Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form
Abstract
1. Introduction
2. Materials and Methods
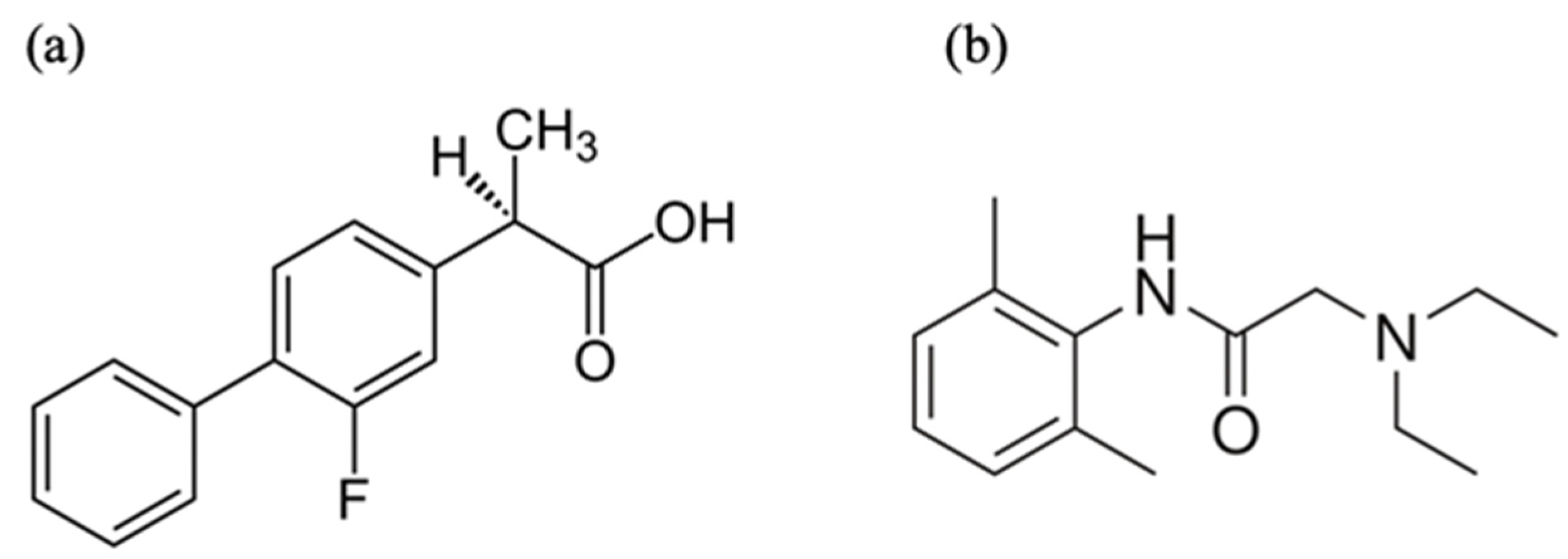
2.1. Materials
2.2. Preparation of NSAIDs/LDC Complexes
2.3. Physicochemical Properties of FLU/LDC Complex
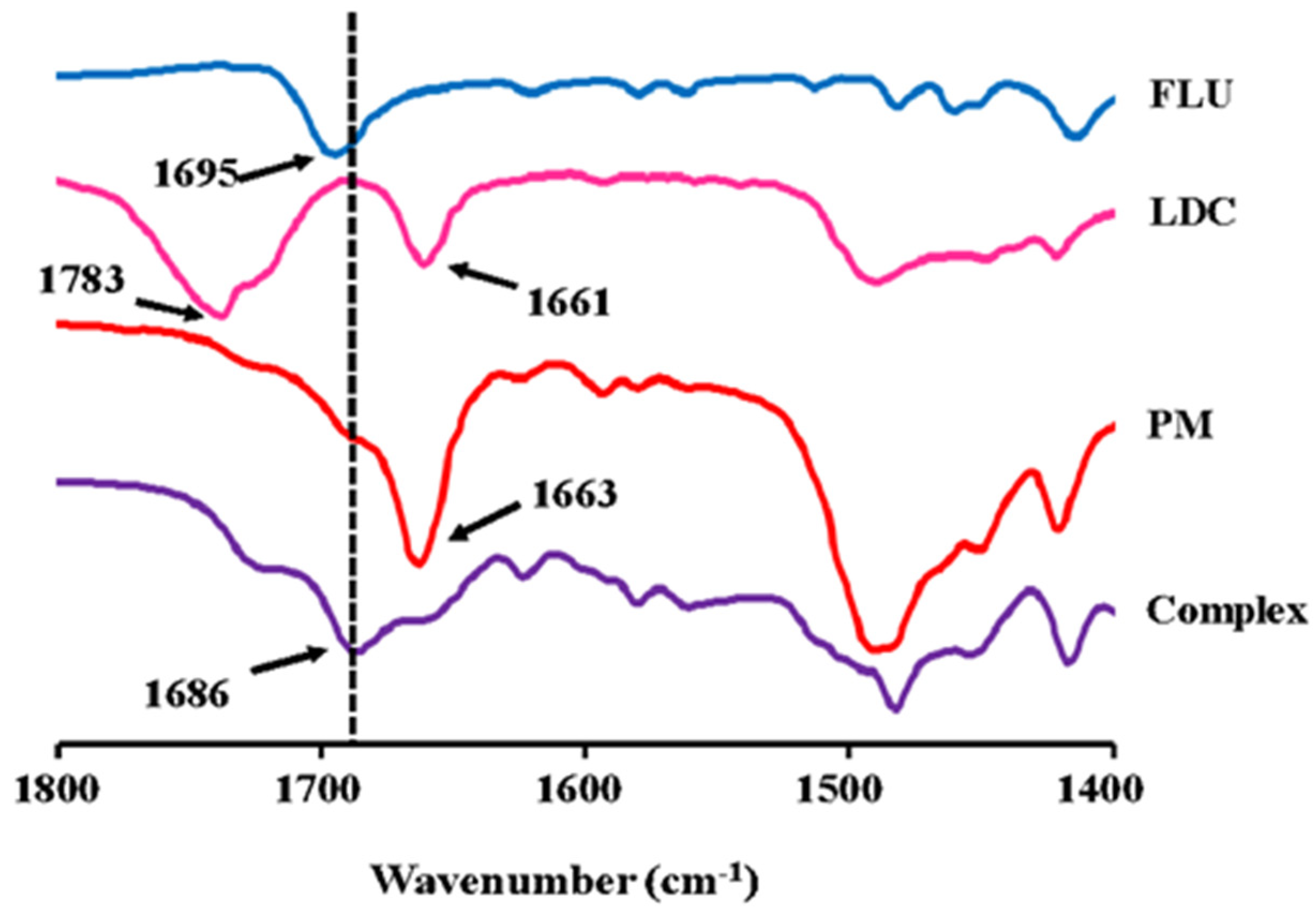
2.3.1. ATR-FTIR Measurements
2.3.2. Differential Scanning Calorimetry (DSC) Measurements
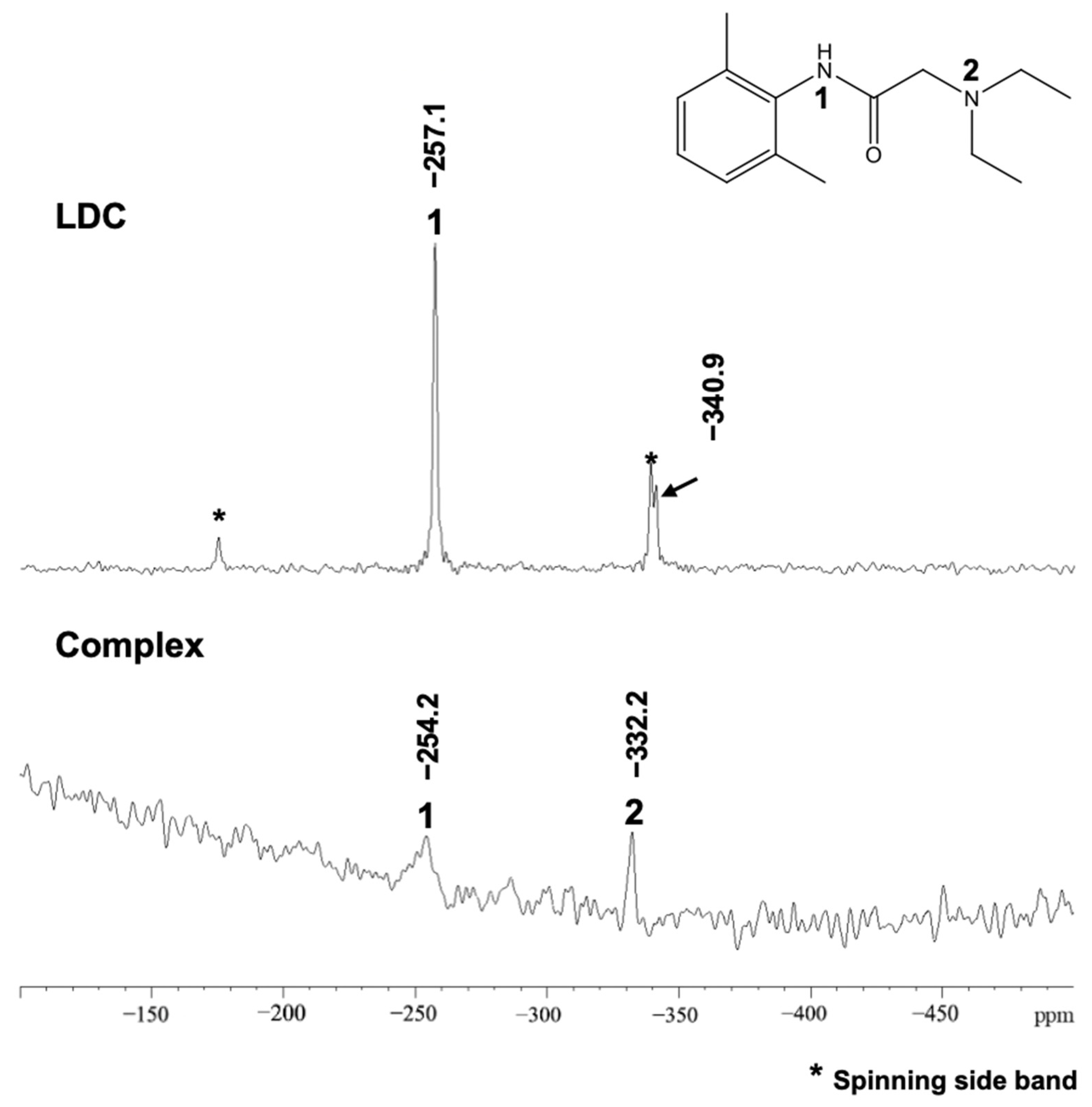
2.3.3. 13C Dipolar Decoupling (13C DD), 15N Cross-Polarization (15N CP), Magic-Angle Spinning (MAS), and Nuclear Magnetic Resonance (NMR) Measurements
2.4. Solubility Test
2.5. In Vitro Skin Permeability Test
2.6. High-Performance Liquid Chromatography (HPLC) Measurements
2.7. Data Analysis of In Vitro Skin Permeability Test
3. Results and Discussion
3.1. Amorphous Molecular Complex Formation Screening between NSAIDs and LDC
3.2. Physicochemical Properties of FLU/LDC Complex
3.2.1. ATR-FTIR Measurements
3.2.2. DSC Measurements
3.2.3. 13C DD and 15N CP/MAS NMR Measurements
3.3. Solubility Test
3.4. Skin Permeation Test of FLU/LDC Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, X.; Capomacchia, A.C. The binary eutectic of NSAIDS and two-phase liquid system for enhanced membrane permeation. Pharm. Dev. Technol. 2005, 10, 1–10. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.; Moinuddin, S.M.; Feng, X.; Ahsan, F. Co-amorphous Drug Delivery Systems: A Review of Physical Stability, In Vitro and In Vivo Performance. AAPS PharmSciTech 2022, 23, 259. [Google Scholar] [CrossRef]
- Zhuang, W.; Hachem, K.; Bokov, D.; Javed Ansari, M.; Taghvaie Nakhjiri, A. Ionic liquids in pharmaceutical industry: A systematic review on applications and future perspectives. J. Mol. Liq. 2022, 349, 118145. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Mannava, M.K.C.; Nangia, A. A novel curcumin–artemisinin coamorphous solid: Physical properties and pharmacokinetic profile. RSC Adv. 2014, 4, 58357–58361. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co amorphous systems: A product development perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Kelley, S.P.; Narita, A.; Holbrey, J.D.; Green, K.D.; Reichert, W.M.; Rogers, R.D. Understanding the Effects of Ionicity in Salts, Solvates, Co-Crystals, Ionic Co-Crystals, and Ionic Liquids, Rather than Nomenclature, Is Critical to Understanding Their Behavior. Cryst. Growth Des. 2013, 13, 965–975. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Duan, L.; Lin, Y.; Cui, X.; Yang, Y.; Wang, C. Deep Eutectic Systems as Novel Vehicles for Assisting Drug Transdermal Delivery. Pharmaceutics 2022, 14, 2265. [Google Scholar] [CrossRef]
- Shimada, Y.; Goto, S.; Uchiro, H.; Hirabayashi, H.; Yamaguchi, K.; Hirota, K.; Terada, H. Features of heat-induced amorphous complex between indomethacin and lidocaine. Colloids Surf. B Biointerfaces 2013, 102, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Essa, E.A.; Elmarakby, A.O.; Donia, A.M.A.; El Maghraby, G.M. Controlled precipitation for enhanced dissolution rate of flurbiprofen: Development of rapidly disintegrating tablets. Drug Dev. Ind. Pharm. 2017, 43, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, R.A.; Essa, E.A.; El Maghraby, G.M. Eutexia for enhanced dissolution rate and anti-inflammatory activity of nonsteroidal anti-inflammatory agents: Caffeine as a melting point modulator. Int. J. Pharm. 2019, 563, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Landau, R.; Achilladelis, B.; Scriabine, A. Pharmaceutical Innovation: Revolutionizing Human Health; Chemical Heritage Press: Philadelphia, PA, USA, 1999. [Google Scholar]
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal Bioavailability in Rats of Lidocaine in the Forms of Ionic Liquids, Salts, and Deep Eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503. [Google Scholar] [CrossRef]
- Umeda, Y.; Fukami, T.; Furuishi, T.; Suzuki, T.; Makimura, M.; Tomono, K. Molecular complex consisting of two typical external medicines: Intermolecular interaction between indomethacin and lidocaine. Chem. Pharm. Bull. 2007, 55, 832–836. [Google Scholar] [CrossRef]
- Umeda, Y.; Kaburagi, M.; Furuishi, T.; Fukami, T.; Suzuki, T.; Tanjou, K.; Tomono, K. Investigation of the Percutaneous Absorption of a Novel Molecular Complex between Indomethacin and Lidocaine. J. Pharm. Sci. Technol. Jpn. 2009, 69, 384–391. [Google Scholar] [CrossRef]
- Mann, S.K.; Pham, T.N.; McQueen, L.L.; Lewandowski, J.R.; Brown, S.P. Revealing Intermolecular Hydrogen Bonding Structure and Dynamics in a Deep Eutectic Pharmaceutical by Magic-Angle Spinning NMR Spectroscopy. Mol. Pharm. 2020, 17, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.; Dalwadi, G.; Wigent, R.; Gupta, P. Ionic liquid formation with deep eutectic forces at an atypical ratio (2:1) of naproxen to lidocaine in the solid-state, thermal characterization and FTIR investigation. Int. J. Pharm. 2020, 575, 118946. [Google Scholar] [CrossRef]
- Moreira, D.N.; Fresno, N.; Pérez-Fernández, R.; Frizzo, C.P.; Goya, P.; Marco, C.; Martins, M.A.P.; Elguero, J. Brønsted acid–base pairs of drugs as dual ionic liquids: NMR ionicity studies. Tetrahedron 2015, 71, 676–685. [Google Scholar] [CrossRef]
- Marei, H.F.; Arafa, M.F.; Essa, E.A.; El Maghraby, G.M. Lidocaine as eutectic forming drug for enhanced transdermal delivery of nonsteroidal anti-inflammatory drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102338. [Google Scholar] [CrossRef]
- Miwa, Y.; Hamamoto, H.; Ishida, T. Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur. J. Pharm. Biopharm. 2016, 102, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Millington, P.F. Skin; Millington, P.F., Wilkinson, R., Eds.; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Waters, C. The development of the rotigotine transdermal patch: A historical perspective. Neurol. Clin. 2013, 31, S37–S50. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Liu, T.; Wang, H.; Wu, C.; Chen, H.; Liu, Z.; Zhang, J. Ionic liquid transdermal delivery system: Progress, prospects, and challenges. J. Mol. Liq. 2022, 351, 118643. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfar, M.; Idkaidek, N.; Al-Remawi, M. A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2020, 22, 4. [Google Scholar] [CrossRef]
- Campos, W.F.; Silva, E.C.; Oliveira, T.J.; Oliveira, J.M., Jr.; Tubino, M.; Pereira, C.; Vila, M.M.; Balcao, V.M. Transdermal permeation of bacteriophage particles by choline oleate: Potential for treatment of soft-tissue infections. Future Microbiol. 2020, 15, 881–896. [Google Scholar] [CrossRef]
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira, J.M., Jr.; Tubino, M.; Vila, M.; et al. Non-invasive Transdermal Delivery of Human Insulin Using Ionic Liquids: In vitro Studies. Front. Pharm. 2020, 11, 243. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Fiala, S.; Jones, S.A.; Brown, M.B. A fundamental investigation into the effects of eutectic formation on transmembrane transport. Int. J. Pharm. 2010, 393, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Nyqvist-Mayer, A.A.; Brodin, A.F.; Frank, S.G. Drug release studies on an oil-water emulsion based on a eutectic mixture of lidocaine and prilocaine as the dispersed phase. J. Pharm. Sci. 1986, 75, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Wertz, P.; Downing, D. Stratum Corneum: Biological and Biochemical Considerations; Hadgraft, J., Guy, R., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1989; Volume 35, pp. 1–22. [Google Scholar]
- Marjukka Suhonen, T.; Bouwstra, J.A.; Urtti, A. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Control. Release 1999, 59, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Furuishi, T.; Takahashi, S.; Ogawa, N.; Gunji, M.; Nagase, H.; Suzuki, T.; Endo, T.; Ueda, H.; Yonemochi, E.; Tomono, K. Enhanced dissolution and skin permeation profiles of epalrestat with beta-cyclodextrin derivatives using a cogrinding method. Eur. J. Pharm. Sci. 2017, 106, 79–86. [Google Scholar] [CrossRef]
- Furuishi, T.; Kunimasu, K.; Fukushima, K.; Ogino, T.; Okamoto, K.; Yonemochi, E.; Tomono, K.; Suzuki, T. Formulation design and evaluation of a transdermal drug delivery system containing a novel eptazocine salt with the Eudragit® E adhesive. J. Drug Deliv. Sci. Technol. 2019, 54, 101298. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Kun, E.; Guo, X.; Yu, Z.; Zhang, F. Influence of lidocaine forms (salt vs. freebase) on properties of drug-eudragit(R) L100-55 extrudates prepared by reactive melt extrusion. Int. J. Pharm. 2018, 547, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H. Masking the bitter taste of injectable lidocaine HCl formulation for dental procedures. AAPS PharmSciTech 2015, 16, 455–465. [Google Scholar] [CrossRef]
- Agarwal, P.; Nieuwoudt, M.K.; Li, S.; Procter, G.; Andrews, G.P.; Jones, D.S.; Svirskis, D. Exploiting hydrogen bonding to enhance lidocaine loading and stability in a poly ethylene-co-vinyl acetate carrier matrix. Int. J. Pharm. 2022, 621, 121819. [Google Scholar] [CrossRef]
- Mrestani-Klaus, C.; Richardt, A.; Wespe, C.; Stark, A.; Humpfer, E.; Bordusa, F. Structural studies on ionic liquid/water/peptide systems by HR-MAS NMR spectroscopy. Chemphyschem 2012, 13, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Rencurosi, A.; Lay, L.; Russo, G.; Prosperi, D.; Poletti, L.; Caneva, E. HRMAS NMR analysis in neat ionic liquids: A powerful tool to investigate complex organic molecules and monitor chemical reactions. Green Chem. 2007, 9, 216–218. [Google Scholar] [CrossRef]
- Warner, L.; Gjersing, E.; Follett, S.E.; Elliott, K.W.; Dzyuba, S.V.; Varga, K. The effects of high concentrations of ionic liquid on GB1 protein structure and dynamics probed by high-resolution magic-angle-spinning NMR spectroscopy. Biochem. Biophys. Rep. 2016, 8, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Shamshina, J.; Hough, W.L.; MacFarlane, D.R.; Rogers, R.D. Liquid forms of pharmaceutical co-crystals: Exploring the boundaries of salt formation. Chem. Commun. 2011, 47, 2267–2269. [Google Scholar] [CrossRef]
- Yataba, I.; Otsuka, N.; Matsushita, I.; Kamezawa, M.; Yamada, I.; Sasaki, S.; Uebaba, K.; Matsumoto, H.; Hoshino, Y. Plasma pharmacokinetics and synovial concentrations of S-flurbiprofen plaster in humans. Eur. J. Clin. Pharm. 2016, 72, 53–59. [Google Scholar] [CrossRef]
- Sun, W.; Yu, J.; Lu, G.; Ye, X.; Fu, J. Clinical therapeutic effects of lidocaine combination with flurbiprofen axetil for reducing propofol-induced pain in adults: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23844. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Furuishi, T.; Fukuzawa, K.; Yonemochi, E. Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form. Pharmaceutics 2023, 15, 318. https://doi.org/10.3390/pharmaceutics15020318
Xu Q, Furuishi T, Fukuzawa K, Yonemochi E. Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form. Pharmaceutics. 2023; 15(2):318. https://doi.org/10.3390/pharmaceutics15020318
Chicago/Turabian StyleXu, Qihui, Takayuki Furuishi, Kaori Fukuzawa, and Etsuo Yonemochi. 2023. "Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form" Pharmaceutics 15, no. 2: 318. https://doi.org/10.3390/pharmaceutics15020318
APA StyleXu, Q., Furuishi, T., Fukuzawa, K., & Yonemochi, E. (2023). Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form. Pharmaceutics, 15(2), 318. https://doi.org/10.3390/pharmaceutics15020318





