Interactions between DMPC Model Membranes, the Drug Naproxen, and the Saponin β-Aescin
Abstract
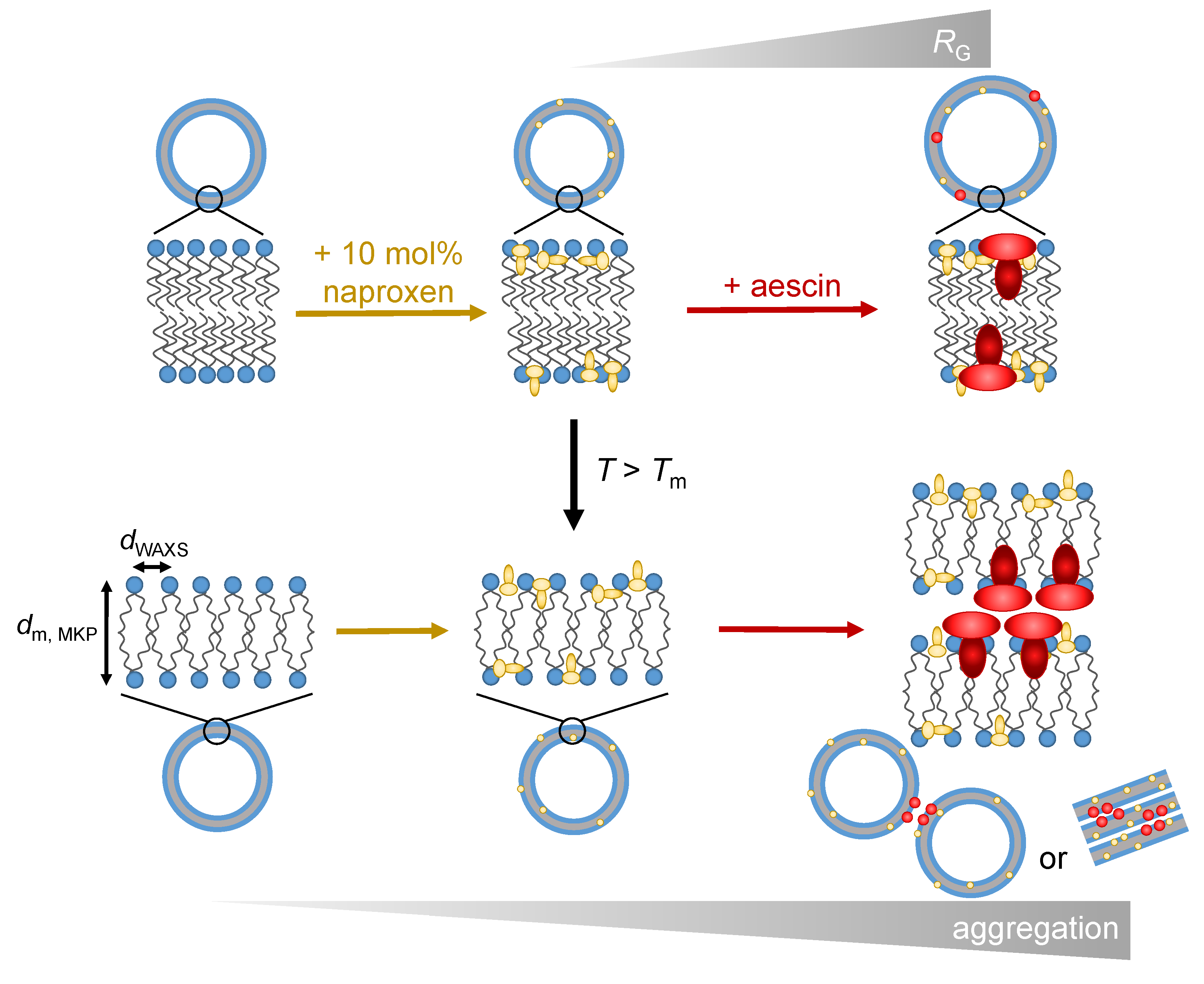
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vesicle Preparation
2.3. Differential Scanning Calorimetry (DSC)
2.4. Small- and Wide-Angle X-ray Scattering (SAXS and WAXS)
2.5. Photon Correlation Spectroscopy (PCS)
3. Results and Discussion
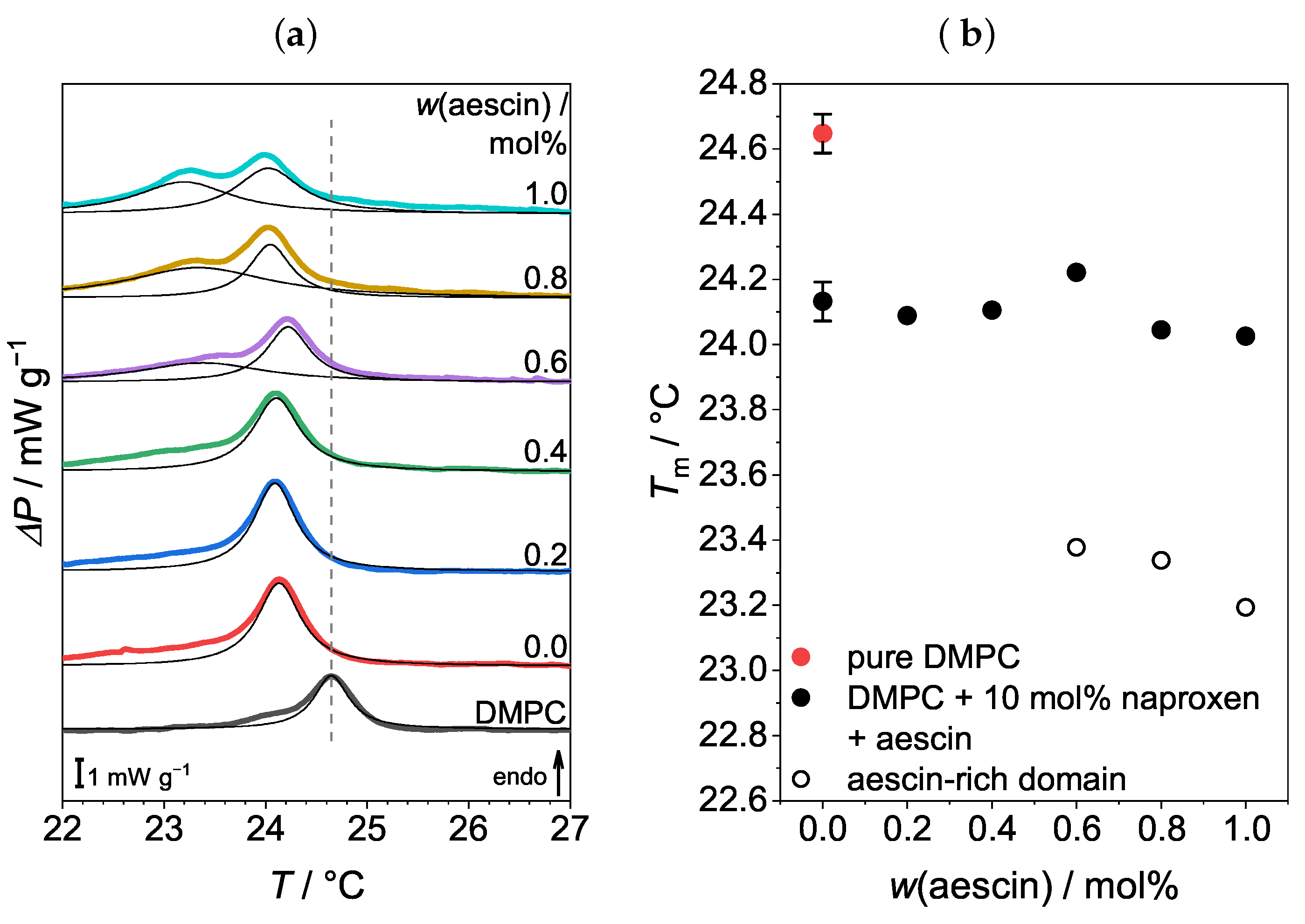
3.1. Differential Scanning Calorimetry (DSC): Determination of
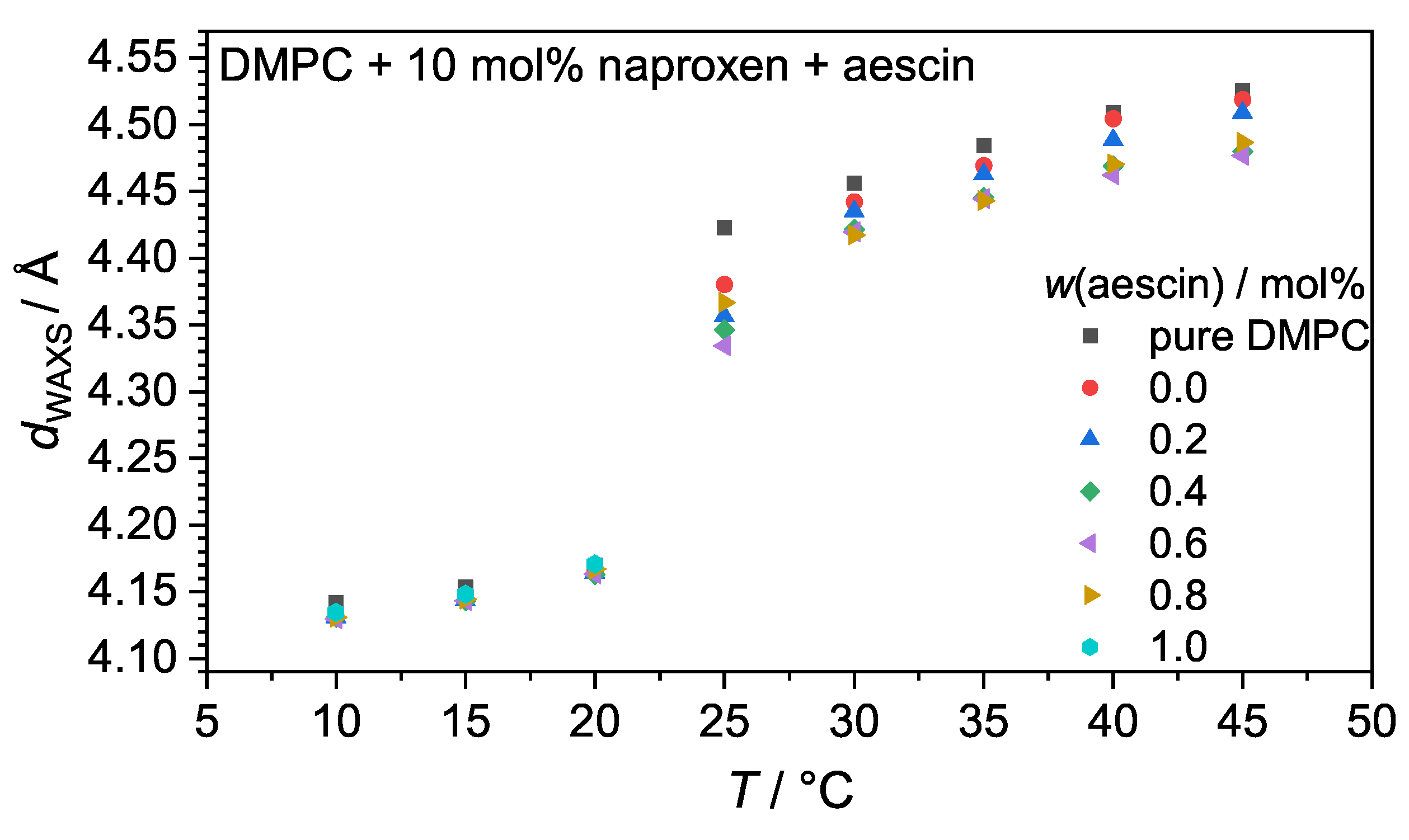
3.2. Wide-Angle X-ray Scattering (WAXS): Determination of
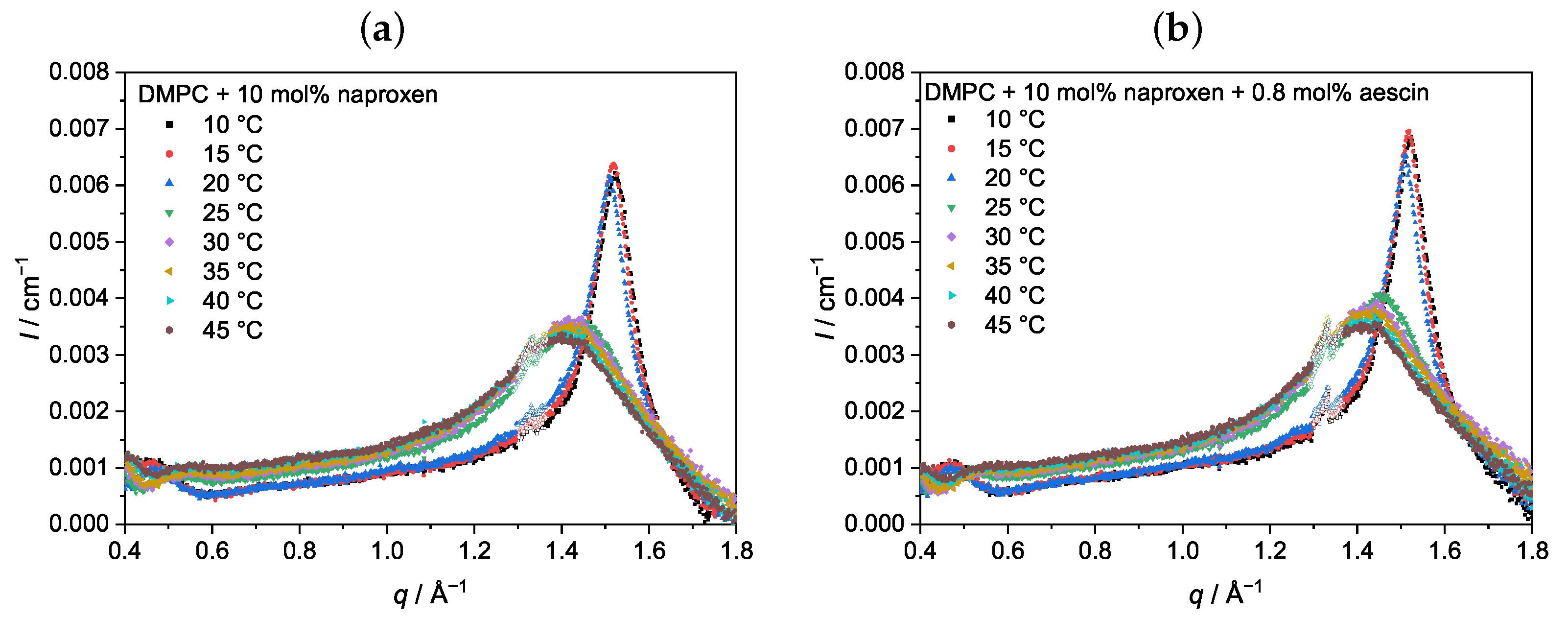
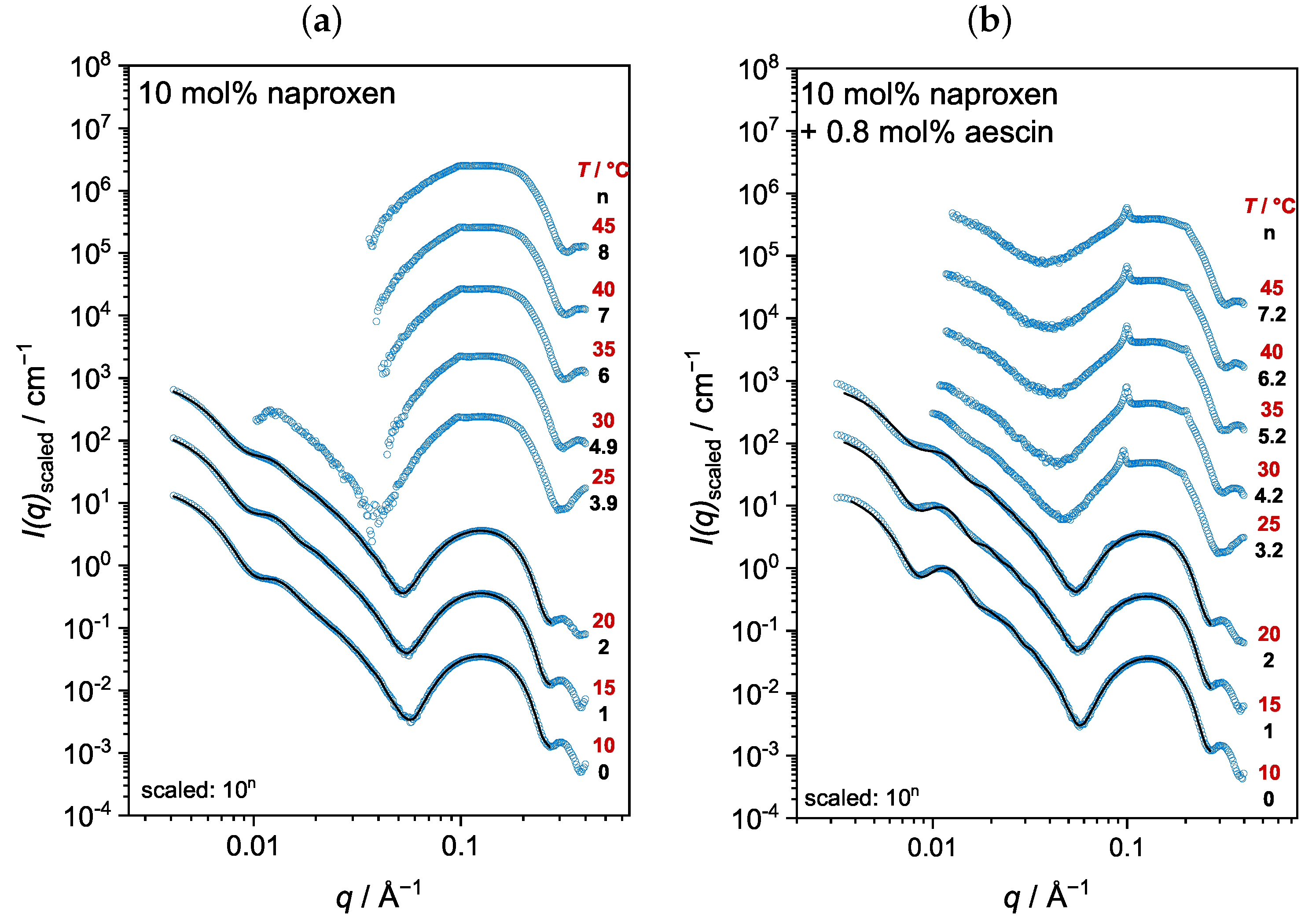
3.3. Analysis by Small-Angle X-ray Scattering (SAXS)
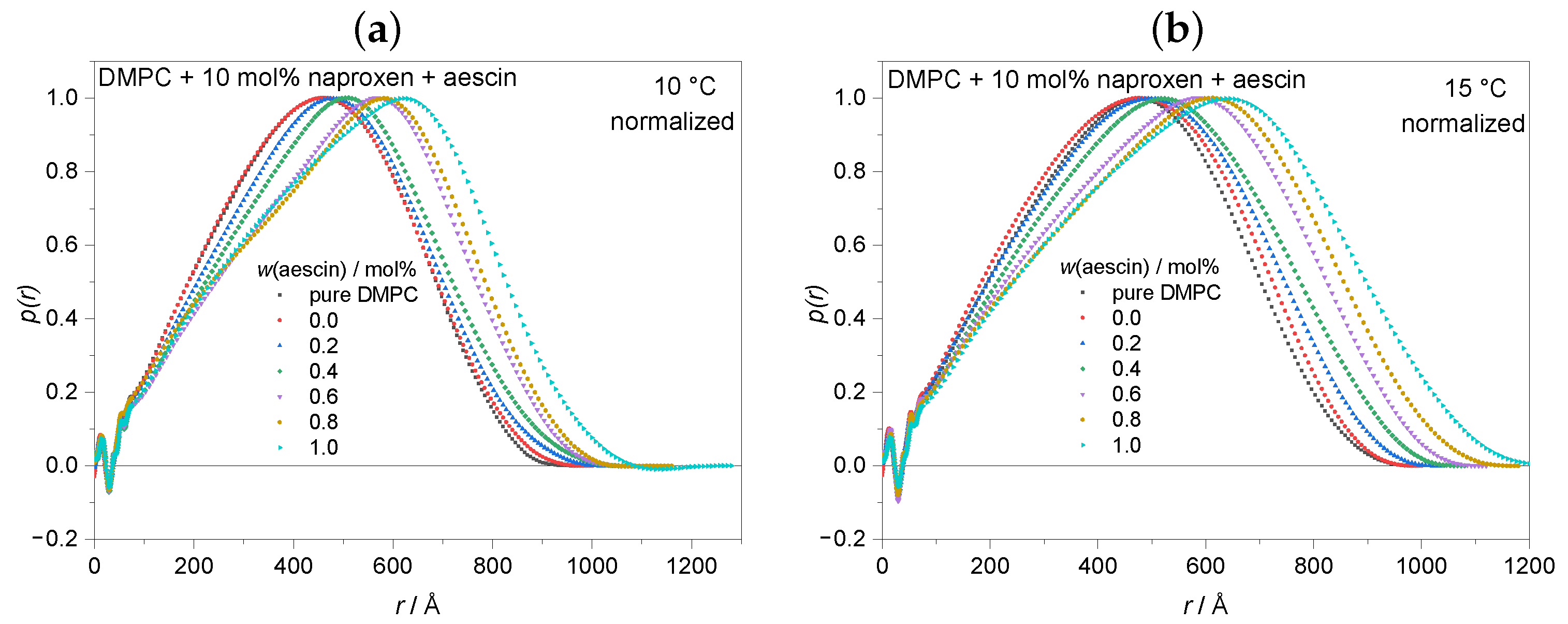
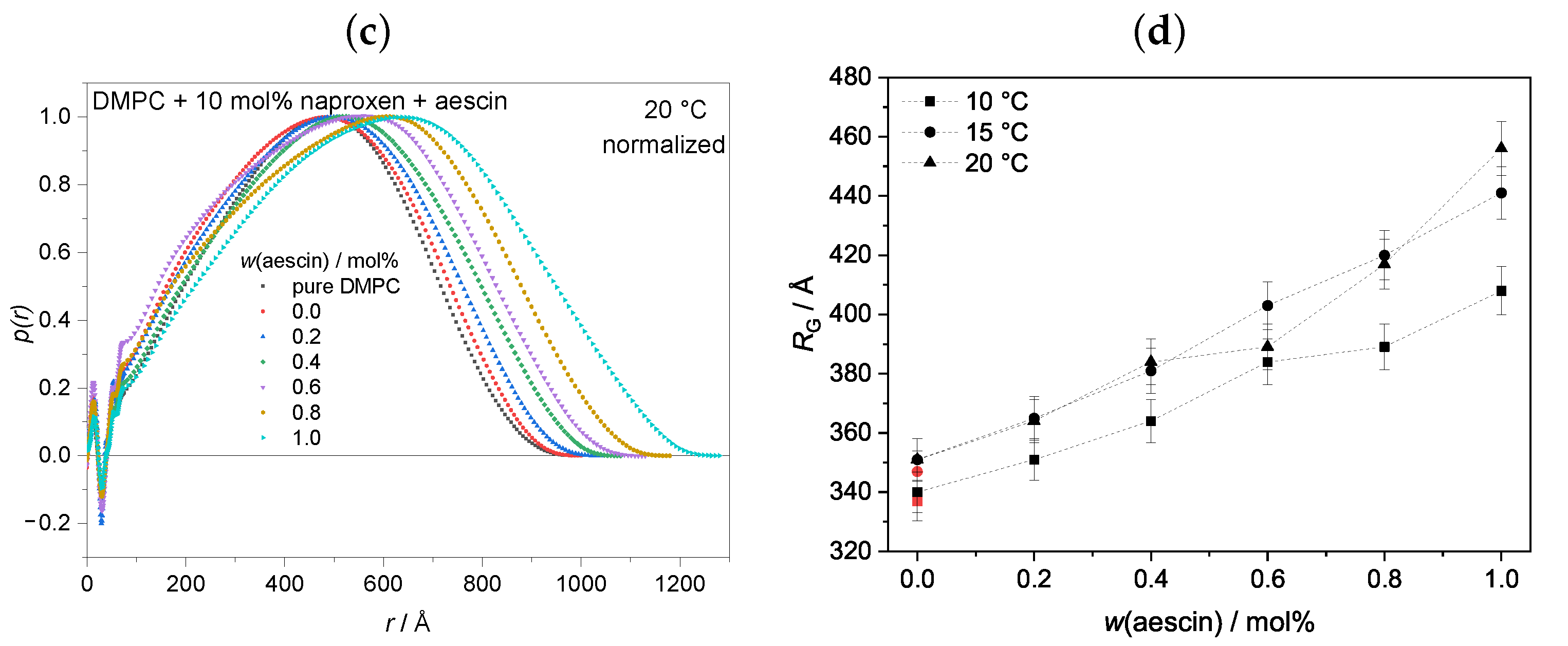
3.3.1. Pair Distance Distribution Function and Radius of Gyration by Indirect Fourier Transformation (IFT) at
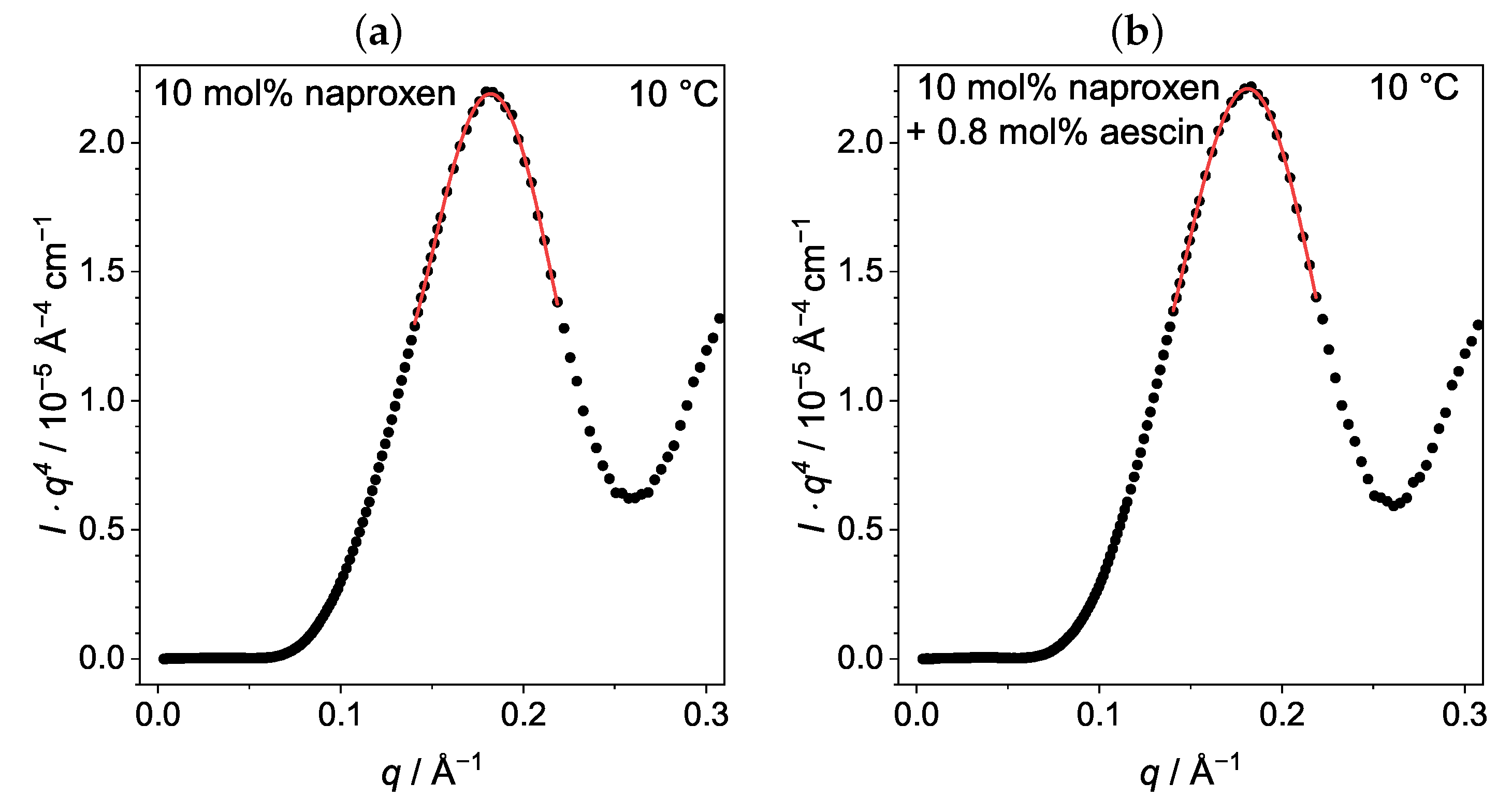
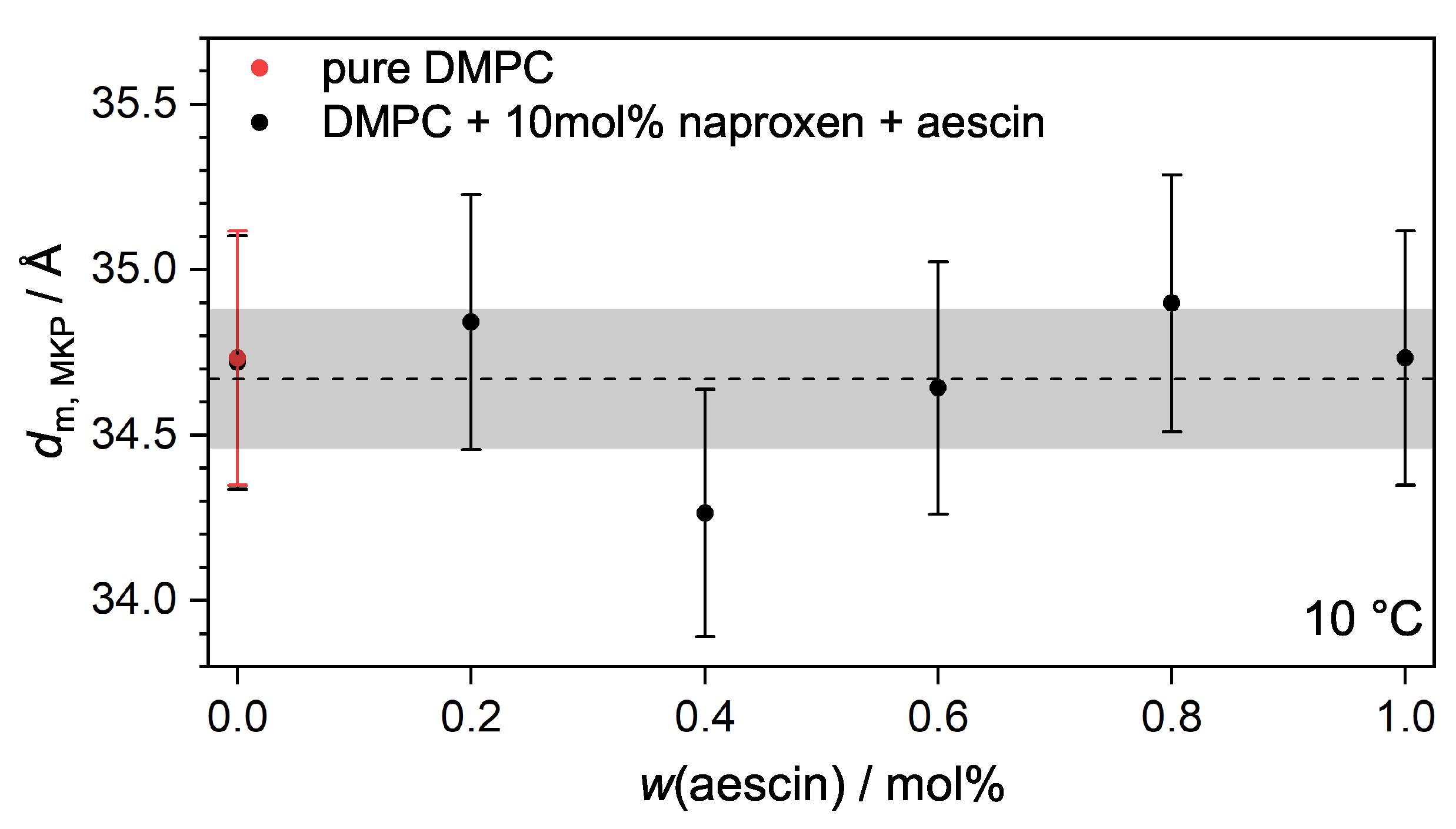
3.3.2. Membrane Thickness by Modified Kratky Porod (MKP) at
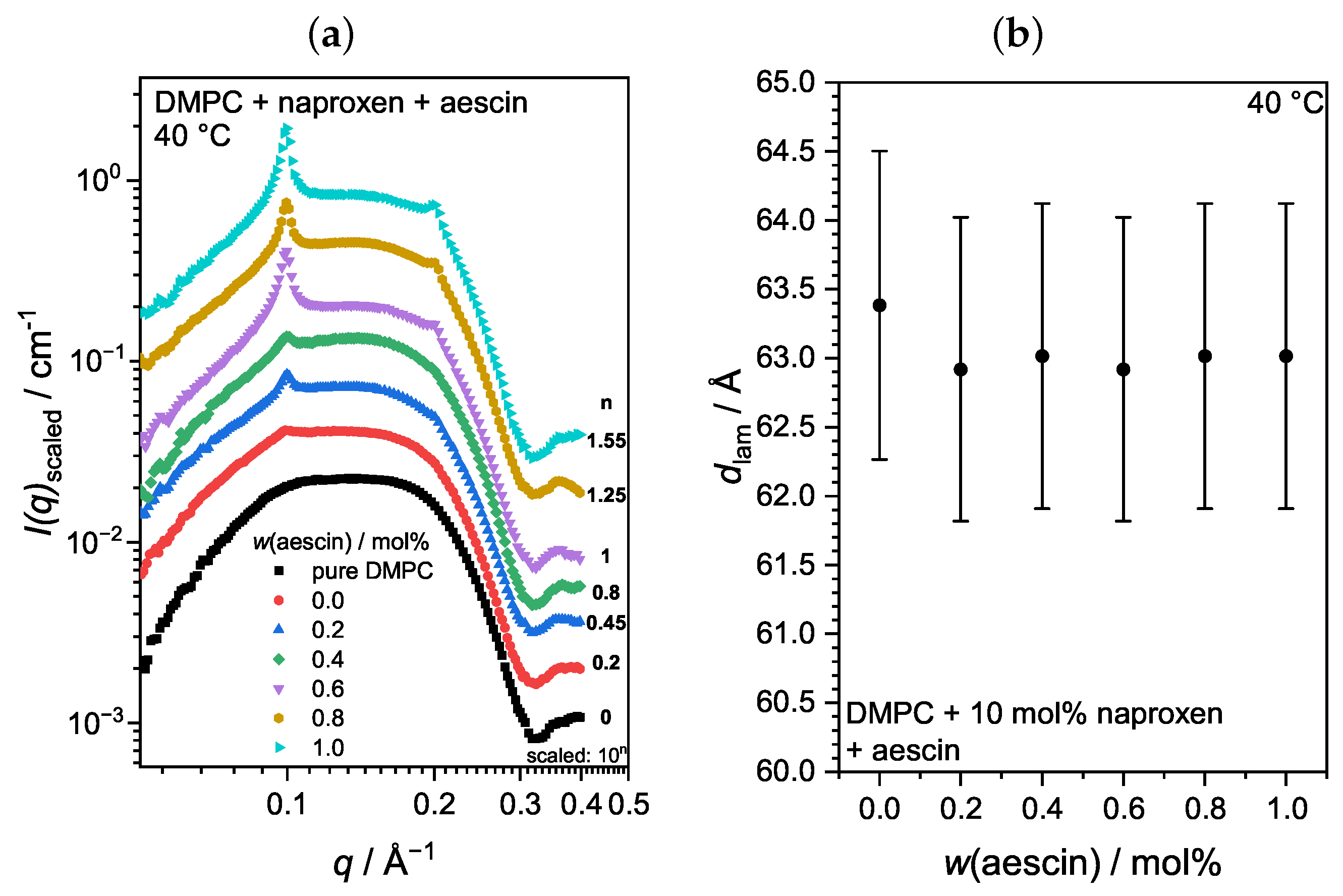
3.3.3. Membrane Structure at
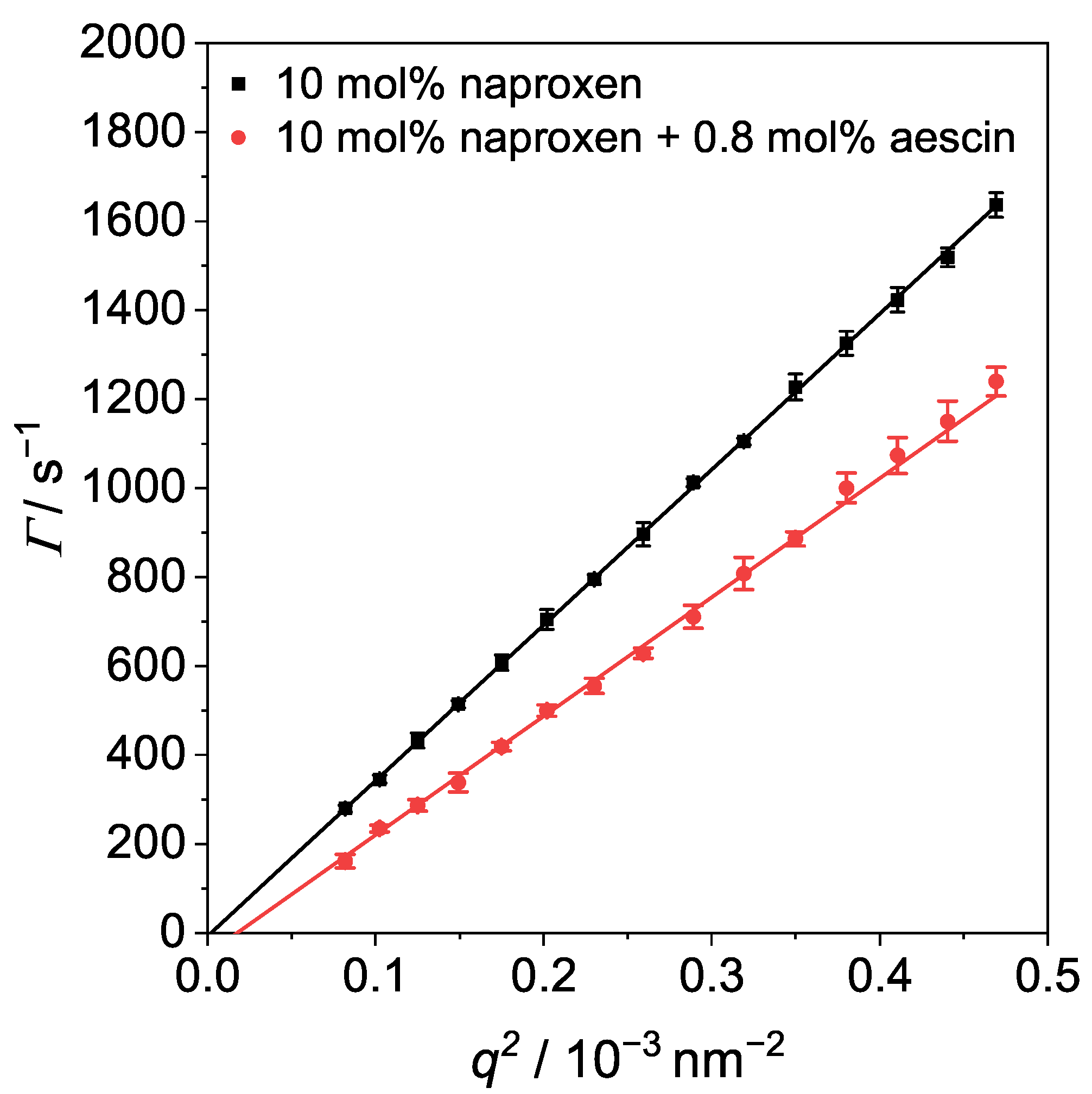
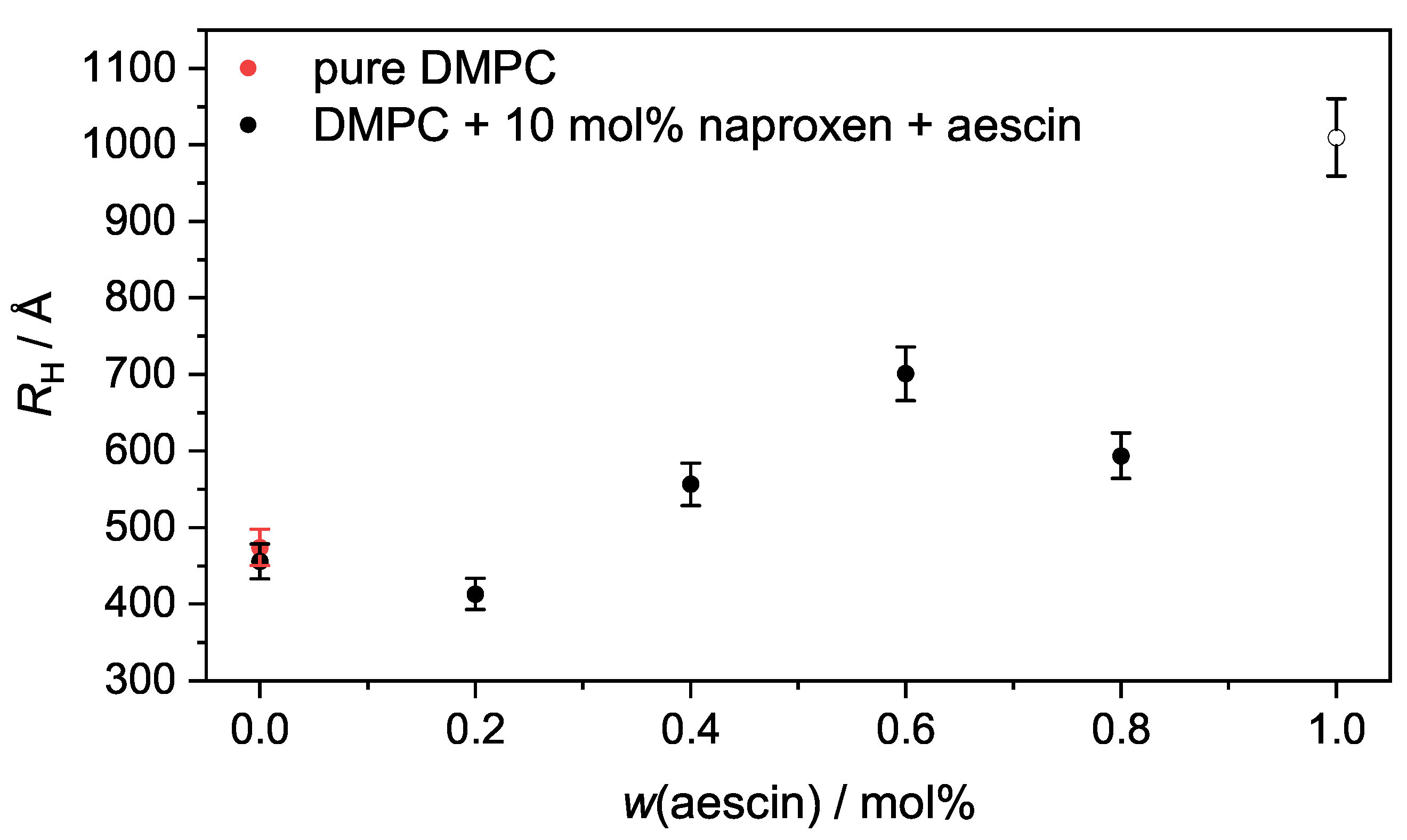
3.4. Photon Correlation Spectroscopy (PCS): Determination of below
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M. Lipids on the frontier: A century of cell-membrane bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef]
- Kiselev, M.A. Methods for Lipid Nanostructure Investigation at Neutron and Synchrotron Sources. Phys. Part. Nucl. 2011, 42, 302–331. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Mayer, L.D.; Janoff, A.S.; Cullis, P.R. Generation of multilamellar and unilamellar phospholipid vesicles. Chem. Phys. Lipids 1986, 40, 89–107. [Google Scholar] [CrossRef]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta (BBA)—Biomembr. 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Ulrich, A.S. Biophysical Aspects of Using Liposomes as Delivery Vehicles. Biosci. Rep. 2002, 22, 129–150. [Google Scholar] [CrossRef]
- Du, L.; Liu, X.; Huang, W.; Wang, E. A study on the interaction between ibuprofen and bilayer lipid membrane. Electrochim. Acta 2006, 51, 5754–5760. [Google Scholar] [CrossRef]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef]
- de Meyer, F.J.M.; Benjamini, A.; Rodgers, J.M.; Misteli, Y.; Smit, B. Molecular Simulation of the DMPC-Cholesterol Phase Diagram. J. Phys. Chem. B 2010, 114, 10451–10461. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Todd, P.A.; Clissold, S.P. Naproxen. A Reappraisal of its Pharmacology, and Therapeutic Use in Rheumatic Diseases and Pain States. Drugs 1990, 40, 91–137. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta 2009, 1788, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Yousefpour, A.; Amjad Iranagh, S.; Nademi, Y.; Modarress, H. Molecular dynamics simulation of nonsteroidal antiinflammatory drugs, naproxen and relafen, in a lipid bilayer membrane. Int. J. Quantum Chem. 2013, 113, 1919–1930. [Google Scholar] [CrossRef]
- Brooks, P. Use and Benefits of Nonsteroidal Anti-inflammatory Drugs. Am. J. Med. 1998, 104, 9S–13S. [Google Scholar] [CrossRef]
- Boggara, M.B.; Krishnamoorti, R. Small-Angle Neutron Scattering Studies of Phospholipid-NSAID Adducts. Langmuir Acs J. Surfaces Colloids 2010, 26, 5734–5745. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Figueiredo, M.; Burdach, K.; Nunes, C.; Reis, S. Unraveling the Role of Drug-Lipid Interactions in NSAIDs-Induced Cardiotoxicity. Membranes 2020, 11, 24. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mamontov, E.; Tyagi, M. Effects of NSAIDs on the Nanoscopic Dynamics of Lipid Membrane. Biochim. Biophys. Acta. Biomembr. 2020, 1862, 183100. [Google Scholar] [CrossRef]
- Przykaza, K.; Jurak, M.; Wiącek, A.E. Effect of naproxen on the model lipid membrane formed on the water-chitosan subphase. Biochim. Biophys. Acta. Biomembr. 2022, 1865, 184099. [Google Scholar] [CrossRef] [PubMed]
- Sreij, R.; Prévost, S.; Dargel, C.; Dattani, R.; Hertle, Y.; Wrede, O.; Hellweg, T. Interaction of the Saponin Aescin with Ibuprofen in DMPC Model Membranes. Mol. Pharm. 2018, 15, 4446–4461. [Google Scholar] [CrossRef] [PubMed]
- Alsop, R.J.; Armstrong, C.L.; Maqbool, A.; Toppozini, L.; Dies, H.; Rheinstädter, M.C. Cholesterol expels ibuprofen from the hydrophobic membrane core and stabilizes lamellar phases in lipid membranes containing ibuprofen. Soft Matter 2015, 11, 4756–4767. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M.; Wang, Z.M.; Romero, J.J.; Ulloa, C.; Perez, J.C.; Giraud, M.N.; Barreto, J.C. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: Insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat. Med. 1995, 1, 154–158. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Böttcher, S.; Drusch, S. Saponins - Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar] [CrossRef]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: A correlation? Bioorg. Med. Chem. 2012, 20, 2822–2828. [Google Scholar] [CrossRef]
- Güçlü-Ustündağ, O.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Sreij, R.; Dargel, C.; Geisler, P.; Hertle, Y.; Radulescu, A.; Pasini, S.; Perez, J.; Moleiro, L.H.; Hellweg, T. DMPC vesicle structure and dynamics in the presence of low amounts of the saponin aescin. Phys. Chem. Chem. Phys. PCCP 2018, 20, 9070–9083. [Google Scholar] [CrossRef]
- Sreij, R.; Dargel, C.; Hannappel, Y.; Jestin, J.; Prévost, S.; Dattani, R.; Wrede, O.; Hellweg, T. Temperature dependent self-organization of DMPC membranes promoted by intermediate amounts of the saponin aescin. Biochim. Biophys. Acta. Biomembr. 2019, 1861, 897–906. [Google Scholar] [CrossRef]
- Dargel, C.; Hannappel, Y.; Hellweg, T. Heating-Induced DMPC/Glycyrrhizin Bicelle-to-Vesicle Transition: A X-Ray Contrast Variation Study. Biophys. J. 2020, 118, 2411–2425. [Google Scholar] [CrossRef]
- Geisler, R.; Prévost, S.; Dattani, R.; Hellweg, T. Effect of Cholesterol and Ibuprofen on DMPC-β-Aescin Bicelles: A Temperature-Dependent Wide-Angle X-ray Scattering Study. Crystals 2020, 10, 401. [Google Scholar] [CrossRef]
- Geisler, R.; Pedersen, M.C.; Preisig, N.; Hannappel, Y.; Prévost, S.; Dattani, R.; Arleth, L.; Hellweg, T. Aescin - a natural soap for the formation of lipid nanodiscs with tunable size. Soft Matter 2021, 17, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Li, Y.; Murakami, T.; Ninomiya, K.; Yamahara, J.; Yoshikawa, M. Effects of Escins Ia, Ib, IIa, and IIb from Horse Chestnut, the Seeds of Aesculus hippocastanum L., on Acute Inflammation in Animals. Biol. Pharm. Bull. 1997, 20, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Pekdemir, T.; Ishigami, Y.; Uchiyama, H. Characterization of Aescin as a Biosurfactant for Environmental Remediation. J. Surfactants Deterg. 1999, 2, 337–341. [Google Scholar] [CrossRef]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef]
- Dargel, C.; Geisler, R.; Hannappel, Y.; Kemker, I.; Sewald, N.; Hellweg, T. Self-Assembly of the Bio-Surfactant Aescin in Solution: A Small-Angle X-ray Scattering and Fluorescence Study. Colloids Interfaces 2019, 3, 47. [Google Scholar] [CrossRef]
- Wilkinson, J.A.; Brown, A.M. Horse chestnut - Aesculus Hippocastanum: Potential applications in cosmetic skin-care products. Int. J. Cosmet. Sci. 1999, 21, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.R.; Rao, C.V. Anti-inflammatory and Anti-cancer Properties of β-Escin, a Triterpene Saponin. Curr. Pharmacol. Rep. 2015, 1, 170–178. [Google Scholar] [CrossRef]
- Pittler, M.H.; Ernst, E. Horse-Chestnut Seed Extract for Chronic Venous Insufficiency. A Criteria-Based Systematic Review. Arch. Dermatol. 1998, 134, 1356–1360. [Google Scholar] [CrossRef]
- Geisler, R.; Dargel, C.; Hellweg, T. The Biosurfactant β-Aescin: A Review on the Physico-Chemical Properties and Its Interaction with Lipid Model Membranes and Langmuir Monolayers. Molecules 2019, 25, 117. [Google Scholar] [CrossRef]
- Sreij, R.; Dargel, C.; Moleiro, L.H.; Monroy, F.; Hellweg, T. Aescin Incorporation and Nanodomain Formation in DMPC Model Membranes. Langmuir ACS J. Surfaces Colloids 2017, 33, 12351–12361. [Google Scholar] [CrossRef]
- Geisler, R.; Pedersen, M.C.; Hannappel, Y.; Schweins, R.; Prévost, S.; Dattani, R.; Arleth, L.; Hellweg, T. Aescin-Induced Conversion of Gel-Phase Lipid Membranes into Bicelle-like Lipid Nanoparticles. Langmuir ACS J. Surfaces Colloids 2019, 35, 16244–16255. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [CrossRef]
- Vree, T.B.; van den Biggelaar-Martea, M.; Verwey-van Wissen, C.P.; Vree, J.B.; Guelen, P.J. Pharmacokinetics of naproxen, its metabolite O-desmethylnaproxen, and their acyl glucuronides in humans. Biopharm. Drug Dispos. 1993, 14, 491–502. [Google Scholar] [CrossRef]
- Albozahid, M.; Naji, H.Z.; Alobad, Z.K.; Wychowaniec, J.K.; Saiani, A. Synthesis and characterization of hard copolymer polyurethane/functionalized graphene nanocomposites: Investigation of morphology, thermal stability, and rheological properties. J. Appl. Polym. Sci. 2022, 139, e53118. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, T.; Konovalov, O. Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research. Materials 2020, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Viguier, G.; Girardot, R.; Perez, J. Foxtrot. 2016. Available online: www.synchrotron-soleil.fr/en/beamlines/swing (accessed on 14 January 2023).
- Marsh, D. Lateral order in gel, subgel and crystalline phases of lipid membranes: Wide-angle X-ray scattering. Chem. Phys. Lipids 2012, 165, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Sztucki, M. SAXSutilities2: A Graphical User Interface for Processing and Analysis of Small-Angle X-ray Scattering Data, Zenodo: Meyrin, Switzerland, 21 September 2021. [CrossRef]
- Glatter, O. A New Method for the Evaluation of Small-Angle Scattering Data. J. Appl. Crystallogr. 1977, 10, 415–421. [Google Scholar] [CrossRef]
- Bergmann, A.; Fritz, G.; Glatter, O. Solving the generalized indirect Fourier transformation (GIFT) by Boltzmann simplex simulated annealing (BSSA). J. Appl. Crystallogr. 2000, 33, 1212–1216. [Google Scholar] [CrossRef]
- Kucerka, N.; Kiselev, M.A.; Balgavý, P. Determination of bilayer thickness and lipid surface area in unilamellar dimyristoylphosphatidylcholine vesicles from small-angle neutron scattering curves: A comparison of evaluation methods. Eur. Biophys. J. EBJ 2004, 33, 328–334. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Cho, C.H.; Urquidi, J.; Singh, S.; Robinson, G.W. Thermal Offset Viscosities of Liquid H2O, D2O, and T2O. J. Phys. Chem. B 1999, 103, 1991–1994. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Villena, F.; Sotomayor, C.P.; Edwards, A.M.; Muñoz, M.A.; Garidel, P.; Suwalsky, M. Human cells and cell membrane molecular models are affected in vitro by the nonsteroidal anti-inflammatory drug ibuprofen. Biochim. Biophys. Acta 2011, 1808, 2656–2664. [Google Scholar] [CrossRef]
- Caracciolo, G.; Pozzi, D.; Caminiti, R.; Marchini, C.; Montani, M.; Amici, A.; Amenitsch, H. Transfection efficiency boost by designer multicomponent lipoplexes. Biochim. Biophys. Acta 2007, 1768, 2280–2292. [Google Scholar] [CrossRef]
- Tristram-Nagle, S. Use of X-Ray and Neutron Scattering Methods with Volume Measurements to Determine Lipid Bilayer Structure and Number of Water Molecules/Lipid. Sub-Cell. Biochem. 2015, 71, 17–43. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Lombardo, D. Structural characterization in mixed lipid membrane systems by neutron and X-ray scattering. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 3700–3717. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.; Scheuermann, R.; Tucker, I.; Mulley, B.P. Dynamics and local environment of an aromatic counterion bound to di-chain cationic surfactant bilayers studied by avoided level crossing muon spin resonance: Evidence for counterion condensation. Phys. Chem. Chem. Phys. 2021, 23, 25542–25549. [Google Scholar] [CrossRef]
- Martyniak, A.; Scheuermann, R.; Dilger, H.; Tucker, I.M.; Burkert, T.; Hashmi, A.; Vujošević, D.; Roduner, E. Solvation thermodynamics of phenylalcohols in lamellar phase surfactant dispersions. Phys. B Condens. Matter 2006, 374–375, 328–331. [Google Scholar] [CrossRef]
- Roduner, E. Muons, soap, and drug delivery—An invitation to enter a new field of research. Phys. B Condens. Matter 2003, 326, 19–24. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Apanasenko, I.E.; Kim, A.V.; Shelepova, E.A.; Khalikov, S.S.; Polyakov, N.E. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surfaces. B Biointerfaces 2016, 147, 459–466. [Google Scholar] [CrossRef]
- Bergström, M.; Pedersen, J.S.; Schurtenberger, P.; Egelhaaf, S.U. Small-Angle Neutron Scattering (SANS) Study of Vesicles and Lamellar Sheets Formed from Mixtures of an Anionic and a Cationic Surfactant. J. Phys. Chem. B 1999, 103, 9888–9897. [Google Scholar] [CrossRef]
- Boggara, M.B.; Faraone, A.; Krishnamoorti, R. Effect of pH and ibuprofen on the phospholipid bilayer bending modulus. J. Phys. Chemistry. B 2010, 114, 8061–8066. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hägerbäumer, P.; Gräbitz-Bräuer, F.; Annegarn, M.; Dargel, C.; Stank, T.J.; Bizien, T.; Hellweg, T. Interactions between DMPC Model Membranes, the Drug Naproxen, and the Saponin β-Aescin. Pharmaceutics 2023, 15, 379. https://doi.org/10.3390/pharmaceutics15020379
Hägerbäumer P, Gräbitz-Bräuer F, Annegarn M, Dargel C, Stank TJ, Bizien T, Hellweg T. Interactions between DMPC Model Membranes, the Drug Naproxen, and the Saponin β-Aescin. Pharmaceutics. 2023; 15(2):379. https://doi.org/10.3390/pharmaceutics15020379
Chicago/Turabian StyleHägerbäumer, Pia, Friederike Gräbitz-Bräuer, Marco Annegarn, Carina Dargel, Tim Julian Stank, Thomas Bizien, and Thomas Hellweg. 2023. "Interactions between DMPC Model Membranes, the Drug Naproxen, and the Saponin β-Aescin" Pharmaceutics 15, no. 2: 379. https://doi.org/10.3390/pharmaceutics15020379
APA StyleHägerbäumer, P., Gräbitz-Bräuer, F., Annegarn, M., Dargel, C., Stank, T. J., Bizien, T., & Hellweg, T. (2023). Interactions between DMPC Model Membranes, the Drug Naproxen, and the Saponin β-Aescin. Pharmaceutics, 15(2), 379. https://doi.org/10.3390/pharmaceutics15020379