Fluorescence-Coupled Techniques for Determining Rose Bengal in Dermatological Formulations and Their Application to Ex Vivo Skin Deposition Studies
Abstract
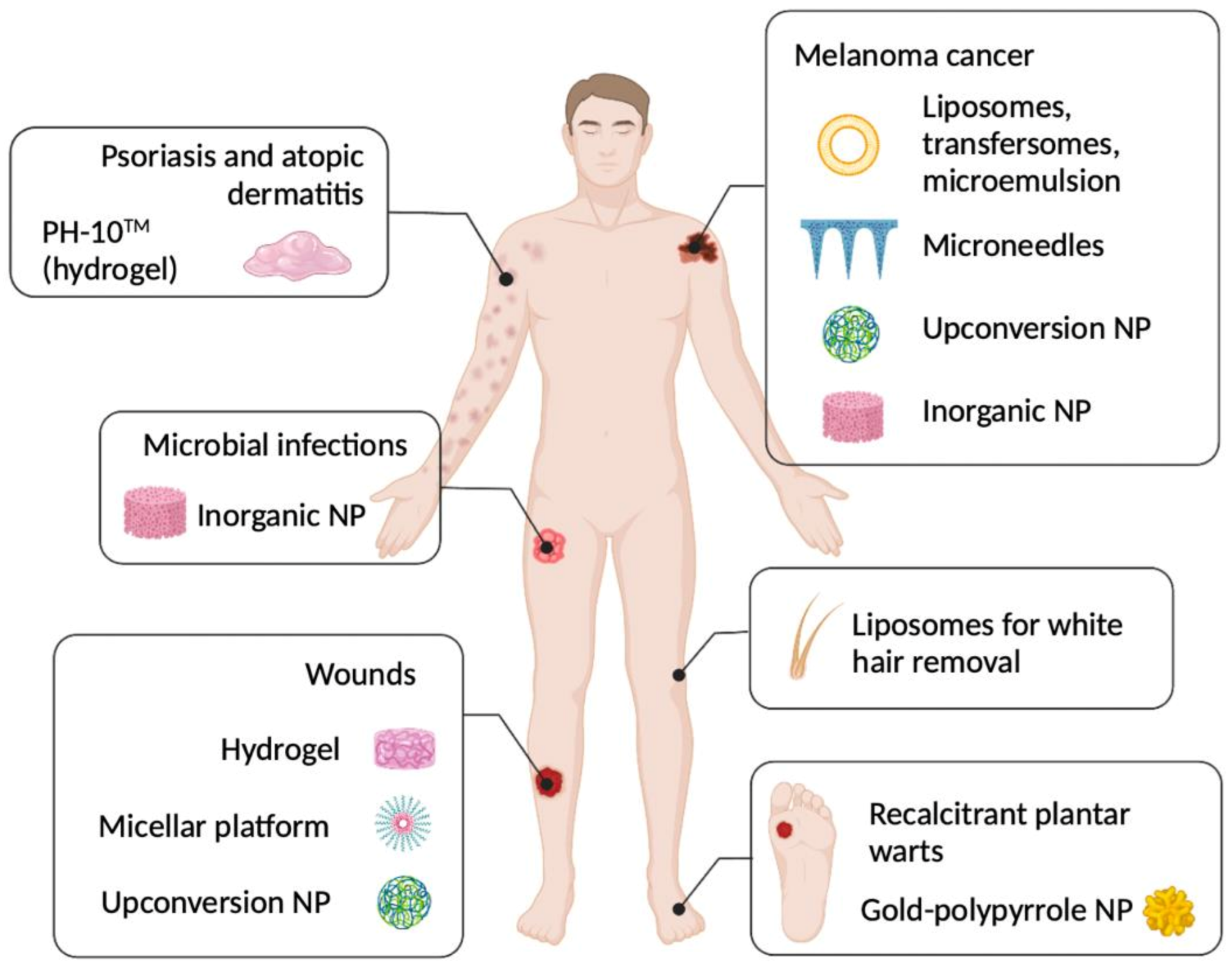
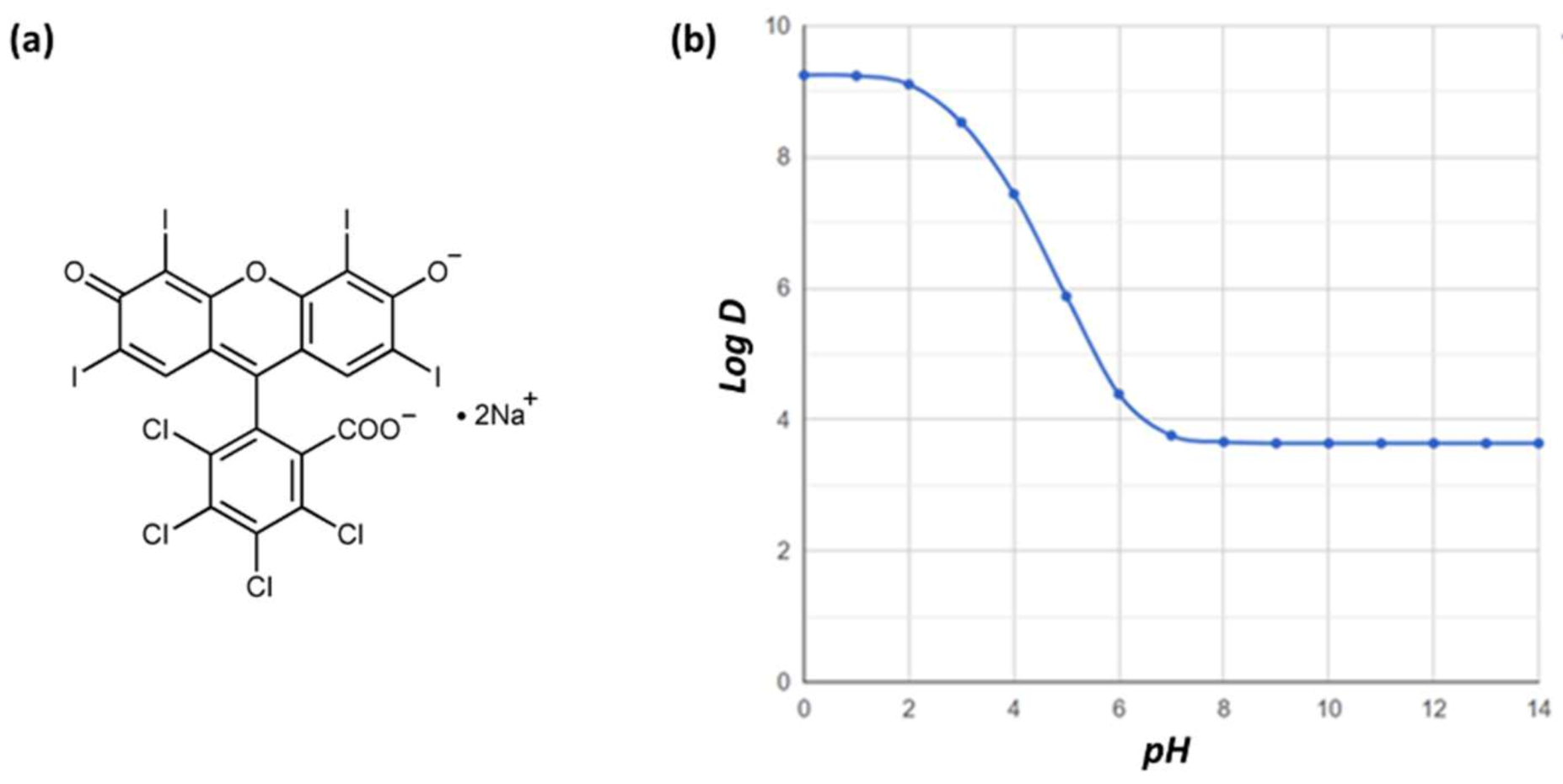
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Chromatographic Conditions
2.3. Standard Stock, Working Solution and Calibration Standards
2.4. Validation Process
2.4.1. HPLC UV-Vis Method Validation
2.4.2. HPLC-FLD Method Validation
Specificity
Linearity, LOD and LLOQ
Inter-Day and Intra-Day Accuracy and Precision
Carry-Over
Dilution Integrity
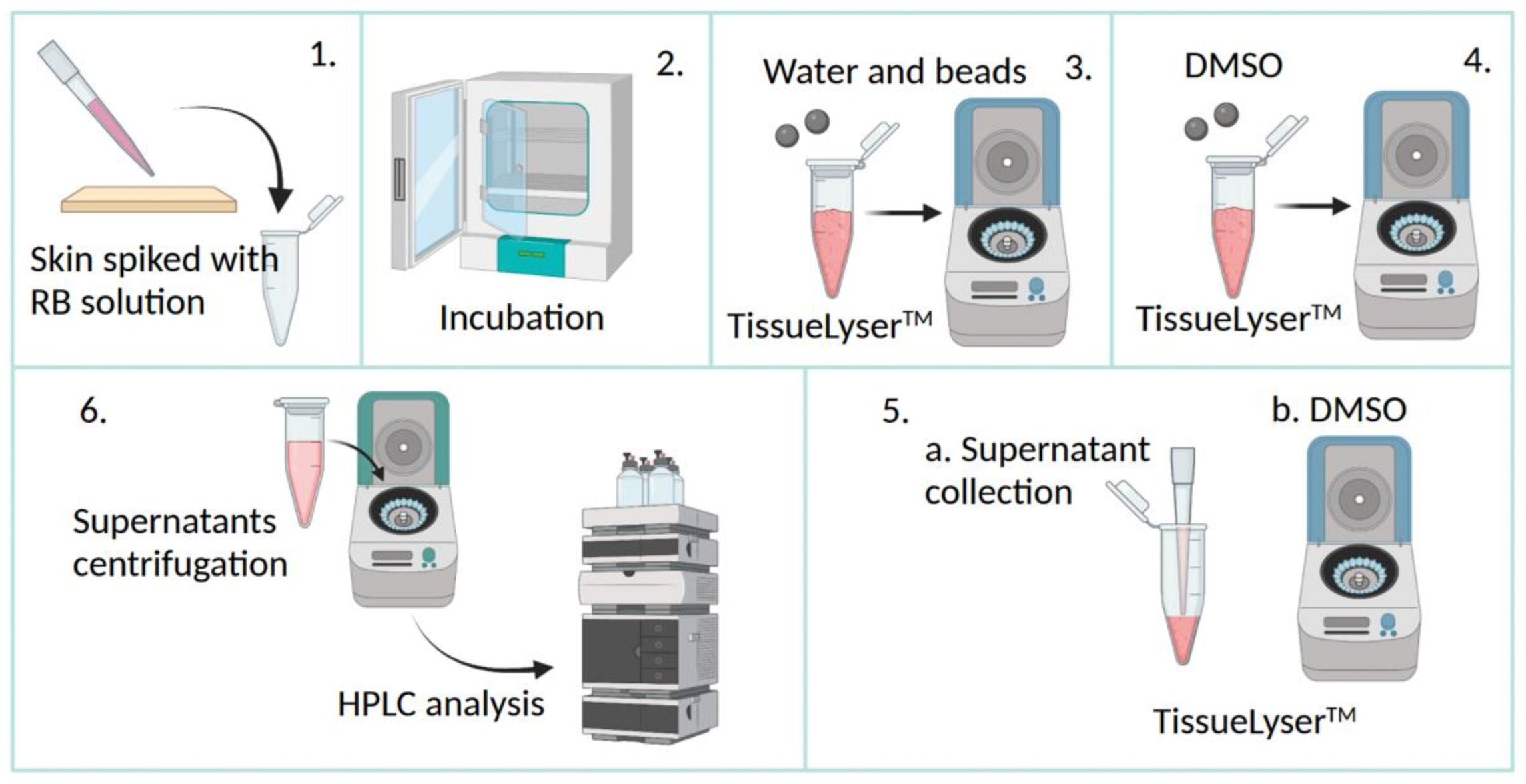
2.5. Skin Extraction Recovery
2.6. Preparation of RB Aqueous Solution, RB-Loaded Cream and RB-Loaded Transfersomes
2.7. Ex Vivo Skin Permeation and Deposition Study
2.8. Multiphoton Microscopy Investigation
2.9. Examination of the Fluorescence Spectra
2.10. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analytical Method
3.1.1. Analytical Method Optimisation
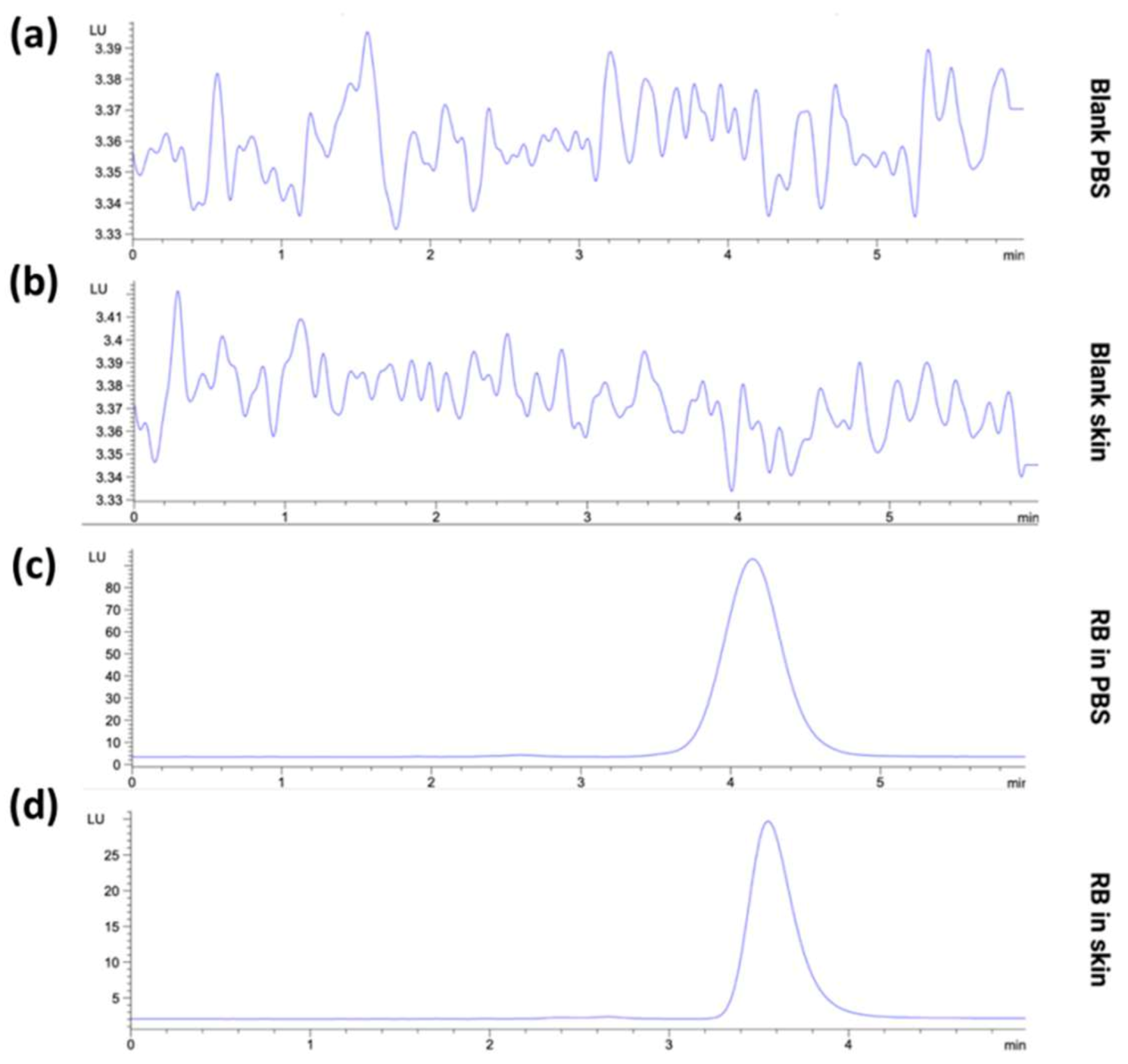
3.1.2. Specificity of the HPLC-FLD Method
3.1.3. Accuracy and Precision of the FLD-HPLC Method
3.1.4. Carry-Over
3.1.5. Dilution Integrity
3.2. Skin Extraction Recovery
3.3. Ex Vivo Skin Permeation and Deposition Study
3.4. Multiphoton Microscopy Investigation
3.5. Examination of the Fluorescence Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-Based Rose Bengal: A Broad-Spectrum Biomedical Tool. Dye. Pigment. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Liu, H.; Innamarato, P.P.; Kodumudi, K.; Weber, A.; Nemoto, S.; Robinson, J.L.; Crago, G.; McCardle, T.; Royster, E.; Sarnaik, A.A.; et al. Intralesional Rose Bengal in Melanoma Elicits Tumor Immunity via Activation of Dendritic Cells by the Release of High Mobility Group Box 1. Oncotarget 2016, 7, 37893–37905. [Google Scholar] [CrossRef] [PubMed]
- Zamani Taghizadeh Rabe, S.; Mousavi, S.H.; Tabasi, N.; Rastin, M.; Zamani Taghizadeh Rabe, S.; Siadat, Z.; Mahmoudi, M. Rose Bengal Suppresses Gastric Cancer Cell Proliferation via Apoptosis and Inhibits Nitric Oxide Formation in Macrophages. J. Immunotoxicol. 2014, 11, 367–375. [Google Scholar] [CrossRef]
- Qin, J.; Kunda, N.; Qiao, G.; Calata, J.F.; Pardiwala, K.; Prabhakar, B.S.; Maker, A.V. Colon Cancer Cell Treatment with Rose Bengal Generates a Protective Immune Response via Immunogenic Cell Death. Cell Death Dis. 2017, 8, e2584. [Google Scholar] [CrossRef]
- Koevary, S.B. Selective Toxicity of Rose Bengal to Ovarian Cancer Cells in Vitro. Int. J. Physiol. Pathophysiol. Pharmacol. 2012, 4, 99–107. Available online: www.ijppp.org (accessed on 15 October 2022).
- Mousavi, S.H.; Tavakkol-Afshari, J.; Brook, A.; Jafari-Anarkooli, I. Direct Toxicity of Rose Bengal in MCF-7 Cell Line: Role of Apoptosis. Food Chem. Toxicol. 2009, 47, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, M.; Mitachi, K.; Yang, J.; Pershing, E.V.; Horowitz, B.D.; Wachter, E.A.; Lacey, J.W.; Ji, Y.; Rodrigues, D.J. Antibacterial Activity of Pharmaceutical-Grade Rose Bengal: An Application of a Synthetic Dye in Antibacterial Therapies. Molecules 2022, 27, 322. [Google Scholar] [CrossRef]
- Slivinschi, B.; Manai, F.; Martinelli, C.; Carriero, F.; D’Amato, C.; Massarotti, M.; Bresciani, G.; Casali, C.; Milanesi, G.; Artal, L.; et al. Enhanced Delivery of Rose Bengal by Amino Acids Starvation and Exosomes Inhibition in Human Astrocytoma Cells to Potentiate Anticancer Photodynamic Therapy Effects. Cells 2022, 11, 2502. [Google Scholar] [CrossRef]
- Amescua, G.; Arboleda, A.; Nikpoor, N.; Durkee, H.; Relhan, N.; Aguilar, M.C.; Flynn, H.W.; Miller, D.; Parel, J.-M. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 2017, 36, 1141–1144. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Porter, S.L.; Rizk, N.; Sheng, Y.; McKaig, T.; Burnett, K.; White, B.; Nesbitt, H.; Matin, R.N.; McHale, A.P.; et al. Rose Bengal–Amphiphilic Peptide Conjugate for Enhanced Photodynamic Therapy of Malignant Melanoma. J. Med. Chem. 2020, 63, 1328–1336. [Google Scholar] [CrossRef]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch. Oral Biol. 2021, 122, 105024. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.; Aebisher, D.; Bartusik-Aebisher, D.; Krupka-Olek, M.; Dynarowicz, K.; Przygoda, M.; Kawczyk-Krupka, A. Photodynamic Therapy-Adjunctive Therapy in the Treatment of Prostate Cancer. Diagnostics 2022, 12, 1113. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic Excitation of Rose Bengal for Eradication of Gram-Positive and Gram-Negative Bacteria. BioMed Res. Int. 2013, 2013, 684930. [Google Scholar] [CrossRef]
- Çalışkan, S.Ö.; Aslan, C.; Duran, Ö.F.; Kaya, İ.; Özen, H. In Vitro Evaluation of the Effect of Sonodynamic Therapy Using Rose Bengal on Leishmania Tropica Promastigotes’. Turk. J. Parasitol. 2022, 46, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Zhou, X.-B.; Wang, A.-L.; Zheng, B.-Y.; Yeh, C.-K.; Huang, J.-D. Synthesis and Biological Characterization of Novel Rose Bengal Derivatives with Improved Amphiphilicity for Sono-Photodynamic Therapy. Eur. J. Med. Chem. 2018, 145, 86–95. [Google Scholar] [CrossRef]
- Mousavi, H.; Zhang, X.; Gillespie, S.; Wachter, E.; Hersey, P. Rose Bengal Induces Dual Modes of Cell Death in Melanoma Cells and Has Clinical Activity against Melanoma. Melanoma Res. 2006, 16, S8. Available online: https://journals.lww.com/melanomaresearch/toc/2006/09001 (accessed on 15 October 2022). [CrossRef]
- Srivastav, A.K.; Mujtaba, S.F.; Dwivedi, A.; Amar, S.K.; Goyal, S.; Verma, A.; Kushwaha, H.N.; Chaturvedi, R.K.; Ray, R.S. Photosensitized Rose Bengal-Induced Phototoxicity on Human Melanoma Cell Line under Natural Sunlight Exposure. J. Photochem. Photobiol. B 2016, 156, 87–99. [Google Scholar] [CrossRef]
- Thompson, J.F.; Hersey, P.; Wachter, E. Chemoablation of Metastatic Melanoma Using Intralesional Rose Bengal. Melanoma Res. 2008, 18, 405–411. [Google Scholar] [CrossRef]
- Wiener, M.; Damian, D.L.; Thompson, J.F. Systemic Phototoxicity Following Intralesional Rose Bengal for Subcutaneous Melanoma Metastases. Dermatology 2008, 216, 361–362. [Google Scholar] [CrossRef]
- Alvarez-López, C.; Aguirre-Soto, A. A Review on Light-Initiated Crosslinking of Biomaterials Stained with Rose Bengal for “Nanosuturing” Macromolecular Interfaces. Mater. Today Proc. 2022, 48, 56–65. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Medical Applications of Rose Bengal- and Riboflavin-Photosensitized Protein Crosslinking. Photochem. Photobiol. 2019, 95, 1097–1115. [Google Scholar] [CrossRef]
- Rosales-Rojas, R.; Zuñiga-Bustos, M.; Salas-Sepúlveda, F.; Galaz-Araya, C.; Zamora, R.A.; Poblete, H. Self-Organization Dynamics of Collagen-like Peptides Crosslinking Is Driven by Rose-Bengal-Mediated Electrostatic Bridges. Pharmaceutics 2022, 14, 1148. [Google Scholar] [CrossRef]
- Samy, N.; Fadel, M. Topical Liposomal Rose Bengal for Photodynamic White Hair Removal: Randomized, Controlled, Double-Blind Study. J. Drugs Dermatol. JDD 2014, 13, 436–442. [Google Scholar] [PubMed]
- Fadel, M.; Fadeel, D.A.; Tawfik, A.; El-Kholy, A.I.; Mosaad, Y.O. Rose Bengal-Gold-Polypyrrole Nanoparticles as a Photothermal/Photodynamic Dual Treatment of Recalcitrant Plantar Warts: Animal and Clinical Study. J. Drug Deliv. Sci. Technol. 2022, 69, 103095. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Samper, S.; Soria-Lozano, P.; Rezusta, A.; Gilaberte, Y. Antimicrobial Photodynamic Activity of Rose Bengal, Alone or in Combination with Gentamicin, against Planktonic and Biofilm Staphylococcus Aureus. Photodiagn. Photodyn. Ther. 2018, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-H.; Lee, C.-N.; Hsu, H.-W.; Ng, I.-S.; Wu, C.-J.; Yu, C.-K.; Lee, N.-Y.; Chang, Y.; Wong, T.-W. Recent Advances in Photodynamic Therapy against Fungal Keratitis. Pharmaceutics 2021, 13, 2011. [Google Scholar] [CrossRef] [PubMed]
- Houang, J.; Halliday, C.; Chen, S.; Ho, C.; Bekmukhametova, A.; Lauto, A. Effective Photodynamic Treatment of Trichophyton Species with Rose Bengal. J. Biophotonics 2021, 14, e202000340. [Google Scholar] [CrossRef] [PubMed]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.M. Topical Delivery and Photodynamic Evaluation of a Multivesicular Liposomal Rose Bengal. Lasers Med. Sci. 2011, 26, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Demartis, S.; Rassu, G.; Murgia, S.; Casula, L.; Giunchedi, P.; Gavini, E. Improving Dermal Delivery of Rose Bengal by Deformable Lipid Nanovesicles for Topical Treatment of Melanoma. Mol. Pharm. 2021, 18, 4046–4057. [Google Scholar] [CrossRef] [PubMed]
- Campanholi, K.D.S.S.; da Silva Junior, R.C.; da Silva, I.C.; dos Santos, R.S.; Vecchi, C.F.; Bruschi, M.L.; dos Santos Pozza, M.S.; de Castro-Hoshino, L.V.; Baesso, M.L.; Hioka, N.; et al. Stimulus-Responsive Phototherapeutic Micellar Platform of Rose Bengal B: A New Perspective for the Treatment of Wounds. J. Drug Deliv. Sci. Technol. 2021, 66, 102739. [Google Scholar] [CrossRef]
- Forouz, F.; Dabbaghi, M.; Namjoshi, S.; Mohammed, Y.; Roberts, M.S.; Grice, J.E. Development of an Oil-in-Water Self-Emulsifying Microemulsion for Cutaneous Delivery of Rose Bengal: Investigation of Anti-Melanoma Properties. Pharmaceutics 2020, 12, 947. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A Rose Bengal/Graphene Oxide/PVA Hybrid Hydrogel with Enhanced Mechanical Properties and Light-Triggered Antibacterial Activity for Wound Treatment. Mater. Sci. Eng. C 2021, 118, 111447. [Google Scholar] [CrossRef]
- Dhal, S.; Verma, P.; Mishra, M.; Giri, S. Oleogel-Mediated Transdermal Delivery of White Emitting NaYF4 Conjugated with Rose Bengal for the Generation of Reactive Oxygen Species through NIR-Upconversion. Colloids Surf. B Biointerfaces 2020, 190, 110945. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hwang, B.W.; Jeon, E.Y.; Jung, D.; Lee, G.H.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Cha, H.J.; Hahn, S.K. Upconversion Nanoparticles/Hyaluronate–Rose Bengal Conjugate Complex for Noninvasive Photochemical Tissue Bonding. ACS Nano 2017, 11, 9979–9988. [Google Scholar] [CrossRef]
- Gurianov, Y.; Meistelman, M.; Albo, Y.; Nisnevitch, M.; Nakonechny, F. Antibacterial Activity of Rose Bengal Entrapped in Organically Modified Silica Matrices. Int. J. Mol. Sci. 2022, 23, 3716. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, E.; Martins Estevão, B.; Cucinotta, F.; Hioka, N.; Rizzi, M.; Renò, F.; Marchese, L. An Efficient Rose Bengal Based Nanoplatform for Photodynamic Therapy. Chem. Eur. J. 2014, 20, 10921–10925. [Google Scholar] [CrossRef]
- Demartis, S.; Anjani, Q.K.; Volpe-Zanutto, F.; Paredes, A.J.; Jahan, S.A.; Vora, L.K.; Donnelly, R.F.; Gavini, E. Trilayer Dissolving Polymeric Microneedle Array Loading Rose Bengal Transfersomes as a Novel Adjuvant in Early-Stage Cutaneous Melanoma Management. Int. J. Pharm. 2022, 627, 122217. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Graham, J.P.; Bukallah, S.B.; Al-Saedi, M.A.S. Solvatochromic Behavior on the Absorption and Fluorescence Spectra of Rose Bengal Dye in Various Solvents. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2009, 72, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Abdul Mannan; Akther Jamal Khan; Mahwish Khan; Ghulam Abass; Sayyad Roohullah; Zahid Khan A Validated RP-HPLC Method for Determination of Rose Bengal in Bulk and Surgical Strips. RADS J. Pharm. Pharm. Sci. 2018, 6, 185–189.
- Egil, A.C.; Carmignani, A.; Battaglini, M.; Sengul, B.S.; Acar, E.; Ciofani, G.; Ozaydin Ince, G. Dual Stimuli-Responsive Nanocarriers via a Facile Batch Emulsion Method for Controlled Release of Rose Bengal. J. Drug Deliv. Sci. Technol. 2022, 74, 103547. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Babu, A.; Kim, S.-J.; Murugesan, R.; Jeyasubramanian, K. Enhanced Photodynamic Efficacy and Efficient Delivery of Rose Bengal Using Nanostructured Poly(Amidoamine) Dendrimers: Potential Application in Photodynamic Therapy of Cancer. Cancer Nanotechnol. 2011, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Wang, X.; Boye, S.; Appelhans, D.; Klajnert-Maculewicz, B. PH-Stable Polymersome as Nanocarrier for Post-Loaded Rose Bengal in Photodynamic Therapy. Colloids Surf. B Biointerfaces 2022, 217, 112662. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Wang, C.; You, J.; Jin, B.; Mo, F.; Chen, J.; Zheng, Y.; Chen, H. Self-Assembled Chitosan/Rose Bengal Derivative Nanoparticles for Targeted Sonodynamic Therapy: Preparation and Tumor Accumulation. RSC Adv. 2015, 5, 17915–17923. [Google Scholar] [CrossRef]
- Repert, S.; Matthes, S.; Rozhon, W. Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography. Molecules 2022, 27, 5673. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, Y.-T.; Yang, J.-C.; Wu, H.-D.; Tsai, T. Absorption and Emission Spectral Shifts of Rose Bengal Associated with DMPC Liposomes. Dye. Pigment. 2008, 79, 170–175. [Google Scholar] [CrossRef]
- El-Bashir, S.M.; Yahia, I.S.; Binhussain, M.A.; AlSalhi, M.S. Designing of PVA/Rose Bengal Long-Pass Optical Window Applications. Results Phys. 2017, 7, 1238–1244. [Google Scholar] [CrossRef]
- Logoyda, L.; Kovalenko, S.; Abdel-Megied, A.M.; Zhulkevych, I.; Drapak, I.; Demchuk, I.; Netsyuk, O. HPLC Method Development for the Analysis of Bisoprolol in Combined Dosage Form Containing Bisoprolol and Enalapril and in Vitro Dissolution Studied. Int. J. Appl. Pharm. 2019, 11, 194–196. [Google Scholar] [CrossRef]
- Alhibah, M.; Kröger, M.; Schanzer, S.; Busch, L.; Lademann, J.; Beckers, I.; Meinke, M.C.; Darvin, M.E. Penetration Depth of Propylene Glycol, Sodium Fluorescein and Nile Red into the Skin Using Non-Invasive Two-Photon Excited FLIM. Pharmaceutics 2022, 14, 1790. [Google Scholar] [CrossRef]
- Mangion, S.E.; Sandiford, L.; Mohammed, Y.; Roberts, M.S.; Holmes, A.M. Multi-Modal Imaging to Assess the Follicular Delivery of Zinc Pyrithione. Pharmaceutics 2022, 14, 1076. [Google Scholar] [CrossRef]
- Wachter, E.A.; Dees, C.; Harkins, J.; Fisher, W.G.; Scott, T. Functional Imaging of Photosensitizers Using Multiphoton Microscopy. In Multiphoton Microscopy in the Biomedical Sciences II; SPIE: San Jose, CA, USA, 2002; Volume 4620, pp. 143–147. [Google Scholar]
- Wachter, E.A.; Dees, C.; Harkins, J.; Fisher, W.G.; Scott, T. Imaging Photosensitizer Distribution and Pharmacology Using Multiphoton Microscopy. In Optical Diagnostics of Living Cells V; Farkas, D.L., Leif, R.C., Eds.; SPIE: San Jose, CA, USA, 2002; pp. 112–118. [Google Scholar] [CrossRef]
- Guy, R.C. International Conference on Harmonisation. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 1070–1072. [Google Scholar] [CrossRef]
- Administration, F.A.D. Bioanalytical Method Validation Guidance for Industry. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 10 October 2022).
- European Medicines Agency. Bioanalytical Method Validation—Scientific Guideline. 2018. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation-scientific-guideline (accessed on 21 December 2022).
- Anjani, Q.K.; Sabri, A.H.B.; Moreno-Castellanos, N.; Utomo, E.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Wardoyo, L.A.H.; Donnelly, R.F. Soluplus®-Based Dissolving Microarray Patches Loaded with Colchicine: Towards a Minimally Invasive Treatment and Management of Gout. Biomater. Sci. 2022, 10, 5838–5855. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Utomo, E.; Wardoyo, L.A.H.; Larrañeta, E.; Donnelly, R.F. Metronidazole Nanosuspension Loaded Dissolving Microarray Patches: An Engineered Composite Pharmaceutical System for the Treatment of Skin and Soft Tissue Infection. Biomater. Adv. 2022, 140, 213073. [Google Scholar] [CrossRef]
- Kang, J.-H.; Chon, J.; Kim, Y.-I.; Lee, H.-J.; Oh, D.-W.; Lee, H.-G.; Han, C.-S.; Kim, D.-W.; Park, C.-W. Preparation and Evaluation of Tacrolimus-Loaded Thermosensitive Solid Lipid Nanoparticles for Improved Dermal Distribution. Int. J. Nanomed. 2019, 14, 5381–5396. [Google Scholar] [CrossRef] [PubMed]
- Anjani, Q.K.; Sabri, A.H.B.; Utomo, E.; Domínguez-Robles, J.; Donnelly, R.F. Elucidating the Impact of Surfactants on the Performance of Dissolving Microneedle Array Patches. Mol. Pharm. 2022, 19, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; de Araujo, D.R.; Padula, C. Validation of a HPLC-UV Method for the Quantification of Budesonide in Skin Layers. J. Chromatogr. B 2021, 1164, 122512. [Google Scholar] [CrossRef]
- Roseboom, I.C.; Rosing, H.; Beijnen, J.H.; Dorlo, T.P.C. Skin Tissue Sample Collection, Sample Homogenization, and Analyte Extraction Strategies for Liquid Chromatographic Mass Spectrometry Quantification of Pharmaceutical Compounds. J. Pharm. Biomed. Anal. 2020, 191, 113590. [Google Scholar] [CrossRef]
- Hill, A.P.; Young, R.J. Getting Physical in Drug Discovery: A Contemporary Perspective on Solubility and Hydrophobicity. Drug Discov. Today 2010, 15, 648–655. [Google Scholar] [CrossRef]
- Ramöller, I.K.; Abbate, M.T.A.; Vora, L.K.; Hutton, A.R.J.; Peng, K.; Volpe-Zanutto, F.; Tekko, I.A.; Moffatt, K.; Paredes, A.J.; McCarthy, H.O.; et al. HPLC-MS Method for Simultaneous Quantification of the Antiretroviral Agents Rilpivirine and Cabotegravir in Rat Plasma and Tissues. J. Pharm. Biomed. Anal. 2022, 213, 114698. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Zawislak, A.A.; Woolfson, A.D. Design and Physicochemical Characterisation of a Bioadhesive Patch for Dose-Controlled Topical Delivery of Imiquimod. Int. J. Pharm. 2006, 307, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z. How the π Conjugation Length Affects the Fluorescence Emission Efficiency. J. Am. Chem. Soc. 2008, 130, 13867–13869. [Google Scholar] [CrossRef]
- González, O.; Blanco, M.E.; Iriarte, G.; Bartolomé, L.; Maguregui, M.I.; Alonso, R.M. Bioanalytical Chromatographic Method Validation According to Current Regulations, with a Special Focus on the Non-Well Defined Parameters Limit of Quantification, Robustness and Matrix Effect. J. Chromatogr. A 2014, 1353, 10–27. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Utomo, E.; Domínguez-Robles, J.; Detamornrat, U.; Donnelly, R.F.; Larrañeta, E. A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings. Molecules 2022, 27, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.C.; Maas, W.J.M.; Nielsen, J.B.; Greaves, L.C.; van de Sandt, J.J.M.; Williams, F.M. Interactions of Skin Thickness and Physicochemical Properties of Test Compounds in Percutaneous Penetration Studies. Int. Arch. Occup. Environ. Health 2006, 79, 405–413. [Google Scholar] [CrossRef]
- Pielesz, A.; Gawłowski, A.; Biniaś, D.; Bobiński, R.; Kawecki, M.; Klama-Baryła, A.; Kitala, D.; Łabuś, W.; Glik, J.; Paluch, J. The Role of Dimethyl Sulfoxide (DMSO) in Ex-Vivo Examination of Human Skin Burn Injury Treatment. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2018, 196, 344–352. [Google Scholar] [CrossRef]
- Otterbach, A.; Lamprecht, A. Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches. Pharmaceutics 2021, 13, 320. [Google Scholar] [CrossRef]
- OECD. Test No. 428: Skin Absorption: In Vitro Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2004; ISBN 978-92-64-07108-7. [Google Scholar] [CrossRef]
- Permana, A.D.; Wahyudin, E.; Ismail; Amir, M.N.; Raihan, M.; Anjani, Q.K.; Utomo, E.; Layadi, P.; Donnelly, R.F. New and Sensitive HPLC-UV Method for Concomitant Quantification of a Combination of Antifilariasis Drugs in Rat Plasma and Organs after Simultaneous Oral Administration. Anal. Methods 2021, 13, 933–945. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Bin Sabri, A.H.; Donnelly, R.F. Development and Validation of Simple and Sensitive HPLC-UV Method for Ethambutol Hydrochloride Detection Following Transdermal Application. Anal. Methods 2022, 14, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 13, 2012. [Google Scholar] [CrossRef] [PubMed]
- Alany, R. Topical and Transdermal Formulation and Drug Delivery. Pharm. Dev. Technol. 2017, 22, 457. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Aqueous Cream BP. Summary of Product Characteristics Updated 12-Sep-2019|Thornton & Ross Ltd. Available online: https://www.medicines.org.uk/emc/product/4820/smpc#gref (accessed on 15 October 2022).
- MHRA UK Public Assessment Report. Aqueous Cream: Contains Sodium Lauryl Sulfate Which May Cause Skin Reactions, Particularly in Children with Eczema. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/852382/Aqueous_cream_contains_sodium_lauryl_sulfate_which_may_cause_skin_reactions__particularly_in_children_with_eczema.pdf (accessed on 15 October 2022).
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B.W. Can Drug-Bearing Liposomes Penetrate Intact Skin? J. Pharm. Pharmacol. 2010, 58, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Neckers, D.C. Aggregation of Rose Bengal Molecules in Solution. J. Photochem. Photobiol. Chem. 1987, 40, 361–370. [Google Scholar] [CrossRef]





| Condition | Starting Method * | UV-Vis HPLC | FLD-HPLC |
|---|---|---|---|
| Mobile phase | MeOH and buffer pH 8 1 50/50 v/v | MeOH and buffer pH 8 2 60/40 v/v | MeOH and buffer pH 8 2 60/40 v/v |
| Pump mode | Isocratic | Isocratic | Isocratic |
| Diluent | Buffer pH 8 1 | PBS pH 7.4 | PBS pH 7.4 |
| Column | C-18 Chromosil 100-5 μm (250 × 4.6 mm) | C-18 Phenomenex SphereClone® (150 × 4.6 m) | C-18 Phenomenex SphereClone® (150 × 4.6 m) |
| Column temperature | Ambient | Ambient | Ambient |
| Excitation wavelength | 262 nm | 549 nm | 556 nm |
| Emission wavelength | - | - | 573 nm |
| Injection volume | 20 μL | 40 μL | 40 μL |
| Flow rate | 1 mL/min | 1 mL/min | 1 mL/min |
| Run time | 10 min | 7 | 6 |
| Retention time | 2.69 min | 3.1 min | 4.1 min |
| Detector | Slope | Intercept | Linearity 1 | LOD 2 | LLOQ 2 |
|---|---|---|---|---|---|
| UV-Vis | 190.5 | 16.84 | 1.0000 | 0.60 | 1.83 |
| FLD | 327.3 | −58.28 | 0.9999 | 0.17 | 0.54 |
| Theor. Concentration (μg/mL) | Exp. Concentration (μg/mL) | Precision (RSD%) | Accuracy (RE%) | |
|---|---|---|---|---|
| Intra-day | 8.00 | 7.89 ± 0.08 | 1.02 | −1.33 |
| 4.00 | 3.95 ± 0.07 | 1.91 | −1.26 | |
| 1.00 | 0.98 ± 0.03 | 2.65 | −2.42 | |
| Inter-day | 8.00 | 7.90 ± 0.08 | 0.98 | −1.17 |
| 4.00 | 3.93 ± 0.10 | 2.57 | −1.84 | |
| 1.00 | 0.98 ± 0.03 | 3.06 | −1.95 |
| Dilution | Recovery (%) | Precision (RSD%) | Accuracy (RE%) |
|---|---|---|---|
| 5 times | 104.70 ± 1.02 | 0.98 | 4.70 |
| 10 times | 104.22 ± 1.60 | 1.53 | 4.22 |
| RB Concentration Added (µg/mL) | ER% ± SD | RSD% |
|---|---|---|
| 1 | 97.18 ± 2.33 | 2.40 |
| 4 | 98.05 ± 8.79 | 8.96 |
| 8 | 100.82 ± 4.53 | 4.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjani, Q.K.; Demartis, S.; Volpe-Zanutto, F.; Li, H.; Sabri, A.H.B.; Gavini, E.; Donnelly, R.F. Fluorescence-Coupled Techniques for Determining Rose Bengal in Dermatological Formulations and Their Application to Ex Vivo Skin Deposition Studies. Pharmaceutics 2023, 15, 408. https://doi.org/10.3390/pharmaceutics15020408
Anjani QK, Demartis S, Volpe-Zanutto F, Li H, Sabri AHB, Gavini E, Donnelly RF. Fluorescence-Coupled Techniques for Determining Rose Bengal in Dermatological Formulations and Their Application to Ex Vivo Skin Deposition Studies. Pharmaceutics. 2023; 15(2):408. https://doi.org/10.3390/pharmaceutics15020408
Chicago/Turabian StyleAnjani, Qonita Kurnia, Sara Demartis, Fabiana Volpe-Zanutto, Huanhuan Li, Akmal Hidayat Bin Sabri, Elisabetta Gavini, and Ryan F. Donnelly. 2023. "Fluorescence-Coupled Techniques for Determining Rose Bengal in Dermatological Formulations and Their Application to Ex Vivo Skin Deposition Studies" Pharmaceutics 15, no. 2: 408. https://doi.org/10.3390/pharmaceutics15020408
APA StyleAnjani, Q. K., Demartis, S., Volpe-Zanutto, F., Li, H., Sabri, A. H. B., Gavini, E., & Donnelly, R. F. (2023). Fluorescence-Coupled Techniques for Determining Rose Bengal in Dermatological Formulations and Their Application to Ex Vivo Skin Deposition Studies. Pharmaceutics, 15(2), 408. https://doi.org/10.3390/pharmaceutics15020408










