Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy
Abstract
1. Introduction
2. PROTAC for Anticancer Therapy
2.1. Structural Characteristics and MOA of PROTACs
2.2. Pharmacokinetic Limitations of PROTACs
3. Cancer-Specific PROTAC Delivery System
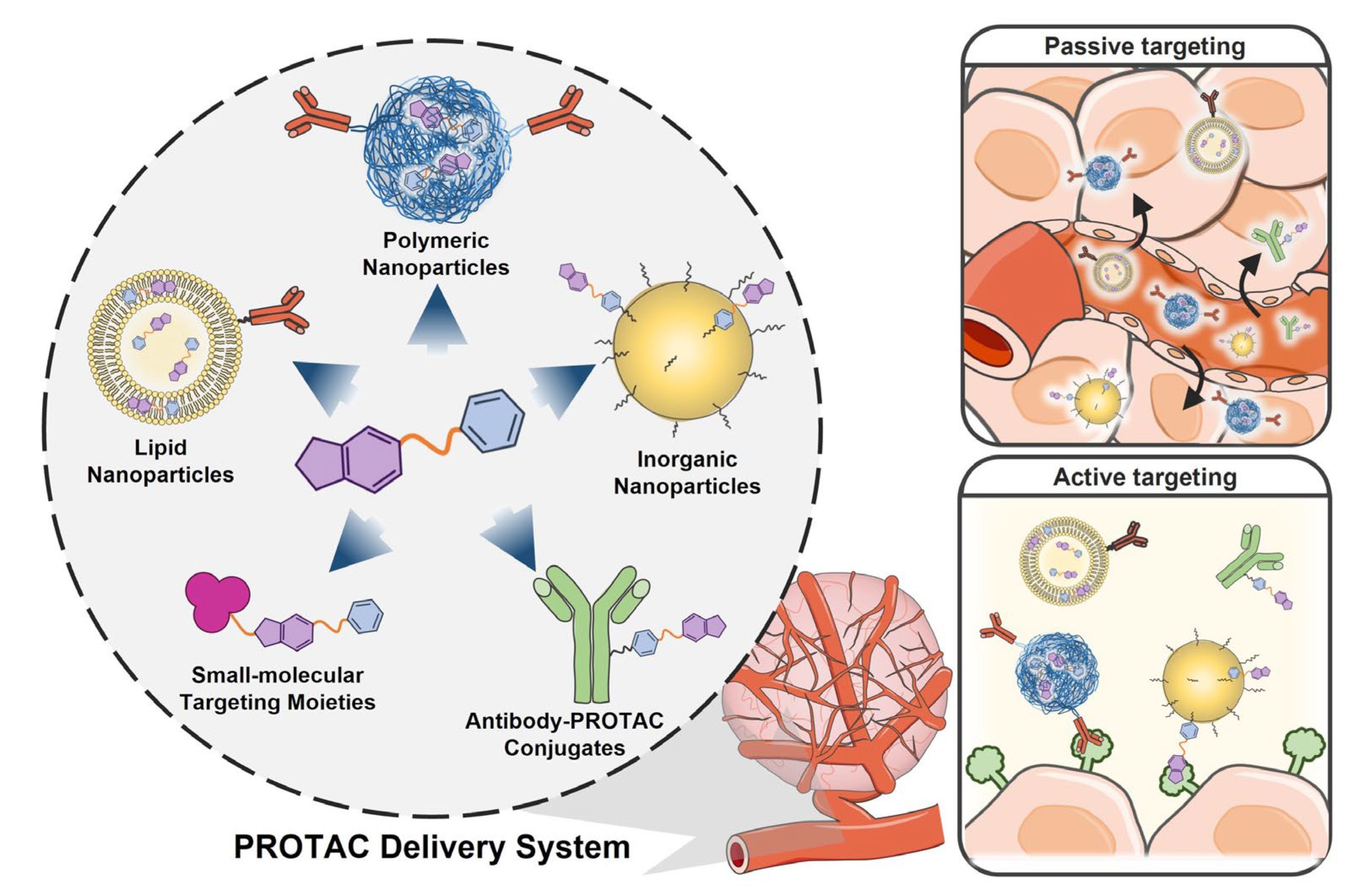
3.1. Nanoparticle-Based Passive Targeting PROTAC Delivery System

3.2. Active Targeting PROTAC Delivery System
3.2.1. Targeting Moiety-PROTAC Conjugates
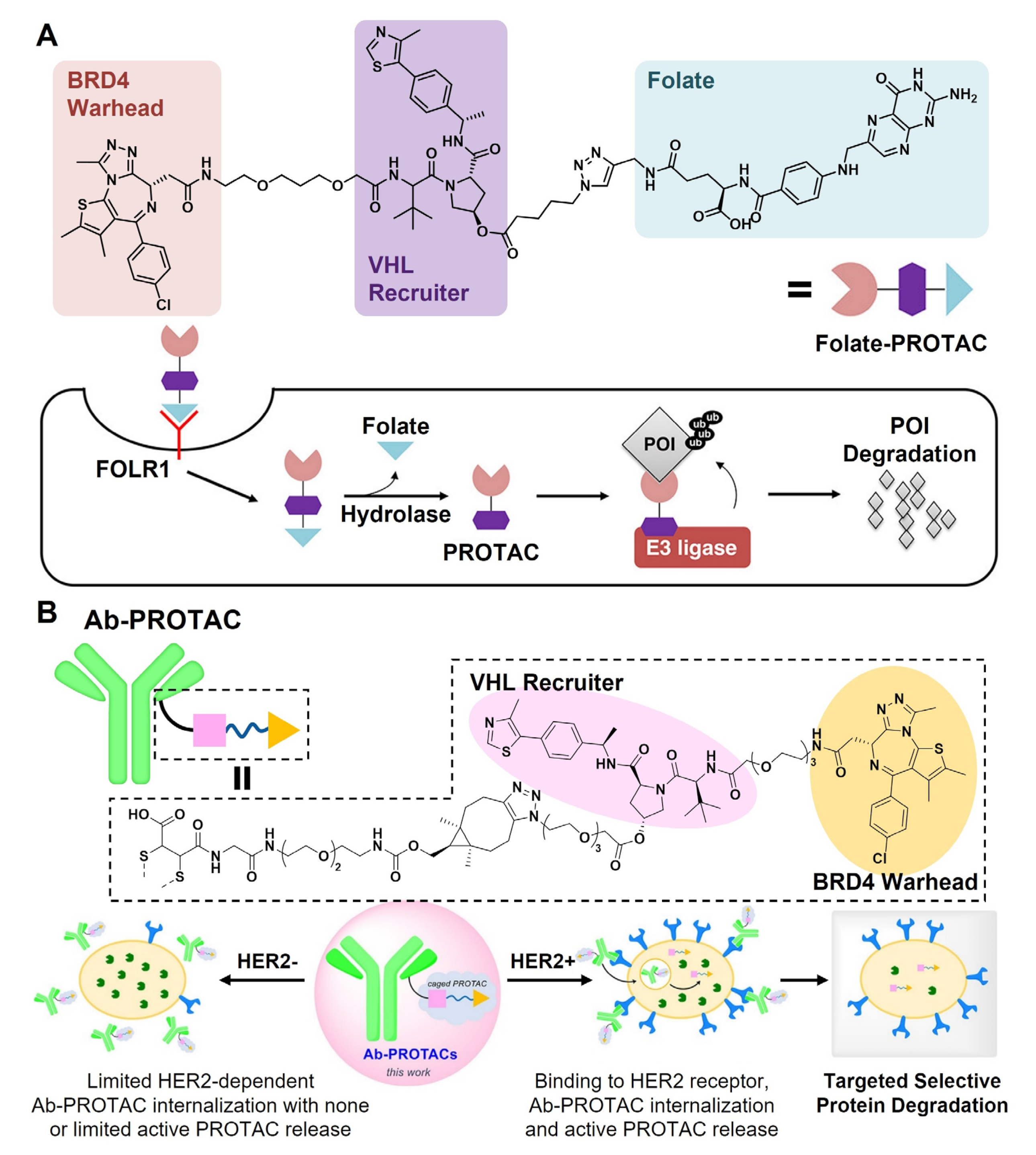
3.2.2. Targeting Moiety-Functionalized Nanoparticles
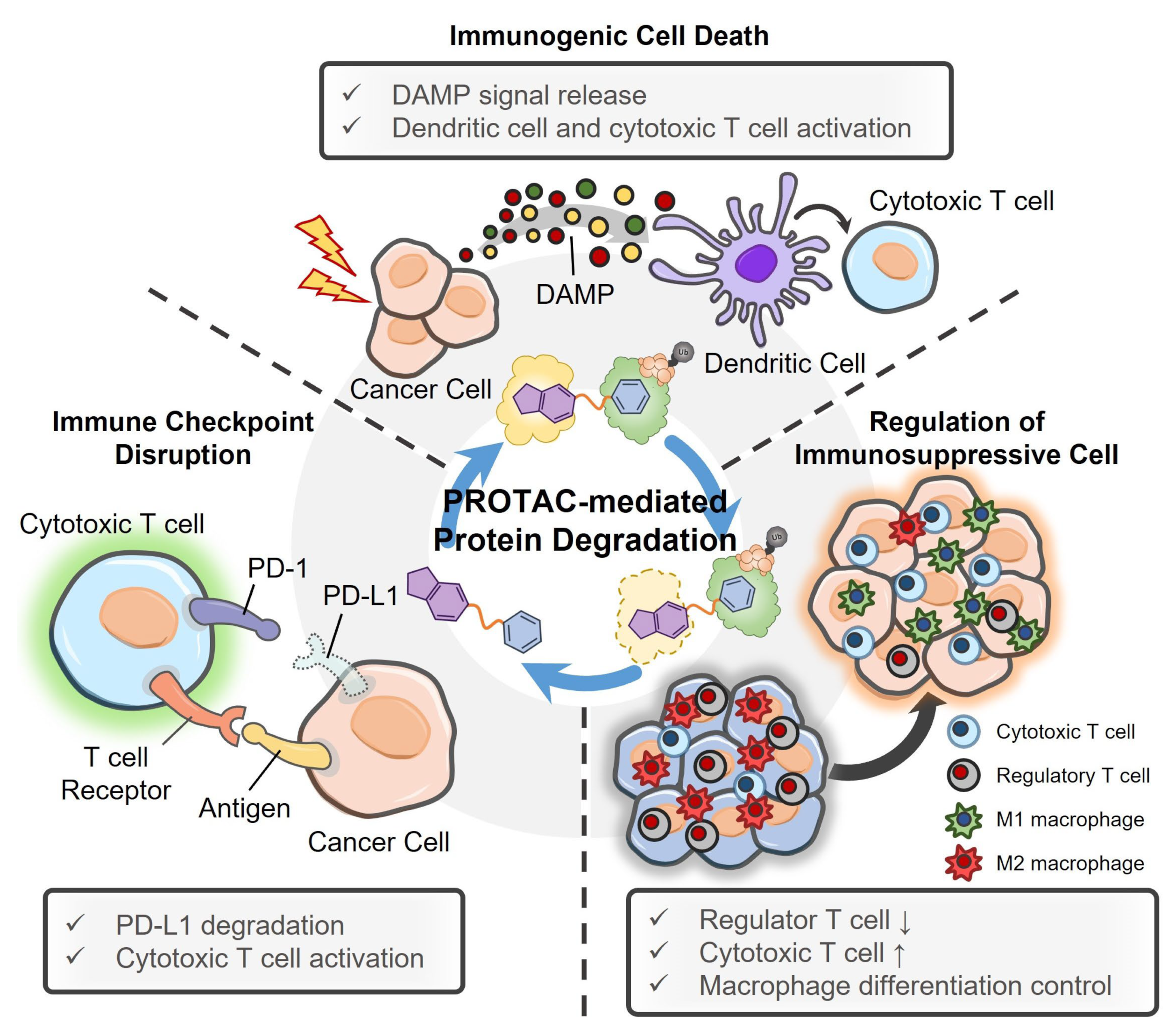
4. PROTACs and Their Delivery System for Cancer Immunotherapy
4.1. PROTACs for Cancer Immunotherapy
4.2. PROTAC Delivery Systems for Cancer Immunotherapy
| POI | E3 Ligase | Results | Ref. |
|---|---|---|---|
| PD-L1 | CRBN | Immune checkpoint degradation,cytotoxic T cell activation | [128] |
| CRBN | [129] | ||
| CRBN, MDM2, cIAP, VHL | [130] | ||
| RNF43 | [131] | ||
| IDO | CRBN | Tryptophan metabolism inhibition,Treg/M2 macrophage inhibition | [139] |
| VHL | |||
| EGFR | VHL | Depletion of IDO and PD-L1 | [145] |
| BET | CRBN | ICD generation | [147] |
| CRBN, MDM2, VHL | MHC-I peptide expression, cytotoxic T cell activation | [148] | |
| NAMPT | VHL | NAM catabolism inhibition, MDSC expansion inhibition | [155] |
| BCL-XL | VHL | Treg inhibition, cytotoxic T cell activation | [158] |
| COX-1/2 | VHL | PGE2 depletion, Treg/M2 macrophage/MDSC inhibition | [160] |
| BRD4 | CRBN | M2 macrophage inhibition | [161] |

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Disis, M.L.; Cheever, M.A. Oncogenic proteins as tumor antigens. Curr. Opin. Immunol. 1996, 8, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Goodell, V.; Salazar, L.G.; Urban, N.; Drescher, C.W.; Gray, H.; Swensen, R.E.; McIntosh, M.W.; Disis, M.L. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J. Clin. Oncol. 2006, 24, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Maddalo, D.; Manchado, E.; Concepcion, C.P.; Bonetti, C.; Vidigal, J.A.; Han, Y.-C.; Ogrodowski, P.; Crippa, A.; Rekhtman, N.; de Stanchina, E. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature 2014, 516, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Lepourcelet, M.; Chen, Y.-N.P.; France, D.S.; Wang, H.; Crews, P.; Petersen, F.; Bruseo, C.; Wood, A.W.; Shivdasani, R.A. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell 2004, 5, 91–102. [Google Scholar] [CrossRef]
- Sioud, M. Therapeutic siRNAs. Trends Pharmacol. Sci. 2004, 25, 22–28. [Google Scholar] [CrossRef]
- Neklesa, T.K.; Winkler, J.D.; Crews, C.M. Targeted protein degradation by PROTACs. Pharmacol. Ther. 2017, 174, 138–144. [Google Scholar] [CrossRef]
- Zhang, B. CRISPR/Cas gene therapy. J. Cell. Physiol. 2021, 236, 2459–2481. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Gao, F.; Han, S.; Cheah, K.S.; Tse, H.-F.; Lian, Q. CRISPR/Cas9 genome-editing system in human stem cells: Current status and future prospects. Mol. Ther.-Nucleic Acids 2017, 9, 230–241. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Gu, S.; Cui, D.; Chen, X.; Xiong, X.; Zhao, Y. PROTACs: An emerging targeting technique for protein degradation in drug discovery. BioEssays 2018, 40, 1700247. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Casement, R.; Bond, A.; Craigon, C.; Ciulli, A. Mechanistic and structural features of PROTAC ternary complexes. In Targeted Protein Degradation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 79–113. [Google Scholar]
- Chen, Y.; Tandon, I.; Heelan, W.; Wang, Y.; Tang, W.; Hu, Q. Proteolysis-targeting chimera (PROTAC) delivery system: Advancing protein degraders towards clinical translation. Chem. Soc. Rev. 2022, 51, 5330–5350. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.; Lartigue, S.; Dong, H.; Qian, Y.; Crews, C.M. MDM2-recruiting PROTAC offers superior, synergistic antiproliferative activity via simultaneous degradation of BRD4 and stabilization of p53. Cancer Res. 2019, 79, 251–262. [Google Scholar] [CrossRef]
- Samarasinghe, K.T.; Crews, C.M. Targeted protein degradation: A promise for undruggable proteins. Cell Chem. Biol. 2021, 28, 934–951. [Google Scholar] [CrossRef]
- Zeng, S.; Huang, W.; Zheng, X.; Zhang, Z.; Wang, J.; Shen, Z. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges. Eur. J. Med. Chem. 2021, 210, 112981. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Lei, Q.; Zhang, Y. Tumor-intrinsic signaling pathways: Key roles in the regulation of the immunosuppressive tumor microenvironment. J. Hematol. Oncol. 2019, 12, 125. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: A review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef]
- Li, X.; Ma, S.; Deng, Y.; Yi, P.; Yu, J. Targeting the RNA m6A modification for cancer immunotherapy. Mol. Cancer 2022, 21, 76. [Google Scholar] [CrossRef]
- Coelho, M.A.; de Carné Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 2017, 47, 1083–1099.e1086. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Xiang, Y.; Kang, X. Small-Molecule PROTACs for Cancer Immunotherapy. Molecules 2022, 27, 5439. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, L.; Tong, D.; Yang, L.; Zhu, H.; Li, Q.; Zhang, F. BET bromodomain inhibitor JQ1 promotes immunogenic cell death in tongue squamous cell carcinoma. Int. Immunopharmacol. 2019, 76, 105921. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, C.; Pannilunghi, S.; Tardy, S.; Scapozza, L. From conception to development: Investigating PROTACs features for improved cell permeability and successful protein degradation. Front. Chem. 2021, 9, 672267. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. The PROTAC gold rush. Nat. Biotechnol 2022, 40, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, M.; Yang, Z.; Li, J.; Gao, Y.; Tan, R. Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy: Present and Future. Molecules 2022, 27, 8828. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.; Cai, X.; Cao, D.; Du, H.; Shen, C.; Deng, Y.; He, Q.; Yang, B.; Li, D.; Hou, T. PROTAC-DB 2.0: An updated database of PROTACs. Nucleic Acids Res. 2022, 51, D1367–D1372. [Google Scholar] [CrossRef]
- Simpson, L.M.; Glennie, L.; Brewer, A.; Zhao, J.-F.; Crooks, J.; Shpiro, N.; Sapkota, G.P. Target protein localization and its impact on PROTAC-mediated degradation. Cell Chem. Biol. 2022, 29, 1482–1504.e1487. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Xu, J.; Tang, L.; Zhang, Y.; Du, F.; Zhao, Y.; Wu, X.; Li, M.; Shen, J. PROTACs in gastrointestinal cancers. Mol. Ther.-Oncolytics 2022, 27, 204–223. [Google Scholar] [CrossRef]
- Ruffilli, C.; Roth, S.; Rodrigo, M.; Boyd, H.; Zelcer, N.; Moreau, K. Proteolysis Targeting Chimeras (PROTACs): A Perspective on Integral Membrane Protein Degradation. ACS Pharmacol. Transl. Sci. 2022, 5, 849–858. [Google Scholar] [CrossRef]
- Salama, A.K.; Trkulja, M.V.; Casanova, E.; Uras, I.Z. Targeted Protein Degradation: Clinical Advances in the Field of Oncology. Int. J. Mol. Sci. 2022, 23, 15440. [Google Scholar] [CrossRef]
- Doroshow, D.; Eder, J.; LoRusso, P. BET inhibitors: A novel epigenetic approach. Ann. Oncol. 2017, 28, 1776–1787. [Google Scholar] [CrossRef]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef]
- Saraswat, A.L.; Vartak, R.; Hegazy, R.; Patel, A.; Patel, K. Drug delivery challenges and formulation aspects of proteolysis targeting chimera (PROTACs). Drug Discov. Today 2022, 28, 103387. [Google Scholar] [CrossRef]
- Rathod, D.; Fu, Y.; Patel, K. BRD4 PROTAC as a novel therapeutic approach for the treatment of vemurafenib resistant melanoma: Preformulation studies, formulation development and in vitro evaluation. Eur. J. Pharm. Sci. 2019, 138, 105039. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, C.; Wang, H.; Zhang, L.; Liu, Z.; Xu, P. The state of the art of PROTAC technologies for drug discovery. Eur. J. Med. Chem. 2022, 114290. [Google Scholar] [CrossRef]
- Klein, V.G.; Bond, A.G.; Craigon, C.; Lokey, R.S.; Ciulli, A. Amide-to-ester substitution as a strategy for optimizing PROTAC permeability and cellular activity. J. Med. Chem. 2021, 64, 18082–18101. [Google Scholar] [CrossRef]
- Jaime-Figueroa, S.; Buhimschi, A.D.; Toure, M.; Hines, J.; Crews, C.M. Design, synthesis and biological evaluation of Proteolysis Targeting Chimeras (PROTACs) as a BTK degraders with improved pharmacokinetic properties. Bioorg. Med. Chem. Lett. 2020, 30, 126877. [Google Scholar] [CrossRef]
- Cecchini, C.; Tardy, S.; Scapozza, L. Linkers as Game-changers in PROTAC Technology: Emphasizing General Trends in PROTAC Pharmacokinetics for their Rational Design. CHIMIA 2022, 76, 341. [Google Scholar] [CrossRef]
- Hu, J.; Johnston, K.P.; Williams, R.O., III. Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev. Ind. Pharm. 2004, 30, 233–245. [Google Scholar] [CrossRef]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C 2017, 78, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Williams, D.; Dash, A.; Leslie-Pelecky, D.; Labhasetwar, V. Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J. Pharm. Sci. 2004, 93, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, M.; Wang, Q.; Li, Y.; Guo, R.; Jiang, X.; Yang, C.; Liu, B. 10-Hydroxycamptothecin loaded nanoparticles: Preparation and antitumor activity in mice. J. Control. Release 2007, 119, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Liu, L.; Xue, X.; Liang, X.-J. Nanoparticle-based drug delivery systems: What can they really do in vivo? F1000Research 2017, 6, 681. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Kim, D.-W.; Chung, J.-Y.; Shin, S.G.; Kim, S.-C.; Heo, D.S.; Kim, N.K.; Bang, Y.-J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef]
- Working, P.; Newman, M.; Huang, S.; Mayhew, E.; Vaage, J.; Lasic, D. Pharmacokinetics, biodistribution and therapeutic efficacy of doxorubicin encapsulated in Stealth® liposomes (Doxil®). J. Liposome Res. 1994, 4, 667–687. [Google Scholar] [CrossRef]
- Soundararajan, A.; Bao, A.; Phillips, W.T.; Perez III, R.; Goins, B.A. [186Re] Liposomal doxorubicin (Doxil): In vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl. Med. Biol. 2009, 36, 515–524. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y. Nanoparticle-based medicines in clinical cancer therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular uptake of nanoparticles versus small molecules: A matter of size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Kleusch, C.; Hersch, N.; Hoffmann, B.; Merkel, R.; Csiszár, A. Fluorescent lipids: Functional parts of fusogenic liposomes and tools for cell membrane labeling and visualization. Molecules 2012, 17, 1055–1073. [Google Scholar] [CrossRef]
- Colson, Y.L.; Grinstaff, M.W. Biologically responsive polymeric nanoparticles for drug delivery. Adv. Mater. 2012, 24, 3878–3886. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, W.S.; Kim, J.H. Surface modification of functional nanoparticles for controlled drug delivery. J. Dispers. Sci. Technol. 2003, 24, 475–487. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Van Vlerken, L.E.; Amiji, M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Eskandari, Z.; Kazdal, F.; Bahadori, F.; Ebrahimi, N. Quality-by-design model in optimization of PEG-PLGA nano micelles for targeted cancer therapy. J. Drug Deliv. Sci. Technol. 2018, 48, 393–402. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA–PEG block copolymer. J. Control. Release 2001, 70, 63–70. [Google Scholar] [CrossRef]
- Saraswat, A.; Patki, M.; Fu, Y.; Barot, S.; Dukhande, V.V.; Patel, K. Nanoformulation of PROteolysis TArgeting Chimera targeting ‘undruggable’c-Myc for the treatment of pancreatic cancer. Nanomedicine 2020, 15, 1761–1777. [Google Scholar] [CrossRef]
- Minko, T. Nanoformulation of BRD4-degrading PROTAC: Improving druggability to target the ‘undruggable’MYC in pancreatic cancer. Trends Pharmacol. Sci. 2020, 41, 684–686. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef]
- Shim, J.; Kim, M.J.; Kim, H.-K.; Kim, D.-H.; Oh, S.G.; Ko, S.Y.; Jang, H.G.; Kim, J.-W. Morphological effect of lipid carriers on permeation of lidocaine hydrochloride through lipid membranes. Int. J. Pharm. 2010, 388, 251–256. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid nanoparticles for drug delivery. Adv. NanoBiomed. Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Vartak, R.; Saraswat, A.; Yang, Y.; Chen, Z.-S.; Patel, K. Susceptibility of Lung Carcinoma Cells to Nanostructured Lipid Carrier of ARV-825, a BRD4 Degrading Proteolysis Targeting Chimera. Pharm. Res. 2022, 39, 2745–2759. [Google Scholar] [CrossRef]
- Fu, Y.; Saraswat, A.; Wei, Z.; Agrawal, M.Y.; Dukhande, V.V.; Reznik, S.E.; Patel, K. Development of Dual ARV-825 and Nintedanib-Loaded PEGylated Nano-Liposomes for Synergistic Efficacy in Vemurafnib-Resistant Melanoma. Pharmaceutics 2021, 13, 1005. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, M.; Ma, F.; Yang, L.; Glass, Z.; Xu, Q. Enhanced protein degradation by intracellular delivery of pre-fused PROTACs using lipid-like nanoparticles. J. Control. Release 2021, 330, 1244–1249. [Google Scholar] [CrossRef]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Liu, F.; Yang, F.; Jiang, X.; Sun, H.; Feng, F.; Xue, J.; Liu, W. Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras. Colloids Surf. B Biointerfaces 2020, 188, 110795. [Google Scholar] [CrossRef]
- Yan, S.; Yan, J.; Liu, D.; Li, X.; Kang, Q.; You, W.; Zhang, J.; Wang, L.; Tian, Z.; Lu, W. A nano-predator of pathological MDMX construct by clearable supramolecular gold (I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics 2021, 11, 6833. [Google Scholar] [CrossRef]
- Atilaw, Y.; Poongavanam, V.; Svensson Nilsson, C.; Nguyen, D.; Giese, A.; Meibom, D.; Erdelyi, M.; Kihlberg, J. Solution conformations shed light on PROTAC cell permeability. ACS Med. Chem. Lett. 2020, 12, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Farnaby, W.; Koegl, M.; Roy, M.J.; Whitworth, C.; Diers, E.; Trainor, N.; Zollman, D.; Steurer, S.; Karolyi-Oezguer, J.; Riedmueller, C. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat. Chem. Biol. 2019, 15, 672–680. [Google Scholar] [CrossRef]
- Verma, R.; Mohl, D.; Deshaies, R.J. Harnessing the power of proteolysis for targeted protein inactivation. Mol. Cell 2020, 77, 446–460. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Drug Deliv. 2010, 197, 3–53. [Google Scholar]
- Liu, J.; Chen, H.; Liu, Y.; Shen, Y.; Meng, F.; Kaniskan, H.U.; Jin, J.; Wei, W. Cancer selective target degradation by folate-caged PROTACs. J. Am. Chem. Soc. 2021, 143, 7380–7387. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Janeway Jr, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. The structure of a typical antibody molecule. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody–drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef]
- Buecheler, J.W.; Winzer, M.; Weber, C.; Gieseler, H. Alteration of physicochemical properties for antibody-drug conjugates and their impact on stability. J. Pharm. Sci. 2020, 109, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody–drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, U.; Parakh, S.; Gan, H.K.; Scott, A.M. Antibody–drug conjugates for cancer therapy. Molecules 2020, 25, 4764. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.T.; Harris, P.W.; Brimble, M.A.; Kavianinia, I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef]
- Chia, C.B. A Patent Review on FDA-Approved Antibody-Drug Conjugates, Their Linkers and Drug Payloads. ChemMedChem 2022, e202200032. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, P.S.; Adhikari, P.; Blake, R.A.; Blaquiere, N.; Chen, J.; Cheng, Y.-X.; den Besten, W.; Han, J.; Hartman, S.J.; He, J. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα). Bioorg. Med. Chem. Lett. 2020, 30, 126907. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Pillow, T.H.; Blake, R.A.; Sadowsky, J.D.; Adaligil, E.; Adhikari, P.; Bhakta, S.; Blaquiere, N.; Chen, J.; dela Cruz-Chuh, J. Antibody-mediated delivery of chimeric BRD4 degraders. Part 1: Exploration of antibody linker, payload loading, and payload molecular properties. J. Med. Chem. 2021, 64, 2534–2575. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Pillow, T.H.; Blake, R.A.; Sadowsky, J.D.; Adaligil, E.; Adhikari, P.; Chen, J.; Corr, N.; dela Cruz-Chuh, J.; Del Rosario, G. Antibody-mediated delivery of chimeric BRD4 degraders. Part 2: Improvement of in vitro antiproliferation activity and in vivo antitumor efficacy. J. Med. Chem. 2021, 64, 2576–2607. [Google Scholar] [CrossRef]
- Maneiro, M.a.; Forte, N.; Shchepinova, M.M.; Kounde, C.S.; Chudasama, V.; Baker, J.R.; Tate, E.W. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem. Biol. 2020, 15, 1306–1312. [Google Scholar] [CrossRef]
- Alshaer, W.; Hillaireau, H.; Fattal, E. Aptamer-guided nanomedicines for anticancer drug delivery. Adv. Drug Deliv. Rev. 2018, 134, 122–137. [Google Scholar] [CrossRef]
- He, F.; Wen, N.; Xiao, D.; Yan, J.; Xiong, H.; Cai, S.; Liu, Z.; Liu, Y. Aptamer-based targeted drug delivery systems: Current potential and challenges. Curr. Med. Chem. 2020, 27, 2189–2219. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Ye, M.; Jiang, J.; Yang, R.; Fu, T.; Chen, Y.; Wang, K.; Liu, C.; Tan, W. Aptamer-conjugated nanomaterials and their applications. Adv. Drug Deliv. Rev. 2011, 63, 1361–1370. [Google Scholar] [CrossRef]
- Nimjee, S.M.; Rusconi, C.P.; Sullenger, B.A. APTAMERS: AN EMERGING CLASS OF. Annu. Rev. Med 2005, 56, 555–583. [Google Scholar] [CrossRef]
- Kruspe, S.; Mittelberger, F.; Szameit, K.; Hahn, U. Aptamers as drug delivery vehicles. ChemMedChem 2014, 9, 1998–2011. [Google Scholar] [CrossRef]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef]
- Zhu, H.; Li, J.; Zhang, X.B.; Ye, M.; Tan, W. Nucleic acid aptamer-mediated drug delivery for targeted cancer therapy. ChemMedChem 2015, 10, 39–45. [Google Scholar] [CrossRef]
- He, S.; Gao, F.; Ma, J.; Ma, H.; Dong, G.; Sheng, C. Aptamer-protac conjugates (apcs) for tumor-specific targeting in breast cancer. Angew. Chem. Int. Ed. 2021, 60, 23299–23305. [Google Scholar] [CrossRef]
- Sun, H.; Zu, Y. A highlight of recent advances in aptamer technology and its application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Wang, X.; Liu, H.; Zhang, Y.; Xie, T.; Zhang, H.; Li, X.; Peng, T.; Sun, X. Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin. Mol. Ther.-Nucleic Acids 2022, 30, 66–79. [Google Scholar] [CrossRef]
- Vasaturo, M.; Cotugno, R.; Fiengo, L.; Vinegoni, C.; Dal Piaz, F.; De Tommasi, N. The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, X. Roles of nucleolin: Focus on cancer and anti-cancer therapy. Saudi Med. J. 2016, 37, 1312. [Google Scholar] [CrossRef] [PubMed]
- Mckertish, C.M.; Kayser, V. Advances and limitations of antibody drug conjugates for cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A. Antibody drug conjugates: Lessons from 20 years of clinical experience. Ann. Oncol. 2016, 27, 2168–2172. [Google Scholar] [CrossRef]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-López, M.d.M.; Bravo, I.; Lara-Sanchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled delivery of BET-PROTACs: In vitro evaluation of MZ1-loaded polymeric antibody conjugated nanoparticles in breast cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef]
- He, Y.; Zan, X.; Miao, J.; Wang, B.; Wu, Y.; Shen, Y.; Chen, X.; Gou, H.; Zheng, S.; Huang, N. Enhanced anti-glioma efficacy of doxorubicin with BRD4 PROTAC degrader using targeted nanoparticles. Mater. Today Bio 2022, 16, 100423. [Google Scholar] [CrossRef]
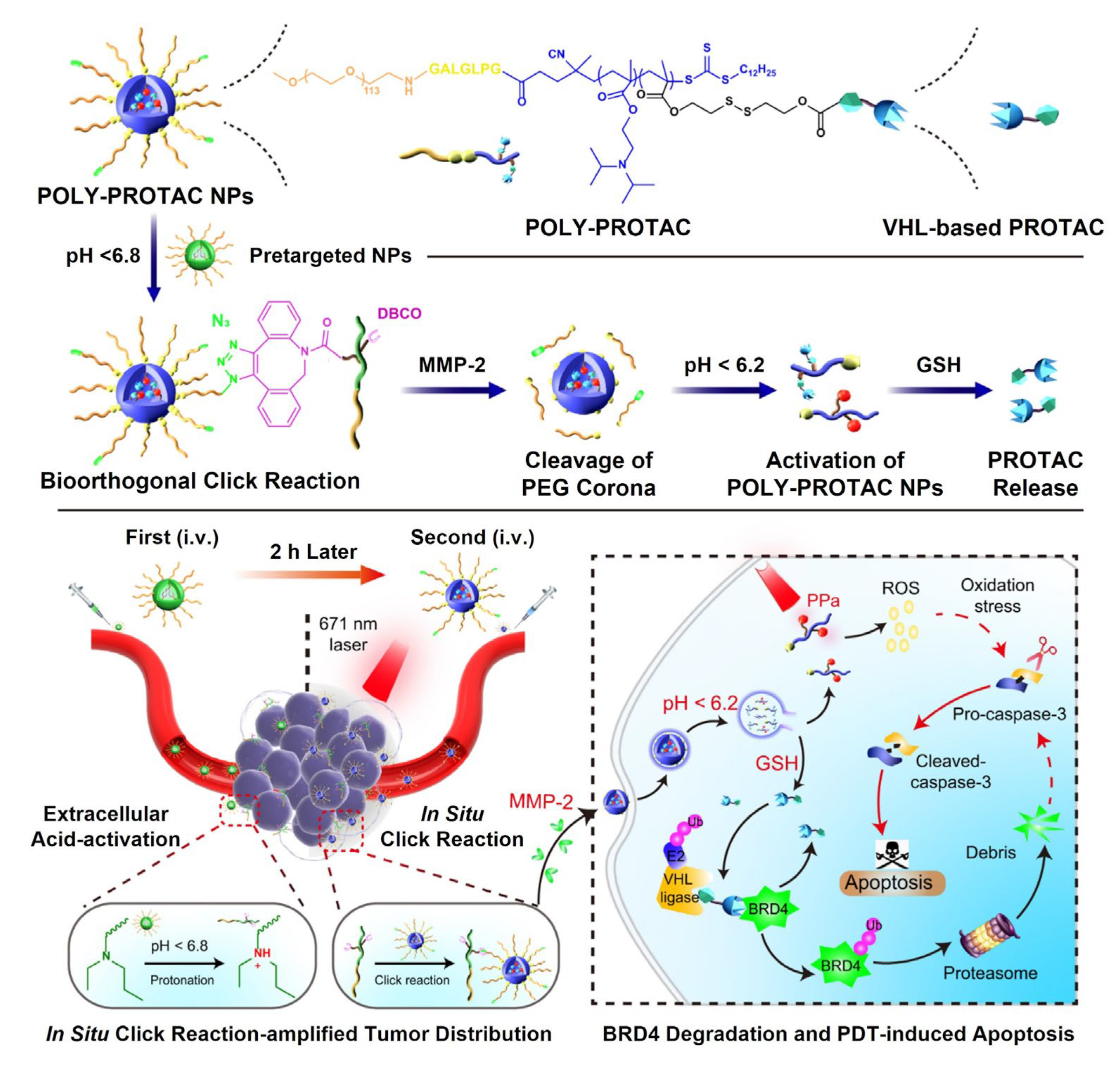
- Gao, J.; Hou, B.; Zhu, Q.; Yang, L.; Jiang, X.; Zou, Z.; Li, X.; Xu, T.; Zheng, M.; Chen, Y.-H. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumour-specific protein degradation and precise cancer therapy. Nat. Commun. 2022, 13, 4318. [Google Scholar] [CrossRef]
- Saraswat, A.; Vemana, H.P.; Dukhande, V.V.; Patel, K. Galactose-decorated liver tumor-specific nanoliposomes incorporating selective BRD4-targeted PROTAC for hepatocellular carcinoma therapy. Heliyon 2022, 8, e08702. [Google Scholar] [CrossRef]
- Fan, R.; He, S.; Wang, Y.; Qiao, J.; Liu, H.; Galstyan, L.; Ghazaryan, A.; Cai, H.; Feng, S.; Ni, P. Targeted delivery of a PROTAC induced PDEδ degrader by a biomimetic drug delivery system for enhanced cytotoxicity against pancreatic cancer cells. Am. J. Cancer Res. 2022, 12, 1027. [Google Scholar]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Lim, S.-O.; Yamaguchi, H. Oncogenic signaling pathways associated with immune evasion and resistance to immune checkpoint inhibitors in cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 51–64. [Google Scholar]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016, 9, 5023. [Google Scholar]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Cheng, B.; Ren, Y.; Cao, H.; Chen, J. Discovery of novel resorcinol diphenyl ether-based PROTAC-like molecules as dual inhibitors and degraders of PD-L1. Eur. J. Med. Chem. 2020, 199, 112377. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Cao, S.; Sun, Y.; Dong, Z.; Li, C.; Wang, H.; Yao, Y.; Yu, H.; Song, X. In vitro and in vivo degradation of programmed cell death ligand 1 (PD-L1) by a proteolysis targeting chimera (PROTAC). Bioorg. Chem. 2021, 111, 104833. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, M.; Ma, Z.; Zhou, Y.; Huo, J.; Zhang, W.; Liu, Y.; Guo, Y.; Zhou, X.; Li, H. Design, synthesis, and evaluation of PD-L1 degraders to enhance T cell killing activity against melanoma. Chin. Chem. Lett. 2022, 107762. [Google Scholar] [CrossRef]
- Cotton, A.D.; Nguyen, D.P.; Gramespacher, J.A.; Seiple, I.B.; Wells, J.A. Development of antibody-based PROTACs for the degradation of the cell-surface immune checkpoint protein PD-L1. J. Am. Chem. Soc. 2021, 143, 593–598. [Google Scholar] [CrossRef]
- Prendergast, G. Immune escape as a fundamental trait of cancer: Focus on IDO. Oncogene 2008, 27, 3889–3900. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Mullard, A. IDO takes a blow. Nat. Rev. Drug Discov. 2018, 17, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Dill, E.A.; Dillon, P.M.; Bullock, T.N.; Mills, A.M. IDO expression in breast cancer: An assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod. Pathol. 2018, 31, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Labadie, B.W.; Bao, R.; Luke, J.J. Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan–Kynurenine–Aryl Hydrocarbon AxisTrp–Kyn–AhR Immunotherapy. Clin. Cancer Res. 2019, 25, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Belladonna, M.L.; Puccetti, P.; Orabona, C.; Fallarino, F.; Vacca, C.; Volpi, C.; Gizzi, S.; Pallotta, M.T.; Fioretti, M.C.; Grohmann, U. Immunosuppression via tryptophan catabolism: The role of kynurenine pathway enzymes. Transplantation 2007, 84, S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural Transm. 2012, 119, 197–209. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, W.; Wang, Y.; Yao, D.; Ye, T.; Yao, Y.; Chen, B.; Liu, G.; Yang, X.; Wang, W. Discovery of the first potent proteolysis targeting chimera (PROTAC) degrader of indoleamine 2, 3-dioxygenase 1. Acta Pharm. Sin. B 2020, 10, 1943–1953. [Google Scholar] [CrossRef]
- Stewart, E.L.; Tan, S.Z.; Liu, G.; Tsao, M.-S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations—A review. Transl. Lung Cancer Res. 2015, 4, 67. [Google Scholar]
- Fraguas, S.; Barberán, S.; Cebrià, F. EGFR signaling regulates cell proliferation, differentiation and morphogenesis during planarian regeneration and homeostasis. Dev. Biol. 2011, 354, 87–101. [Google Scholar] [CrossRef]
- Regales, L.; Gong, Y.; Shen, R.; de Stanchina, E.; Vivanco, I.; Goel, A.; Koutcher, J.A.; Spassova, M.; Ouerfelli, O.; Mellinghoff, I.K. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J. Clin. Investig. 2009, 119, 3000–3010. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, W.; Zhang, Y.; Hong, S.; Kang, S.; Yan, Y.; Chen, N.; Zhan, J.; He, X.; Qin, T. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015, 6, 14209. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Cheng, L.; Jin, J.; Kong, F.-M.S. Coexpression patterns of IDO-1, PD-L1 and EGFR in non-small cell lung cancer. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, H. Proteolysis targeting chimera (PROTAC) for epidermal growth factor receptor enhances anti-tumor immunity in non-small cell lung cancer. Drug Dev. Res. 2021, 82, 422–429. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Qin, C.; Bai, L.; Wang, S. Small-molecule PROTAC degraders of the Bromodomain and Extra Terminal (BET) proteins—A review. Drug Discov. Today Technol. 2019, 31, 43–51. [Google Scholar] [CrossRef]
- Tong, J.; Tan, X.; Risnik, D.; Gao, M.; Song, X.; Ermine, K.; Shen, L.; Wang, S.; Yu, J.; Zhang, L. BET protein degradation triggers DR5-mediated immunogenic cell death to suppress colorectal cancer and potentiate immune checkpoint blockade. Oncogene 2021, 40, 6566–6578. [Google Scholar] [CrossRef]
- Jensen, S.M.; Potts, G.K.; Ready, D.B.; Patterson, M.J. Specific MHC-I peptides are induced using PROTACs. Front. Immunol. 2018, 9, 2697. [Google Scholar] [CrossRef]
- Taylor, B.C.; Balko, J.M. Mechanisms of MHC-I Downregulation and Role in Immunotherapy Response. Front. Immunol. 2022, 13, 844866. [Google Scholar] [CrossRef]
- Zhu, H.; Bengsch, F.; Svoronos, N.; Rutkowski, M.R.; Bitler, B.G.; Allegrezza, M.J.; Yokoyama, Y.; Kossenkov, A.V.; Bradner, J.E.; Conejo-Garcia, J.R. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016, 16, 2829–2837. [Google Scholar] [CrossRef]
- Hogg, S.J.; Vervoort, S.J.; Deswal, S.; Ott, C.J.; Li, J.; Cluse, L.A.; Beavis, P.A.; Darcy, P.K.; Martin, B.P.; Spencer, A. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep. 2017, 18, 2162–2174. [Google Scholar] [CrossRef]
- Imai, S.-I. Nicotinamide phosphoribosyltransferase (Nampt): A link between NAD biology, metabolism, and diseases. Curr. Pharm. Des. 2009, 15, 20–28. [Google Scholar] [CrossRef]
- Shackelford, R.E.; Mayhall, K.; Maxwell, N.M.; Kandil, E.; Coppola, D. Nicotinamide phosphoribosyltransferase in malignancy: A review. Genes Cancer 2013, 4, 447–456. [Google Scholar] [CrossRef]
- Galli, U.; Colombo, G.; Travelli, C.; Tron, G.C.; Genazzani, A.A.; Grolla, A.A. Recent advances in NAMPT inhibitors: A novel immunotherapic strategy. Front. Pharmacol. 2020, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pu, C.; Fu, Y.; Dong, G.; Huang, M.; Sheng, C. NAMPT-targeting PROTAC promotes antitumor immunity via suppressing myeloid-derived suppressor cell expansion. Acta Pharm. Sin. B 2022, 12, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yang, Y.; Xing, D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011, 278, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Susnow, N.; Zeng, L.; Margineantu, D.; Hockenbery, D.M. Bcl-2 family proteins as regulators of oxidative stress. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2009; pp. 42–49. [Google Scholar]
- Kolb, R.; De, U.; Khan, S.; Luo, Y.; Kim, M.-C.; Yu, H.; Wu, C.; Mo, J.; Zhang, X.; Zhang, P. Proteolysis-targeting chimera against BCL-XL destroys tumor-infiltrating regulatory T cells. Nat. Commun. 2021, 12, 1281. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, Z.; Cui, D.; He, S.; Jiang, Y.; Li, J.; Huang, J.; Pu, K. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat. Commun. 2021, 12, 2934. [Google Scholar] [CrossRef]
- Zhang, C.; He, S.; Zeng, Z.; Cheng, P.; Pu, K. Smart Nano-PROTACs Reprogram Tumor Microenvironment for Activatable Photo-metabolic Cancer Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202114957. [Google Scholar]
- Yang, T.; Hu, Y.; Miao, J.; Chen, J.; Liu, J.; Cheng, Y.; Gao, X. A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation, apoptosis and M2 macrophages polarization. Acta Pharm. Sin. B 2022, 12, 2658–2671. [Google Scholar] [CrossRef]

| POI | Name | E3 Ligase | Indication | Clinical Phase | Ref. |
|---|---|---|---|---|---|
| Androgen receptor(AR) | AC176 | N/A | Prostate cancer | Phase I | NCT05241613 |
| ARV-110 | CRBN | Phase II | NCT03888612 | ||
| ARV-766 | VHL | Phase II | NCT05067140 | ||
| CC-94676 | CRBN | Phase I | NCT04428788 | ||
| HP518 | N/A | Phase I | NCT05252364 | ||
| B-cell lymphoma-extra large (BCL-XL) | DT2216 | VHL | Solid tumor, Hematologic malignancy | Phase I | NCT04886622 |
| Bromodomain 4 (BRD4) | RNK05047 | N/A | Advanced solid tumor, Diffuse large B cell lymphoma (DLBCL) | Phase I/II | NCT05487170 |
| Bromodomain 9 (BRD9) | CFT8634 | CRBN | Synovial sarcoma, Soft tissue sarcoma | Phase I/II | NCT05355753 |
| FHD-609 | CRBN | Phase I | NCT04965753 | ||
| Bruton’s tyrosine kinase (BTK) | BGB-16673 | N/A | B cell malignancies | Phase I | NCT05006716 |
| HSK29116 | CRBN | Phase I | NCT04861779 | ||
| NX-2127 | CRBN | Phase I | NCT04830137 | ||
| NX-5948 | CRBN | Phase I | NCT05131022 | ||
| Epidermal growth factor receptor (EGFR) | CFT8919 | CRBN | Non-small cell lung cancer (NSCLC) | IND-e | N/A |
| Estrogen receptor (ER) | ARV-471 | CRBN | Breast cancer | Phase II | NCT04072952 |
| AC682 | CRBN | Phase I | NCT05080842 | ||
| Interleukin-1 receptor-associated kinase 4 (IRAK4) | KT-413 | CRBN | DLBCL | Phase I | NCT05233033 |
| KRAS-G12D | ASP3082 | N/A | Solid tumor | Phase I | NCT05382559 |
| Signal transducer and activator of transcription 3 (STAT3) | KT-333 | N/A | Liquid and solid tumors | Phase I | NCT05225584 |
| Tyrosine receptor kinase (TRK) | CG001419 | CRBN | Cancers and other indications | IND-e | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.; Jeon, S.I.; Shim, M.K.; Kim, K. Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy. Pharmaceutics 2023, 15, 411. https://doi.org/10.3390/pharmaceutics15020411
Moon Y, Jeon SI, Shim MK, Kim K. Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy. Pharmaceutics. 2023; 15(2):411. https://doi.org/10.3390/pharmaceutics15020411
Chicago/Turabian StyleMoon, Yujeong, Seong Ik Jeon, Man Kyu Shim, and Kwangmeyung Kim. 2023. "Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy" Pharmaceutics 15, no. 2: 411. https://doi.org/10.3390/pharmaceutics15020411
APA StyleMoon, Y., Jeon, S. I., Shim, M. K., & Kim, K. (2023). Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy. Pharmaceutics, 15(2), 411. https://doi.org/10.3390/pharmaceutics15020411







