Silk Fibroin-Modified Liposome/Gene Editing System Knocks out the PLK1 Gene to Suppress the Growth of Lung Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
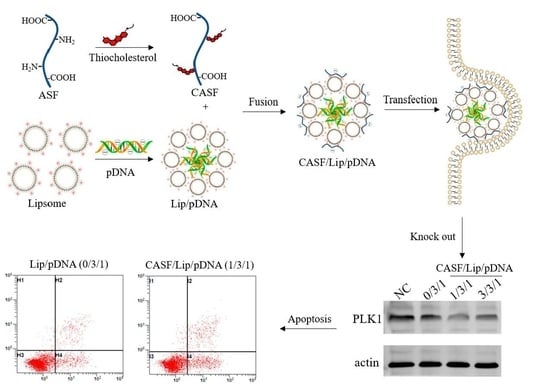
2.1. Preparation of ASF
2.2. Synthesis of CASF
2.3. Characterization of CASF
2.4. Plasmid DNA Production
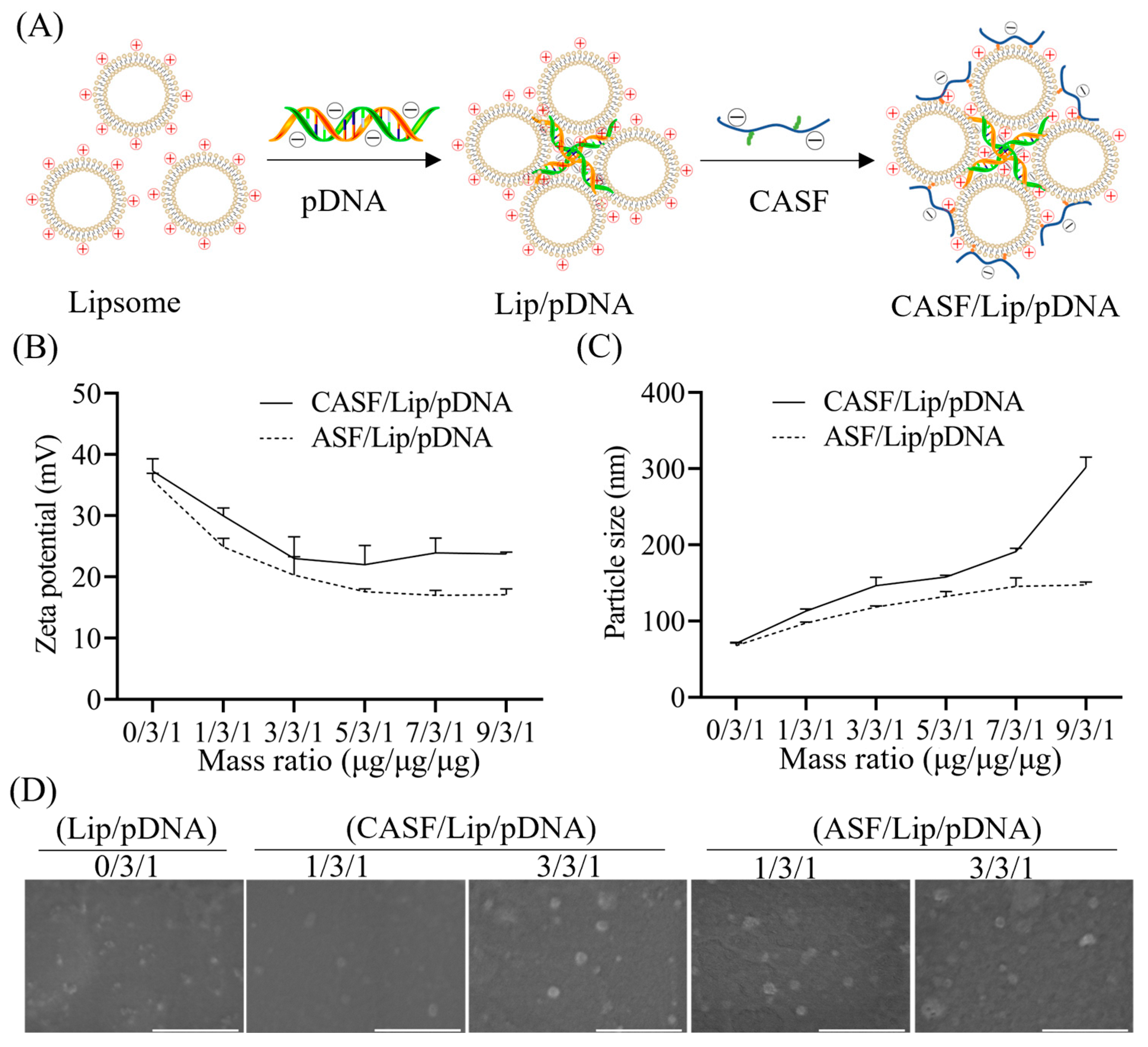
2.5. Preparation of CASF/Lip/pDNA Complex
2.6. Characterization of the CASF/Lip/pDNA Complex
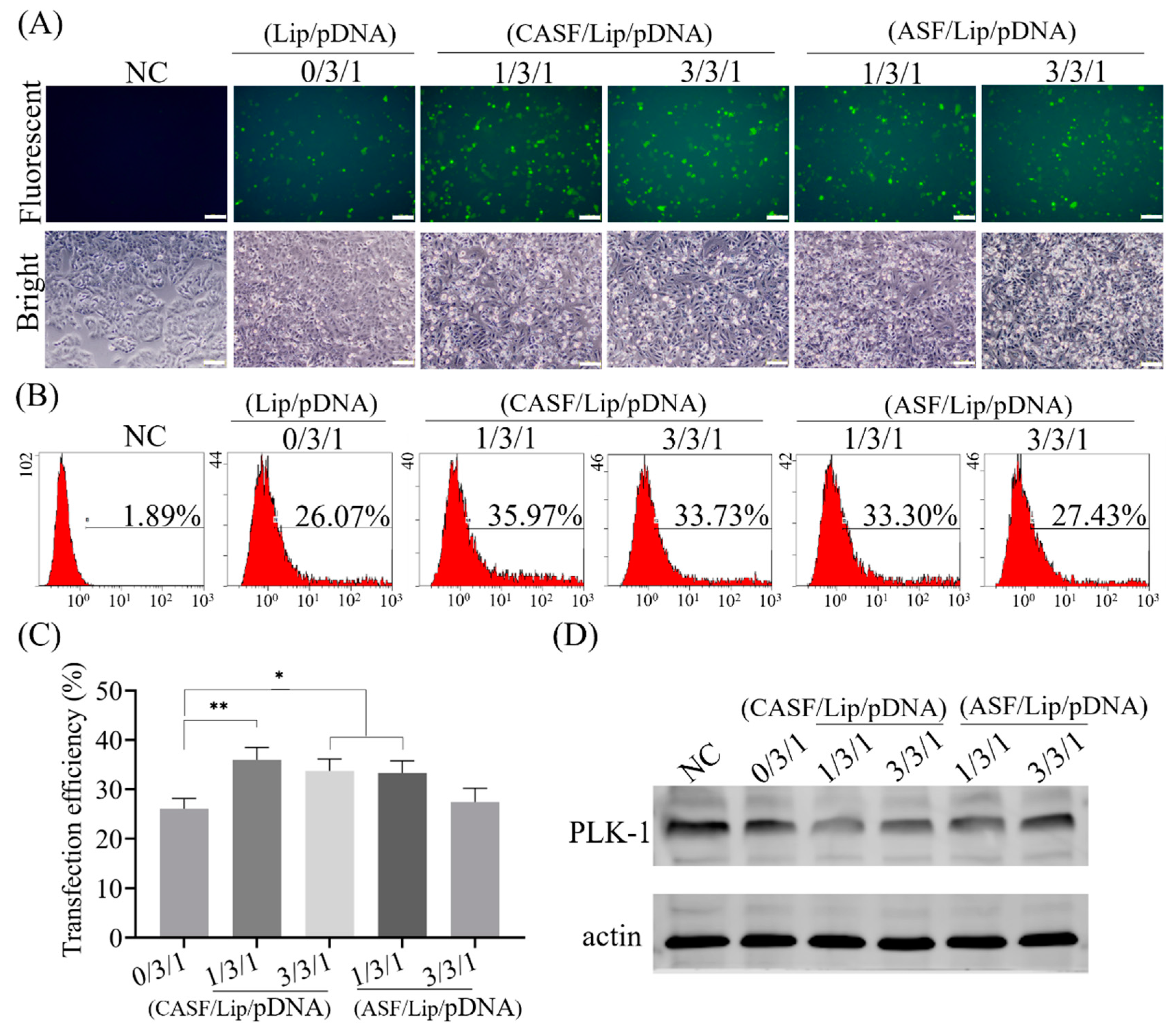
2.7. Gene Transfection of A549 Cells
2.8. Western Blot Assay of PLK1 Gene Expression
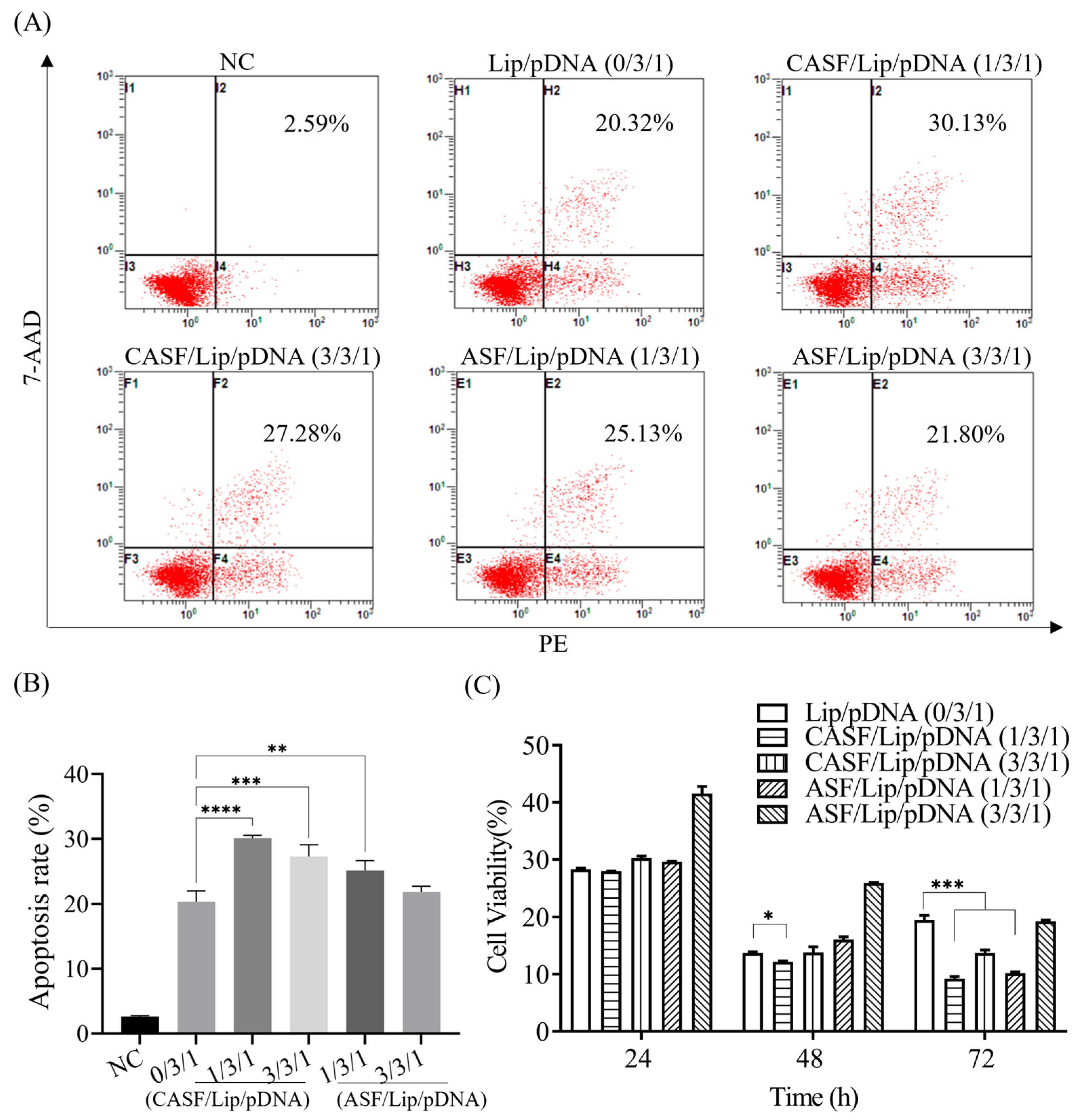
2.9. Apoptosis Assay of A549 Cells
2.10. Viability of A549 Cells
2.11. Cytotoxicity to BEAS2B Cells
2.12. Statistical Analysis
3. Results
3.1. Characterization of CASF
3.2. Characterization of CASF/Lip/pDNA Complexes
3.3. Transfection of Lung Cancer Cells A549 with CASF/Lip/pDNA Complexes
3.4. Apoptosis Rate and Proliferation Activity of A549 Cells
3.5. Transfection of Normal Lung Cells BEAS2B with CASF/Lip/pDNA Complexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Uras, I.Z.; Moll, H.P.; Casanova, E. Targeting KRAS Mutant Non-Small-Cell Lung Cancer: Past, Present and Future. Int. J. Mol. Sci. 2020, 21, 4325. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Meng, W.; Huang, X.; Zhu, W.; Yin, C.; Wang, C.; Fassan, M.; Yu, Y.; Kudo, M.; Xiao, S.; et al. MiR-196b-5p–Mediated Downregulation of TSPAN12 and GATA6 Promotes Tumor Progression in Non-Small Cell Lung Cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 4347–4357. [Google Scholar] [CrossRef]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward Personalized Treatment Approaches for Non-Small-Cell Lung Cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, G.; Felip, E. Non-Small-Cell Lung Cancer: ESMO Clinical Recommendations for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2009, 20 (Suppl. S4), 68–70. [Google Scholar] [CrossRef]
- Edwards, B.K.; Noone, A.-M.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Henley, S.J.; Jemal, A.; Cho, H.; Anderson, R.N.; Kohler, B.A.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2010, Featuring Prevalence of Comorbidity and Impact on Survival among Persons with Lung, Colorectal, Breast, or Prostate Cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Q.; Wang, X. PLK1, a Potential Target for Cancer Therapy. Transl. Oncol. 2016, 10, 22–32. [Google Scholar] [CrossRef]
- Van Vugt, M.A.; Van de Weerdt, B.C.; Vader, G.; Janssen, H.; Calafat, J.; Klompmaker, R.; Wolthuis, R.M.; Medema, R.H. Polo-like Kinase-1 Is Required for Bipolar Spindle Formation but Is Dispensable for Anaphase Promoting Complex/Cdc20 Activation and Initiation of Cytokinesis. J. Biol. Chem. 2004, 279, 36841–36854. [Google Scholar] [CrossRef]
- Iliaki, S.; Beyaert, R.; Afonina, I.S. Polo-like Kinase 1 (PLK1) Signaling in Cancer and Beyond. Biochem. Pharmacol. 2021, 193, 114747. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, X.; Zhao, Z. Applications of CRISPR/Cas9 Technology in the Treatment of Lung Cancer. Trends Mol. Med. 2019, 25, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Balon, K.; Sheriff, A.; Jacków, J.; Łaczmański, Ł. Targeting Cancer with CRISPR/Cas9-Based Therapy. Int. J. Mol. Sci. 2022, 23, 573. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Xie, Y.; Wang, N.; Tang, R.; Zheng, W.; Jiang, X. Genome Editing for Cancer Therapy: Delivery of Cas9 Protein/sgRNA Plasmid via a Gold Nanocluster/Lipid Core-Shell Nanocarrier. Adv. Sci. 2017, 4, 1700175. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 Genome Editing Using Targeted Lipid Nanoparticles for Cancer Therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Q.T.; Liu, H.; Zhang, Z.Z.; Huang, W.L. Advancement and Prospects of Tumor Gene Therapy. Chin. J. Cancer 2011, 30, 182–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, X.; Liu, C.; Wang, N.; Huang, H.; He, S.; Gong, C.; Wei, Y. Delivery of CRISPR/Cas Systems for Cancer Gene Therapy and Immunotherapy. Adv. Drug Deliver. Rev. 2021, 168, 158–180. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lee, C.M.; Hurley, A.E.; Jarrett, K.E.; De Giorgi, M.; Lu, W.; Balderrama, K.S.; Doerfler, A.M.; Deshmukh, H.; Ray, A.; et al. A Self-Deleting AAV-CRISPR System for In Vivo Genome Editing. Mol. Ther. Methods Clin. Dev. 2018, 12, 111–122. [Google Scholar] [CrossRef]
- Maggio, I.; Zittersteijn, H.A.; Wang, Q.; Liu, J.; Janssen, J.M.; Ojeda, I.T.; Van der Maarel, S.M.; Lankester, A.C.; Hoeben, R.C.; Gonçalves, M.A.F.V. Integrating Gene Delivery and Gene-Editing Technologies by Adenoviral Vector Transfer of Optimized CRISPR-Cas9 Components. Gene Ther. 2020, 27, 209–225. [Google Scholar] [CrossRef]
- Huo, W.; Zhao, G.; Yin, J.; Ouyang, X.; Wang, Y.; Yang, C.; Wang, B.; Dong, P.; Wang, Z.; Watari, H.; et al. Lentiviral CRISPR/Cas9 Vector Mediated MiR-21 Gene Editing Inhibits the Epithelial to Mesenchymal Transition in Ovarian Cancer Cells. J. Cancer 2017, 8, 57–64. [Google Scholar] [CrossRef]
- Zhou, H.; He, Y.; Xiong, W.; Jing, S.; Duan, X.; Huang, Z.; Nahal, G.S.; Peng, Y.; Li, M.; Zhu, Y.; et al. MSC Based Gene Delivery Methods and Strategies Improve the Therapeutic Efficacy of Neurological Diseases. Bioact. Mater. 2022, 23, 409–437. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Xin, H.; Wan, T.; Ping, Y. Near-Infrared Optogenetic Engineering of Photothermal NanoCRISPR for Programmable Genome Editing. Proc. Natl. Acad. Sci. USA 2020, 117, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Niu, D.; Wu, C.; Xu, F.J.; Church, G.; Ping, Y. Material Solutions for Delivery of CRISPR/Cas-Based Genome Editing Tools: Current Status and Future Outlook. Mater. Today 2019, 26, 40–66. [Google Scholar] [CrossRef]
- Ayad, C.; Libeau, P.; Lacroix-Gimon, C.; Ladavière, C.; Verrier, B. LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro MRNA Transfection Compared to Liposomes. Pharmaceutics 2021, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, J.; Wei, Z. Strategies for Liposome Drug Delivery Systems to Improve Tumor Treatment Efficacy. AAPS PharmSciTech 2021, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lu, Y.; Xie, F.; Zou, H.; Fan, X.; Li, B.; Li, W.; Zhang, W.; Mei, L.; Feng, S.S.; et al. Cationic Liposomes Induce Cell Necrosis through Lysosomal Dysfunction and Late-Stage Autophagic Flux Inhibition. Nanomedicine 2016, 11, 3117–3137. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Aramaki, Y.; Tsuchiya, S. Physicochemical Properties of Liposomes Affecting Apoptosis Induced by Cationic Liposomes in Macrophages. Pharm. Res. 2003, 20, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, B.; He, Z.; Ye, T.; Luo, M.; Sang, Y.; Liang, X.; Wang, W.; Luo, S.; Yang, S.; et al. Cationic Nanocarriers Induce Cell Necrosis through Impairment of Na(+)/K(+)-ATPase and Cause Subsequent Inflammatory Response. Cell Res. 2015, 25, 237–253. [Google Scholar] [CrossRef]
- Wang, M.Z.; Xu, Y.; Xie, J.F.; Jiang, Z.H.; Peng, L.H. Ginsenoside as a New Stabilizer Enhances the Transfection Efficiency and Biocompatibility of Cationic Liposome. Biomater. Sci. 2021, 9, 8373–8385. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Feng, Q.; Wang, N.; Chen, Z.; Huang, Y.; Zheng, W.; Jiang, X. Lipid Nanoparticle-Mediated Efficient Delivery of CRISPR/Cas9 for Tumor Therapy. NPG Asia Mater. 2017, 9, e441. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.U.D.; Zargar, M.I.; Masoodi, M.H.; Alshehri, S.; Alam, P.; Ghoneim, M.M.; Alshlowi, A.; Shivakumar, H.G.; Ali, M.; Shakeel, F. Silk Fibroin as an Efficient Biomaterial for Drug Delivery, Gene Therapy, and Wound Healing. Int. J. Mol. Sci. 2022, 23, 14421. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk Fibroin Nanoparticles for Cellular Uptake and Control Release. Int. J. Pharmaceut. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Nasrine, A.; Ahmed, M.G.; Narayana, S. Silk Fibroin-Anastrozole Loaded Prolonged-Release Biodegradable Nanomedicine: A Promising Drug Delivery System for Breast Cancer Therapy. Mater. Today Proc. 2022, 68, 56–65. [Google Scholar] [CrossRef]
- Niu, L.; Chen, G.; Feng, Y.; Liu, X.; Pan, P.; Huang, L.; Guo, Y.; Li, M. Polyethylenimine-Modified Bombyx Mori Silk Fibroin as a Delivery Carrier of the ING4-IL-24 Coexpression Plasmid. Polymers 2021, 13, 3592. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, J.; Qu, J.; Sheng, W.; Yang, J.; Li, M. Cationized Bombyx Mori Silk Fibroin as a Delivery Carrier of the VEGF165–Ang-1 Coexpression Plasmid for Dermal Tissue Regeneration. J. Mater. Chem. B 2018, 7, 80–94. [Google Scholar] [CrossRef]
- Luo, H.; Chen, Y.; Niu, L.; Liang, A.; Yang, J.; Li, M. Hepatoma Cell-Targeted Cationized Silk Fibroin as a Carrier for the Inhibitor of Growth 4-Interleukin-24 Double Gene Plasmid. J. Biomed. Nanotechnol. 2019, 15, 1622–1635. [Google Scholar] [CrossRef]
- Gobin, A.S.; Rhea, R.; Newman, R.A.; Mathur, A.B. Silk-Fibroin-Coated Liposomes for Long-Term and Targeted Drug Delivery. Int. J. Nanomed. 2006, 1, 81–87. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, P.; Huang, D.; Mei, L.; Xia, Y.; Wang, Z.; Pan, X.; Li, G.; Wu, C. Fabrication and Characterization of Silk Fibroin-Coated Liposomes for Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 91, 82–90. [Google Scholar] [CrossRef]
- Qu, J.; Wang, W.; Feng, Y.; Niu, L.; Li, M.; Yang, J.; Xie, Y. Cationic Antheraea Pernyi Silk Fibroin-Modified Adenovirus-Mediated ING4 and IL-24 Dual Gene Coexpression Vector Suppresses the Growth of Hepatoma Carcinoma Cells. Int. J. Nanomed. 2019, 14, 9745–9761. [Google Scholar] [CrossRef] [PubMed]
- Byram, P.K.; Sunka, K.C.; Barik, A.; Kaushal, M.; Dhara, S.; Chakravorty, N. Biomimetic Silk Fibroin and Xanthan Gum Blended Hydrogels for Connective Tissue Regeneration. Int. J. Biol. Macromol. 2020, 165, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zheng, J.; Liu, J.; Carr, C.M. Fourier Transform Raman and Fourier Transform Infrared Spectroscopy Studies of Silk Fibroin. J. Appl. Polym. Sci. 2005, 96, 1999–2004. [Google Scholar] [CrossRef]
- Schulz, H.; Schrader, B.; Quilitzsch, R.; Pfeffer, S.; Krüger, H. Rapid Classification of Basil Chemotypes by Various Vibrational Spectroscopy Methods. J. Agric. Food Chem. 2003, 51, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Yu, D. Lung cancer gene therapy: Transferrin and Hyaluronic Acid Dual Ligand-Decorated Novel Lipid Carriers for Targeted Gene Delivery. Oncol. Rep. 2017, 37, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Maslov, M.A.; Kabilova, T.O.; Petukhov, I.A.; Morozova, N.G.; Serebrennikova, G.A.; Vlassov, V.V.; Zenkova, M.A. Novel Cholesterol Spermine Conjugates Provide Efficient Cellular Delivery of Plasmid DNA and Small Interfering RNA. J. Control. Release 2012, 160, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, G. Lung Cancer Targeted Therapy: Folate and Transferrin Dual Targeted, Glutathione Responsive Nanocarriers for the Delivery of Cisplatin. Biomed. Pharmacother. 2018, 102, 55–63. [Google Scholar] [CrossRef]
- Dan, N.; Danino, D. Structure and Kinetics of Lipid–Nucleic Acid Complexes. Adv. Colloid Interface Sci. 2014, 205, 230–239. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Shen, F.; Long, D.; Yu, T.; Lin, X. Introduction of Exogenous Wild-Type P53 Mediates the Regulation of Oncoprotein 18/Stathmin Signaling Via Nuclear Factor-ΚB in Non-Small Cell Lung Cancer NCI-H1299 Cells. Oncol. Rep. 2019, 41, 2051–2059. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Shen, F.; Long, D.; Yu, T.; Lin, X. In Vitro Neutralization of Autocrine IL-10 Affects Op18/Stathmin Signaling in Non-Small Cell Lung Cancer Cells. Oncol. Rep. 2019, 41, 501–511. [Google Scholar] [CrossRef]
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering Liposomal Nanoparticles for Targeted Gene Therapy. Gene Ther. 2017, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, B.; Mohammadi, A.; Shamekhi, S.; Majidazar, N.; Dilmaghani, A.; Soofiyani, S.R.; McMillan, N.A.; Lotfipour, F.; Hallaj-Nezhadi, S. A Novel Method for the Development of Plasmid DNA-Loaded Nanoliposomes for Cancer Gene Therapy. Drug Deliv. Transl. Res. 2022, 12, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, R.; Liu, G.; Li, X.; Sheng, W.; Yang, J.; Li, M. Antheraea Pernyi Silk Fibroin-Coated PEI/DNA Complexes for Targeted Gene Delivery in HEK 293 and HCT 116 Cells. Int. J. Mol. Sci. 2014, 15, 7049. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, Y.; Li, X.; Liu, Y.; Li, M.; Yang, J.; Sheng, W. Spermine-Modified Antheraea Pernyi Silk Fibroin as a Gene Delivery Carrier. Int. J. Nanomed. 2016, 11, 1013–1023. [Google Scholar]
- Sezutsu, H.; Yukuhiro, K. Dynamic Rearrangement within the Antheraea pernyi Silk Fibroin Gene Is Associated with Four Types of Repetitive Units. J. Mol. Evol. 2000, 51, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Kundu, B.; Lu, S.; Reis, R.L.; Kundu, S.C. Chinese Oak Tasar Silkworm Antheraea pernyi Silk Proteins: Current Strategies and Future Perspectives for Biomedical Applications. Macromol. Biosci. 2019, 19, e1800252. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shi, Y.W.; Li, B.X.; Xu, W.C.; Shi, Z.L.; Zhou, C.; Fu, S. Photo-Thermal Effect Enhances the Efficiency of Radiotherapy Using Arg-Gly-Asp Peptides-Conjugated Gold Nanorods That Target AVΒ3 In Melanoma Cancer Cells. J. Nanobiotechnol. 2015, 13, 52. [Google Scholar] [CrossRef]
- Wang, H.X.; Song, Z.; Lao, Y.H.; Xu, X.; Gong, J.; Cheng, D.; Chakraborty, S.; Park, J.S.; Li, M.; Huang, D.; et al. Nonviral Gene Editing Via CRISPR/Cas9 Delivery by Membrane-Disruptive and Endosomolytic Helical Polypeptid. Proc. Natl. Acad. Sci. USA 2018, 115, 4903–4908. [Google Scholar] [CrossRef]
- Filion, M.C.; Phillips, N.C. Major Limitations in the Use of Cationic Liposomes for DNA Delivery. Int. J. Pharmaceut. 1998, 162, 159–170. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The Systemic Toxicity of Positively Charged Lipid Nanoparticles and the Role of Toll-like Receptor 4 in Immune Activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.L.; Mohseni, R.; Hamidieh, A.A.; MacLaren, R.E.; Habib, N.; Seifalian, A.M. Limitations in Clinical Translation of Nanoparticle-Based Gene Therapy. Trends Biotechnol. 2017, 35, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Liang, X.; Yang, J.; Zhao, C.; Nie, W.; Liu, L.; Yi, T.; Jiang, Y.; Geng, J.; Zhao, X.; et al. Hyaluronan Reduces Cationic Liposome-Induced Toxicity and Enhances the Antitumor Effect of Targeted Gene Delivery in Mice. ACS Appl. Mater. Inter. 2018, 10, 32006–32016. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thuy, L.T.; Lee, J.; Choi, J.S. Second-Generation Polyamidoamine Dendrimer Conjugated with Oligopeptides Can Enhance Plasmid DNA Delivery In Vitro. Molecules 2023, 28, 7644. [Google Scholar] [CrossRef]
- Sabir, F.; Qindeel, M.; Zeeshan, M.; Ul Ain, Q.; Rahdar, A.; Barani, M.; Aboudzadeh, M.A. Onco-Receptors Targeting in Lung Cancer via Application of Surface-Modified and Hybrid Nanoparticles: A Cross-Disciplinary Review. Processes 2021, 9, 621. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, P.; Liu, X.; Fang, M.; Yang, S.; Zhang, Y.; Li, M.; Liu, Y. Silk Fibroin-Modified Liposome/Gene Editing System Knocks out the PLK1 Gene to Suppress the Growth of Lung Cancer Cells. Pharmaceutics 2023, 15, 2756. https://doi.org/10.3390/pharmaceutics15122756
Pan P, Liu X, Fang M, Yang S, Zhang Y, Li M, Liu Y. Silk Fibroin-Modified Liposome/Gene Editing System Knocks out the PLK1 Gene to Suppress the Growth of Lung Cancer Cells. Pharmaceutics. 2023; 15(12):2756. https://doi.org/10.3390/pharmaceutics15122756
Chicago/Turabian StylePan, Peng, Xueping Liu, Mengqi Fang, Shanlong Yang, Yadong Zhang, Mingzhong Li, and Yu Liu. 2023. "Silk Fibroin-Modified Liposome/Gene Editing System Knocks out the PLK1 Gene to Suppress the Growth of Lung Cancer Cells" Pharmaceutics 15, no. 12: 2756. https://doi.org/10.3390/pharmaceutics15122756
APA StylePan, P., Liu, X., Fang, M., Yang, S., Zhang, Y., Li, M., & Liu, Y. (2023). Silk Fibroin-Modified Liposome/Gene Editing System Knocks out the PLK1 Gene to Suppress the Growth of Lung Cancer Cells. Pharmaceutics, 15(12), 2756. https://doi.org/10.3390/pharmaceutics15122756