Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression
Abstract
:1. Introduction
2. The Transdermal Route as an Alternative for Biomacromolecules Delivery
3. Types of Microneedles and the Use of the MN Platform to Deliver Genetic Materials, Stem Cells, and Secretome
| Microneedle Type | Material | Application | Advantages | Drawbacks |
|---|---|---|---|---|
| Solid Microneedles | Silicon, silica glass, and metals, such as stainless steel and titanium | Two-step application |
|
|
| Coated Microneedles | Ceramics, silicon, silica glass, and metals, such as stainless steel and titanium | Single-step application |
|
|
| Hollow Microneedles | Ceramics, glass, and metals, such as stainless steel and titanium | Single-step application |
|
|
| Dissolving Microneedles | Biodegradable and biocompatible polymers | Single-step application |
|
|
| Hydrogel-Forming Microneedles | Crosslinking biocompatible polymers | Single-step application |
|
|
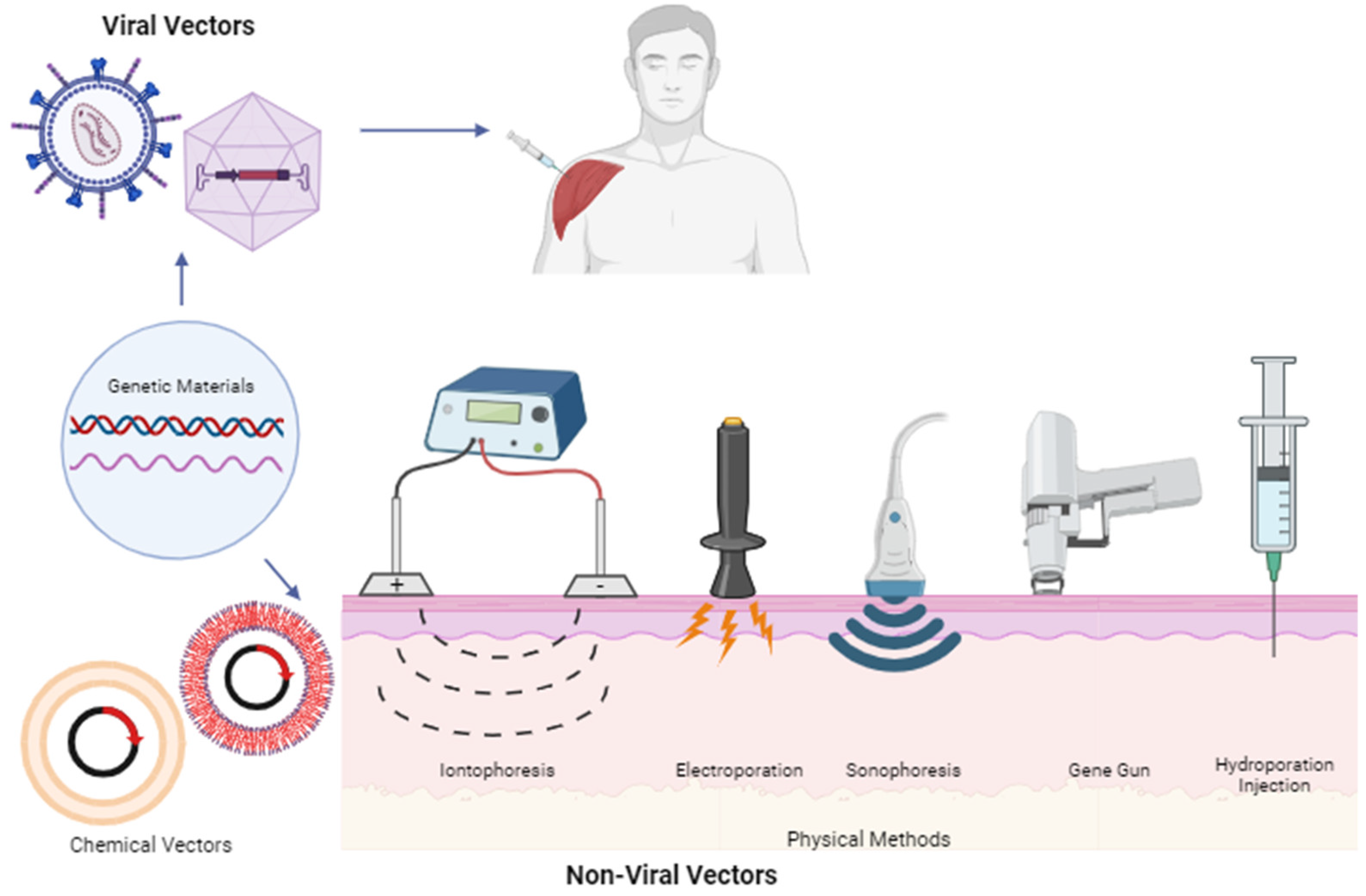
4. Genetic Material-Based Therapy
5. Therapies Based on Stem Cells and Their Products
6. Future Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Food and Drug Administration (FDA). Approved Cellular and Gene Therapy Products; U.S Food and Drug Administration: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 23 October 2023).
- Agyei, D.; Tan, K.X.; Pan, S.; Udenigwe, C.C.; Danquah, M.K. Peptides for biopharmaceutical applications. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–251. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.H.J.; Ahmed, M.G.; Husain, A. Transferosomal in situ gel administered through umbilical skin tissues for improved systemic bioavailability of drugs: A novel strategy to replace conventional transdermal route. Med. Hypotheses 2022, 161, 110805. [Google Scholar] [CrossRef]
- Shah, B.; Surti, N.; Misra, A. Other Routes of Protein and Peptide Delivery: Transdermal, Topical, Uterine, and Rectal. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 623–671. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- MRawas-Qalaji, M.; Thu, H.E.; Hussain, Z. Oromucosal delivery of macromolecules: Challenges and recent developments to improve bioavailability. J. Control. Release 2022, 352, 726–746. [Google Scholar] [CrossRef]
- Kapoor, Y.; Milewski, M.; Dick, L.; Zhang, J.; Bothe, J.R.; Gehrt, M.; Manser, K.; Nissley, B.; Petrescu, I.; Johnson, P.; et al. Coated microneedles for transdermal delivery of a potent pharmaceutical peptide. Biomed. Microdevices 2020, 22, 7. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Wiley-Blackwell: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Merino, V. Current Technologies to Increase the Transdermal Delivery of Drugs; Bentham Science Publishers Ltd.: Sharjah, United Arab Emirates, 2010. [Google Scholar]
- Donnelly, R.F.; Singh, T.R.R. Novel Delivery Systems for Transdermal and Intradermal Drug Delivery; John Wiley & Sons, Ltd.: West Sussex, UK, 2015. [Google Scholar]
- Kulkarni, V.S. Handbook of Non-Invasive Drug Delivery Systems; William Andrew: Burlington, ON, Canada, 2009. [Google Scholar]
- Donnelly, R.F.; Larraneta, E.; Singh, T.R.R.; McCrudden, M.T.C. Microneedles for Drug and Vaccine Delivery and Patient Monitoring; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Ita, K. Basic principles of transdermal drug delivery. In Transdermal Drug Delivery; Elsevier: London, UK, 2020; pp. 19–36. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Watkinson, A.C. Topical and Transdermal Drug Delivery: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Banga, A.K. Transdermal and Intradermal Delivery of Therapeutic Agents; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Escobar-Chávez, J.J. Current Technologies to Increase the Transdermal Delivery of Drugs. In Physical Penetration Enhancers: Therapeutic Applications and Devices; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 2. [Google Scholar]
- Moffatt, K.; Donnelly, R.F. Microneedle technology. In Drug Delivery Devices Therapeutic Systems; Elsevier: London, UK, 2020; pp. 345–366. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Ramadon, D.; Ulayya, F.; Qur’ani, A.S.; Iskandarsyah, I.; Harahap, Y.; Anjani, Q.K.; Aileen, V.; Hartrianti, P.; Donnelly, R.F. Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen. Pharmaceuticals 2023, 16, 378. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, S.; Ma, Y.; Chen, Y.; Yang, G.; Zhou, Z.; Gao, Y. Preparation and evaluation of dissolving microneedle loaded with azelaic acid for acne vulgaris therapy. J. Drug Deliv. Sci. Technol. 2022, 75, 103667. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Gu, X.; Wei, F.; Cao, W.; Zheng, L.; Li, Y.; Ma, T.; Wu, C.; Wang, Q. Celecoxib nanocrystal-loaded dissolving microneedles with highly efficient for osteoarthritis treatment. Int. J. Pharm. 2022, 625, 122108. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Vora, L.K.; Tekko, I.A.; Permana, A.D.; Domínguez-Robles, J.; Ramadon, D.; Chambers, P.; McCarthy, H.O.; Larrañeta, E.; Donnelly, R.F. Dissolving microneedle patches loaded with amphotericin B microparticles for localised and sustained intradermal delivery: Potential for enhanced treatment of cutaneous fungal infections. J. Control. Release 2021, 339, 361–380. [Google Scholar] [CrossRef]
- Lee, Y.; Li, W.; Tang, J.; Schwendeman, S.P.; Prausnitz, M.R. Immediate detachment of microneedles by interfacial fracture for sustained delivery of a contraceptive hormone in the skin. J. Control. Release 2021, 337, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.D.; Son, S.; Xavier, W.; Nguyen, V.Q.; Jung, J.M.; Lee, J.; Shin, S.; Um, W.; An, J.Y.; Kim, C.H.; et al. Dissolving microneedles for long-term storage and transdermal delivery of extracellular vesicles. Biomaterials 2022, 287, 121644. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Bin Sabri, A.H.; Moreno-Castellanos, N.; Utomo, E.; Cárcamo-Martínez, N.; Domínguez-Robles, J.; Wardoyo, L.A.H.; Donnelly, R.F. Soluplus®-based dissolving microarray patches loaded with colchicine: Towards a minimally invasive treatment and management of gout. Biomater. Sci. 2022, 10, 5838–5855. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Fallows, S.J.; Birkhäuer, L.L.; McCrudden, M.T.; Woolfson, A.D.; Donnelly, R.F. A facile system to evaluate in vitro drug release from dissolving microneedle arrays. Int. J. Pharm. 2016, 497, 62–69. [Google Scholar] [CrossRef]
- Miranda, B.; Battisti, M.; De Martino, S.; Nocerino, V.; Dardano, P.; De Stefano, L.; Cangiano, G. Hollow Microneedle-based Plasmonic Sensor for on Patch Detection of Molecules in Dermal Interstitial Fluid. Adv. Mater. Technol. 2023, 8, 2300037. [Google Scholar] [CrossRef]
- Davidson, A.; Al-Qallaf, B.; Das, D.B. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 2008, 86, 1196–1206. [Google Scholar] [CrossRef]
- Le, Z.; Yu, J.; Quek, Y.J.; Bai, B.; Li, X.; Shou, Y.; Myint, B.; Xu, C.; Tay, A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today 2023, 63, 137–169. [Google Scholar] [CrossRef]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Ramadon, D.; Sutrisna, L.F.P.; Harahap, Y.; Putri, K.S.S.; Ulayya, F.; Hartrianti, P.; Anjani, Q.K.; Donnelly, R.F. Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers. Pharmaceutics 2023, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; Permana, A.D.; Courtenay, A.J.; McCrudden, M.T.C.; Tekko, I.A.; McAlister, E.; Anjani, Q.K.; Utomo, E.; McCarthy, H.O.; Donnelly, R.F. Development, Evaluation, and Pharmacokinetic Assessment of Polymeric Microarray Patches for Transdermal Delivery of Vancomycin Hydrochloride. Mol. Pharm. 2020, 17, 3353–3368. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Andranilla, R.K.; Anjani, Q.K.; Hartrianti, P.; Donnelly, R.F.; Ramadon, D. Fabrication of dissolving microneedles for transdermal delivery of protein and peptide drugs: Polymer materials and solvent casting micromoulding method. Pharm. Dev. Technol. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.E.; Vucen, S.; Moore, A.C. Trends in drug- and vaccine-based dissolvable microneedle materials and methods of fabrication. Eur. J. Pharm. Biopharm. 2022, 173, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, e2000307. [Google Scholar] [CrossRef] [PubMed]
- De Martino, S.; Battisti, M.; Napolitano, F.; Palladino, A.; Serpico, L.; Amendola, E.; Martone, A.; De Girolamo, P.; Squillace, A.; Dardano, P.; et al. Effect of microneedles shape on skin penetration and transdermal drug administration. Biomater. Adv. 2022, 142, 213169. [Google Scholar] [CrossRef]
- Cole, G.; Ali, A.A.; McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; Robson, T.; Kett, V.L.; Dunne, N.J.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur. J. Pharm. Biopharm. 2018, 127, 288–297. [Google Scholar] [CrossRef]
- Dalvi, M.; Kharat, P.; Thakor, P.; Bhavana, V.; Singh, S.B.; Mehra, N.K. Panorama of dissolving microneedles for transdermal drug delivery. Life Sci. 2021, 284, 119877. [Google Scholar] [CrossRef]
- Golchin, A.; Farahany, T.Z. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev. Rep. 2019, 15, 166–175. [Google Scholar] [CrossRef]
- Shams, S.; Silva, E.A. Bioengineering Strategies for Gene Delivery. In Engineering Strategies for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–148. [Google Scholar] [CrossRef]
- Sung, Y.; Kim, S. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Worgall, S.; Crystal, R.G. Gene Therapy. In Principles of Tissue Engineering; Elsevier: London, UK, 2020; pp. 493–518. [Google Scholar] [CrossRef]
- Schaffer, D.V.; Zhou, W. Gene Therapy and Gene Delivery Systems. Springer: Berlin/Heidelberg, Germany, 2005; Volume 99. [Google Scholar] [CrossRef]
- Huang, L.; Liu, D.; Wagner, E. Advances in Genetics: Nonviral Vectors for Gene Therapy; Elsevier: Waltham, MA, USA, 2015; Volume 89. [Google Scholar]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of covid-19 viral vector-based vaccines and covid-19 variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- South, E.; Cox, E.; Meader, N.; Woolacott, N.; Griffin, S. Strimvelis® for Treating Severe Combined Immunodeficiency Caused by Adenosine Deaminase Deficiency: An Evidence Review Group Perspective of a NICE Highly Specialised Technology Evaluation. PharmacoEconomics-Open 2019, 3, 151–161. [Google Scholar] [CrossRef]
- Lopes, R.M.; Fonseca, N.; Cruz, A.; Gregório, A.; Valério-Fernandes, Â.; Moura, V.; Simões, S.; Moreira, J. Advances on Nucleic Acid Delivery with Nonviral Vectors; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Morini, M.; Albini, A.; Lorusso, G.; Moelling, K.; Lu, B.; Cilli, M.; Ferrini, S.; Noonan, D. Prevention of angiogenesis by naked DNA IL-12 gene transfer: Angioprevention by immunogene therapy. Gene Ther. 2004, 11, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, L.; Li, N.; She, F. Enhancement of Helicobacter pylori outer inflammatory protein DNA vaccine efficacy by co-delivery of interleukin-2 and B subunit heat-labile toxin gene encoded plasmids. Microbiol. Immunol. 2012, 56, 85–92. [Google Scholar] [CrossRef]
- Vasan, S.; Hurley, A.; Schlesinger, S.J.; Hannaman, D.; Gardiner, D.F.; Dugin, D.P.; Boente-Carrera, M.; Vittorino, R.; Caskey, M.; Andersen, J.; et al. In Vivo Electroporation Enhances the Immunogenicity of an HIV-1 DNA Vaccine Candidate in Healthy Volunteers. PLoS ONE 2011, 6, e19252. [Google Scholar] [CrossRef]
- Zhang, N.; Foiret, J.; Kheirolomoom, A.; Liu, P.; Feng, Y.; Tumbale, S.; Raie, M.; Wu, B.; Wang, J.; Fite, B.Z.; et al. Optimization of Microbubble-Based DNA Vaccination with Low-Frequency Ultrasound for Enhanced Cancer Immunotherapy. Adv. Ther. 2021, 4, 2100033. [Google Scholar] [CrossRef]
- Singh, J.; Mohanty, I.; Rattan, S. In vivo magnetofection: A novel approach for targeted topical delivery of nucleic acids for rectoanal motility disorders. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G109–G118. [Google Scholar] [CrossRef] [PubMed]
- Viecelli, H.M.; Harbottle, R.P.; Wong, S.P.; Schlegel, A.; Chuah, M.K.; VandenDriessche, T.; Harding, C.O.; Thöny, B. Treatment of phenylketonuria using minicircle-based naked-DNA gene transfer to murine liver. Hepatology 2014, 60, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zong, Z.-M.; Liu, Q.; Jiang, S.-S.; Zhang, Q.; Cen, L.-Q.; Gao, J.; Gao, X.-G.; Huang, J.-D.; Liu, Y.; et al. A novel galactose-PEG-conjugated biodegradable copolymer is an efficient gene delivery vector for immunotherapy of hepatocellular carcinoma. Biomaterials 2018, 184, 20–30. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Zhang, P.; Wang, Y. Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J. Mater. Chem. B 2020, 8, 4331–4339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, Y.; Ye, W.; Wang, X.; Huai, Y.; Huang, Q.; Chu, X.; Deng, X.; Qian, A. A lipid–polymer hybrid nanoparticle (LPN)-loaded dissolving microneedle patch for promoting hair regrowth by transdermal miR-218 delivery. Biomater. Sci. 2022, 11, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.J.; Liu, Y.; Lim, S.H.; Loh, X.J.; Kang, L.; Lim, C.Y.; Phua, K.K.L. Formulation, characterization and evaluation of mRNA-loaded dissolvable polymeric microneedles (RNApatch). Sci. Rep. 2018, 8, 11842. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Kim, B.S.; Jeong, J.H.; Lee, D.S. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy. J. Mater. Chem. B 2020, 8, 1171–1181. [Google Scholar] [CrossRef]
- Yan, Q.; Cheng, Z.; Liu, H.; Shan, W.; Cheng, Z.; Dai, X.; Xue, Y.; Chen, F. Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice. Vaccine 2018, 36, 4471–4476. [Google Scholar] [CrossRef]
- Pan, J.; Ruan, W.; Qin, M.; Long, Y.; Wan, T.; Yu, K.; Zhai, Y.; Wu, C.; Xu, Y. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci. Rep. 2018, 8, 1117. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Zhang, P.; Zhao, X.; Wang, Y. Cutaneous microenvironment responsive microneedle patch for rapid gene release to treat subdermal tumor. J. Control. Release 2019, 314, 72–80. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, G.S.; Li, S.; Lee, J.W.; Mixson-Hayden, T.; Woo, J.; Xia, D.; Prausnitz, M.R.; Kamili, S.; Purdy, M.A.; et al. Hepatitis B vaccine delivered by microneedle patch: Immunogenicity in mice and rhesus macaques. Vaccine 2023, 41, 3663–3672. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Zhang, F.; Liu, X.; Xie, Q.; Liu, Q.; Yuan, L.; Zhao, T.; Xie, S.; Xu, Q.; et al. A microneedle-based delivery system for broad-protection seasonal influenza A DNA nanovaccines. Cell Rep. Phys. Sci. 2023, 4, 101430. [Google Scholar] [CrossRef]
- Pham, P.V. Stem Cell Drugs—A New Generation of Biopharmaceuticals; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Praveen Kumar, L.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Bakalorz, K.; Los, L.D.; Wiecheć, E. Introduction and historic perspective. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Asal, M.; Güven, S. Stem cells: Sources, properties, and cell types. In Biomaterials for Organ and Tissue Regeneration New Technologies and Future Prospects; Woodhead Publishing: Sawston, UK, 2020; pp. 177–196. [Google Scholar] [CrossRef]
- Kalra, K.; Tomar, P.C. Stem Cell: Basics, Classification and Applications. Am. J. Phytomedicine Clin. Ther. 2014, 2, 919–930. [Google Scholar]
- Ota, K.I. Fuel Cells: Past, Present and Future. IEEJ Trans. Fundam. Mater. 2008, 128, 329–332. [Google Scholar] [CrossRef]
- Ramalingam, M.; Ramakrishna, S.; Best, S. Biomaterials and Stem Cells in Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- El-Hashash, A.H.K. Mesenchymal stem cells in human health and diseases: General discussion, remarks and future directions. In Mesenchymal Stem Cells in Human Health and Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 179–199. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.R. Stem Cells and Regenerative Medicine. In Goodman’s Medical Cell Biology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.-H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Mendes-Pinheiro, B.; Marote, A.; Marques, C.R.; Teixeira, F.G.; Ribeiro, J.C.; Salgado, A.J. Applications of the stem cell secretome in regenerative medicine. In Mesenchymal Stem Cells in Human Health and Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 79–114. [Google Scholar] [CrossRef]
- Srinivasan, A.; Fults, M.L.; Supronowicz, P.; Esquivel, R.; Zamilpa, R. Mesenchymal Stem Cell e Derived Products for Tissue Repair and Regeneration; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Vu, D.M.; Nguyen, V.-T.; Nguyen, T.H.; Do, P.T.X.; Dao, H.H.; Hai, D.X.; Le, N.T.; Nguyen, X.-H.; Than, U.T.T. Effects of Extracellular Vesicles Secreted by TGFβ-Stimulated Umbilical Cord Mesenchymal Stem Cells on Skin Fibroblasts by Promoting Fibroblast Migration and ECM Protein Production. Biomedicines 2022, 10, 1810. [Google Scholar] [CrossRef]
- Gualeni, B.; Coulman, S.A.; Shah, D.; Eng, P.F.; Ashraf, H.; Vescovo, P.; Blayney, G.J.; Piveteau, L.-D.; Guy, O.J.; Birchall, J.C. Minimally invasive and targeted therapeutic cell delivery to the skin using microneedle devices. Br. J. Dermatol. 2018, 178, 731–739. [Google Scholar] [CrossRef]
- Chellathurai, M.S.; Ling, V.W.T.; Palanirajan, V.K. Fabrication and evaluation of transdermal microneedles for a recombinant human keratinocyte growth factor. Turkish J. Pharm. Sci. 2021, 18, 96–103. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Q.; Wen, D.; Chen, Z.; Wang, J.; Chen, G.; Wang, Z.; Zhang, X.; Zhang, Y.; Hu, Q.; et al. A Therapeutic Microneedle Patch Made from Hair-Derived Keratin for Promoting Hair Regrowth. ACS Nano 2019, 13, 4354–4360. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Xue, Y.; Lee, J.; Kim, H.; Liu, Y.; Tebon, P.; Sarikhani, E.; Sun, W.; Zhang, S.; Haghniaz, R.; et al. A Patch of Detachable Hybrid Microneedle Depot for Localized Delivery of Mesenchymal Stem Cells in Regeneration Therapy. Adv. Funct. Mater. 2020, 30, 2000086. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Zhang, X.; Ma, W.; Zhao, Y.; Sun, L. Antibacterial, adhesive, and MSC exosomes encapsulated microneedles with spatio-temporal variation functions for diabetic wound healing. Nano Today 2022, 47, 101630. [Google Scholar] [CrossRef]
- Yuan, A.; Gu, Y.; Bian, Q.; Wang, R.; Xu, Y.; Ma, X.; Zhou, Y.; Gao, J. Conditioned media-integrated microneedles for hair regeneration through perifollicular angiogenesis. J. Control. Release 2022, 350, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jin, S.; Wang, P.; He, Q.; Yang, Y.; Gao, Z.; Wang, X. Microneedle based adipose derived stem cells-derived extracellular vesicles therapy ameliorates UV-induced photoaging in SKH-1 mice. J. Biomed. Mater. Res. Part A 2021, 109, 1849–1857. [Google Scholar] [CrossRef]
- Wang, X.; Shu, X.; Huo, W.; Zou, L.; Li, L. Efficacy of protein extracts from medium of Adipose-derived stem cells via microneedles on Asian skin. J. Cosmet. Laser Ther. 2018, 20, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yusharyahya, S.N.; Japranata, V.V.; Sitohang, I.B.S. A Comparative Study on Adipose-Derived Mesenchymal Stem Cells Secretome Delivery Using Microneedling and Fractional CO2 Laser for Facial Skin Rejuvenation A Comparative Study on Adipose-Derived Mesenchymal Stem Cells Secretome Delivery Using Microneedlin. Clin. Cosmet. Investig. Dermatol. 2023, 16, 387–395. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, J.; Li, H.; Yu, M.; Zhang, W.; Qin, D.; Qiu, K.; Chen, X.; Kong, M. A Drug-Free, Hair Follicle Cycling Regulatable, Separable, Antibacterial Microneedle Patch for Hair Regeneration Therapy. Adv. Healthc. Mater. 2022, 11, e2200908. [Google Scholar] [CrossRef]
- Chandran, R.; Tohit, E.R.M.; Stanslas, J.; Tuan Mahmood, T.M. Recent Advances and Challenges in Microneedle-Mediated Transdermal Protein and Peptide Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Dul, M.; Alali, M.; Ameri, M.; Burke, M.D.; Craig, C.M.; Creelman, B.P.; Dick, L.; Donnelly, R.F.; Eakins, M.N.; Frivold, C.; et al. Assessing the risk of a clinically significant infection from a Microneedle Array Patch (MAP) product. J. Control. Release 2023, 361, 236–245. [Google Scholar] [CrossRef]
- Forster, A.H.; Witham, K.; Depelsenaire, A.C.I.; Veitch, M.; Wells, J.W.; Wheatley, A.; Pryor, M.; Lickliter, J.D.; Francis, B.; Rockman, S.; et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase i clinical trial. PLoS Med. 2020, 17, e1003024. [Google Scholar] [CrossRef]
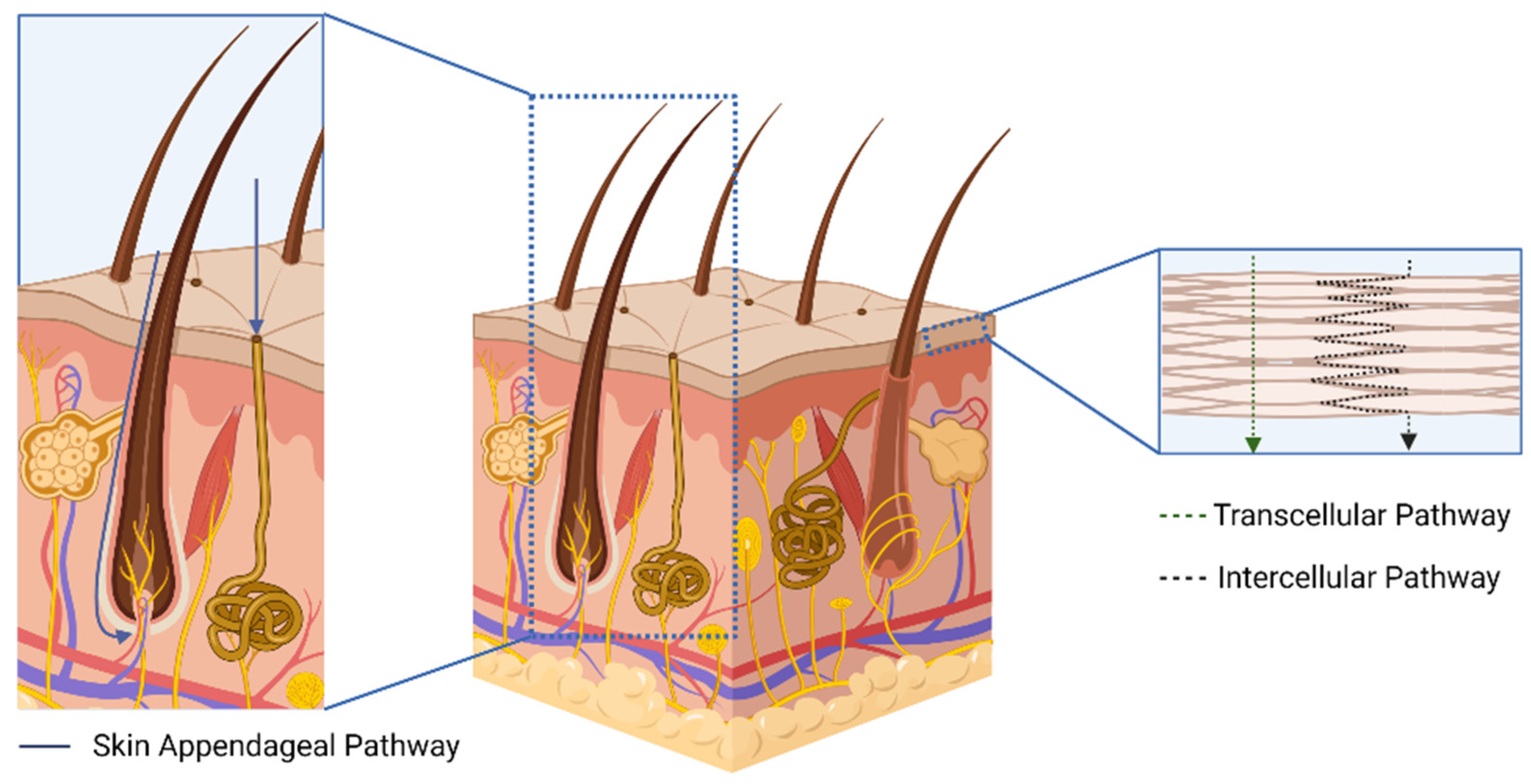


| Microneedle Design | Material | Compounds | Outcome | Ref. |
|---|---|---|---|---|
| 5 × 5 microneedle arrays with each needle’s height being 1000 μm | Hyaluronic acid | p53 DNA and IR820 | Dissolving microneedle patch containing p53 DNA and IR820 application with near-infrared laser irradiation could minimize tumor volumes in epidermoid carcinoma model mice compared to caudal vein injection treatment by promoting endosomal membrane obstruction and enhancing pDNA transfection efficiency. | [62] |
| 19 × 19 microneedle arrays with each needle’s height being 600 μm | Poly(vinyl alcohol) | pDNA | A third immunization using a dissolving microneedle patch containing lyophilized RALA/pDNA nanoparticles could decrease tumor mass in cervical cancer model mice by increasing anti-E6/E7 IgG serum levels, which are higher levels than the intramuscular injection treatment group. | [41] |
| 10 × 10 microneedle arrays with each needle’s height being 800 μm | Hyaluronic acid | miR-218 | A dissolving microneedle patch containing miR-218 incorporated in lipid/polymer hybrid nanoparticles (LPNs) is applied every four days on shaved model mice compared to the gel formulation group, which results in fast-onset hair regrowth by promoting the proliferation of dermal papilla cells. | [63] |
| 10 × 10 microneedle arrays with each needle’s height being 800 μm | Poly(vinylpyrrolidone) | Ovalbumin mRNA | After three immunizations by three dissolving microneedle patches carrying naked Ovalbumin (OVA) mRNA, tumor progression in E.G7-OVA carcinoma model mice have similar inhibition results with the subcutaneous injection method by increasing anti-OVA antibody production. | [64] |
| 15 × 15 microneedle arrays with each needle’s height being 475 μm | Polypeptide copolymer matrix (mPEG5K-PN2LG30) | pOVA and poly(I:C) | Fourteen days after the fourth vaccination using a dissolving microneedle containing nanoplex pOVA and poly(I:C) loaded in a polypeptide copolymer matrix, B16-OVA melanoma model mice have an induced anti-OVA antibody IgG1 level higher than subcutaneous injection due to the contact of OVA antigens with antigen-presenting cells (APCs) located on dermal skin. | [65] |
| 8 × 8 microneedle arrays with each needle’s height being 500 μm | Sodium hyaluronate | Ag85B DNA | Humoral immunities were formed on tuberculosis model mice which applied three dissolving microneedle patches containing Ag85B DNA before infection due to increasing IgG1 and IgG2a antibodies same level with intramuscular injection model mice. | [66] |
| 12 × 12 microneedle arrays with each needle’s height being 650 μm | Dextran, poly(vinylpyrrolidone) and hyaluronic acid | STAT3 siRNA | A dissolving microneedle patch containing STAT3 siRNA encapsulated in polyethyleneimine (PEI) carrier could better result in reduced tumor volume and mass in B16F10 melanoma model mice than no treatment group by lowering STAT3 mRNA expression. | [67] |
| 6 × 6 microneedle arrays with each needle’s height being 993 μm | Polycaprolactone, dimethulmaleic anhydride-modified polylysine (PLL-DMA), and polyethyleneimine (PEI) | p53 DNA | Oral carcinoma model mice have lower tumor growth rate after three applications of the coated microneedle patch containing p53 DNA with a stimulus-responsive transition layer (PLL-DMA) than the intravenous injection model due to highly expressed P53 protein, which could disrupt cancer cell proliferation. | [68] |
| 10 × 10 microneedle arrays with each needle’s height being 600 μm | Maltodextrin, sucrose, and fish gelatin | HBsAg | Seven weeks after the third immunization of the hepatitis B vaccine by dissolving microneedle patch, 11-week-old female mice have robust humoral and cellular immune responses similar levels with intramuscular injection due to the induction of the hepatitis B surface antigen (HBsAg) to dendritic cells, which processes and lead it to T cells. | [69] |
| 76 microneedle arrays with each needle’s height being 1000 μm | Sucrose, poly(vinyl alcohol), deoxycholic acid (DCA), and polyetherimide (PEI) | DNA | The third immunization using a dissolving microneedle patch consists of a DNA vaccine (ligation of antigens mH1 and mH3 with an internal ribosome-entry site (IRES)) encapsulated on a DCA-PEI nanomaterial has better protective immunity than an intramuscular injection in mice against influenza A H1N1 and H3N2 infection by introducing the DNA vaccine to Langerhans cells, which can trigger T cells and B cells. | [70] |
| Microneedle Design | Material | Compounds | Outcome | Ref. |
|---|---|---|---|---|
| 11 × 11 microneedle arrays with each needle’s height being 600 μm | Poly(lactic-co-glycolic-acid) (PLGA), poly(vinylpyrrolidone) (PVP), and poly(vinyl alcohol) (PVA) | rHuKGF | Dissolving microneedles could deliver recombinant human keratinocyte growth factor (rHuKGF) by in vitro evaluation using Parafilm® M (Bemis Company, Inc., Sheboygan Falls, WI, USA) exposed in a phosphate-buffered saline solution (PBS). | [86] |
| 15 × 15 microneedle arrays with each needle’s height being 600 μm | Keratin, cysteine, hyaluronic acid, and poly(lactic-co-glycolic-acid) (PLGA) | Exosomes and UK5099 | Exosomes derived from human bone marrow mesenchymal stem cells and UK5099 drugs loaded in PLGA nanoparticles incorporated on hydrogel microneedles have a higher hair regrowth effect than topical subcutaneous injection on hairless model mice after two rounds of application by activating hair follicle stem cells (HFSCs). | [87] |
| 8 × 8 microneedle arrays with each needle’s height being 700 μm | Gelatin methacryloyl (GelMA) and poly(lactic-co-glycolic-acid) (PLGA) | MSCs | Detachable microneedles containing human bone marrow mesenchymal stem cells regenerate skin wound model mice by enhancing re-epithelialization and angiogenesis, which is compared to the intradermal injection model. | [88] |
| 20 × 20 microneedle arrays with each needle’s height being 600 μm | Gelatin methacrylate (GelMA) and silk fibroin-methacryloyl (SilMA) | AgNPs and Exosomes | The single use of hydrogel microneedles containing exosomes derived from human umbilical cord mesenchymal stem cells and Ag nanoparticles has a faster wound healing process than injection at the wound site and antiinfection effect on wound-infected diabetic rats by improving the vascularization process and reducing inflammatory response. | [89] |
| 15 × 15 microneedle arrays with each needle’s height being 600 μm | Hyaluronic acid, trehalose, and poly(vinylpyrrolidone) (PVP) | Secretome | After three applications of dissolving microneedles containing secretome from rat bone marrow mesenchymal stem cells, hairless model mice show higher hair regeneration effects than intradermal injection by enhancing angiogenesis around hair follicles. | [90] |
| 15 × 15 microneedle arrays with each needle’s height being 600 μm | Hyaluronic acid | Extracellular vesicles | Dissolving microneedles could retain extracellular vesicles derived from human adipose stem cells longer in dermal fibroblasts in healthy mice than intradermal injection, which promotes collagen synthesis and fibroblast proliferation. | [26] |
| MN roller device | Not described | Extracellular vesicles | A combination solid microneedle roller with an extracellular vesicles solution derived from adipose stem cells topically applied on photoaging hairless model mice shows a better skin regeneration effect than the no treatment group by promoting the proliferation and migration of epidermal cells and fibroblasts. | [91] |
| Derma-Q® (Dongbang Medi-Care Inc., Seongnam-si, Republic of Korea) device | Stainless steel | Secretome | Six topical administrations of secretome obtained from adipose stem cells after solid microneedle application on middle-aged Asian women provides anti-aging and whitening effects by increasing type I collagen expression and inhibiting melanin synthesis more than no treatment group. | [92] |
| 36 microneedle arrays with each needle’s height being 150 μm | Not described | Secretome | Two weeks after facial treatment with topical concentrated secretome extracted from adipose stem cells assisted by solid microneedles reduced the wrinkle area in middle-aged Indonesian women due to extending procollagen type I production and had fewer side effects than those assisted by a fractional laser. | [93] |
| 40 × 40 microneedle arrays with each needle’s height being 600 μm | Poly(vinyl alcohol) (PVA) and hyaluronic acid | Exosome and chitosan lactate | Two applications of dissolving microneedles containing calcium lactate and exosomes acquired from adipose stem cells results in better hair regeneration than the subcutaneous injection method on hair-shaved model mice by hair follicle stromal cell activation and modulation. | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nainggolan, A.D.C.; Anjani, Q.K.; Hartrianti, P.; Donnelly, R.F.; Kurniawan, A.; Ramadon, D. Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression. Pharmaceutics 2023, 15, 2767. https://doi.org/10.3390/pharmaceutics15122767
Nainggolan ADC, Anjani QK, Hartrianti P, Donnelly RF, Kurniawan A, Ramadon D. Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression. Pharmaceutics. 2023; 15(12):2767. https://doi.org/10.3390/pharmaceutics15122767
Chicago/Turabian StyleNainggolan, Avelia Devina Calista, Qonita Kurnia Anjani, Pietradewi Hartrianti, Ryan F. Donnelly, Arief Kurniawan, and Delly Ramadon. 2023. "Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression" Pharmaceutics 15, no. 12: 2767. https://doi.org/10.3390/pharmaceutics15122767
APA StyleNainggolan, A. D. C., Anjani, Q. K., Hartrianti, P., Donnelly, R. F., Kurniawan, A., & Ramadon, D. (2023). Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression. Pharmaceutics, 15(12), 2767. https://doi.org/10.3390/pharmaceutics15122767








