Immobilization of Papain in Chitosan Membranes as a Potential Alternative for Skin Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Chitosan
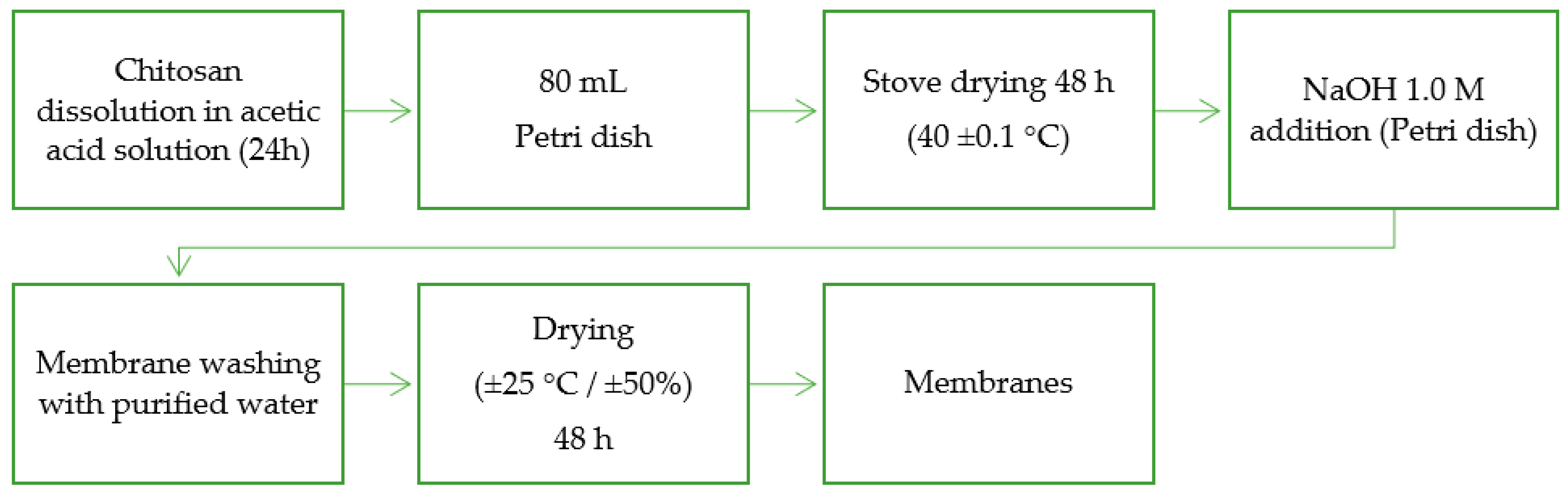
2.3. Preparation of Free Papain Chitosan Membrane
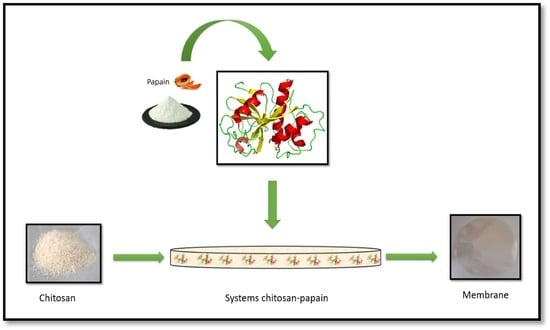
2.4. Papain Immobilization in Chitosan Membranes
2.5. Analysis of Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
2.6. pH Analysis of Membranes
2.7. Mechanical Properties
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
2.9. X-ray Diffraction (XRD) Analyses
2.10. Thermal Analyses
2.11. Swelling Assay and Water Contact Angle Measurements
2.12. Enzymatic Activity Assay
2.13. In Vitro Papain Kinetic Release Studies
2.14. Statistical Analysis
3. Results and Discussion
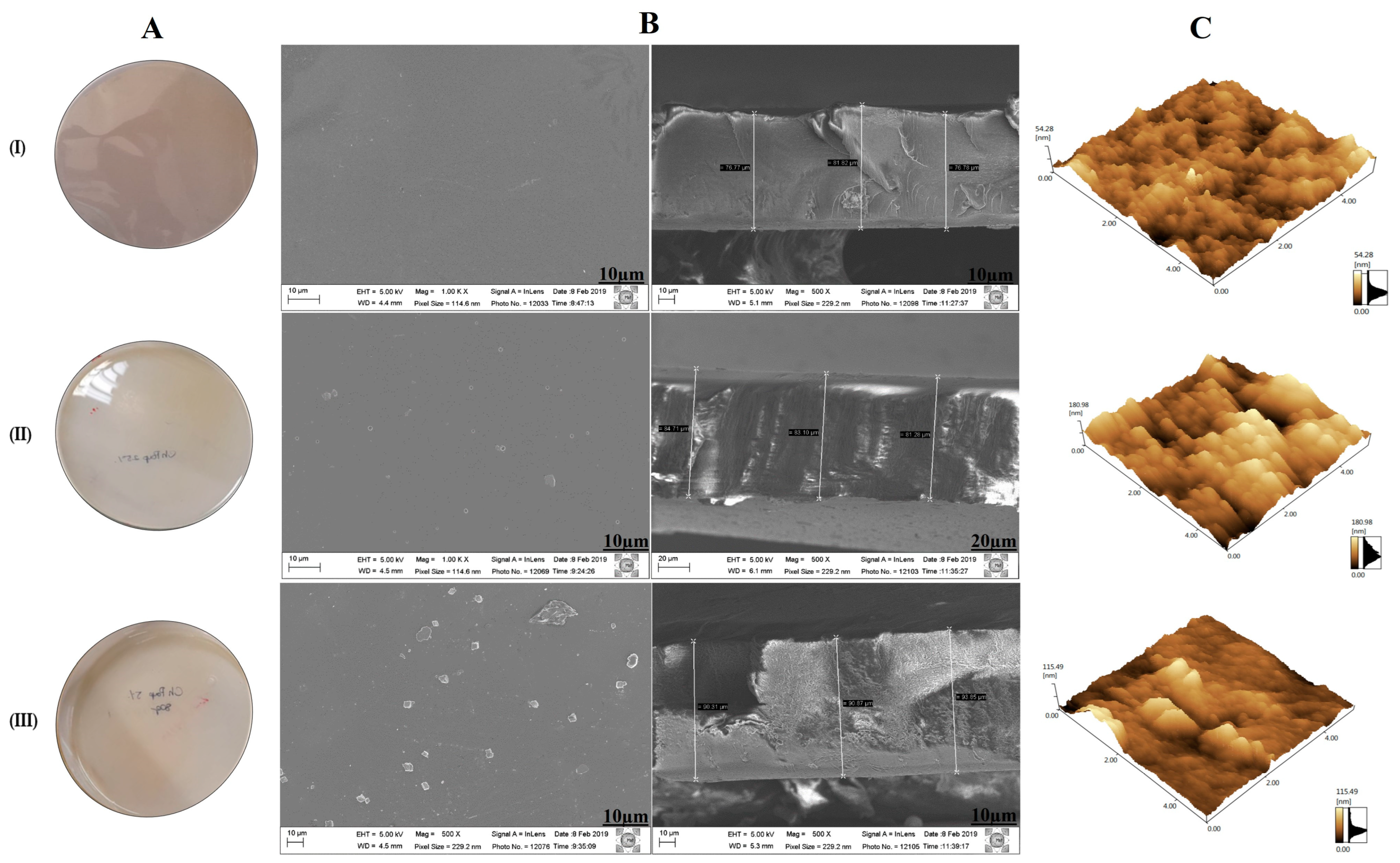
3.1. Preparation of Membranes and Morphology
3.2. Surface pH Measurements and Mechanical Properties
3.3. Papain Interactions with Chitosan by X-ray Diffraction, Thermal Analyses and FTIR
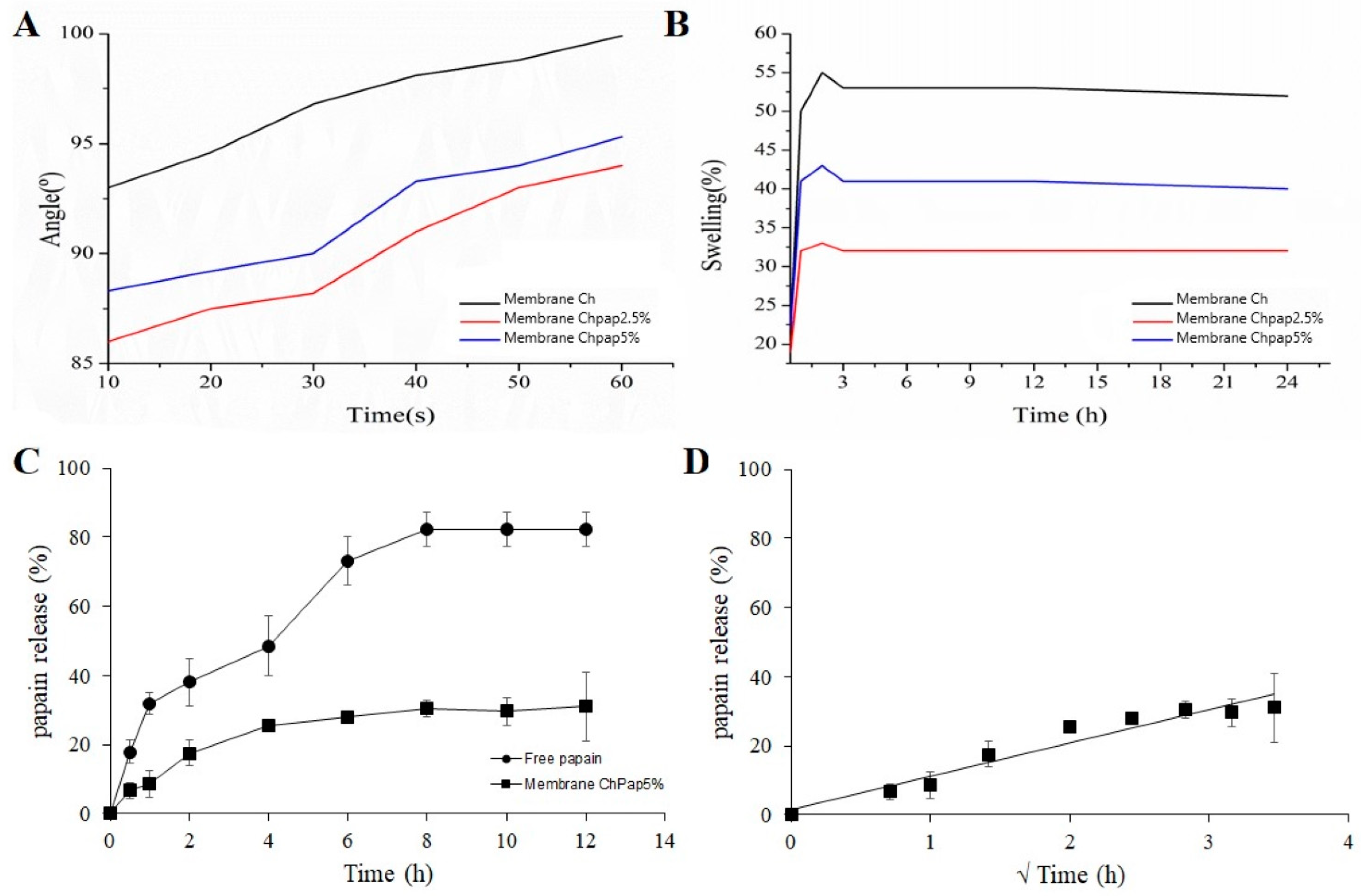
3.4. Water Contact Angle, Swelling Assay, and In Vitro Papain Release
3.5. Enzymatic Activity Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajiteru, O.; Lee, O.J.; Kim, J.H.; Lee, Y.J.; Lee, J.S.; Lee, H.; Sultan, M.T.; Park, C.H. Fabrication and characterization of a myrrh hydrocolloid dressing for dermal wound healing. Colloids Interface Sci. Commun. 2022, 48, 100617. [Google Scholar] [CrossRef]
- Castaño, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.Á.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Alpay, P.; Uygun, D.A. Usage of immobilized papain for enzymatic hydrolysis of proteins. J. Mol. Catal. B Enzym. 2015, 111, 56–63. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605. [Google Scholar] [CrossRef]
- Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar]
- Liu, X.; Xu, Y.; An, M.; Zeng, Q. The risk factors for diabetic peripheral neuropathy: A meta-analysis. PLoS ONE 2019, 14, e0212574. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2017, 81, 366–372. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sheng, W.; Xi, Y.; Zhang, L.; Ye, T.; Zhao, X. Enhanced activity and stability of papain by covalent immobilization on porous magnetic nanoparticles. Int. J. Biol. Macromol. 2018, 114, 143–148. [Google Scholar] [CrossRef]
- Ajlia, S.A.S.H.; Majid, F.A.A.; Suvik, A.; Effendy, M.A.W.; Serati Nouri, H. Efficacy of papain-based wound cleanser in promoting wound regeneration. Pakistan J. Biol. Sci. 2010, 13, 596–603. [Google Scholar] [CrossRef]
- Thompson, E. Debridement Techniques and Non–Negative Pressure Wound Therapy Wound Management. Vet. Clin. North Am. -Small Anim. Pract. 2017, 47, 1181–1202. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Cunha, A.P.; Ricardo, N.M.P.S.; Freire, R.S.; Vieira, L.D.A.P.; Santa Brigida, A.I.; de Fátima Borges, M.; de Freitas Rosa, M.; Vieira, R.S.; Andrade, F.K. Papain immobilization on heterofunctional membrane bacterial cellulose as a potential strategy for the debridement of skin wounds. Int. J. Biol. Macromol. 2020, 165, 3065–3077. [Google Scholar] [CrossRef]
- Singh, T.P.; Siddiqi, R.A.; Sogi, D.S. Statistical optimization of enzymatic hydrolysis of rice bran protein concentrate for enhanced hydrolysate production by papain. Lwt 2019, 99, 77–83. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S.I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Nascimento, E.G.; Caland, L.B.; Medeiros, A.S.A.; Fernandes-Pedrosa, M.F.; Soares-Sobrinho, J.L.; Santos, K.S.C.R.; Silva-Júnior, A.A. Tailoring drug release properties by gradual changes in the particle engineering of polysaccharide chitosan based powders. Polymers 2017, 9, 253. [Google Scholar] [CrossRef]
- do Nascimento, E.G.; de Azevedo, E.P.; Alves-Silva, M.F.; Aragão, C.F.S.; Fernandes-Pedrosa, M.F.; da Silva-Junior, A.A. Supramolecular aggregates of cyclodextrins with co-solvent modulate drug dispersion and release behavior of poorly soluble corticosteroid from chitosan membranes. Carbohydr. Polym. 2020, 248, 116724. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-based membranes with different ionic crosslinking density for pharmaceutical and industrial applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Saldanha, T.D.P.; Navarro, M.V.M.; Medeiros, A.C.; Ginani, M.F.; Raffin, F.N. Mechanical properties and release studies of chitosan films impregnated with silver sulfadiazine. J. Appl. Polym. Sci. 2006, 102, 3462–3470. [Google Scholar] [CrossRef]
- Cupp-Enyard, C. Sigma’s Non-specific Protease Activity Assay—Casein as a Substrate. J. Vis. Exp. 2008, 19, e899. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Araujo, V.H.S.; dos Santos, A.M.; Duarte, J.L.; Silvestre, A.L.P.; Fonseca-Santos, B.; Villanova, J.C.O.; Gremião, M.P.D.; Chorilli, M. Advances and challenges in nanocarriers and nanomedicines for veterinary application. Int. J. Pharm. 2020, 580, 119214. [Google Scholar] [CrossRef]
- Dos Santos-Silva, A.M.; De Caland, L.B.; Do Nascimento, E.G.; Oliveira, A.L.C.D.S.L.; De Araújo-Júnior, R.F.; Cornélio, A.M.; Fernandes-Pedrosa, M.F.; Da Silva-Júnior, A.A. Self-assembled benznidazole-loaded cationic nanoparticles containing cholesterol/sialic acid: Physicochemical properties, in vitro drug release and in vitro anticancer effcacy. Int. J. Mol. Sci. 2019, 20, 2350. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, X.; Li, S. A novel method to get methotrexatum/layered double hydroxides intercalation compounds and their release properties. J. Phys. Chem. Solids 2013, 74, 1101–1108. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC -Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Yan, C.; Lu, Y. Study on the interaction between gold nanoparticles and papain by spectroscopic methods. J. Lumin. 2015, 157, 229–234. [Google Scholar] [CrossRef]
- Silva, D.S.; Menezes, J.E.S.A.; Santos, H.S.; Ferreira, M.K.A.; Magalh, F.E.A.; Bandeira, P.N.; Saraiva, G.D.; Pessoa, O.D.L.; Ricardo, N.M.P.S.; Cruz, B.G.; et al. Preparation, structural and spectroscopic characterization of chitosan membranes containing allantoin. J. Mol. Struct. 2020, 1199, 126968. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Nath, G.; Bansal, M.; Mishra, B. Development and Evaluation of Biodegradable Chitosan Films of Metronidazole and Levofloxacin for the Management of Periodontitis. AAPS Pharmscitech 2016, 17, 1312–1325. [Google Scholar] [CrossRef]
- Marques, J.S.; Chagas, J.A.O.D.; Fonseca, J.L.C.; Pereira, M.R. Comparing homogeneous and heterogeneous routes for ionic crosslinking of chitosan membranes. React. Funct. Polym. 2016, 103, 156–161. [Google Scholar] [CrossRef]
- Nowacki, K.; Galiński, M.; Stępniak, I. Synthesis and characterization of modified chitosan membranes for applications in electrochemical capacitor. Electrochim. Acta 2019, 320, 134632. [Google Scholar] [CrossRef]
- Sathish, H.A.; Kumar, P.R.; Prakash, V. Mechanism of solvent induced thermal stabilization of papain. Int. J. Biol. Macromol. 2007, 41, 383–390. [Google Scholar] [CrossRef]
- Moreira Filho, R.N.F.; Vasconcelos, N.F.; Andrade, F.K.; de Freitas Rosa, M.; Vieira, R.S. Papain immobilized on alginate membrane for wound dressing application. Colloids Surf. B Biointerfaces 2020, 194, 111222. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Oliveira, A.L.B.; Mota, G.F.; da Silva Souza, J.E.; de Aguiar Falcão, I.R.; et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A new look towards the thermal decomposition of chitins and chitosans with di ff erent degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Fráguas, R.M.; Rocha, D.A.; Queiroz, E.D.R.; Abreu, C.M.P.D.; Sousa, R.V.D.; Oliveira Júnior, E.N.D. Chemical characterization and healing effect of chitosan with low molar mass and acetylation degree in skin lesions T Caracterização química e efeito cicatrizante de quitosana, com baixos valores de massa molar e grau de acetilação, em lesões cutâneas. Polímeros Ciência e Tecnol. 2015, 25, 205–211. [Google Scholar] [CrossRef]
- Campelo, M.J.M.; Freire, P.T.C.; Filho, J.M.; de Toledo, T.A.; Teixeira, A.M.R.; Coutinho, H.D.M. Synthesis, crystal structure, vibrational spectra and theoretical calculations of quantum chemistry of a potential antimicrobial Meldrum’s acid derivative. J. Mol. Struct. 2017, 1146, 828–836. [Google Scholar] [CrossRef]
- Peres, R.S.; Armelin, E.; Moreno-Martínez, J.A.; Alemán, C.; Ferreira, C.A. Transport and antifouling properties of papain-based antifouling coatings. Appl. Surf. Sci. 2015, 341, 75–85. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, V.; Panda, A.K.; Majumdar, D.K. Development of enteric submicron particle formulation of papain for oral delivery. Int. J. Nanomed. 2011, 6, 2097–2111. [Google Scholar]
- Ferreira, I.C.; Araújo, D.; Voisin, P.; Alves, V.D.; Rosatella, A.A.; Afonso, C.A.M.; Freitas, F.; Neves, L.A. Chitin-glucan complex—Based biopolymeric structures using biocompatible ionic liquids. Carbohydr. Polym. 2020, 247, 116679. [Google Scholar] [CrossRef]
- Jothi Prakash, C.G.; Prasanth, R. Approaches to design a surface with tunable wettability: A review on surface properties. J. Mater. Sci. 2021, 56, 108–135. [Google Scholar] [CrossRef]
- Kamel, N.A.; Abd El-messieh, S.L.; Saleh, N.M. Chitosan/banana peel powder nanocomposites for wound dressing application: Preparation and characterization. Mater. Sci. Eng. C 2017, 72, 543–550. [Google Scholar] [CrossRef]
- Xu, W.; Song, Q.; Xu, J.F.; Serpe, M.J.; Zhang, X. Supramolecular Hydrogels Fabricated from Supramonomers: A Novel Wound Dressing Material. ACS Appl. Mater. Interfaces 2017, 9, 11368–11372. [Google Scholar] [CrossRef]
- Rafati, A.E.A.A. Development of Novel Biodegradable Enrofloxacin—Silica Composite for In Vitro Drug Release Kinetic Studies. J. Polym. Environ. 2018, 26, 3404–3411. [Google Scholar] [CrossRef]
- Arafa, E.G.; Sabaa, M.W.; Mohamed, R.R.; Kamel, E.M.; Elzanaty, A.M.; Mahmoud, A.M.; Abdel-gawad, O.F. Eco-friendly and biodegradable sodium alginate / quaternized chitosan hydrogel for controlled release of urea and its antimicrobial activity. Carbohydr. Polym. 2022, 291, 119555. [Google Scholar] [CrossRef]
- Altunkaynak, F.; Okur, M.; Saracoglu, N. Journal of Drug Delivery Science and Technology Controlled release of paroxetine from chitosan/montmorillonite composite films. J. Drug Deliv. Sci. Technol. 2022, 68, 103099. [Google Scholar] [CrossRef]
- Onnainty, R.; Onida, B.; Páez, P.; Longhi, M.; Barresi, A.; Granero, G. Targeted chitosan-based bionanocomposites for controlled oral mucosal delivery of chlorhexidine. Int. J. Pharm. 2016, 509, 408–418. [Google Scholar] [CrossRef]
- Cheikh, D.; García-villén, F.; Majdoub, H.; Viseras, C.; Benna, M. International Journal of Biological Macromolecules Chitosan / beidellite nanocomposite as diclofenac carrier. Int. J. Biol. Macromol. 2019, 126, 44–53. [Google Scholar] [CrossRef]
- Soares, A.M.B.F.; Gonçalves, L.M.O.; Ferreira, R.D.S.; de Souza, J.M.; Fangueiro, R.; Alves, M.M.M.; Carvalho, F.A.A.; Mendes, A.N.; Cantanhêde, W. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications. Carbohydr. Polym. 2020, 243, 116498. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis of carbonate doped chlorapatite—ZnO nanocomposite with negative zeta potential. Ceram. Int. 2015, 41, 12323–12330. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]

| Membrane Formulations | Surface pH | Tensile Strength (N) | Elongation at Break (%) |
|---|---|---|---|
| Membrane Ch | 7.1 ± 0.22 (a,b) | 33.8 ± 11.15 (a,b) | 2.5 ± 3.5 (a,b) |
| Membrane ChPap2.5% | 7.5 ± 0.32 (a,b) | 33.2 ± 10.07 (a,b) | 2.3 ± 2.8 (a,b) |
| Membrane ChPap5% | 7.4 ± 0.17 (a,b) | 20.2 ± 14.34 (a,b) | 1.1 ± 1.38 (a,b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Melo, A.E.C.; de Sousa, F.S.R.; dos Santos-Silva, A.M.; do Nascimento, E.G.; Fernandes-Pedrosa, M.F.; de Medeiros, C.A.C.X.; da Silva-Junior, A.A. Immobilization of Papain in Chitosan Membranes as a Potential Alternative for Skin Wounds. Pharmaceutics 2023, 15, 2649. https://doi.org/10.3390/pharmaceutics15122649
da Silva Melo AEC, de Sousa FSR, dos Santos-Silva AM, do Nascimento EG, Fernandes-Pedrosa MF, de Medeiros CACX, da Silva-Junior AA. Immobilization of Papain in Chitosan Membranes as a Potential Alternative for Skin Wounds. Pharmaceutics. 2023; 15(12):2649. https://doi.org/10.3390/pharmaceutics15122649
Chicago/Turabian Styleda Silva Melo, Anne Emmanuelle Câmara, Felipe Sanderson Ribeiro de Sousa, Alaine M. dos Santos-Silva, Ednaldo Gomes do Nascimento, Matheus F. Fernandes-Pedrosa, Caroline Addison Carvalho Xavier de Medeiros, and Arnóbio Antônio da Silva-Junior. 2023. "Immobilization of Papain in Chitosan Membranes as a Potential Alternative for Skin Wounds" Pharmaceutics 15, no. 12: 2649. https://doi.org/10.3390/pharmaceutics15122649
APA Styleda Silva Melo, A. E. C., de Sousa, F. S. R., dos Santos-Silva, A. M., do Nascimento, E. G., Fernandes-Pedrosa, M. F., de Medeiros, C. A. C. X., & da Silva-Junior, A. A. (2023). Immobilization of Papain in Chitosan Membranes as a Potential Alternative for Skin Wounds. Pharmaceutics, 15(12), 2649. https://doi.org/10.3390/pharmaceutics15122649