Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulgel Preparation
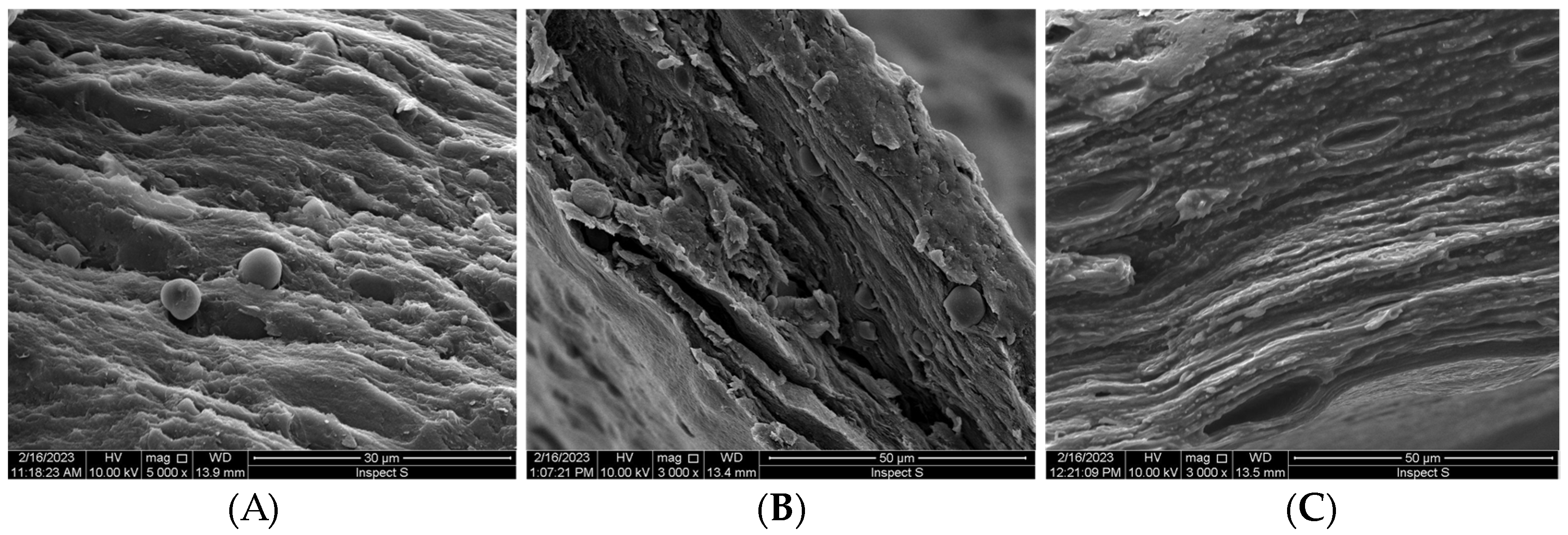
2.3. SEM Analysis
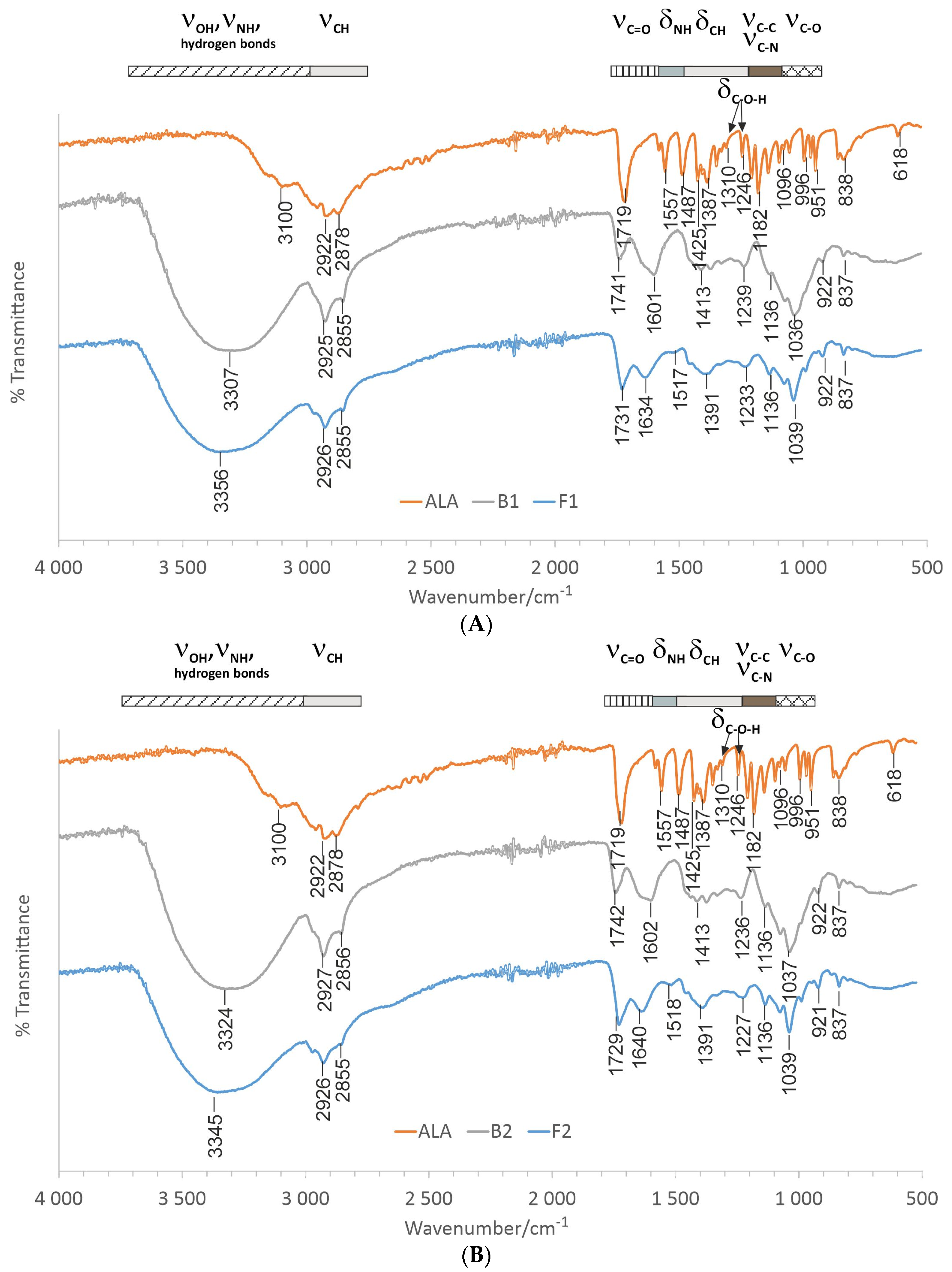
2.4. Fourier Transform Infrared—Attenuated Total Reflectance Spectroscopy (ATR-FTIR)
2.5. HPLC Analysis
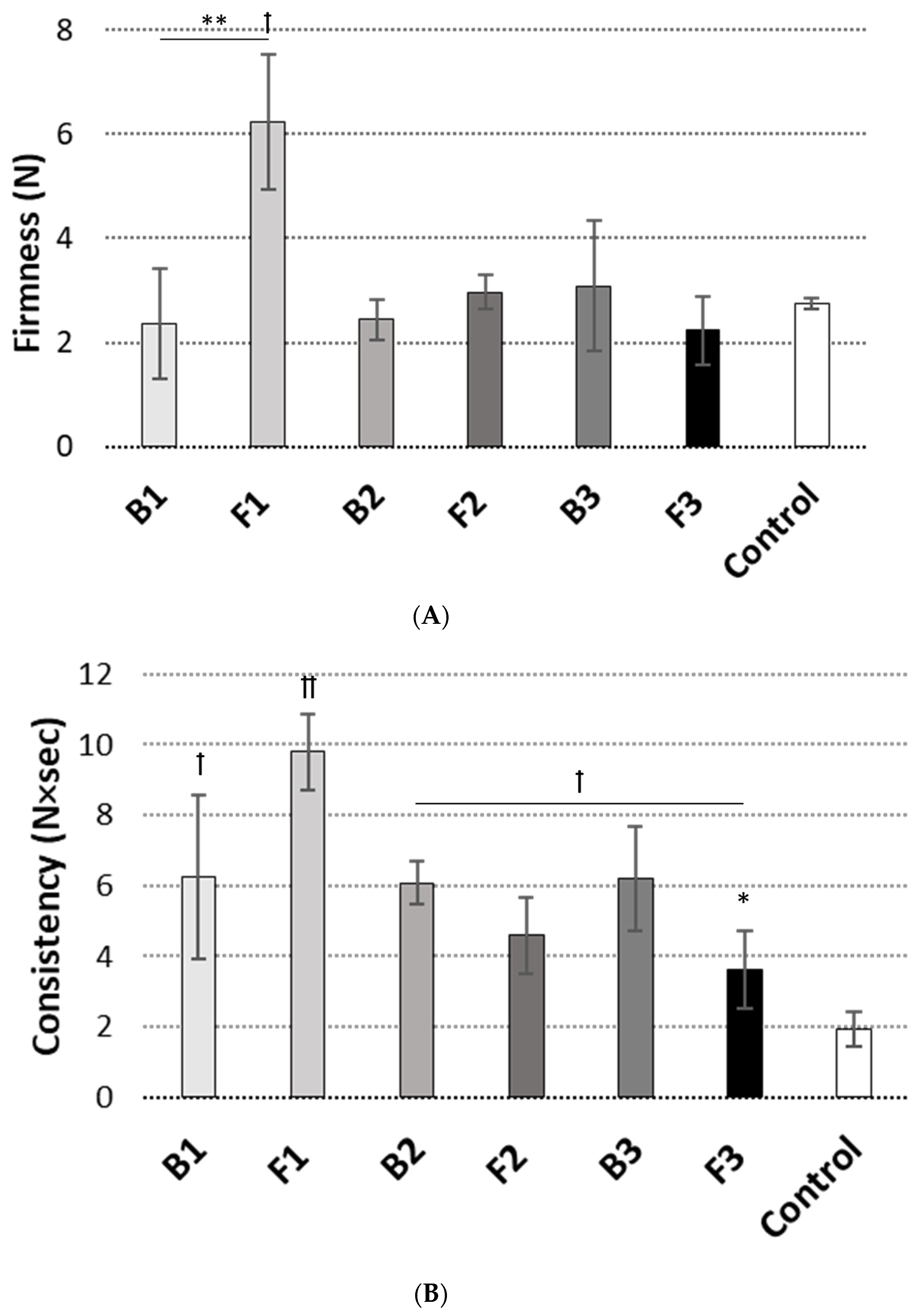
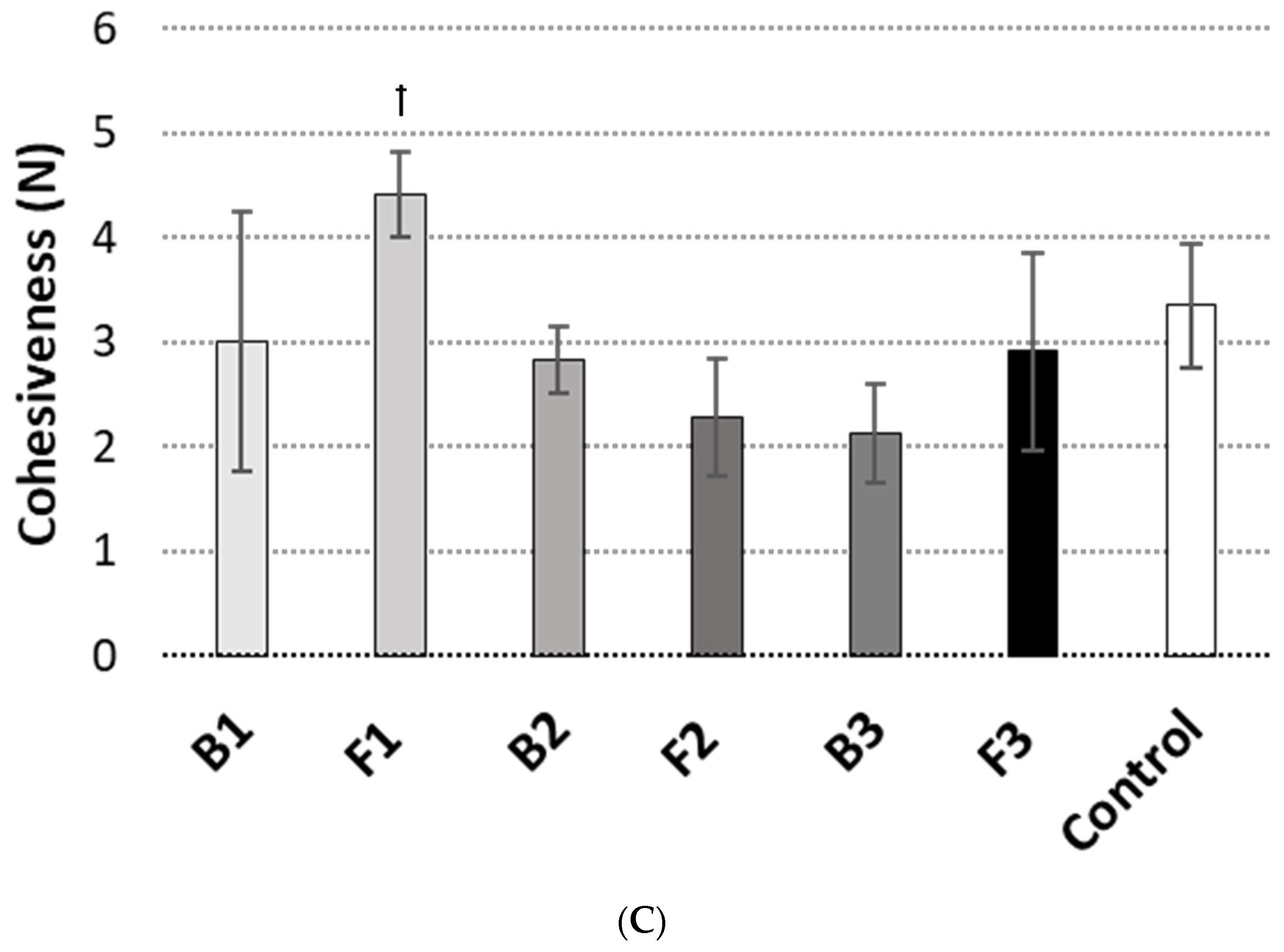
2.6. Textural Properties
2.7. Ex Vivo Mucoadhesive Studies
2.8. Dissolution Studies
2.9. Ex Vivo Penetration Studies
2.10. Safety Profile Using HOE Tissue
2.10.1. Human Cultured Oral Mucosa Model
2.10.2. Irritation Assay
2.10.3. IL-1β Release Assay
2.10.4. Histopathological Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Emulgels’ Characterization
3.2. Texture Analysis
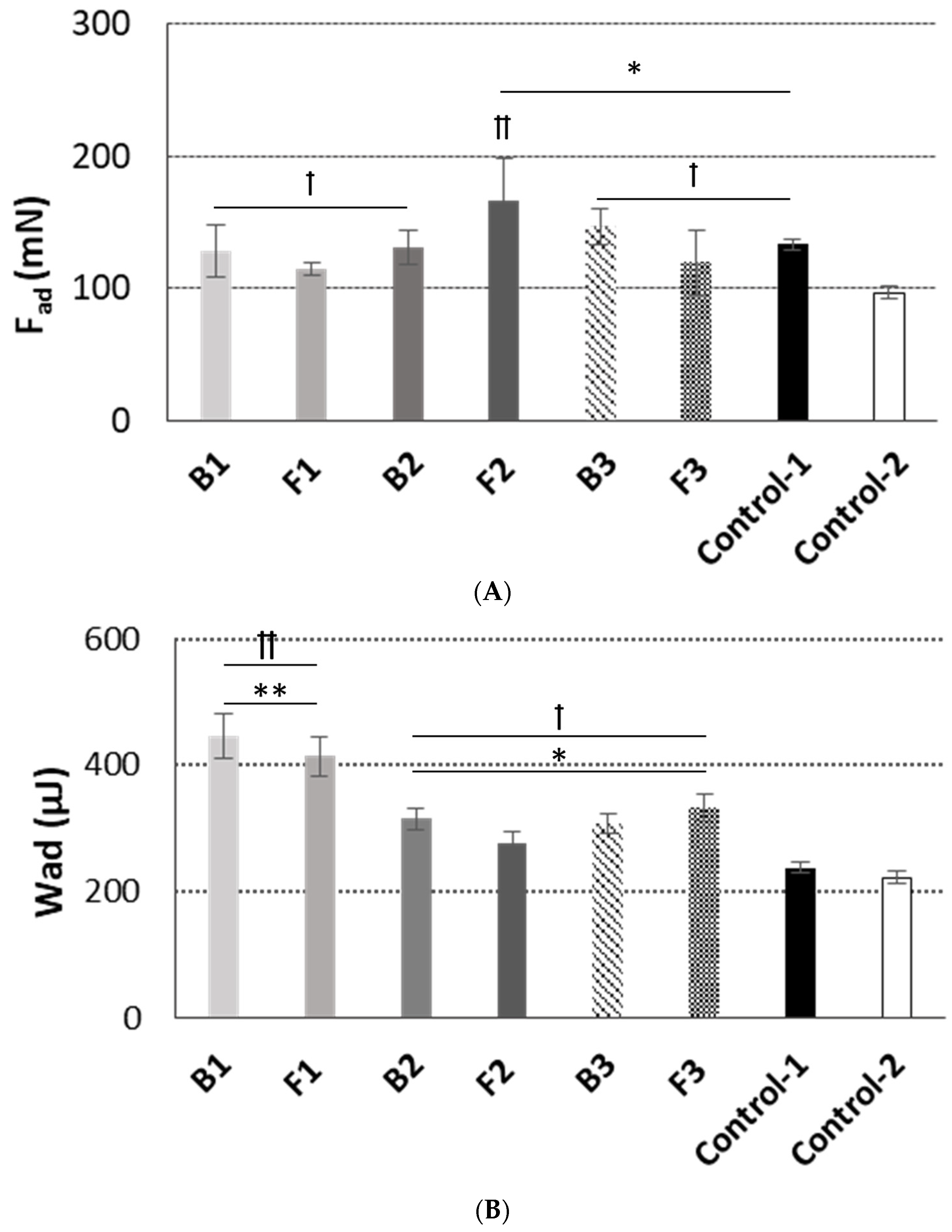
3.3. Mucoadhesive Behavior
3.4. Dissolution Studies
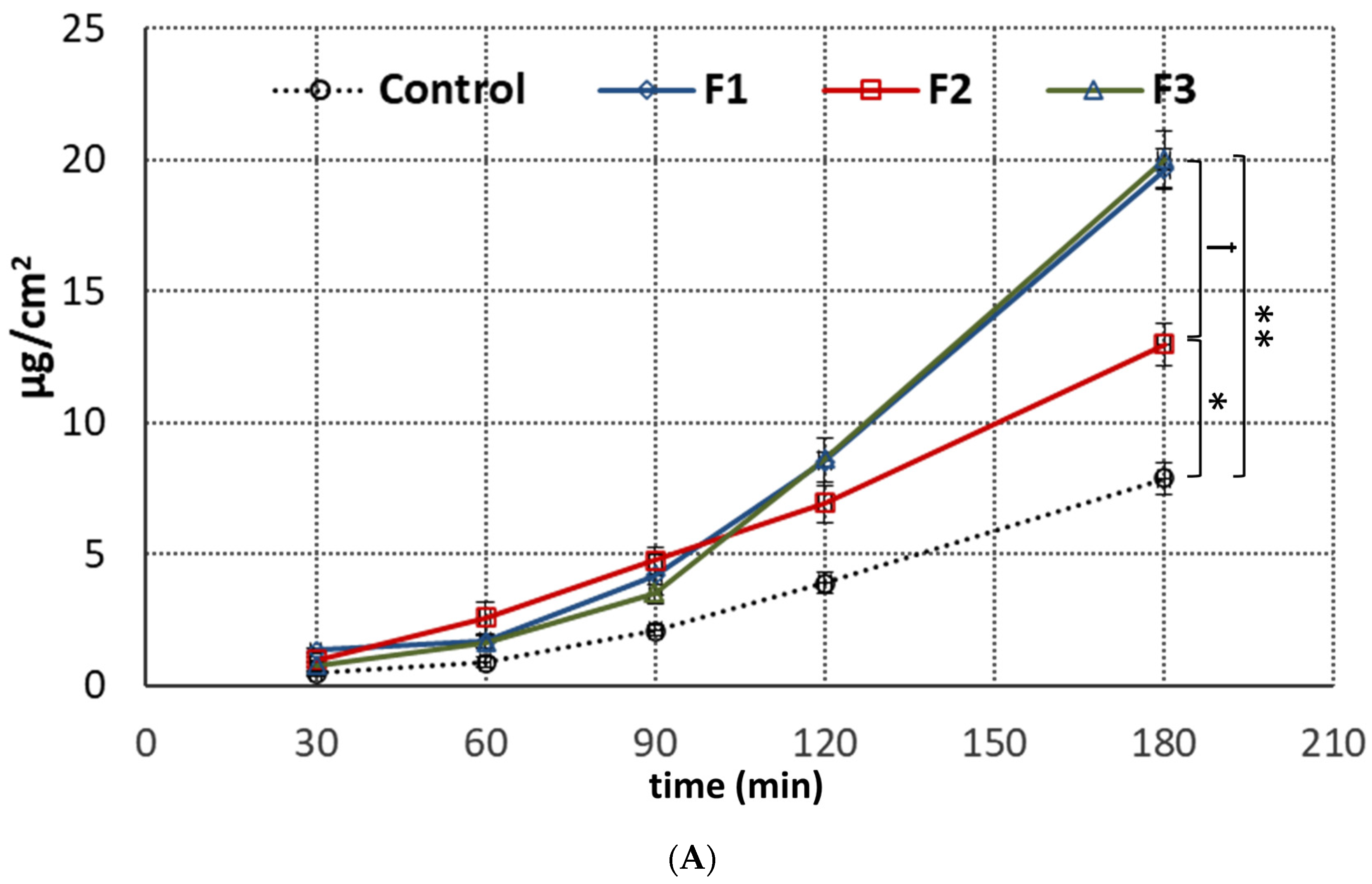
3.5. Penetration Studies
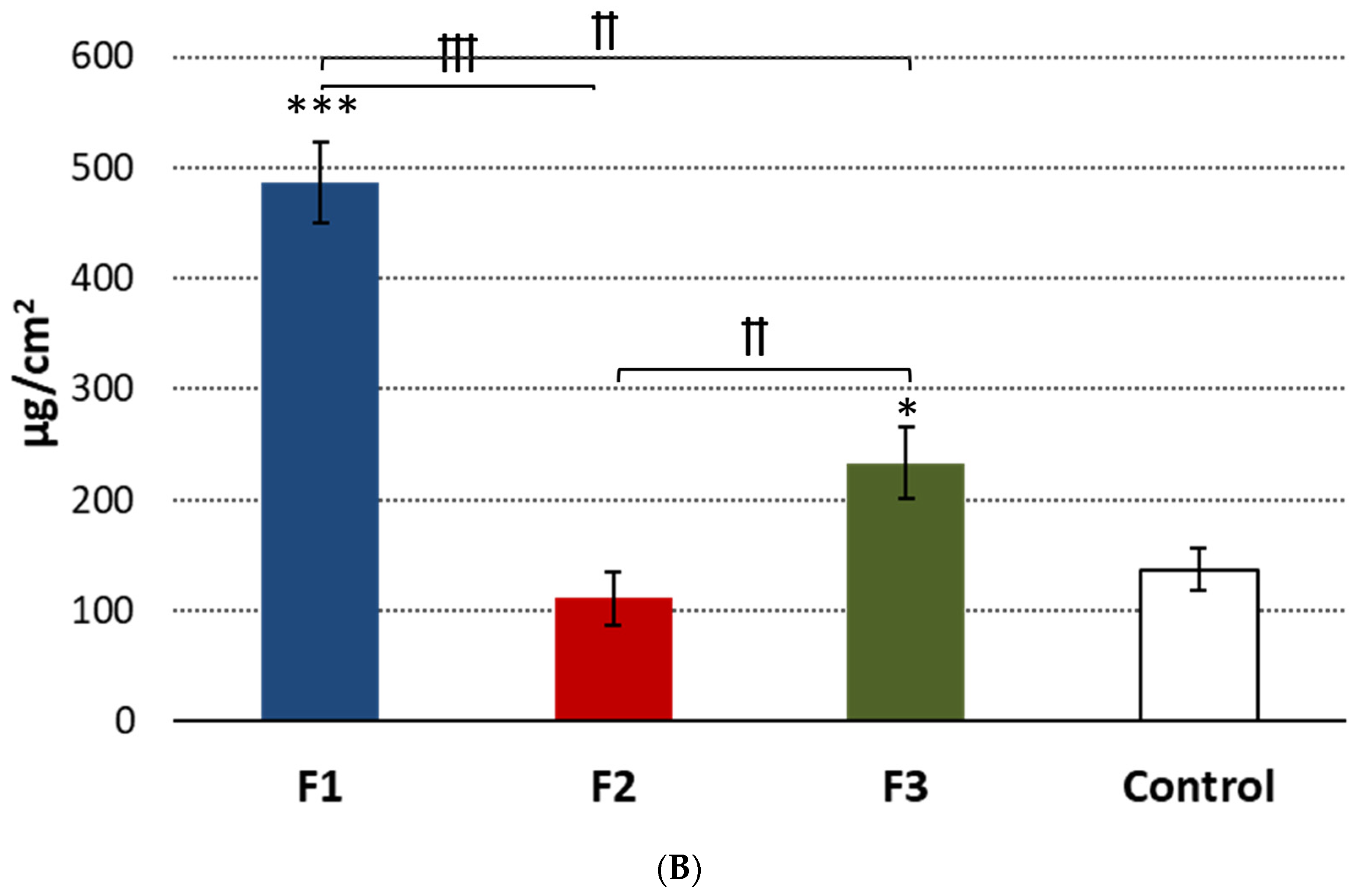
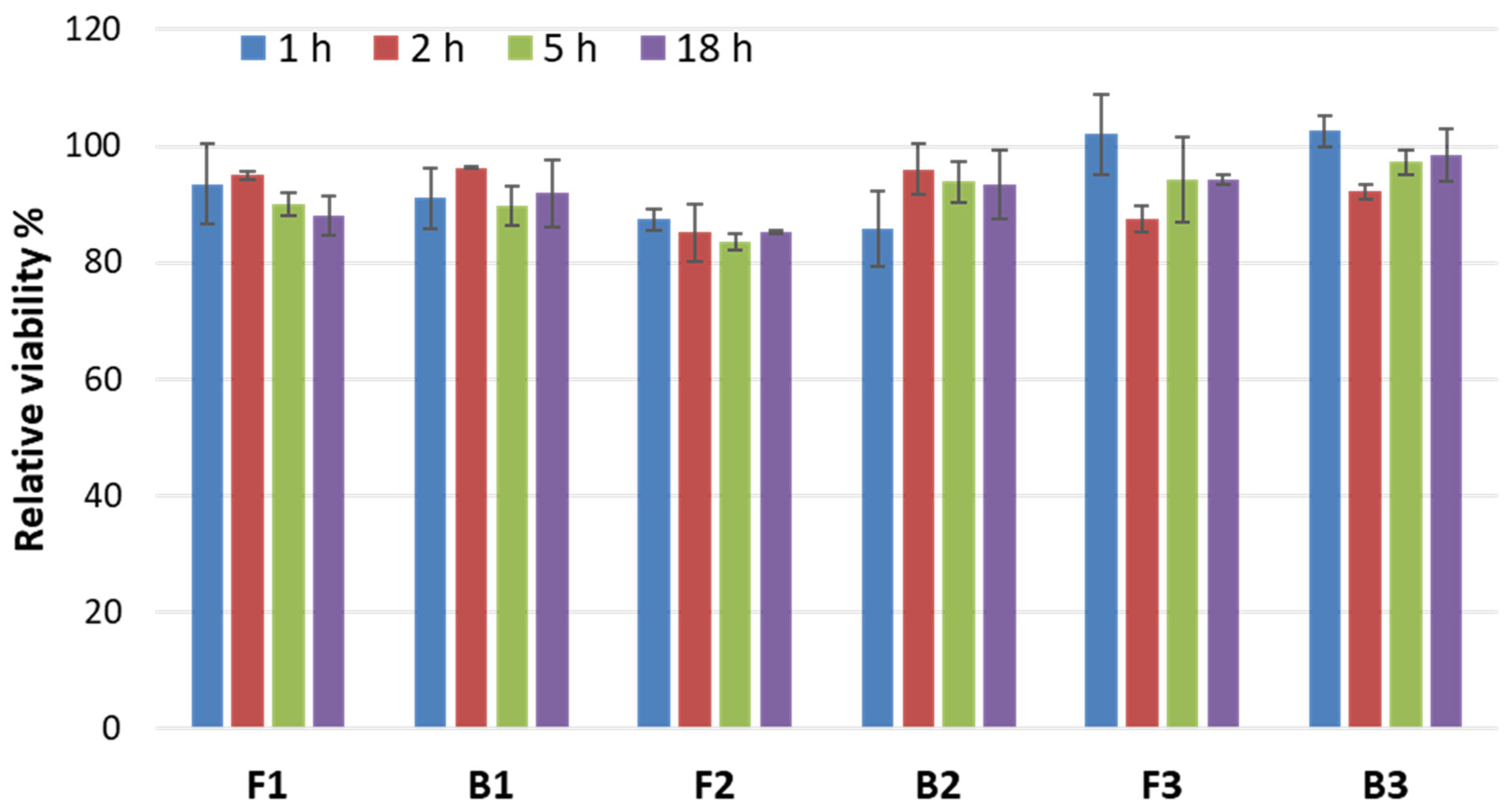
3.6. Safety Profile
3.6.1. Irritation Studies
3.6.2. IL-1β Release Assay
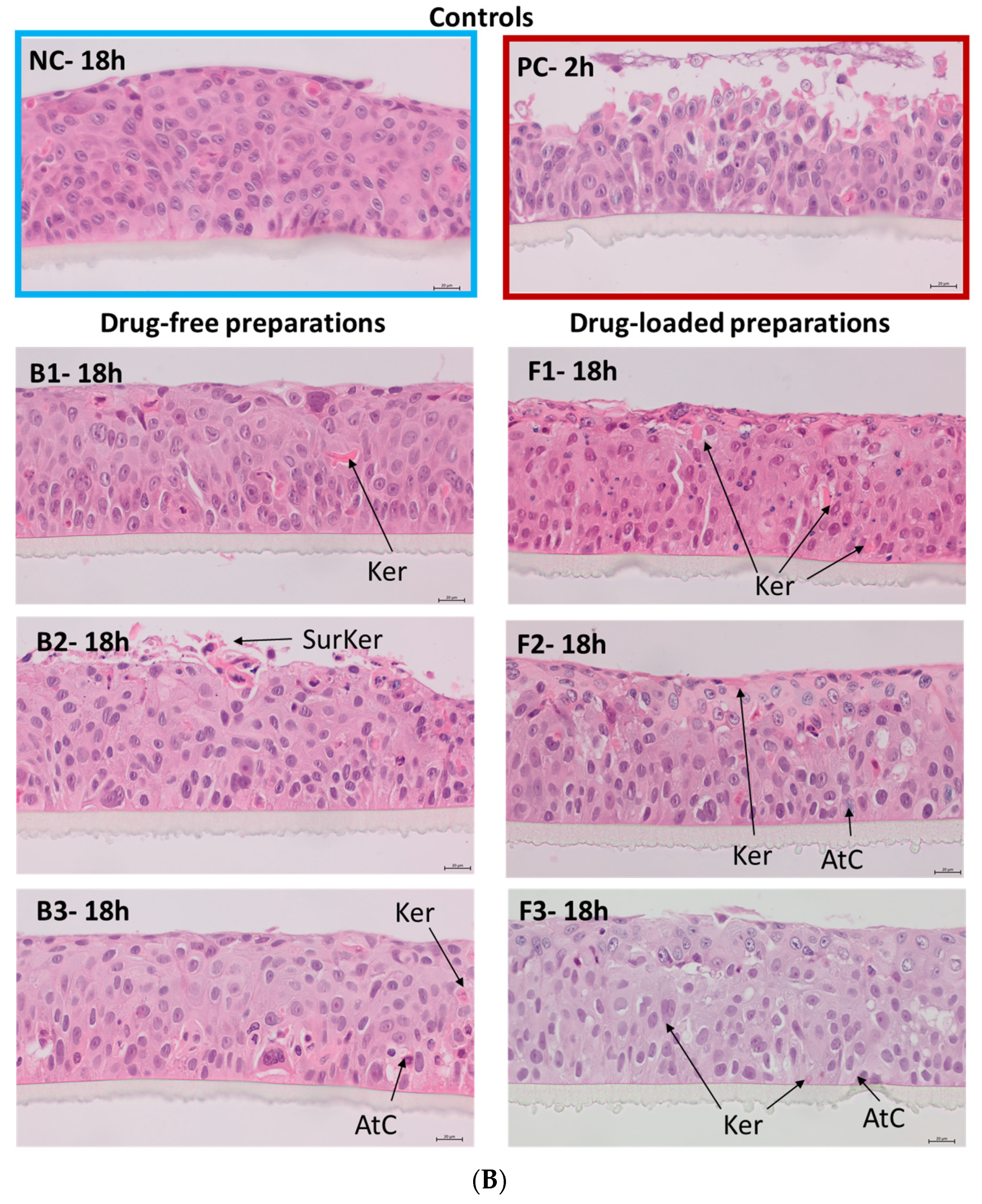
3.6.3. Histological Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Szeimies, R.-M.; Sidoroff, A.; Braathen, L.R. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications-actinic keratoses, Bowen’s disease, basal cell carcinoma: European PDT Guidelines. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Mamardashvili, G.M.; Maltceva, O.V.; Lazovskiy, D.A.; Khodov, I.A.; Borovkov, V.; Mamardashvili, N.Z.; Koifman, O.I. Medium viscosity effect on fluorescent properties of Sn(IV)-tetra(4-sulfonatophenyl)porphyrin complexes in buffer solutions. J. Mol. Liq. 2019, 277, 1047–1053. [Google Scholar] [CrossRef]
- Cramers, P.; Ruevekamp, M.; Oppelaar, H.; Dalesio, O.; Baas, P.; Stewart, F.A. Foscan® uptake and tissue distribution in relation to photodynamic efficacy. Br. J. Cancer 2003, 88, 283–290. [Google Scholar] [CrossRef]
- Casas, A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Tewari, K.M.; Eggleston, I.M. Chemical approaches for the enhancement of 5-aminolevulinic acid-based photodynamic therapy and photodiagnosis. Photochem. Photobiol. Sci. 2018, 17, 1553–1572. [Google Scholar] [CrossRef]
- Romano, A.; Di Stasio, D.; Gentile, E.; Petruzzi, M.; Serpico, R.; Lucchese, A. The potential role of Photodynamic therapy in oral premalignant and malignant lesions: A systematic review. J. Oral Pathol. Med. 2021, 50, 333–344. [Google Scholar] [CrossRef]
- Sulewska, M.; Duraj, E.; Sobaniec, S.; Graczyk, A.; Milewski, R.; Wróblewska, M.; Pietruski, J.; Pietruska, M. A clinical evaluation of the efficacy of photodynamic therapy in the treatment of erosive oral lichen planus: A case series. Photodiagnosis Photodyn. Ther. 2017, 18, 12–19. [Google Scholar] [CrossRef]
- Choudhary, R.; Reddy, S.; Nagi, R.; Nagaraju, R.; Kunjumon, S.; Sen, R. The Effect of Photodynamic Therapy on Oral-Premalignant Lesions: A Systematic Review. J. Clin. Exp. Dent. 2022, e285–e292. [Google Scholar] [CrossRef]
- Casas, A.; Fukuda, H.; Di Venosa, G.; Del, C. Batlle, A.M. The influence of the vehicle on the synthesis of porphyrins after topical application of 5-aminolaevulinic acid. Implications in cutaneous photodynamic sensitization: TOPICAL ALA and PHOTODYNAMIC THERAPY. Br. J. Dermatol. 2000, 143, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Chinna Reddy, P.; Chaitanya, K.S.C.; Madhusudan Rao, Y. A review on bioadhesive buccal drug delivery systems: Current status of formulation and evaluation methods. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 385–403. [Google Scholar]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers: Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials 2020, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Villar-Padilla, A.; Del Prado-Audelo, M.L.; González-Torres, M.; Giraldo-Gomez, D.M.; Caballero-Florán, I.H.; González-Del Carmen, M.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H.; et al. Development of a xanthan gum film for the possible treatment of vaginal infections. Cell. Mol. Biol. 2021, 67, 80–88. [Google Scholar] [CrossRef]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Arslan, D.; Akbal Dağıstan, Ö.; Sagirli, O.; Mulazimoglu, L.; Cevher, E.; Yildiz-Pekoz, A. Development and Evaluation of Combined Effect Buccal Films for Treatment of Oral Candidiasis. AAPS PharmSciTech 2022, 24, 23. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Hancock, B.C.; Moss, G.P.; Goldfarb, D.J. (Eds.) Handbook of Pharmaceutical Excipients; Pharmaceutical Press (PhP): London, UK, 2020; ISBN 978-0-85711-375-7. [Google Scholar]
- The United States Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed on 23 March 2023).
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Namjoshi, S.; Caccetta, R.; Edwards, J.; Benson, H.A.E. Liquid chromatography assay for 5-aminolevulinic acid: Application to in vitro assessment of skin penetration via Dermaportation. J. Chromatogr. B 2007, 852, 49–55. [Google Scholar] [CrossRef]
- Szymańska, E.; Wojasiński, M.; Dąbrowska, J.; Krzyżowska, M.; Nowicka, M.; Ciach, T.; Winnicka, K. Chitosan-poly(ethylene oxide) nanofibrous mat as a vaginal platform for tenofovir disoproxyl fumarate–The effect of vaginal pH on drug carrier performance. Int. J. Biol. Macromol. 2022, 222, 856–867. [Google Scholar] [CrossRef] [PubMed]
- EpiSkin. Human Oral Epithelium Model: EpiSkin_Direction for Use_ HOE. 2023. Available online: http://www.episkin.com/ (accessed on 7 September 2022).
- EpiSkin. In Vitro Mucosal Irritation Testing on Reconstituted Human Oral Epithelium. 2023. Available online: http://www.episkin.com/ (accessed on 7 September 2022).
- Colman, K.; Andrews, R.N.; Atkins, H.; Boulineau, T.; Bradley, A.; Braendli-Baiocco, A.; Capobianco, R.; Caudell, D.; Cline, M.; Doi, T.; et al. International Harmonization of Nomenclature and Diagnostic Criteria (INHAND): Non-proliferative and Proliferative Lesions of the Non-human Primate (M. fascicularis). J. Toxicol. Pathol. 2021, 34, 1S–182S. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; D’Amico, E.; Pierfelice, T.V.; Aceto, G.M.; Karaban, M.; Felice, P.; Piattelli, A.; Barone, A.; Iezzi, G. Photodynamic Therapy with Aminolevulinic Acid Enhances the Cellular Activity of Cells Cultured on Porcine Acellular Dermal Matrix Membranes Used in Periodontology. Gels 2023, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Warloe, T.; Jordan, O.; Gurny, R.; Lange, N. Clinical evaluation of bioadhesive hydrogels for topical delivery of hexylaminolevulinate to Barrett’s esophagus. J. Control. Release Off. J. Control. Release Soc. 2007, 123, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Z.; Tao, A.; Wang, P.; Xie, W.; Yang, S.; Huang, J.; Wen, N. Hydrogel-based patient-friendly photodynamic therapy of oral potentially malignant disorders. Biomaterials 2022, 281, 121377. [Google Scholar] [CrossRef]
- The European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2020; Volume 1.
- Chen, J.Y.; Peng, Q.; Jodl, H.-J. Infrared spectral comparison of 5-aminolevulinic acid and its hexyl ester. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2003, 59, 2571–2576. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Serpe, L.; Martinelli, C.C.M.; da Silva, C.B.; Santos, C.P.D.; Novaes, P.D.; Volpato, M.C.; de Paula, E.; Lopez, R.F.V.; Groppo, F.C. Evaluation of different pig oral mucosa sites as permeability barrier models for drug permeation studies. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 81, 52–59. [Google Scholar] [CrossRef]
- Frum, Y.; Khan, G.M.; Sefcik, J.; Rouse, J.; Eccleston, G.M.; Meidan, V.M. Towards a correlation between drug properties and in vitro transdermal flux variability. Int. J. Pharm. 2007, 336, 140–147. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- European Medicine Agency Draft Guideline on Quality and Equivalence of Topical Products. 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 18 November 2022).
- Shiledar, R.R.; Tagalpallewar, A.A.; Kokare, C.R. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr. Polym. 2014, 101, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez Moretti, M.; Correa García, S.; Perotti, C.; Batlle, A.; Casas, A. Delta-Aminolevulinic acid transport in murine mammary adenocarcinoma cells is mediated by beta transporters. Br. J. Cancer 2002, 87, 471–474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rud, E.; Gederaas, O.; Høgset, A.; Berg, K. 5-aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem. Photobiol. 2000, 71, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Gederaas, O.A.; Holroyd, A.; Brown, S.B.; Vernon, D.; Moan, J.; Berg, K. 5-Aminolaevulinic acid methyl ester transport on amino acid carriers in a human colon adenocarcinoma cell line. Photochem. Photobiol. 2001, 73, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-W.; Fang, Y.-P.; Fang, J.-Y. Enhancement techniques for improving 5-aminolevulinic acid delivery through the skin. Dermatol. Sin. 2011, 29, 1–7. [Google Scholar] [CrossRef]
- EU Reference Laboratory for alternatives to animal testing (EURL ECVAM. EVCAM/EURL Skin Irritation: SkinEthic Skin Irritation Test (SIT). Available online: https://joint-research-centre.ec.europa.eu/eu-reference-laboratory-alternatives-animal-testing-eurl-ecvam/alternative-methods-toxicity-testing/validated-test-methods-health-effects/skin-irritation/skin-irritation-skinethic-skin-irritation-test-sit_en (accessed on 30 September 2022).
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Pyrillou, K.; Burzynski, L.C.; Clarke, M.C.H. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Front. Immunol. 2020, 11, 613170. [Google Scholar] [CrossRef]
- Gümüş, P.; Nizam, N.; Nalbantsoy, A.; Özçaka, Ö.; Buduneli, N. Saliva and serum levels of pentraxin-3 and interleukin-1β in generalized aggressive or chronic periodontitis. J. Periodontol. 2014, 85, e40–e46. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Ogden, S.; Eaton, L.H.; Dearman, R.J.; Kimber, I.; Griffiths, C.E.M. Lower levels of interleukin-1β gene expression are associated with impaired Langerhans’ cell migration in aged human skin. Immunology 2018, 153, 60–70. [Google Scholar] [CrossRef] [PubMed]




| Name of Chemical | Company |
|---|---|
| Acetonitryl (HLPLC grade) | Avantor Performance (Gliwice, Poland) |
| Castor oil (pharmaceutical grade) | Coel (Kraków, Poland) |
| Delta-aminolevulinic acid hydrochloride (purity ≥ 99%, serial number S005/syntal/19022020) | Syntal Chemicals Sp. Z o.o. (Gliwice, Poland) |
| Delta-aminolevulinic acid hydrochloride (internal standard) | Sigma Aldrich (Steinheim, Germany) |
| Disodium dihydrogen ethylenediaminetetraacetate | ChemPur (Piekary Śląskie, Poland) |
| Fluorescamine | Sigma Aldrich (Steinheim, Germany) |
| Phosphate buffer saline (PBS) | BTL (Łódź, Poland) |
| Propylene glycol | Avantor Performance (Gliwice, Poland) |
| Sodium benzoate | ChemPur (Piekary Śląskie, Poland) |
| Sodium chloride | Polpharma (Starogard Gdański, Poland) |
| Sodium dodecyl sulfate (SDS) | Avantor Performance (Gliwice, Poland) |
| Soybean phosphatidylcholine (Phospholipon 90) | Lipoid (Kőln, Germany) |
| Trichloroacetic acid (HPLC grade) | Avantor Performance (Gliwice, Poland) |
| Compound | Concentration (%, w/w) | |||||
|---|---|---|---|---|---|---|
| B1 | B2 | B3 | F1 | F2 | F3 | |
| 5-aminolevulinic acid | - | - | - | 5.0 | 5.0 | 5.0 |
| Tragacanth gum | 5.0 | 5.0 | - | 5.0 | 5.0 | - |
| Xanthan gum | 1.0 | - | 2.0 | 1.0 | - | 2.0 |
| Gellan gum | - | - | 0.7 | - | - | 0.7 |
| Lecithin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Castor oil | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Disodium dihydrogen ethylenediaminetetraacetate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium benzoate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Propylene glycol | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Purified water | up to 100.0 | up to 100.0 | up to 100.0 | up to 100.0 | up to 100.0 | up to 100.0 |
| Emulgel F1 | Emulgel F2 | Emulgel F3 | |
|---|---|---|---|
| After preparation | |||
| Drug content (%) | 94.2 ± 2.2 | 95.3 ± 1.8 | 91.6 ± 1.5 |
| pH | 3.75 ± 0.02 | 3.73 ± 0.03 | 3.91 ± 0.01 |
| Upon 3-month storage at 4 °C | |||
| Drug content (%) | 92.4 ± 1.3 | 94.2 ± 3.2 | 92.0 ± 1.7 |
| pH | 3.78 ± 0.04 | 3.77 ± 0.02 | 3.86 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, E.; Potaś, J.; Baranowski, M.; Czarnomysy, R.; Sulewska, M.E.; Basa, A.; Pietruska, M.; Bielawski, K.; Winnicka, K. Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions. Pharmaceutics 2023, 15, 2512. https://doi.org/10.3390/pharmaceutics15102512
Szymańska E, Potaś J, Baranowski M, Czarnomysy R, Sulewska ME, Basa A, Pietruska M, Bielawski K, Winnicka K. Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions. Pharmaceutics. 2023; 15(10):2512. https://doi.org/10.3390/pharmaceutics15102512
Chicago/Turabian StyleSzymańska, Emilia, Joanna Potaś, Marcin Baranowski, Robert Czarnomysy, Magdalena Ewa Sulewska, Anna Basa, Małgorzata Pietruska, Krzysztof Bielawski, and Katarzyna Winnicka. 2023. "Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions" Pharmaceutics 15, no. 10: 2512. https://doi.org/10.3390/pharmaceutics15102512
APA StyleSzymańska, E., Potaś, J., Baranowski, M., Czarnomysy, R., Sulewska, M. E., Basa, A., Pietruska, M., Bielawski, K., & Winnicka, K. (2023). Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions. Pharmaceutics, 15(10), 2512. https://doi.org/10.3390/pharmaceutics15102512







