Liau, I.; Huang, G.S. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- Pompa, P.P.; Vecchio, G.; Galeone, A.; Brunetti, V.; Sabella, S.; Maiorano, G.; Falqui, A.; Bertoni, G.; Cingolani, R. In Vivo toxicity assessment of gold nanoparticles in Drosophila melanogaster. Nano Res. 2011, 4, 405–413. [Google Scholar] [CrossRef]
- Vecchio, G.; Galeone, A.; Brunetti, V.; Maiorano, G.; Rizzello, L.; Sabella, S.; Cingolani, R.; Pompa, P.P. Mutagenic effects of gold nanoparticles induce aberrant phenotypes in Drosophila melanogaster. Nanomedicine 2012, 8, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]

| Nanomaterial | Size and Shape | Targeted Bacteria | Efficacy | Remarks | Ref. |
|---|---|---|---|---|---|
| Vancomycin-AuNPs | Polygonal | Vancomycin-resistant Enterococci | MIC50 of Vancomycin-AuNPs was 2 µg/mL, which was significantly lower than free vancomycin | AuNPs developed have the ability of photothermal killing by irradiation | [17] |
| Ampicillin- AuNPs | 25–50 nm and spherical | S. aureus, E. coli, B. subtilis, Flavobacteria devorans | 4- and 16-fold increase in activity of ampicillin- AuNPs against amp-resistant and amp-sensitive strains, respectively | Ampicillin-mediated AuNP synthesis, where β−lactam ring remains intact for its action after attachment | [18] |
| Colistin- AuNPs | 5 nm and spherical | E. coli | 6-times reduction in MIC concentration compared to free colistin | Anionic AuNPs were used to deliver colistin that showed activity at very low dose compared to colistin alone | [20] |
| Azithromycin/Streptomycin with AuNPs | 20–40 nm and spherical | Clinical Staphylococcus spp. | Significant improvement in antibacterial activity in comparison to free antibiotics | Synergistic effect was observed after combining AuNPs with antibiotics | [21] |
| Ciprofloxacin- AuNPs | 10–20 nm and spherical | MDR K. pneumoniae MDR E. coli | Synergistic antibacterial effect | Bacterial efflux pump is targeted along with usual antibacterial action | [22,23] |
| Cefotaxime- AuNPs | 65 nm and spherical | E. coli, K. oxytoca, S. aureus, P. aeruginosa | Marked reduction in MIC concentration was observed against all the tested strains in comparison to free cefotaxime | Cefotaxime-mediated AuNP synthesis, where more than 80% of cefotaxime was loaded and showed potent activity against both gm + ve and gm -ve bacteria | [24] |
| Ceftriaxone- AuNPs | 21 nm and spherical | E. coli, S. aureus, S. abony, K. pneumoniae | Significantly (2-fold) better potential against the tested strains in comparison to free ceftriaxone | Ceftriaxone-mediated AuNP synthesis, where 79% of ceftriaxone was loaded and showed potent activity against both gm +ve and gm -ve bacteria | [25] |
| Cefoxitin- AuNPs | 2–12 nm and spherical | ESBL +ve E. coli, K. pneumoniae | Marked potency against cefoxitin-resistant strains of E. coli and K. pneumoniae | Cefoxitin-mediated AuNP synthesis, where 70% of cefoxitin was loaded and showed potent activity against uropathogenic resistant gram -ve strains | [26] |
| Delafloxacin -AuNPs | 16 nm and spherical | E. coli, P. aeruginosa S. aureus B. subtilis | Potent antibacterial activity against all the tested gram +ve and -ve strains in comparison to free delafloxacin | Delafloxacin-mediated AuNP synthesis, where around 90% of delafloxacin was loaded and showed more potent activity against gram -ve strains as compared to gram +ve strains | [27] |
| Cefaclor- AuNPs | 22 nm and spherical | S. aureus, E. coli | Marked increase in potency against both the tested strains | Cefaclor-mediated AuNP synthesis, where AuNPs form pores in the cell wall and ample cefaclor was available for its antibacterial action | [28] |
| Cefotaxime- AuNPs | 17.55 nm And spherical | CTX-M-15 positive E. coli, K. pneumoniae | Increased potency against CTX-M-15 positive resistant bacterial strains | Cefotaxime was conjugated on bromelain synthesized AuNPs with the help of coupling agent EDC and showed potent activity against resistant gram -ve strains | [29] |
| Imipenem/Meropenem- AuNPs | 35–200 nm | K.pneumoniae, P.mirabilis, A. baumanii | Marked augmentation in antibacterial activity against all the tested strains | Carbapenem antibiotics were loaded on citrate stabilized AuNPs, and reduced the MIC of Imipenem by 4-fold and meropenem by 3-fold | [30] |
| Amoxicillin- AuNPs | 33.9 nm | Methicillin-resistant S. aureus | Enhanced potency against MRSA, and less cytototoxicy in in vivo study | Amoxicillin was loaded on herbal synthesized AuNPs and showed potency to overcome β-lactamase-mediated resistance in MRSA | [31] |
| Vancomycin- AuNPs and Vancomycin AgNPs | 11 nm | Methicillin-resistant S. aureus | 2.4- to 4.8-fold increase in antibacterial activity of AgNPs than AuNPs against MRSA | Vancomycin AgNPs were more effective than vancomycin AuNPs against MRSA | [32] |
| Daptomycin-AuNPs | 80 nm | E. coli, S. aureus | Antibacterial inhibition rates were 64% and 52% for S. aureus and E. coli, respectively | Near-infra-red radiation caused significant photothermal inhibition of bacterial growth | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, S.M.D.; Lila, A.S.A.; Moin, A.; Hussain, T.; Kamal, M.A.; Sonbol, H.; Khafagy, E.-S. Antibiotic-Loaded Gold Nanoparticles: A Nano-Arsenal against ESBL Producer-Resistant Pathogens. Pharmaceutics 2023, 15, 430. https://doi.org/10.3390/pharmaceutics15020430
Rizvi SMD, Lila ASA, Moin A, Hussain T, Kamal MA, Sonbol H, Khafagy E-S. Antibiotic-Loaded Gold Nanoparticles: A Nano-Arsenal against ESBL Producer-Resistant Pathogens. Pharmaceutics. 2023; 15(2):430. https://doi.org/10.3390/pharmaceutics15020430
Chicago/Turabian StyleRizvi, Syed Mohd Danish, Amr Selim Abu Lila, Afrasim Moin, Talib Hussain, Mohammad Amjad Kamal, Hana Sonbol, and El-Sayed Khafagy. 2023. "Antibiotic-Loaded Gold Nanoparticles: A Nano-Arsenal against ESBL Producer-Resistant Pathogens" Pharmaceutics 15, no. 2: 430. https://doi.org/10.3390/pharmaceutics15020430
APA StyleRizvi, S. M. D., Lila, A. S. A., Moin, A., Hussain, T., Kamal, M. A., Sonbol, H., & Khafagy, E.-S. (2023). Antibiotic-Loaded Gold Nanoparticles: A Nano-Arsenal against ESBL Producer-Resistant Pathogens. Pharmaceutics, 15(2), 430. https://doi.org/10.3390/pharmaceutics15020430










