Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Vesicles from Tomato Fruit by Differential Ultracentrifugation
2.2. Separation of Nanovesicles into Subpopulations by Density Gradient Ultracentrifugation
2.3. Protein Quantification and SDS-PAGE Analysis
2.4. Density Determination
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Lysis of Vesicles and Proteolytic Digestion
2.7. LC-ESI-MS/MS
2.8. Bioinformatics
2.9. Determination of Lipid Content
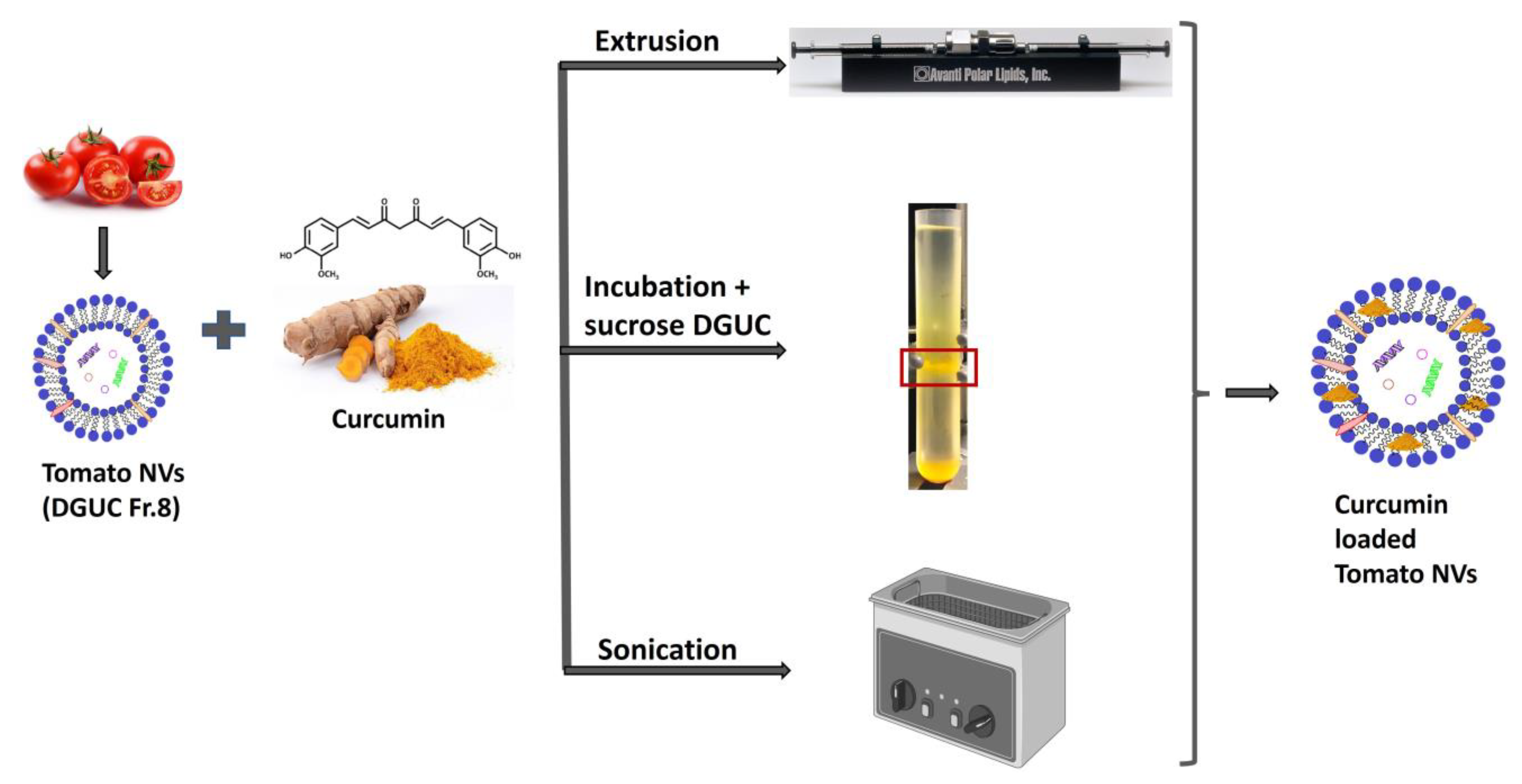
2.10. Preparation of Curcumin-Loaded Small Unilamellar Vesicles
2.10.1. Cargo Loading by Extrusion
2.10.2. Cargo Loading by Sonication
2.10.3. Passive Cargo Loading
2.11. Cell Cultures
2.12. MTT Assay and Trypan Blue Staining
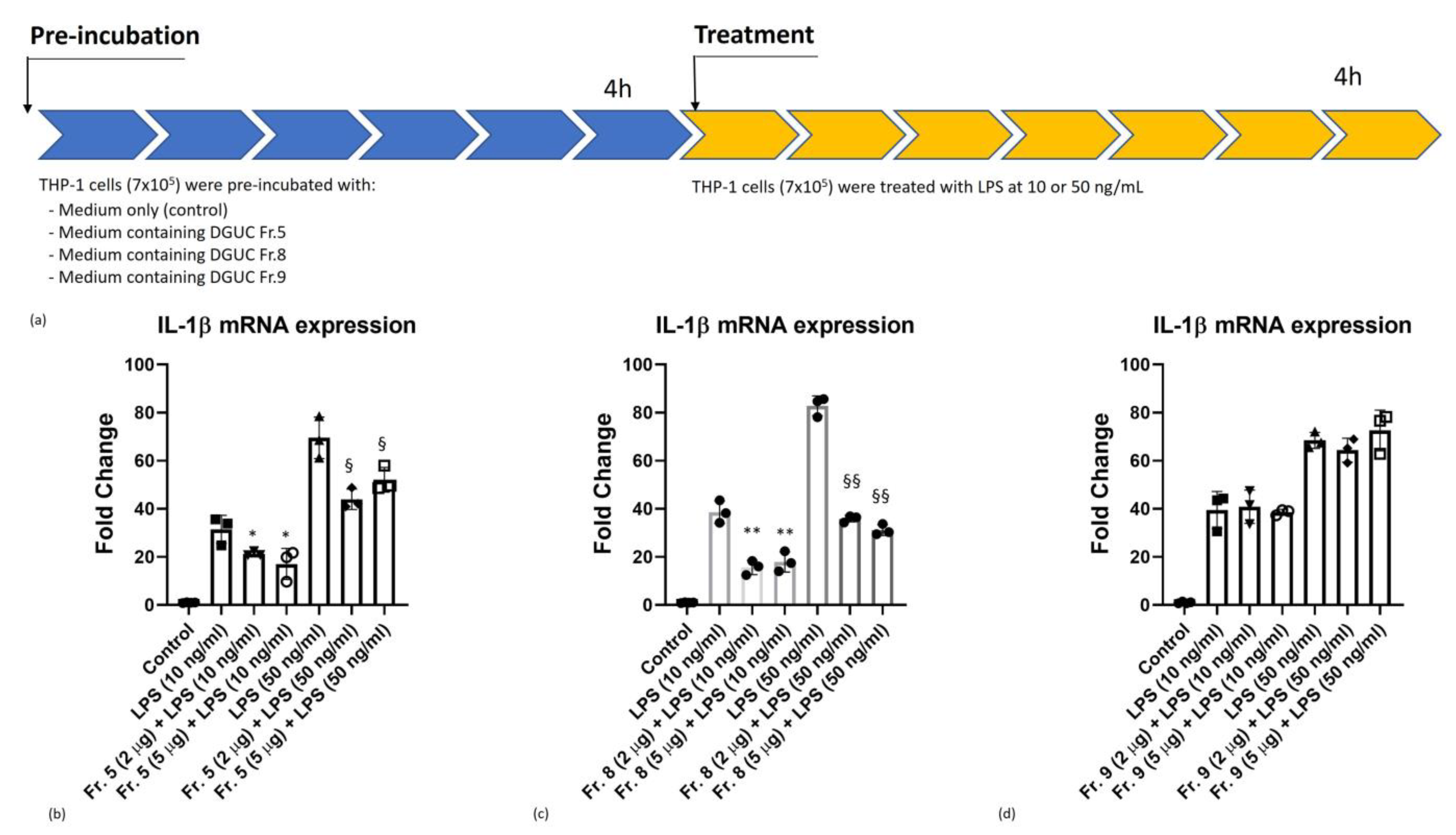
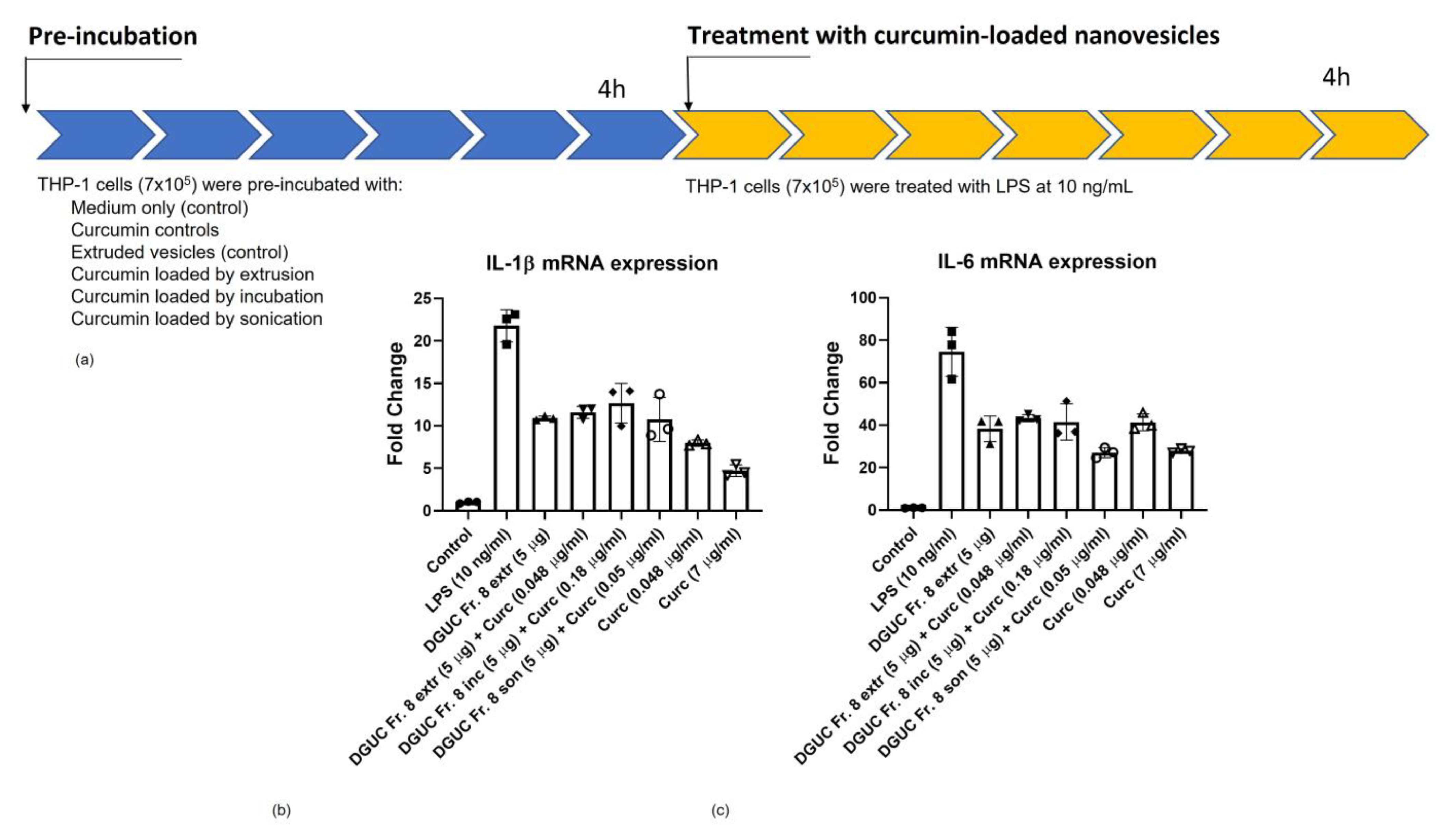
2.13. Anti-Inflammatory Activity Assay
2.14. Inflammatory Cytokine Test
2.15. Statistics
3. Results
3.1. Tomato-Derived Vesicles Isolation, Separation Based on Density and Characterization
3.2. Proteomic Characterization of Tomato Fruit-Derived NVs and DGUC Fractions
3.3. Cytotoxicity of Tomato-Derived NVs
3.4. Anti-Inflammatory Activity of Native Tomato NVs on THP-1 Cell Line
3.5. Curcumin Loading into Tomato Vesicles
3.6. Anti-Inflammatory Activity of Curcumin Loaded Tomato NVs on THP-1 Cell Line
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bokka, R.; Ramos, A.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Mammadova, R.; Fiume, I.; Bokka, R.; Kralj-Iglič, V.; Božič, D.; Kisovec, M.; Podobnik, M.; Zavec, A.; Hočevar, M.; Gellén, G.; et al. Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials 2021, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L.; Pocsfalvi, G. Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles. Int. J. Mol. Sci. 2019, 20, 6205. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Dico, A.L.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Stanly, C.; Kim, H.; Antonucci, G.; Fiume, I.; Guescini, M.; Kim, K.P.; Ciardiello, M.A.; Giangrieco, I.; Mari, A.; Pocsfalvi, G. Crosstalk Between the Immune System and Plant-Derived Nanovesicles: A Study of Allergen Transporting. Front. Bioeng. Biotechnol. 2021, 9, 760730. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Cho, E.-G.; Choi, S.-Y.; Kim, H.; Choi, E.-J.; Lee, E.-J.; Park, P.-J.; Ko, J.; Kim, K.; Baek, H. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef]
- Pinedo, M.; de la Canal, L.; Lousa, C.D.M. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Raimondo, S.; Urzì, O.; Meraviglia, S.; Di Simone, M.; Corsale, A.M.; Ganji, N.R.; Piccionello, A.P.; Polito, G.; Presti, E.L.; Dieli, F.; et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J. Cell. Mol. Med. 2022, 26, 4195–4209. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peerj 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Guerriero, G.; Hausman, J.-F.; Renaut, J.; Leclercq, C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M. Plant Extracellular Vesicles and Nanovesicles: Focus on Secondary Metabolites, Proteins and Lipids with Perspectives on Their Potential and Sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An insilico approach. J. Transl. Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef]
- Marcus, M.E.; Leonard, J.N. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals 2013, 6, 659–680. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.-G.; Qiao, H. Technology insight: Plant-derived vesicles—How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Rome, S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Oskouie, M.N.; Moghaddam, N.S.A.; Butler, A.E.; Zamani, P.; Sahebkar, A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J. Cell. Physiol. 2018, 234, 8182–8191. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Chaturvedi, P.; Tyagi, S.C.; Tyagi, N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014, 107, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Stöckl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; König, M.A.; Johnstone, B.; Grässel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef]
- Arab-Tehrany, E.; ElKhoury, K.; Francius, G.; Jierry, L.; Mano, J.F.; Kahn, C.; Linder, M. Curcumin Loaded Nanoliposomes Localization by Nanoscale Characterization. Int. J. Mol. Sci. 2020, 21, 7276. [Google Scholar] [CrossRef]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Božič, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing extracellular vesicles produced by microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef] [PubMed]
- Visnovitz, T.; Osteikoetxea, X.; Sódar, B.W.; Mihály, J.; Lőrincz, P.; Vukman, K.V.; Tóth, E.; Koncz, A.; Székács, I.; Horváth, R.; et al. An improved 96 well plate format lipid quantification assay for standardisation of experiments with extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565263. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Chen, G.; Hackett, R.; Walker, D.; Taylor, A.; Lin, Z.; Grierson, D. Identification of a Specific Isoform of Tomato Lipoxygenase (TomloxC) Involved in the Generation of Fatty Acid-Derived Flavor Compounds. Plant Physiol. 2004, 136, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W.; MacDonald, I.A.; Zeisel, S.H. Present Knowledge in Nutrition: Tenth Edition; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Hedi, H.; Norbert, G. 5-Lipoxygenase Pathway, Dendritic Cells, and Adaptive Immunity. J. Biomed. Biotechnol. 2004, 2004, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Munier, C.C.; Ottmann, C.; Perry, M.W. 14-3-3 modulation of the inflammatory response. Pharmacol. Res. 2021, 163, 105236. [Google Scholar] [CrossRef]
- Panayi, G.; Corrigall, V. BiP regulates autoimmune inflammation and tissue damage. Autoimmun. Rev. 2006, 5, 140–142. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef]
- Mohri, S.; Takahashi, H.; Sakai, M.; Takahashi, S.; Waki, N.; Aizawa, K.; Suganuma, H.; Ara, T.; Matsumura, Y.; Shibata, D.; et al. Wide-range screening of anti-inflammatory compounds in tomato using LC-MS and elucidating the mechanism of their functions. PLoS ONE 2018, 13, 4–6. [Google Scholar] [CrossRef]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine Towards Cancer: 111In-Labeled Nanoparticles. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef]

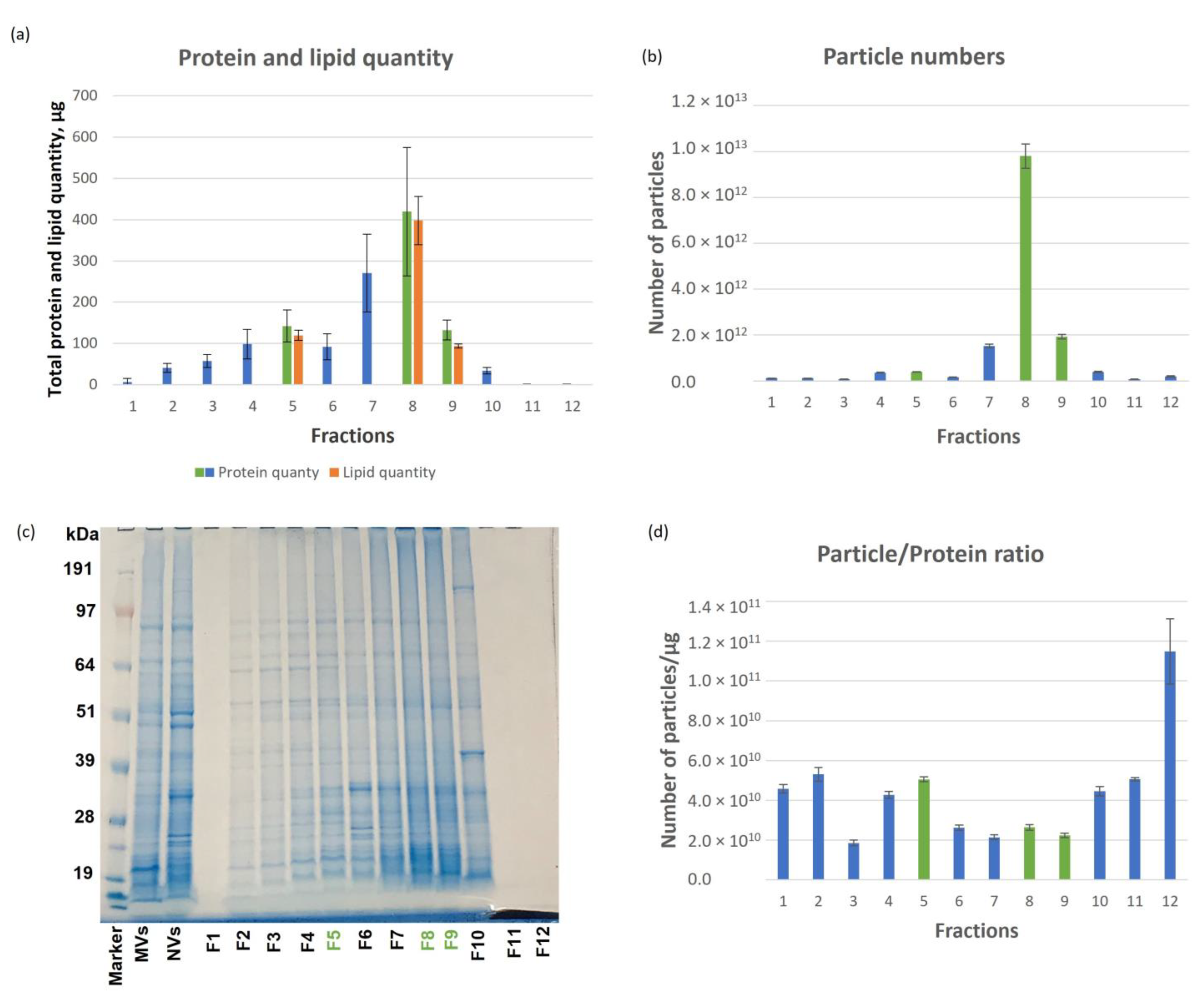
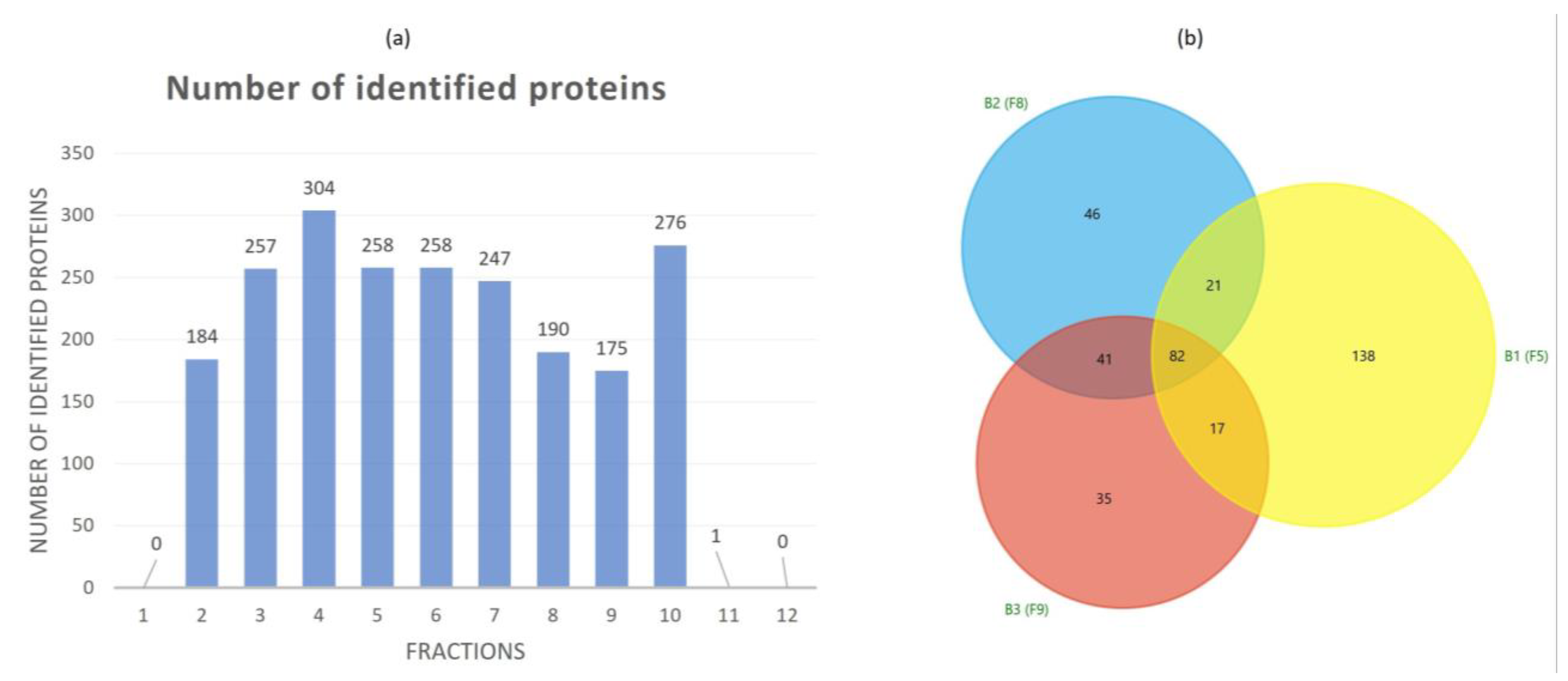
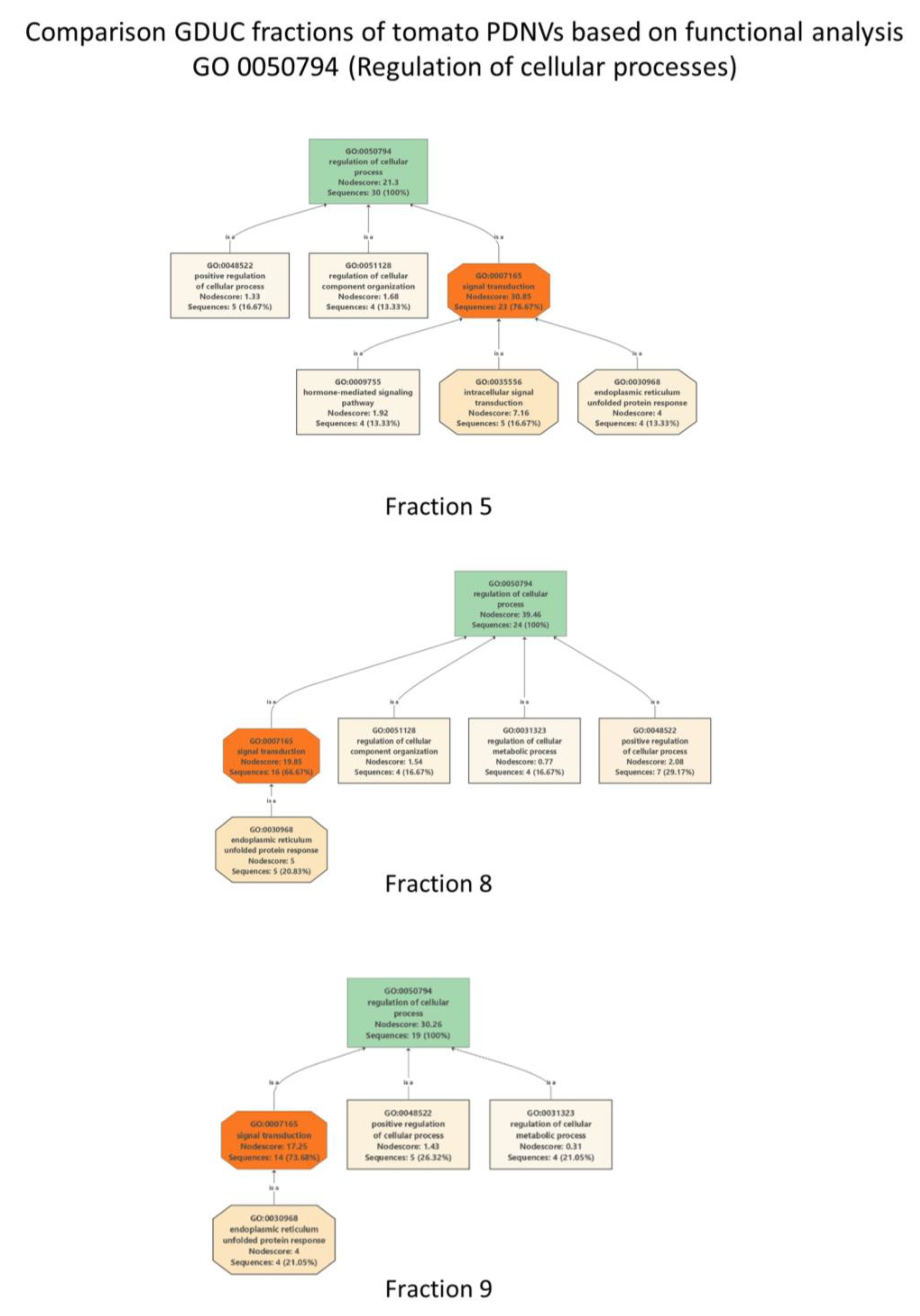
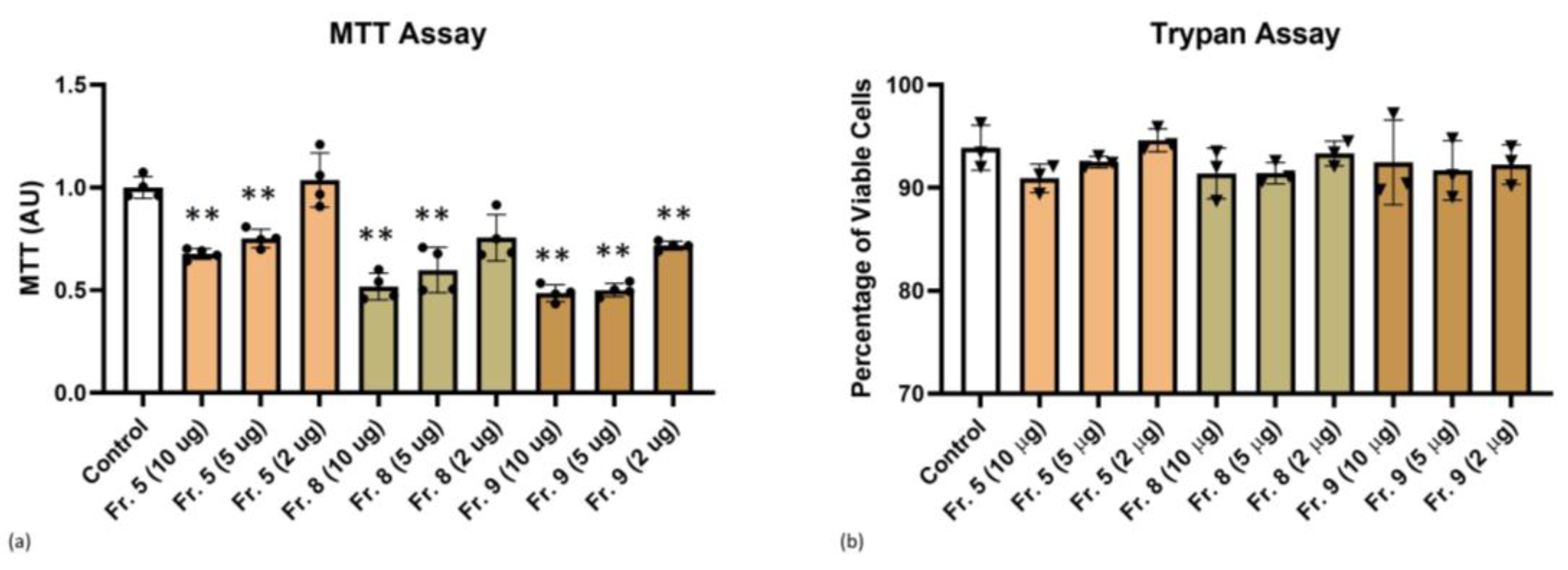
| Cluster ID UniRef | Cluster Name | Ranking in Dataset | mW (Da) | PLGS Score | Coverage (%) | Precursor RMS Mass Error (ppm) | Products | Products RMS Mass Error (ppm) |
|---|---|---|---|---|---|---|---|---|
| A0A1U8H482 | Alcohol dehydrogenase 1 | 6 | 41,275 | 23,279 | 50.3 | 0.9 | 534 | 24.4 |
| A0A3Q7GTI1 | Luminal-binding protein 5 | 85 | 60,108 | 2618 | 18.1 | 3.3 | 196 | 36.6 |
| A0A1S4AVH1 | GTP-binding protein SAR1A | 94 | 22,021 | 7487 | 48.2 | 4.3 | 167 | 29.5 |
| Q9FSY7 | Endoplasmic reticulum chaperone BiP | 28 | 73,444 | 17,203 | 29.0 | 5.7 | 325 | 33.7 |
| P93209 | 14-3-3 protein 3 | 97 | 30,420 | 6509 | 17.9 | 6.8 | 125 | 29.8 |
| Q03685 | Luminal-binding protein 5 | 43 | 73,859 | 16,665 | 23.8 | 4.9 | 319 | 35.9 |
| Q7Y240 | Glutaredoxin-dependent peroxiredoxin | 161 | 17,425 | 4178 | 22.2 | 3.3 | 88 | 34.1 |
| P93207 | 14-3-3 protein 10 | 101 | 33,680 | 4477 | 16.3 | 1.6 | 161 | 34.6 |
| P93214 | 14-3-3 protein 9 | 135 | 29,413 | 4033 | 13.8 | 1.8 | 158 | 37.3 |
| P49118 | Luminal-binding protein | 42 | 73,189 | 17,054 | 22.5 | 4.9 | 311 | 33.4 |
| P93206 | 14-3-3 protein 1 | 100 | 28,183 | 4550 | 19.3 | 3.8 | 152 | 34.1 |
| P25858 | Glyceraldehyde-3-phosphate dehydrogenase GAPC1, cytosolic | 96 | 36,650 | 6664 | 22.8 | 1.9 | 189 | 33.4 |
| Q41418 | 14-3-3-like protein | 63 | 29,320 | 8262 | 38.5 | 4.5 | 248 | 32.3 |
| A0A3Q7EAX2 | 14-3-3 domain-containing protein | 153 | 28,176 | 2311 | 6.4 | 1.6 | 103 | 35.5 |
| P93212 | 14-3-3 protein 7 | 134 | 28,796 | 4212 | 17.5 | 2.0 | 159 | 36.5 |
| A0A3Q7ETU0 | AAA domain-containing protein | 184 | 48,803 | 2396 | 6.7 | 31.2 | 137 | 39.2 |
| A0A3Q7EH12 | GTP-binding protein SAR1A | 110 | 21,910 | 5624 | 40.4 | 1.6 | 135 | 31.9 |
| A0A3Q7F894 | AAA domain-containing protein | 162 | 51,696 | 4109 | 6.1 | 5.6 | 165 | 39.7 |
| A0A3Q7HZY2 | Senescence-associated protein | 149 | 30,101 | 3726 | 19.2 | 0.8 | 90 | 29.4 |
| A0A3Q7GX91 | mitogen-activated protein kinase | 48 | 142,212 | 6454 | 12.0 | 5.6 | 405 | 36.6 |
| A0A3Q7JBH3 | 14-3-3 domain-containing protein | 70 | 45,120 | 7041 | 16.7 | 17.3 | 160 | 29.0 |
| W1P062 | Ras-related protein RABH1b | 167 | 23,083 | 3665 | 9.1 | 6.3 | 136 | 39.4 |
| A0A1S3YWY0 | Mediator of RNA polymerase II transcription subunit 37a-like | 155 | 74,724 | 13,707 | 2.5 | 18.5 | 92 | 35.2 |
| M1CBH0 | Alcohol dehydrogenase 1 | 139 | 41,124 | 3025 | 4.5 | 6.4 | 73 | 21.7 |
| Sample | Loading Method | Ratio PDNVs (µg of Protein:µg of Curcumin) | Quantity of Loaded Curcumin (µg) | Quantity of Loaded PDNVs (by Protein Content µg) | EE% | DL% | Curcumin Quantity Used for In Vitro Assay (µg) |
|---|---|---|---|---|---|---|---|
| Blank (MVs) | Extrusion | 0 | 77 | - | - | - | |
| Blank (DGUC Fr. 8) | Extrusion | 0 | 924 | - | - | - | |
| MVs | Extrusion | 1:2 | 0.78 | 94 | 0.08 | 0.82 | 0.08 |
| DGUC Fr. 8 | Extrusion | 1:10 | 5.36 | 540 | 0.03 | 0.98 | 0.05 |
| DGUC Fr. 8 | Incubation | 1:5 | 15.63 | 418 | 0.22 | 3.60 | 0.18 |
| DGUC Fr. 8 | Sonication | 1:4 | 2.78 | 254 | 0.10 | 1.10 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammadova, R.; Maggio, S.; Fiume, I.; Bokka, R.; Moubarak, M.; Gellén, G.; Schlosser, G.; Adamo, G.; Bongiovanni, A.; Trepiccione, F.; et al. Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin. Pharmaceutics 2023, 15, 333. https://doi.org/10.3390/pharmaceutics15020333
Mammadova R, Maggio S, Fiume I, Bokka R, Moubarak M, Gellén G, Schlosser G, Adamo G, Bongiovanni A, Trepiccione F, et al. Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin. Pharmaceutics. 2023; 15(2):333. https://doi.org/10.3390/pharmaceutics15020333
Chicago/Turabian StyleMammadova, Ramila, Serena Maggio, Immacolata Fiume, Ramesh Bokka, Maneea Moubarak, Gabriella Gellén, Gitta Schlosser, Giorgia Adamo, Antonella Bongiovanni, Francesco Trepiccione, and et al. 2023. "Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin" Pharmaceutics 15, no. 2: 333. https://doi.org/10.3390/pharmaceutics15020333
APA StyleMammadova, R., Maggio, S., Fiume, I., Bokka, R., Moubarak, M., Gellén, G., Schlosser, G., Adamo, G., Bongiovanni, A., Trepiccione, F., Guescini, M., & Pocsfalvi, G. (2023). Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin. Pharmaceutics, 15(2), 333. https://doi.org/10.3390/pharmaceutics15020333










