Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Selection of Excipients
2.2.2. Critical Quality Attributes (CQAs) and Optimization
2.2.3. Preparation of Nanoemulsions
2.2.4. Quantification of Etodolac
2.2.5. Characterization Studies
Determination of Droplet Size, Polydispersity Index and Zeta Potential
Morphological Analysis
Measurement of Encapsulation Efficiency (EE) and Loading Capacity (LC)
Fourier Transform Infrared (FTIR) Analysis
Measurement of Viscosity and pH
Stability Study
In Vitro Drug Release Study
Ex Vivo Permeation Study
2.2.6. Preparation of Etodolac Nanoemulsion-Based Gel
2.2.7. In Vivo Study
Animals
Induction of Inflammation
In Vivo Experimental Group Design
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Selection of Excipients
3.2. Optimization of Nanoemulsions
3.3. Quantification of Etodolac and Analytical Method Validation
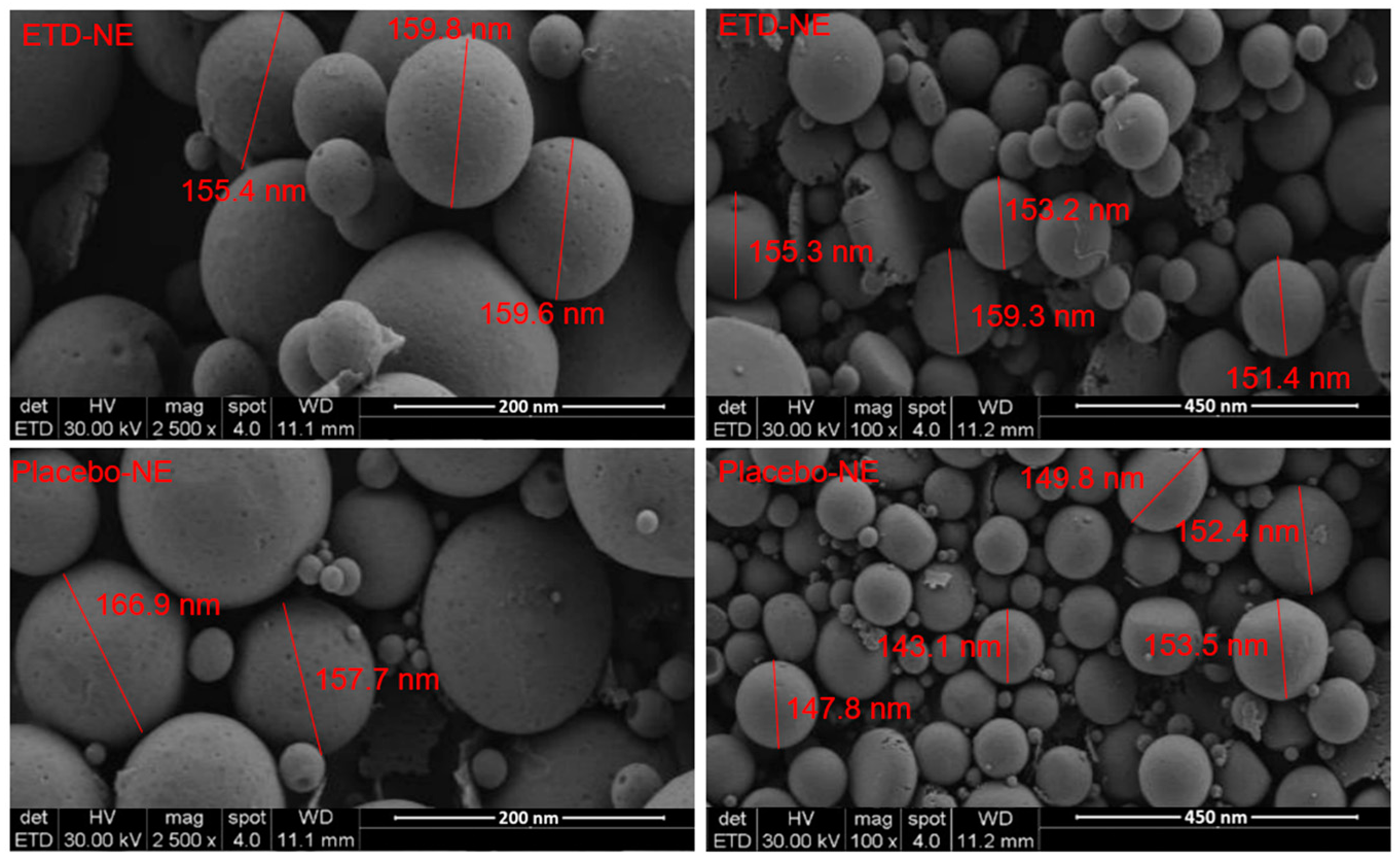
3.4. Morphological Evaluation
3.5. FTIR Analysis
3.6. In Vitro and Ex Vivo Release Studies
3.7. Stability Studies
3.8. In Vivo Studies
3.9. Comparison of Current Work to Previous Reports
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation Enhancers in Transdermal Drug Delivery: Benefits and Limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as Pharmaceutical Carrier for Dermal and Transdermal Drug Delivery: Formulation Development, Stability Issues, Basic Considerations and Applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Jones, R.A. Etodolac: An Overview of a Selective COX-2 Inhibitor. Inflammopharmacology 1999, 7, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, N.; Sudhakar, B.; Reddy, K.V.N.S.; Ratna, J.V. Design by Optimization and Comparative Evaluation of Vesicular Gels of Etodolac for Transdermal Delivery. Drug Dev. Ind. Pharm. 2019, 45, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Karakucuk, A.; Tort, S.; Han, S.; Oktay, A.N.; Celebi, N. Etodolac Nanosuspension Based Gel for Enhanced Dermal Delivery: In Vitro and in Vivo Evaluation. J. Microencapsul. 2021, 38, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Kesharwani, R.; Kumar, V. Etodolac Loaded Solid Lipid Nanoparticle Based Topical Gel for Enhanced Skin Delivery. Biocatal. Agric. Biotechnol. 2020, 29, 101810. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szymańska, E.; Czarnomysy, R.; Jacyna, J.; Markuszewski, M.; Basa, A.; Winnicka, K. Nanostructured Lipid Carriers Engineered as Topical Delivery of Etodolac: Optimization and Cytotoxicity Studies. Materials 2021, 14, 596. [Google Scholar] [CrossRef] [PubMed]
- Shilakari Asthana, G.; Asthana, A.; Singh, D.; Sharma, P.K. Etodolac Containing Topical Niosomal Gel: Formulation Development and Evaluation. J. Drug Deliv. 2016, 2016. [Google Scholar] [CrossRef]
- Özdemir, S.; Celik, B.; Sümer, E.; Acar, E.T.; Üner, M. Eplerenone Nanoemulsions for Treatment of Hypertension. Part II: Physical Stability Assessment and in Vivo Study. J. Drug Deliv. Sci. Technol. 2018, 45, 287–295. [Google Scholar] [CrossRef]
- Yu, L.X. Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Design, Characterization and in Vivo Evaluation of Nanostructured Lipid Carriers (NLC) as a New Drug Delivery System for Hydrochlorothiazide Oral Administration in Pediatric Therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, S.; Çelik, B.; Türköz Acar, E.; Duman, G.; Üner, M. Eplerenone Nanoemulsions for Treatment of Hypertension. Part I: Experimental Design for Optimization of Formulations and Physical Characterization. J. Drug Deliv. Sci. Technol. 2018, 45, 357–366. [Google Scholar] [CrossRef]
- Uner, B.; Ozdemir, S.; Tas, C.; Uner, M.; Ozsoy, Y. Loteprednol-Loaded Nanoformulations for Corneal Delivery by Quality-by-Design Concepts: Optimization, Characterization, and Anti-Inflammatory Activity. AAPS PharmSciTech 2023, 24, 92. [Google Scholar] [CrossRef]
- Herneisey, M.; Liu, L.; Lambert, E.; Schmitz, N.; Loftus, S.; Janjic, J.M. Development of Theranostic Perfluorocarbon Nanoemulsions as a Model Non-Opioid Pain Nanomedicine Using a Quality by Design (QbD) Approach. AAPS PharmSciTech 2019, 20, 65. [Google Scholar] [CrossRef]
- Tas, C.; Ozkan, Y.; Okyar, A.; Savaser, A. In Vitro and Ex Vivo Permeation Studies of Etodolac from Hydrophilic Gels and Effect of Terpenes as Enhancers. Drug Deliv. 2007, 14, 453–459. [Google Scholar] [CrossRef]
- Borman, P.; Elder, D. Q2(R1) Validation of Analytical Procedures. In ICH Quality Guidelines; Wiley: Hoboken, NJ, USA, 2017; pp. 127–166. ISBN 9781118971147. [Google Scholar]
- Üner, B.; Özdemir, S.; Taş, Ç.; Özsoy, Y.; Üner, M. Development of Lipid Nanoparticles for Transdermal Loteprednol Etabonate Delivery. J. Microencapsul. 2022, 39, 327–340. [Google Scholar] [CrossRef]
- Özdemir, S.; Üner, B.; Karaküçük, A. Preparation and Characterization Studies of Etodolac Suppositories: Investigation on Oleaginous Blends of Witepsol® H15. J. Res. Pharm. 2023, 27, 837–847. [Google Scholar] [CrossRef]
- Kahraman, E.; Neşetoğlu, N.; Güngör, S.; Ünal, D.Ş.; Özsoy, Y. The Combination of Nanomicelles with Terpenes for Enhancement of Skin Drug Delivery. Int. J. Pharm. 2018, 551, 133–140. [Google Scholar] [CrossRef]
- Marchianti, A.C.N.; Sakinah, E.N.; Elfiah, U.; Putri, N.K.S.; Wahyuliswari, D.I.; Maulana, M.; Ulfa, E.U. Gel Formulations of Merremia Mammosa (Lour.) Accelerated Wound Healing of the Wound in Diabetic Rats. J. Tradit. Complement. Med. 2021, 11, 38–45. [Google Scholar] [CrossRef]
- Simmons, P.A.; Carlisle-Wilcox, C.; Chen, R.; Liu, H.; Vehige, J.G. Efficacy, Safety, and Acceptability of a Lipid-Based Artificial Tear Formulation: A Randomized, Controlled, Multicenter Clinical Trial. Clin. Ther. 2015, 37, 858–868. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for Planning Animal Research and Testing. Lab. Anim. 2017, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Uluata, S.; Decker, E.A.; McClements, D.J. Optimization of Nanoemulsion Fabrication Using Microfluidization: Role of Surfactant Concentration on Formation and Stability. Food Biophys. 2016, 11, 52–59. [Google Scholar] [CrossRef]
- Barakat, N.S. In Vitro and in Vivo Characteristics of a Thermogelling Rectal Delivery System of Etodolac. AAPS PharmSciTech 2009, 10, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for Dermal Controlled Release of Oleanolic and Ursolic Acids: In Vitro, Ex Vivo and in Vivo Characterization. Colloids Surfaces B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef]
- Amul, B.; Muthu, S.; Raja, M.; Sevvanthi, S. Spectral, DFT and Molecular Docking Investigations on Etodolac. J. Mol. Struct. 2019, 1195, 747–761. [Google Scholar] [CrossRef]
- Sherje, A.P.; Kulkarni, V.; Murahari, M.; Nayak, U.Y.; Bhat, P.; Suvarna, V.; Dravyakar, B. Inclusion Complexation of Etodolac with Hydroxypropyl-Beta-Cyclodextrin and Auxiliary Agents: Formulation Characterization and Molecular Modeling Studies. Mol. Pharm. 2017, 14, 1231–1242. [Google Scholar] [CrossRef]
- Karakucuk, A.; Celebi, N. Investigation of Formulation and Process Parameters of Wet Media Milling to Develop Etodolac Nanosuspensions. Pharm. Res. 2020, 37, 111. [Google Scholar] [CrossRef]
- Ali, F.R.; Shoaib, M.H.; Ali, S.A.; Yousuf, R.I.; Siddiqui, F.; Raja, R.; Jamal, H.S.; Saleem, M.T.; Ahmed, K.; Imtiaz, M.S.; et al. A Nanoemulsion Based Transdermal Delivery of Insulin: Formulation Development, Optimization, in-Vitro Permeation across Strat-M® Membrane and Its Pharmacokinetic/Pharmacodynamic Evaluation. J. Drug Deliv. Sci. Technol. 2022, 71, 103338. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac Transdermal Cubosomes for the Treatment of Rheumatoid Arthritis: Ex Vivo Permeation and in Vivo Pharmacokinetic Studies. Drug Deliv. 2017, 24, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of Skin Integrity Tests for Dermal Absorption Studies in Vitro. Toxicol. Vitr. 2015, 29, 113–123. [Google Scholar] [CrossRef]
- Schoenfelder, H.; Liu, Y.; Lunter, D.J. Systematic Investigation of Factors, Such as the Impact of Emulsifiers, Which Influence the Measurement of Skin Barrier Integrity by in-Vitro Trans-Epidermal Water Loss (TEWL). Int. J. Pharm. 2023, 638, 122930. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Schwarz, J.C.; Haberfeld, S.; Xiao, P.; Wirth, M.; Valenta, C. Skin Integrity Testing and Monitoring of in Vitro Tape Stripping by Capacitance-Based Sensor Imaging. Ski. Res. Technol. 2013, 19, e259–e272. [Google Scholar] [CrossRef] [PubMed]
- Uner, B.; Ozdemir, S.; Yildirim, E.; Yaba, A.; Tas, C.; Uner, M.; Ozsoy, Y. Loteprednol Loaded Nanoformulations for Corneal Delivery: Ex-Vivo Permeation Study, Ocular Safety Assessment and Stability Studies. J. Drug Deliv. Sci. Technol. 2023, 81, 104252. [Google Scholar] [CrossRef]
- Çelik, B.; Sağıroğlu, A.A.; Özdemir, S. Design, Optimization and Characterization of Coenzyme Q10- and D-Panthenyl Triacetate-Loaded Liposomes. Int. J. Nanomed. 2017, 12, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J. Carrageenan-Induced Paw Edema in the Rat and Mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar] [CrossRef]
- Salunkhe, S.S.; Bhatia, N.M.; Thorat, J.D.; Choudhari, P.B.; Bhatia, M.S. Formulation, Development and Evaluation of Ibuprofen Loaded Nanoemulsion Prepared by Nanoprecipitation Technique: Use of Factorial Design Approach as a Tool of Optimization Methodology. J. Pharm. Investig. 2014, 44, 273–290. [Google Scholar] [CrossRef]
- Goindi, S.; Kaur, R.; Kaur, R. An Ionic Liquid-in-Water Microemulsion as a Potential Carrier for Topical Delivery of Poorly Water Soluble Drug: Development, Ex-Vivo and in-Vivo Evaluation. Int. J. Pharm. 2015, 495, 913–923. [Google Scholar] [CrossRef]





| Phase 1—Assessment of all generated formulations | |
| DS (nm) | 120–180 nm |
| PDI | ≤0.2 |
| ZP (mV) | ≥|−30| mV |
| EE (%) | ≥90% |
| Phase 2—Assessment of selected formulation (90 day stability study) | |
| DS (nm) | Droplet size change 20 nm (<10%) |
| PDI | ≤0.2 (<10%) |
| ZP (mV) | ≥|−30| mV (<10%) |
| EE (%) | ≥90% (<10%) |
| pH | 6.5–7.5 (<10%) |
| Viscosity (cP) | 15.5–17.5 cP (<10%) |
| Formulation | Oil (%; X1) | Surfactant (%; X2) | ZP (mV; Y1) | PDI (Y2) | DS (nm; Y3) |
|---|---|---|---|---|---|
| F1 | 8 | 1.5 | −42.4 ± 2.4 | 0.233 ± 0.03 | 198.6 ± 2.6 |
| F2 | 6.6 | 3.3 | −39.4 ± 0.9 | 0.351 ± 0.04 | 249.8 ± 3.3 |
| F3 | 8 | 3.1 | −33.7 ± 0.6 | 0.150 ± 0.04 | 164.6 ± 1.9 |
| F4 | 5 | 2.8 | −34.4 ± 1.3 | 0.184 ± 0.01 | 158.9 ± 0.9 |
| F5 | 8 | 5 | −36.0 ± 1.6 | 0.176 ± 0.03 | 159.2 ± 1.2 |
| F6 | 5.6 | 3.2 | −36.7 ± 2.1 | 0.355 ± 0.04 | 224.7 ± 2.4 |
| F7 | 5.3 | 5 | −38.1 ± 0.7 | 0.407 ± 0.03 | 240.3 ± 3.1 |
| F8 | 6.8 | 4.7 | −39.2 ± 1.5 | 0.178 ± 0.02 | 171.6 ± 2.7 |
| F9 | 5 | 1 | −33.4 ± 1.8 | 0.239 ± 0.04 | 196.8 ± 1.3 |
| F10 | 6.5 | 1 | −34.7 ± 0.5 | 0.193 ± 0.01 | 188.7 ± 1.4 |
| F11 | 5 | 4 | −31.6 ± 2.7 | 0.182 ± 0.04 | 176.8 ± 1.5 |
| F12 | 6.6 | 4.2 | −31.8 ± 2.5 | 0.190 ± 0.02 | 173.8 ± 1.5 |
| F13 | 6.5 | 1 | −34.9 ± 1.1 | 0.249 ± 0.01 | 221.5 ± 2.9 |
| Formulation | ETD (%) | DS (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|---|
| F1 | 3.2 | 175.5 ± 1.4 | 0.176 ± 0.03 | −40.1 ± 2.4 | 73.01 ± 1.92 |
| F2 | 1 | 163.5 ± 2.2 | 0.141 ± 0.02 | −33.1 ± 1.7 | 92.30 ± 1.05 |
| F3 | 2 | 179.5 ± 0.7 | 0.204 ± 0.04 | −39.4 ± 1.7 | 91.02 ± 0.95 |
| F4 | 1.8 | 184.7 ± 0.9 | 0.218 ± 0.03 | −29.6 ± 1.6 | 92.00 ± 1.33 |
| F5 | 4 | 230.0 ± 1.8 | 0.315 ± 0.04 | −37.5 ± 0.9 | 73.52 ± 3.47 |
| F6 | 3 | 215.6 ± 2.6 | 0.291 ± 0.03 | −38.7 ± 1.5 | 80.19 ± 2.58 |
| F7 | 4.1 | 239.3 ± 3.5 | 0.167 ± 0.01 | −37.2 ± 0.7 | 73.51 ± 1.46 |
| F8 | 5 | 203.8 ± 2.7 | 0.264 ± 0.01 | −37.4 ± 2.2 | 72.46 ± 0.76 |
| F9 | 2.6 | 235.1 ± 2.8 | 0.184 ± 0.02 | −36.9 ± 1.6 | 88.70 ± 2.33 |
| Formulation | Zero Order (R2) | First Order (R2) | Higuchi (R2) | Hixson Crowell (R2) | Korsmeyer Peppas (R2/n) |
|---|---|---|---|---|---|
| Control group | 0.9975 | 0.8519 | 0.9666 | 0.7995 | 0.9554/0.201 |
| ETD-NE-CMC | 0.9340 | 0.9707 | 0.9865 | 0.9600 | 0.9914/0.769 |
| Formulation | Flux (Jss) (µg/cm2 h) | Permeability Coefficient (Kp) (cm/h) | Permeability Coefficient (LogKp) | Permeated Amount at 8 h (µg × cm2) |
|---|---|---|---|---|
| Dialysis membrane | ||||
| Control group | 783.4 ± 56.2 | 0.041 ± 0.009 | −1.03 ± 0.06 | 8793.6 ± 630.0 |
| ETD-NE-CMC | 1068.4 ± 71.5 | 0.069 ± 0.005 | −1.99 ± 0.03 | 14,336.9 ± 613.1 |
| Porcine skin | ||||
| Control group | 59.7± 15.2 | 0.004 ± 0.001 | −0.72 ± 0.09 | 45.4 ± 1449.3 |
| ETD-NE-CMC | 165.7 ± 11.7 | 0.011 ± 0.001 | −2.55 ± 0.17 | 357.2 ± 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, S.; Üner, B.; Karaküçük, A.; Çelik, B.; Sümer, E.; Taş, Ç. Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization. Pharmaceutics 2023, 15, 2510. https://doi.org/10.3390/pharmaceutics15102510
Özdemir S, Üner B, Karaküçük A, Çelik B, Sümer E, Taş Ç. Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization. Pharmaceutics. 2023; 15(10):2510. https://doi.org/10.3390/pharmaceutics15102510
Chicago/Turabian StyleÖzdemir, Samet, Burcu Üner, Alptuğ Karaküçük, Burak Çelik, Engin Sümer, and Çetin Taş. 2023. "Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization" Pharmaceutics 15, no. 10: 2510. https://doi.org/10.3390/pharmaceutics15102510
APA StyleÖzdemir, S., Üner, B., Karaküçük, A., Çelik, B., Sümer, E., & Taş, Ç. (2023). Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization. Pharmaceutics, 15(10), 2510. https://doi.org/10.3390/pharmaceutics15102510









