Recent Strategies for Cancer Therapy: Polymer Nanoparticles Carrying Medicinally Important Phytochemicals and Their Cellular Targets
Abstract
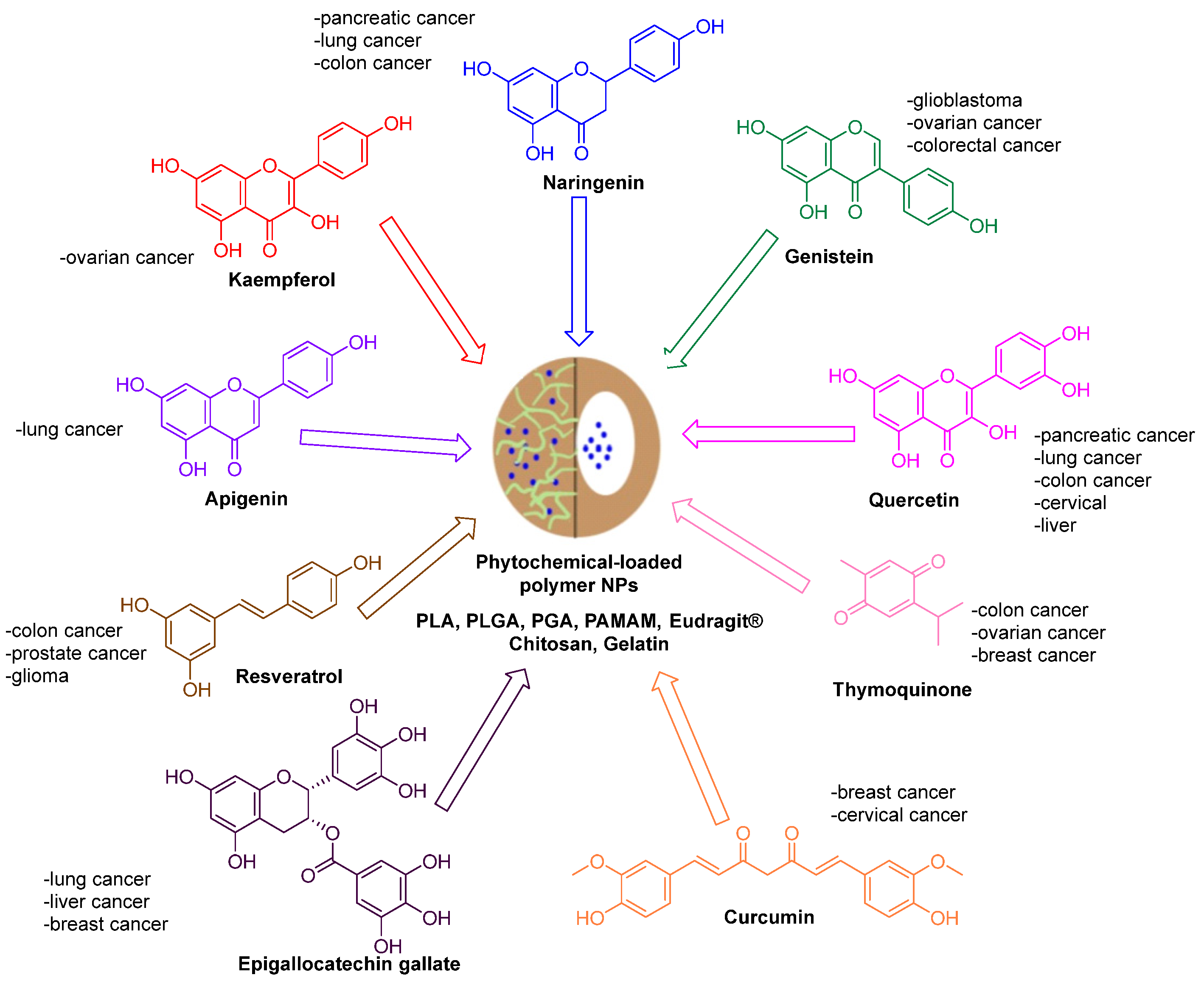
:1. Introduction
2. Nanoparticle Preparation and Characterization
3. Curcumin
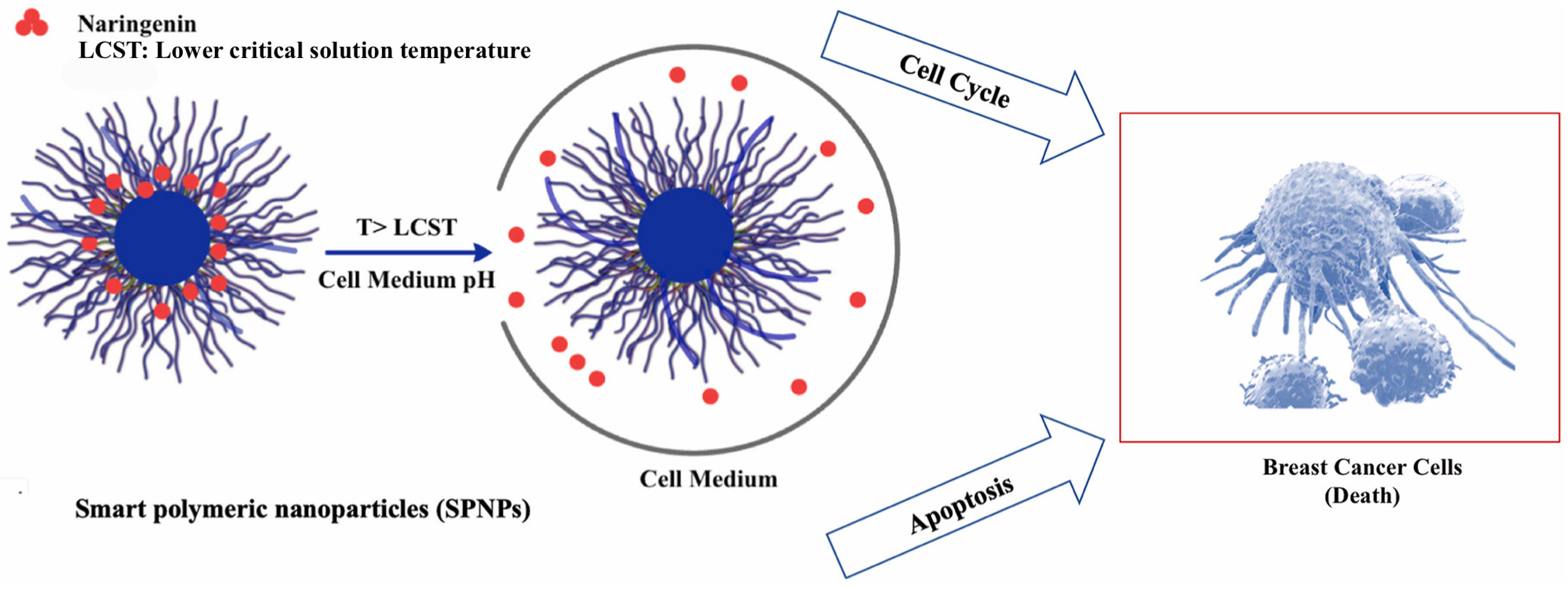
4. Naringenin
5. Quercetin
6. Epigallocatechin Gallate
7. Thymoquinone
8. Kaempferol
9. Resveratrol
10. Genistein
11. Apigenin
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavas, S.; Quazi, S.; Karpinski, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Bachar, S.C.; Bachar, R.; Jannat, K.; Jahan, R.; Rahmatullah, M. Hepatoprotective natural products. Ann. Rep. Med. Chem. 2020, 55, 207–249. [Google Scholar]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.M.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Bunkar, N.; Shandilya, R.; Bhargava, A.; Samarth, R.M.; Tiwari, R.; Mishra, D.K.; Srivastava, R.K.; Sharma, R.S.; Lohiya, N.K.; Mishra, P.K. Nano-engineered flavonoids for cancer protection. Front. Biosci. 2019, 24, 1097–1157. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Pal, S.L.; Jana, U.; Manna, P.K.; Mohanta, G.P.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. [Google Scholar]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Ahlin Grabnar, P.; Kristl, J. The manufacturing techniques of drug-loaded polymeric nanoparticles from preformed polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym. Plast. Tech. Mater. 2021, 60, 1996–2024. [Google Scholar] [CrossRef]
- Weiss, A.V.; Schorr, D.; Metz, J.K.; Yildirim, M.; Khan, S.A.; Schneider, M. Gelatin nanoparticles with tunable mechanical properties: Effect of crosslinking time and loading. Beilstein J. Nanotech. 2022, 13, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Gasilova, E.R.; Skorik, Y.A. Nano-sized fucoidan interpolyelectrolyte complexes: Recent advances in design and prospects for biomedical applications. Int. J. Mol. Sci. 2023, 24, 2615. [Google Scholar] [CrossRef]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef]
- Bell, C.G.; Treder, K.P.; Kim, J.S.; Schuster, M.E.; Kirkland, A.I.; Slater, T.J.A. Trainable segmentation for transmission electron microscope images of inorganic nanoparticles. J. Microsc. 2022, 288, 169–184. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A review on preparation, properties and applications of polymeric nanoparticle-based materials. Polym-Plast. Technol. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- de Castro, K.C.; Costa, J.M.; Campos, M.G.N. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Po 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Karam, M.; Fahs, D.; Maatouk, B.; Safi, B.; Jaffa, A.A.; Mhanna, R. Polymeric nanoparticles in the diagnosis and treatment of myocardial infarction: Challenges and future prospects. Mater. Today Bio 2022, 14, 100249. [Google Scholar] [CrossRef]
- Yao, H.; Ma, J. Dendrimer-paclitaxel complexes for efficient treatment in ovarian cancer: Study on OVCAR-3 and HEK293T cells. Acta Biochim. Pol. 2018, 65, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Umerska, A.; Gaucher, C.; Oyarzun-Ampuero, F.; Fries-Raeth, I.; Colin, F.; Villamizar-Sarmiento, M.G.; Maincent, P.; Sapin-Minet, A. Polymeric nanoparticles for increasing oral bioavailability of curcumin. Antioxidants 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hawthorne, S.; Jha, S.K.; Jha, N.K.; Kumar, D.; Girgis, S.; Goswami, V.K.; Gupta, G.; Singh, S.; Dureja, H.; et al. Effects of curcumin-loaded poly(lactic-co-glycolic acid) nanoparticles in MDA-MB231 human breast cancer cells. Nanomedicine 2021, 16, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, M.; Nourbakhsh, M.S.; Saber, R.; Hashemzadeh, H.; Darvishi, M.H. Preparation and evaluation of doxorubicin-loaded PLA-PEG-FA copolymer containing superparamagnetic iron oxide nanoparticles (SPIONs) for cancer treatment: Combination therapy with hyperthermia and chemotherapy. Int. J. Nanomed. 2020, 15, 6167–6182. [Google Scholar] [CrossRef]
- Kumar, S.S.; Surianarayanan, M.; Vijayaraghavan, R.; Mandal, A.B.; MacFarlane, D.R. Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid–in vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.A.; Abdel-Haseb, O.M.; Abdel-Ghany, S.E.; Cevik, E.; Sabit, H. Doxorubicin loaded on chitosan-protamine nanoparticles triggers apoptosis via downregulating Bcl-2 in breast cancer cells. J. Drug Deliv. Sci. Tec. 2020, 55, 101423. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, D.H.; Chung, C.W.; Yoo, J.J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b- poly(DL-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011, 6, 1415–1427. [Google Scholar] [CrossRef]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; McCarron, P.A.; Tambuwala, M.M. Polymeric nano-encapsulation of curcumin enhances its anti-cancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of HIF-1α and nuclear p65 (Rel A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef]
- Verderio, P.; Bonetti, P.; Colombo, M.; Pandolfi, L.; Prosperi, D. Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules 2013, 14, 672–682. [Google Scholar] [CrossRef]
- Lin, X.P.; Wang, Q.; Du, S.; Guan, Y.C.; Qiu, J.M.; Chen, X.J.; Yuan, D.S.; Chen, T.K. Nanoparticles for co-delivery of paclitaxel and curcumin to overcome chemoresistance against breast cancer. J. Drug Deliv. Sci. Tec. 2023, 79, 104050. [Google Scholar] [CrossRef]
- Kumari, P.; Muddineti, O.S.; Rompicharla, S.V.; Ghanta, P.; Karthik, B.B.N.A.; Ghosh, B.; Biswas, S. Cholesterol-conjugated poly(D, L-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017, 24, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Mollazade, M.; Nejati-Koshki, K.; Akbarzadeh, A.; Zarghami, N.; Nasiri, M.; Jahanban-Esfahlan, R.; Alibakhshi, A. PAMAM dendrimers augment inhibitory effects of curcumin on cancer cell proliferation: Possible inhibition of telomerase. Asian Pac. J. Cancer Prev. 2013, 14, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Esthar, S.; Rajesh, J.; Prakash, N.; Ayyanaar, S.; Bhaskar, R.; Thanigaivel, S.; Webster, T.J.; Rajagopal, G. An effective biodegradable curcumin loaded magnetic microsphere: Applications for drug delivery and cancer treatment. Pharmacol. Res.—Mod. Chin. Med. 2023, 6, 100219. [Google Scholar] [CrossRef]
- Akhter, M.H.; Kumar, S.; Nomani, S. Sonication tailored enhance cytotoxicity of naringenin nanoparticle in pancreatic cancer: Design, optimization, and in vitro studies. Drug Dev. Ind. Pharm. 2020, 46, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Yildirim, M.; Acet, O.; Yetkin, D.; Acet, B.O.; Karakoc, V.; Odabasi, M. Anti-cancer activity of naringenin loaded smart polymeric nanoparticles in breast cancer. J. Drug Deliv. Sci. Technol. 2022, 74, 103552. [Google Scholar] [CrossRef]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic acid decorated naringenin nanoparticles: Appraisal of chemopreventive and curative potential for lung cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ye, X.W.; Fang, Y.T.; Cheng, H.; Xu, Y.; Wang, X.Q. Development and in vitro evaluation of pH-sensitive naringenin@ZIF-8 polymeric micelles mediated by aptamer. J. Drug Deliv. Sci. Tec. 2021, 65, 102702. [Google Scholar] [CrossRef]
- Sunogrot, S.; Abujamous, L. pH-sensitive polymeric nanoparticles of quercetin as a potential colon cancer-targeted nanomedicine. J. Drug Deliv. Sci. Tech. 2019, 52, 670–676. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Yadav, N.; Tripathi, A.K.; Parveen, A. PLGA-quercetin nano-formulation inhibits cancer progression via mitochondrial dependent caspase-3,7 and independent FoxO1 activation with concomitant PI3K/AKT suppression. Pharmaceutics 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, D.; Xue, G.; Yu, S.; Yuan, C.; Huang, M.; Jiang, L. Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv. 2020, 10, 34517–34526. [Google Scholar] [CrossRef] [PubMed]
- Colpan, R.D.; Erdemir, A. Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. J. Microencapsul. 2021, 38, 381–393. [Google Scholar] [CrossRef]
- Ren, K.W.; Li, Y.H.; Wu, G.; Ren, J.Z.; Lu, H.B.; Li, Z.M.; Han, X.W. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef]
- Alserihi, R.F.; Mohammed, M.R.S.; Kaleem, M.; Khan, M.I.; Sechi, M.; Sanna, V.; Zughaibi, T.A.; Abuzenadah, A.M.; Tabrez, S. Development of (–)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment. Nanotechnol. Rev. 2022, 11, 298–311. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, W.S.; Tu, G.H.; Chen, X.Y.; Lu, Y.G.; Wu, L.X.; Zheng, D.L. Enhanced chemotherapeutic efficacy of PLGA-encapsulated epigallocatechin gallate (EGCG) against human lung cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Hu, S.Y.; Feng, Y.S.; Zou, P.; Wang, Y.; Qin, P.; Yue, J.; Liang, Y.T.; Wang, H.; Liu, L.W. Preparation of chitosan-epigallocatechin-3-O-gallate nanoparticles and their inhibitory effect on the growth of breast cancer cells. J. Innov. Opt. Health Sci. 2018, 11, 1850018. [Google Scholar] [CrossRef]
- Moghaddam, F.A.; Ebrahimian, M.; Oroojalian, F.; Yazdian-Robati, R.; Kalalinia, F.; Tayebi, L.; Hashemi, M. Effect of thymoquinone-loaded lipid-polymer nanoparticles as an oral delivery system on anticancer efficiency of doxorubicin. J. Nanostruct. Chem. 2022, 12, 33–44. [Google Scholar] [CrossRef]
- Noor, N.S.; Kaus, N.H.M.; Szewczuk, M.R.; Hamid, S.B.S. Formulation, characterization and cytotoxicity effects of novel thymoquinone-PLGA-PF68 nanoparticles. Int. J. Mol. Sci. 2021, 22, 9420. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Fakhri, K.U.; Rizvi, M.M.A.; Mahdi, W.; Kazi, M. Effect of chitosan coating on PLGA nanoparticles for oral delivery of thymoquinone: In vitro, ex vivo, and cancer cell line assessments. Coatings 2021, 11, 6. [Google Scholar] [CrossRef]
- Upadhyay, P.; Sarker, S.; Ghosh, A.; Gupta, P.; Das, S.; Ahir, M.; Bhattacharya, S.; Chattopadhyay, S.; Ghosh, S.; Adhikary, A. Transferrin-decorated thymoquinone-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater. Sci. 2019, 7, 4325–4344. [Google Scholar] [CrossRef] [PubMed]
- Ince, I.; Yıldırım, Y.; Güler, G.; Medine, E.I.; Ballica, G.; Kuşdemir, B.C.; Göker, E. Synthesis and characterization of folic acid-chitosan nanoparticles loaded with thymoquinone to target ovarian cancer cells. J. Radioanal. Nucl. Chem. 2020, 324, 71–85. [Google Scholar] [CrossRef]
- Ibrahim, W.N.; Muizzuddin Bin Mohd Rosli, L.; Doolaanea, A.A. Formulation, cellular uptake and cytotoxicity of thymoquinone-loaded PLGA nanoparticles in malignant melanoma cancer cells. Int. J. Nanomed. 2020, 15, 8059–8074. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.T.; Jiang, B.B.; Li, B.Y.; Li, Z.L.; Jiang, B.H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 2015, 478, 251–257. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-loaded nanoparticles based on poly(epsilon-caprolactone) and poly(D,L-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Kosuru, R.; Singh, S.K.; Prasad, C.B.; Narayan, G.; Muthu, M.S.; Singh, S. Resveratrol loaded PLGA: D-alpha-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv. 2016, 6, 74254–74268. [Google Scholar] [CrossRef]
- Yee, Y.J.; Benson, H.A.E.; Dass, C.R.; Chen, Y. Evaluation of novel conjugated resveratrol polymeric nanoparticles in reduction of plasma degradation, hepatic metabolism and its augmentation of anticancer activity in vitro and in vivo. Int. J. Pharm. 2022, 615, 121499. [Google Scholar] [CrossRef]
- Legette, L.L.; Lee, W.H.; Martin, B.R.; Story, J.A.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Genistein, a phytoestrogen, improves total cholesterol, and Synergy, a prebiotic, improves calcium utilization, but there were no synergistic effects. Menopause 2011, 18, 923–931. [Google Scholar] [CrossRef]
- Meteoglu, I.; Erdemir, A. Genistein and temozolomide-loaded polymeric nanoparticles: A synergistic approach for improved anti-tumor efficacy against glioblastoma. Process Biochem. 2021, 110, 9–18. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Naik, P.K.; Kazi, M.; Hussain, M.D. Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2022, 50, 228–239. [Google Scholar] [CrossRef] [PubMed]
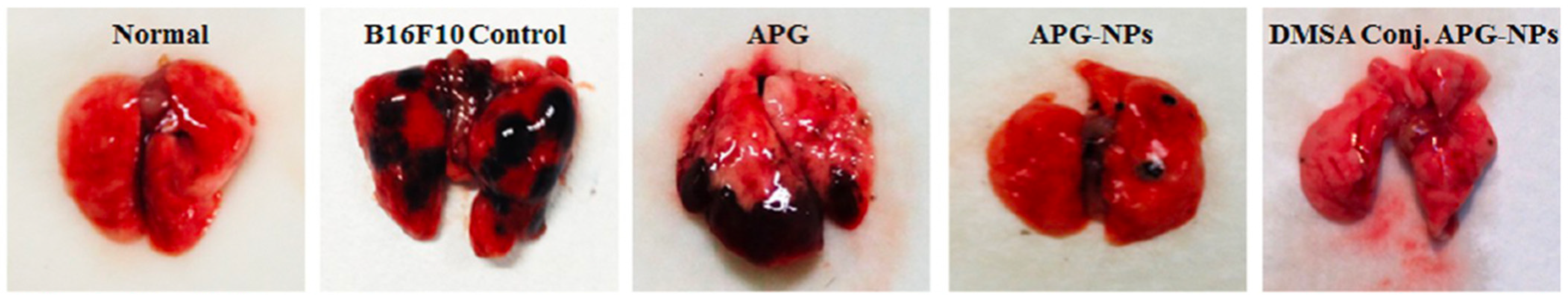
- Sen, R.; Ganguly, S.; Ganguly, S.; Debnath, M.C.; Chakraborty, S.; Mukherjee, B.; Chattopadhyay, D. Apigenin-loaded PLGA-DMSA nanoparticles: A novel strategy to treat melanoma lung metastasis. Mol. Pharm. 2021, 18, 1920–1938. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Jagannathan, R.; Abraham, P.M.; Poddar, P. Temperature-dependent spectroscopic evidences of curcumin in aqueous medium: A mechanistic study of its solubility and stability. J. Phys. Chem. B 2012, 116, 14533–14540. [Google Scholar] [CrossRef]
- Chamani, S.; Moossavi, M.; Naghizadeh, A.; Abbasifard, M.; Kesharwani, P.; Sathyapalan, T.; Sahebkar, A. Modulatory properties of curcumin in cancer: A narrative review on the role of interferons. Phytother. Res. 2023, 37, 1003–1014. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Aly, S.M.; Khan, M.A.; Aldebasi, Y.H. Role of curcumin in disease prevention and treatment. Adv. Biomed. Res. 2018, 7, 38. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.D.; Yang, C.; Li, Y.S.; Shao, M.L.; Su, W.; Song, N. Curcumin regulates cancer progression: Focus on ncRNAs and molecular signaling pathways. Front. Oncol. 2021, 11, 660712. [Google Scholar] [CrossRef]
- Vadukoot, A.K.; Mottemmal, S.; Vekaria, P.H. Curcumin as a potential therapeutic agent in certain cancer types. Cureus 2022, 14, e22825. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.J.; Zhang, J.F.; Dai, W.J.; Li, R.L.; Lu, Z.Y.; Duan, J.L.; Li, J.P.; Chen, X.P.; Fan, J.P.; et al. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget 2017, 8, 50747–50760. [Google Scholar] [CrossRef]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sandoughdaran, S.; Razzaghdoust, A.; Tabibi, A.; Basiri, A.; Simforoosh, N.; Mofid, B. Randomized, double-blind pilot study of nanocurcumin in bladder cancer patients receiving induction chemotherapy. Urol. J. 2021, 18, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Hatamipour, M.; Sahebkar, A.; Alavizadeh, S.H.; Dorri, M.; Jaafari, M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic. Med. Sci. 2019, 22, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Manchope, M.F.; Casagrande, R.; Verri, W.A., Jr. Naringenin: An analgesic and anti-inflammatory citrus flavanone. Oncotarget 2017, 8, 3766–3767. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 12, 99. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, Y.J.; Lee, J.H.; Nam, M.J. Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem. Toxicol. 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Faramarzi, F.; Alimohammadi, M.; Rahimi, A.; Alizadeh-Navaei, R.; Shakib, R.J.; Rafiei, A. Naringenin induces intrinsic and extrinsic apoptotic signaling pathways in cancer cells: A systematic review and meta-analysis of in vitro and in vivo data. Nutr. Res. 2022, 105, 33–52. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Song, H.M.; Park, G.H.; Eo, H.J.; Jeong, J.B. Naringenin-mediated ATF3 expression contributes to apoptosis in human colon cancer. Biomol. Ther. 2016, 24, 140–146. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J. Cell Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Kong, S.; Zhao, S.; Tang, Q. Naringenin inhibits human breast cancer cells (MDA-MB-231) by inducing programmed cell death, caspase stimulation, G2/M phase cell cycle arrest and suppresses cancer metastasis. Cell Mol. Biol. 2021, 67, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.H.; Jang, H.; Park, S.Y.; Seol, J.W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological effects of quercetin: Q literature-based review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-Induced diabetic rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef]
- Fortunato, L.R.; Alves, C.D.; Teixeira, M.M.; Rogerio, A.P. Quercetin: A flavonoid with the potential to treat asthma. Braz. J. Pharm. Sci. 2012, 48, 589–599. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhagat, M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020, 9, 9181–9192. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The potential of epigallocatechin gallate (EGCG) in targeting autophagy for cancer treatment: A narrative review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG inhibits tumor growth in melanoma by targeting JAK-STAT signaling and its downstream PD-L1/PD-L2-PD1 axis in tumors and enhancing cytotoxic T-cell responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Wen, L.H.; Wu, D.; Tan, X.D.; Zhong, M.Q.; Xing, J.B.; Li, W.; Li, D.; Cao, F.R. The role of catechins in regulating diabetes: An update review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Ibrahim, A.M.; Alammar, A.; Alsinan, R.; Aleid, M.; Alshehhi, A.; Alshehri, M.; Mishra, S.; Alhajri, N. Thymoquinone: Review of its potential in the treatment of neurological diseases. Pharmaceuticals 2022, 15, 408. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef]
- Ojha, S.; Azimullah, S.; Mohanraj, R.; Sharma, C.; Yasin, J.; Arya, D.S.; Adem, A. Thymoquinone protects against myocardial ischemic injury by mitigating oxidative stress and inflammation. Evid.-Based Compl. Alt. 2015, 2015, 143629. [Google Scholar] [CrossRef]
- Kalemci, S.; Micili, S.C.; Acar, T.; Senol, T.; Dirican, N.; Omeroglu, G.; Bagriyanik, A.; Kamaci, G.; Yilmaz, O. Effectiveness of thymoquinone in the treatment of experimental asthma. Clin. Ter. 2013, 164, E155–E158. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Fu, S.Y.; Fu, J.J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Wadher, K.; Umekar, M. Development of topical thymoquinone loaded polymer-lipid hybrid vesicular gel: In-vitro and ex-vivo evaluation. J. Liposome Res. 2022, 32, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a dietary anti-inflammatory Agent: Current therapeutic standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.Q.; Liu, D.M. The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Yao, H.; Sun, J.Y.; Wei, J.; Zhang, X.; Chen, B.; Lin, Y.J. Kaempferol protects blood vessels from damage induced by oxidative stress and inflammation in association with the Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Da, J.; Xu, M.X.; Wang, Y.W.; Li, W.F.; Lu, M.J.; Wang, Z. Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Anal. Cell Pathol. 2019, 2019, 1907698. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; Ong, C.S.; et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell Physiol. 2003, 197, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.T.; Rankin, G.O.; Li, Z.L.; DePriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A review on its anti-inflammatory properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A potent anti-breast cancer agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Lee, S.C.; Song, Y.S. Involvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.Z.; Du, G.J.; Qi, L.W.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, F.; Pagliacci, M.C.; Migliorati, G.; Moraca, R.; Grignani, F.; Riccardi, C.; Nicoletti, I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk. Res. 1994, 18, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Miao, Q.; Li, J.G.; Miao, S.; Hu, N.; Zhang, J.; Zhang, S.; Xie, Y.H.; Wang, J.B.; Wang, S.W. The bone-protective effect of genistein in the animal model of bilateral ovariectomy: Roles of phytoestrogens and PTH/PTHR1 against post-menopausal osteoporosis. Int. J. Mol. Sci. 2012, 13, 56–70. [Google Scholar] [CrossRef]
- Rahmani, F.; Karimi, E.; Oskoueian, E. Synthesis and characterisation of chitosan-encapsulated genistein: Its anti-proliferative and anti-angiogenic activities. J. Microencapsul. 2020, 37, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced phytochemical-based nanocarrier systems for the treatment of breast cancer. Cancers 2023, 15, 1023. [Google Scholar] [CrossRef]


| Drug Carrier | Cancer | Cellular Effect | Cell Line | Particle Size | Method | References | |
|---|---|---|---|---|---|---|---|
| Curcumin | PLGA | Breast | Decreases HIF-1α and Nuclear p65 activity | MDA-MB-231 | 606 nm | Solvent evaporation | [28] |
| Curcumin | PLGA | Breast | G2/M block of cell cycle progression | MCF-7 | 116.9 ± 3.8 | Single emulsion | [29] |
| Curcumin | PLGA | Breast | Suppresses the NF-κB signaling pathway and inhibits the production of P-glycoprotein | MDA-MB-231 | 85.8 ± 0.21 nm | Antisolvent precipitation | [30] |
| Curcumin | PLA | Breast | Inhibits cell proliferation | T47D | 169.3 ± 1.52 nm | Thin-film hydration method | [31] |
| Curcumin | PAMAM dendrimers | Breast | Increases telomerase activity | T47D | NI * | Ionic gelation | [32] |
| Curcumin | Magnetic microsphere | Lung and cervical | Inhibits cell proliferation | A549 and Hela | NI * | Dual-emulsion solvent washout | [33] |
| Naringenin | PLGA | Pancreatic | Inhibits cell proliferation | NI * | 150.45 ± 12.45 nm | Emulsion diffusion evaporation | [34] |
| Naringenin | Chitosan | Lung | Inhibits cell proliferation | A549 | 407.47 nm | Ionic gelation | [35] |
| Naringenin | PHEMA | Breast | Cell cycle arrest in G1 phase and induces early apoptosis | MCF-7 | 53 ± 1.1 nm | Mini-emulsion polymerization | [36] |
| Naringenin | PCL | Lung | Cell cycle arrest in G2-M phase | A549 | 251.6 ± 3.22 nm | Nano-precipitation | [37] |
| Naringenin | Polymeric nano micelles | Lung and breast | Inhibits cell proliferation | A549 and MCF-7 | 239.8 ± 0.76 nm | Membrane hydration method | [38] |
| Quercetin | Eudragit® S100 | Colon | Inhibits cell proliferation | CT26 | 66.8 nm | Nano-precipitation | [39] |
| Quercetin | Chitosan | Lung and breast | Increases cytotoxic activity | A549 and MCF-7 | 339.37 nm | Ionic gelation | [40] |
| Quercetin | PLGA | Breast and cervical | Induces apoptosis, mitochondrial damage, caspase activation, and cell cycle arrest | MCF-7 and Hela | 89.8 ± 5.9 nm | Solvent evaporation | [41] |
| Quercetin | PLGA/TPGS | Breast | Decreases migration and invasion | MDA-MB-231 | 198.4 ± 7.8 nm | Nanoprecipitation | [42] |
| Quercetin | PLGA | Colon | Increases Cas-3 and Cas-9 expression | HT-29 | 237.8 ± 9.67 nm | Solvent evaporation | [43] |
| Quercetin | PLGA | Liver | Enhances Cyt-c/caspase and Akt/ERK1/2, AP-2β/hTERT, and p65/COX-2 signal inactivation | MHCC97H, Hep3B, HCCLM3, and Bel7402 | 106.7 nm | NI * | [44] |
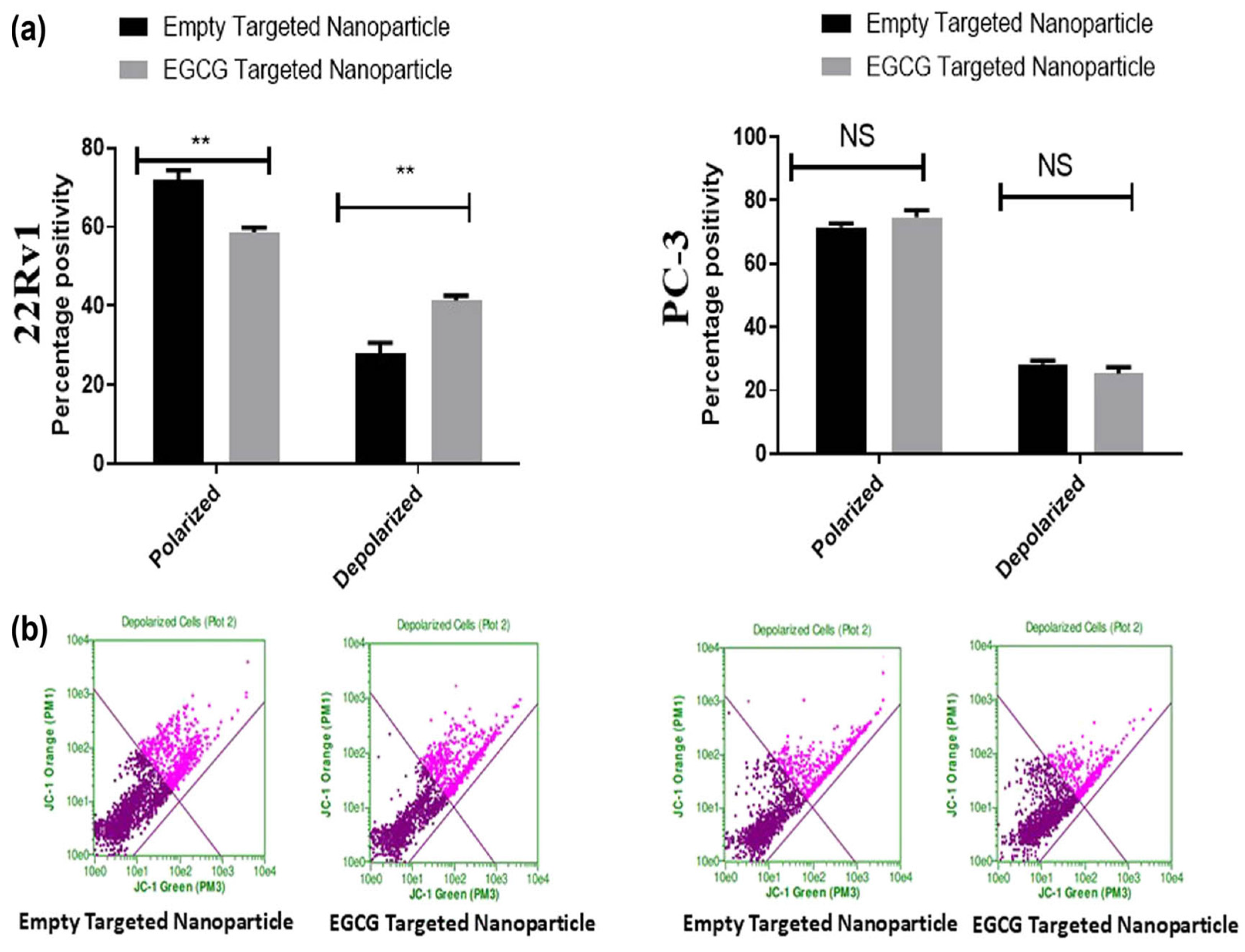
| Epigallo-catechin gallate | PLGA | Prostate | Increases apoptotic cells and mitochondrial depolarization | PC-3 and 22Rv1 | 115 to 130 nm | Nanoprecipitation | [45] |
| Epigallo-catechin gallate | PLGA | Lung | Blocks NF-κB activity and suppresses genes regulated by NF-κB such as BCL2, BCL-XL, COX-2, TNF-a, cyclinD1, C-MYC, TWIST, and MMP2 | A549 and H1299 | 175.8 ± 3.8 nm | Oil-in-water emulsion solvent evaporation | [46] |
| Epigallo-catechin gallate | Chitosan | Breast | Inhibits cell proliferation | MCF-7 | 342 nm | Ionic gelation | [47] |
| Thymoquinone | PLGA | Colon | Inhibits cell proliferation | C26 and Caco-2 | 184 nm | Single-emulsion-solvent evaporation | [48] |
| Thymoquinone | PLGA-PEG | Breast | Inhibits cell proliferation | MDA-MB-231 and MCF-7 | 6.92 ± 27.38 nm | Emulsion-solvent evaporation | [49] |
| Thymoquinone | PLGA | Breast | Greater muco-adhesion, intestinal permeability, and enhanced antioxidant potential and cytotoxicity | MDA-MB-231 and MCF-7 | 126.03–196.71 nm | Emulsion evaporation | [50] |
| Thymoquinone | PLGA-PEG | Lung | TF-TQ-Np-mediated p53 upregulation and activated miR-34a and miR-16 expression levels | A549 | 77.50 ± 6.35 nm | Nanoprecipitation | [51] |
| Thymoquinone | Chitosan | Ovarian | Inhibits cell proliferation | SKOV-3 | 250 to 350 nm | Ionic gelation | [52] |
| Thymoquinone | PLGA | Melanoma | Inhibits cell proliferation | A375 | 147.2 nm | Multiple-emulsion-solvent diffusion | [53] |
| Kaempferol | PEO-PPO-PEO, PLGA, PLGA-PEI, chitosan, and PAMAM | Ovarian | Inhibits cell proliferation | A2780/CP70 and OVCAR-3 | 160, 210, 220, 230, and 250 nm | Nanoprecipitation | [54] |
| Resveratrol | PEG-PLA | Colon | Induced apoptosis | CT26 | 119.9 nm | Solvent-evaporation method | [55] |
| Resveratrol | PLGA | Prostate | G1-S transition phase, externalization of phosphatidylserine, DNA nicking, loss of mitochondrial membrane potential, and reactive oxygen species generation | LNCaP | 202.8 nm | Nanoprecipitation | [56] |
| Resveratrol | PLGA | Prostate | Inhibits cell proliferation | DU-145, PC-3, and LNCaP | 150 nm | Nanoprecipitation | [57] |
| Resveratrol | PLGA-TPGS | Brain | Inhibits cell proliferation | C6 | 175.5 nm | Single-emulsion-solvent- evaporation | [58] |
| Resveratrol | mPEG-PLA | Melanoma | Inhibits cell proliferation | B16-F10 | 162.2 ± 2.9 nm | Solvent evaporation | [59] |
| Genistein | PLGA | Glioblastoma | Increases levels of Cyt-c, Cas-3, Cas-9, and BAX gene and Cas-3 and Cas-9 protein expression | U87MG | 135.5 to 180.7 nm | Single-emulsion (o/w) solvent evaporation | [60] |
| Genistein | PLGA-PEG | Ovarian | Inhibits cell proliferation | SKOV-3 | 104.17 to 125.41 nm | Nanoprecipitation | [61] |
| Genistein | Chitosan | Colorectal | Inhibits cell proliferation | HT-29 | 788 nm | Ionic gelation | [62] |
| Apigenin | PLGA | Melanoma and lung | Depolarizes mitochondrial membrane potential, enhances caspase activity | B16-F10 and A549 | 92.18 nm | Nanoprecipitation | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldırım, M.; Sessevmez, M.; Poyraz, S.; Düzgüneş, N. Recent Strategies for Cancer Therapy: Polymer Nanoparticles Carrying Medicinally Important Phytochemicals and Their Cellular Targets. Pharmaceutics 2023, 15, 2566. https://doi.org/10.3390/pharmaceutics15112566
Yıldırım M, Sessevmez M, Poyraz S, Düzgüneş N. Recent Strategies for Cancer Therapy: Polymer Nanoparticles Carrying Medicinally Important Phytochemicals and Their Cellular Targets. Pharmaceutics. 2023; 15(11):2566. https://doi.org/10.3390/pharmaceutics15112566
Chicago/Turabian StyleYıldırım, Metin, Melike Sessevmez, Samet Poyraz, and Nejat Düzgüneş. 2023. "Recent Strategies for Cancer Therapy: Polymer Nanoparticles Carrying Medicinally Important Phytochemicals and Their Cellular Targets" Pharmaceutics 15, no. 11: 2566. https://doi.org/10.3390/pharmaceutics15112566
APA StyleYıldırım, M., Sessevmez, M., Poyraz, S., & Düzgüneş, N. (2023). Recent Strategies for Cancer Therapy: Polymer Nanoparticles Carrying Medicinally Important Phytochemicals and Their Cellular Targets. Pharmaceutics, 15(11), 2566. https://doi.org/10.3390/pharmaceutics15112566









